Abstract
Atrial fibrillation (AF) is the most common arrhythmia and its prevalence is increasing due to the progressive aging of the population. About 20% of strokes are attributable to AF and AF patients are at five-fold increased risk of stroke. The mainstay of treatment of AF is the prevention of thromboembolic complications with oral anticoagulation therapy. Drug treatment for many years has been based on the use of vitamin K antagonists, but recently newer and safer molecules have been introduced (dabigatran etexilate, rivaroxaban, apixaban, and edoxaban). Despite these advances, many patients still do not receive adequate anticoagulation therapy because of contraindications (relative and absolute) to this treatment. Over the last decade, percutaneous closure of left atrial appendage, main site of thrombus formation during AF, proved effective in reducing thromboembolic complications, thus offering a valid medical treatment especially in patients at increased bleeding risk. The aim of this consensus document is to review the main aspects of left atrial appendage occlusion (selection and multidisciplinary assessment of patients, currently available methods and devices, requirements for centres and operators, associated therapies and follow-up modalities) having as a ground the significant evolution of techniques and the available relevant clinical data.
Keywords: Atrial fibrillation, Left atrial appendage occlusion
Revised by Piero Maglia, Gennaro Santoro
Consensus Document Approval Faculty in appendix
Table of contents
Introduction
Stroke and thromboembolic risk layering: risk of haemorrhage associated to oral anticoagulant treatment
Current antithrombotic treatment recommendations
Specific clinical situations associated to high risk of haemorrhage
Anatomy of the left atrial appendage
-
Surgical closure (occlusion) of the left atrial appendage
-
Results
Echocardiogram assessment
Indications for surgical occlusion of the left atrial appendage
Requirements for staff and Centres
-
-
Percutaneous occlusion of the left atrial appendage
Left atrial appendage occlusion devices currently available
Devices currently under development
Cost-effectiveness studies
Indications for left atrial appendage occlusion
Screening of patients who are possible candidates for left atrial appendage occlusion
Echocardiogram assessment and echocardiogram anatomy of the left atrial appendage
-
‘Step by step’ procedure
Choice of anaesthesia
Choice of vascular access
Transseptal puncture
Intraprocedural antithrombotic therapy
Insertion of the deployment system into the left atrium
Choice of device
Preparation of the system and device
Implantation of the device
Deployment of the device
Management of antithrombotic therapy after the procedure
-
Recommended organisational and operating standards
General requirements
Staff training and preparation
Requirements for the centre and operating room
Procedural recommendations and minimum skill levels
Requirements for the facility and staff
-
Management of complications
Pericardial effusion and cardiac tamponade
Gaseous embolism
Embolization of the device
Conclusions
Acknowledgments
Annex
References
Introduction
Non-valvular atrial fibrillation (AF) is the most common form of cardiac arrhythmia and its incidence and prevalence are constantly increasing1 due to the ageing of the population and the higher survival rates of patients with conditions such as ischaemic cardiopathy, valve defects, congenital heart defects, and cardiac insufficiency. The estimated prevalence in the general population is about 2% and increases with age.1 The lifetime risk of onset of AF is 25% in men and women with age > 40 with heart defects, while for those without heart defects the risk is about 15%.2,3 A diagnosis of AF features in about 3–6% of hospitalizations for acute heart conditions,3 especially coronary heart diseases and congestive cardiac insufficiency. In the general population, arterial hypertension is the most common condition associated with AF.3,4 Moreover, AF is a common post-operative complication, especially after cardiothoracic surgery.3,4 However, there are also isolated forms of AF, diagnosed mainly by exclusion once concomitant cardiac diseases have been ruled out.
Most patients are asymptomatic and AF is an occasional occurrence. In some patients, it may occur with minor symptoms (palpitations) while in others the first onset may be a complication of AF such as ischaemic stroke. The presence of AF is an independent risk factor for increased mortality and morbidity, and is a cause of stroke and/or thromboembolism, congestive cardiac insufficiency, and seriously reduced quality of life, with a consequent increase in health-care costs.3,4 The risk of stroke in patients with non-valvular AF is ∼5% per year and at least 15–20% of all ischaemic strokes are associated with AF.5 AF is also an independent risk factor for the recurrence of stroke6 and is associated with higher mortality and morbidity than stroke not correlated to AF, underlining the need for more effective stroke prevention in these patients.5,6
AF, subdivided into ‘valvular’ (patients with mechanical valve prostheses or mitral stenosis) and ‘non valvular’, is classified in four different categories depending on the mode of presentation and duration of the arrhythmia:
paroxystic AF (ends spontaneously or after therapy within 7 days after onset),
persistent AF (lasts more than 7 days),
persistent or long-standing AF (continues for more than 1 year), and
permanent AF (continual, and the patient and physician have decided together against attempts at cardioversion and maintenance of the sinus rhythm).
The risk of stroke is basically the same for all four types of AF.4
Stroke and thromboembolic risk layering: risk of haemorrhage associated to oral anticoagulant treatment
The presence of AF is an independent risk factor for ischaemic stroke and thromboembolic events, significantly increases mortality and morbidity and can cause serious disabilities, as well as longer hospitalization.
The treatment of choice for the prevention of ischaemic stroke is oral anticoagulant therapy (OAT) with warfarin or, in the case of non-valvular AF, with the new oral anticoagulants (NOACs). In the case of non-valvular AF, warfarin has been shown to reduce the risk of stroke by about 62%, but it increases the risk of haemorrhage in percentages which vary from 1.2% to 3.4% per year.7 NOACs have proved equally effective with a lower risk of cerebral haemorrhage.4
In about 90% of non-valvular AF patients, the thrombi form in the left atrial appendage (LAA);8 however, it is fundamental to bear in mind that, even in AF patients, ischaemic stroke can be correlated to other factors, such as hypercoagulability or atherosclerosis of the ascending aorta and carotids.
Basically, the management of AF can be summed up in two main steps:
identification and treatment of predisposing factors and concomitant pathologies and
assessment of the thromboembolic risk and haemorrhagic risk for choice of the best therapeutic strategy.
In AF patients, risk layering is crucial. In fact, in spite of the plentiful, universally accepted evidence of the benefits of OAT in reducing the risk of ischaemic stroke, many studies have revealed that this therapy is underused, especially in high-risk patients.8,9 A large number of stroke risk layering methods have been produced on the basis of risk factors identified in the control arms of clinical studies on warfarin, cohort studies and consent documents. These methods vary in complexity and the number of risk factors assessed, but they have almost all divided AF patients, by convention, into low, moderate and high risk.4,10
CHA2DS2-VASc10 is the thromboembolic risk assessment score recommended by the European Society of Cardiology (ESC), the American Heart Association (AHA) and the American College of Cardiology (ACC), which considers gender, vascular diseases, cardiac insufficiency, hypertension, age (divided into two classes), diabetes and ischaemic stroke as risk factors.
The HAS-BLED score is the most widely used for the layering of haemorrhage risk.11 HAS-BLED has been compared with the HEMORR2HAGES method and with ATRIA and, although, like the others, it has shown only a moderate ability to predict the risk of bleeding, it has proved the most effective in significantly predicting the risk of intracranial haemorrhage, and is also the simplest.12 A HAS-BLED score ≥ 3 identifies patients with high risk of haemorrhage but is not a criterion for exclusion from OAT.
Tables 1 and 2 summarize the risk factors assessed in CHA2DS2-VASc and HAS-BLED with the relative scores.
Table 1.
CHA2DS2-VASc score and incidence of stroke
| Risk factors for stroke and thromboembolism in non-valvular AF | |
|---|---|
| ‘Major’ risk factors | ‘Significant’ risk factors |
| Previous stroke, TIA or systemic embolism | Cardiac insufficiency or moderate or severe systolic dysfunction (EF < 40%) |
| Age > 75 years | Arterial hypertension, diabetes mellitus, female gender, age 65–74 years, peripheral vascular disease |
| Risk factors included in the CHA2DS2-VASc score | |
| Risk factors | Score |
| Cardiac insufficiency/ systolic dysfunction | 1 |
| Arterial hypertension | 1 |
| Age 65–74 years. | 1 |
| Age > 75 years | 2 |
| Diabetes mellitus | 1 |
| Stroke/TIA/thromboembolism | 2 |
| Peripheral vascular disease | 1 |
| Female gender | 1 |
EF, ejection fraction; TIA, transient ischaemic attack.
Table 2.
HAS-BLED score
| Letter | Clinical characteristics | Score |
|---|---|---|
| H | Hypertension | 1 |
| A | Impaired kidney or liver function (1 point each) | 1 or 2 |
| S | Stroke | 1 |
| B | Bleeding | 1 |
| L | Labile INR | 1 |
| E | Old age (>65 years) | 1 |
| D | Drugs or alcohol (1 point each) | 1 or 2 |
INR, international normalized ratio.
The final decision on the type of antithrombotic treatment in AF patients must therefore balance the degree of reduction of the risk of thromboembolism against the minimization of serious haemorrhagic complications (in other words intracranial haemorrhage) associated with these therapies.
Current antithrombotic treatment recommendations
An analysis of the net clinical benefit (balancing the reduction of ischaemic and haemorrhagic events) of vitamin K antagonists was conducted on a large population of AF patients in the real world (n = 130 000) using the CHA2DS2-VASc and HAS-BLED scores. This study revealed a net benefit for anticoagulant therapy in patients with CHA2DS2-VASc ≥2. The effect was neutral in patients with CHA2DS2-VASc =1, while there was no net benefit in patients with CHA2DS2-VASc =0. Moreover, this study further confirmed the absence of benefits from aspirin alone in any risk layer.13,14
The most recent guidelines (2014) on the treatment of AF are those of the AHA/ACC,15 which suggest, in AF patients with CHA2DS2-VASc ≥2, the use of warfarin with class I and level of evidence A and of NOACs with class I and level of evidence B, while the 2012 ESC guidelines16 recommend class I with level of evidence A for both warfarin and NOACs in the same patients. In patients with CHA2DS2-VASc =1, the US guidelines basically advise against antithrombotic therapy (IIb, C), while the European ones are more in favour (IIa, A). All guidelines advise against any anticoagulant therapy in patients with CHA2DS2-VASc =0.
Warfarin or NOACs are definitely the most widely used therapy with the highest level of evidence in the prevention of AF-related thromboembolism. NOACs, in particular, have been shown to be as effective as warfarin [in patients with international normalized ratio (INR) on target], and are associated with a lower risk of haemorrhagic events.17
Although OAT with warfarin has been shown to significantly reduce the incidence of AF-related stroke, a high percentage of patients with AF (∼40%) do not receive appropriate prophylactic therapy.18,19 Moreover, about 20% of patients have contraindications for OAT and a further 30–40% of patients receiving OAT are unable to maintain a TTR of ≥65%, meaning that the risk of inefficacy of the therapy is high. Furthermore, a considerable proportion of patients suspend anticoagulant treatment within 1 year.20 In addition, although NOACs are more manageable and apparently safer than warfarin, they are still associated with a risk of haemorrhagic events (1.6–3.6% haemorrhagic events/year). Moreover, in the trials which tested these new drugs, between 20% and 35% of patients were forced to suspend administration due to the onset of side effects, and a high risk of bleeding was one of the exclusion criteria in all trials.
Specific clinical situations associated to high risk of haemorrhage
The decision with regards to any antithrombotic treatment must necessarily involve an assessment of the risk of stroke as against the risk of major bleeding, especially cerebral haemorrhage, which is the most dangerous complication, associated with a high rate of mortality and disability.21 However, the risk of cerebral haemorrhage in AF patients receiving OAT with vitamin K antagonists is currently quite low, between 0.1% and 0.6%. The percentage of intracranial haemorrhages increases for INR values around 3.5–4.0, while there is no difference in haemorrhage risk in patients with INR between 2.0 and 3.0 compared to those with lower INR.21
In the general population of AF patients, there are some categories with particularly high risk of haemorrhage, specifically:
elderly patients: it is known that the prevalence of AF is dramatically higher in patients with age > 75 years and that in these patients the risk of thromboembolism and haemorrhage is often much higher than in younger patients;
patients with a history of major bleeding episodes, especially intracranial;
patients with gastrointestinal diseases (liver diseases, angiodysplasias) or with peptic ulcer, a condition which is a significant problem for the use of NOACs, which are associated with a higher number of gastrointestinal haemorrhages than warfarin16–18;
patients with chronic renal failure, who account for about 3% of AF patients; in 90% of cases, this population of patients with kidney disease has a CHA2DS2-VASc >2 and has a HAS-BLED ≥ 3 in 40% of cases.16–18 Moreover, there is no concrete proof on the efficacy-safety ratio of anticoagulants in patients undergoing dialysis or with glomerular filtration rate <30 mL/min.22 In dialysis patients, the risk of ischaemic stroke is higher than that of the general population, even in the absence of AF,23 as is the risk of haemorrhage.24 Moreover, in haemodialysis patients an association has been suggested between the use of warfarin and an increase in vascular calcification25–27;
patients with coagulation disorders;
patients with acute coronary diseases who have received stent implants and require prolonged dual antiplatelet therapy (DAPT). AF may, for example, occur in 5–10% of patients with acute coronary syndrome and is associated with a significant increase in mortality.20 In view of the fact that the vast majority of patients with acute coronary syndrome undergo coronary angioplasty with stent implant, the population of patients requiring DAPT + anticoagulant therapy (triple therapy) is presumably very large. It is known and also logical that the combination of antiplatelet agents with anticoagulants increases the risk of bleeding in the case of both warfarin and NOACs. In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study, the risk of major bleeding increased by 2.8% to 4.8% per year when antiplatelet agents were added to warfarin and from 2.6% to 4.4%/year with dabigatran 150 mg18; and
patients with mental disabilities or at risk of frequent falls.
All these categories represent a large segment of patients for whom randomized trials fail to provide clear evidence with regard to the best therapeutic strategy.
Anatomy of the left atrial appendage
The LAA is an embryonic remnant of the primordial left atrium, a small offshoot opening off the side wall of the atrium, with variable, elongated, trabecular, often multi-lobed shape. The appendage’s orifice on the atrial cavity is oval in form and is located between the left upper pulmonary vein, from which it is separated by the fibrous ligament of Marshall, and the mitral valve. On the outside the LAA extends antero-laterally parallel with the left atrioventricular sulcus, just above the proximal part of the circumflex artery. On the inside, the distal part is trabecular, with the distinctive uneven surface of the endocardium, while the proximal end has a smooth surface. It varies in length (16–51 mm), volume (0.7–19.2 mL) and orifice diameter (5–40 mm).28
The LAA consists of a main body onto which one or more lobes may be attached and its variable in both shape and size. The study performed by Di Biase et al.,29 using computed tomography and magnetic resonance, gathered information about the morphology of the LAA and also correlated the various forms with risk of ischaemic stroke/transient ischaemic attack (TIA). Four main forms emerged: Cactus (30%), Chicken Wing (48%), Windsock (19%), and Cauliflower (3%). The correlation with thromboembolic events revealed a diverse, significant (P < 0.001) distribution, of 12, 4, 10, and 18%, respectively, in patients with Cactus, Chicken Wing, Windsock, and Cauliflower morphology. The Chicken Wing morphology showed a significantly lower prevalence of events compared with the other morphologies; in particular, patients with Cactus morphology showed a risk of cerebral ischaemic events 4 times higher, those with Windsock morphology 4.5 times higher and those with Cauliflower morphology 8 times higher than patients with Chicken Wing morphology.
Surgical closure (occlusion) of the left atrial appendage
Surgical closure of the LAA has been proposed since the ’40s as prophylaxis for thromboembolism in patients with mitral valve disease.30–32 Even then, the observation that in this condition about 50% of thrombi were located in the LAA pointed to its occlusion as prophylaxis for the risk of stroke.33 During the same period, Belcher and Somerville32 noted that after mitral surgery LAA thrombosis occurred in 64% of patients who experienced embolic events. In the ’80s, further to the findings of a review of the mitral commissurotomy procedure, Halseth et al.34 suggested that surgical occlusion was only indicated in cases of large-sized LAA.
In modern times, LAA amputation has been reintroduced as an integral part of the surgical procedure for AF ablation, initially proposed by Cox35 in the ’90s and still performed with various technologies.
The history of the surgical treatment of the LAA originated in 1949, when Madden31 performed a complete amputation of the LAA on 2 patients with rheumatic mitral disease. An analysis of the literature only reveals 7 studies with detailed assessments (Table 3).36–43 These 7 studies recruited a total of 3653 patients with an LAA occlusion group (LAAO) (1716 patients) compared to a non LAAO group (1937 patients). In three works,37,39,42 the main operation was mitral valve surgery, in one39 it was coronary surgery and in the other three40,41,43 there was a mix of aortocoronary bypass and valve procedures. Four studies used surgical sutures for the LAAO37,38,41,42 while in two the LAA was amputated with a Stapler.38,43
Table 3.
Studies in the literature which assessed surgical treatment of the left atrial appendage
| Author | Period | Type of study | Number of LAA | Number of non-LAA | LAA occlusion method |
|---|---|---|---|---|---|
| Lee et al.39 | 1999–2011 | Propensity-score matched | 119 | 119 | LAA amputation during crio-Maze |
| Whitlock et al.43 | 2009–2010 | Miscellaneous | 26 | 25 | Amputation and stapler |
| Kim et al.40 | 2001–2010 | Propensity-score matched | 631 | 631 | Amputation or closure of LAA |
| Zapolanski et al.41 | 2005–2012 | Observational | 808 | 969 | Double ligature with suture |
| Nagpal et al.37 | 2007 | RCT | 22 | 21 | Amputation, suture |
| Healey et al.38 | 2001–2002 | RCT | 52 | 25 | Suture or stapler |
| Garcia-Fernandez et al.42 | 1996–2001 | Observational | 58 | 147 | Ligature of LAA with endocardial suture |
LAA, left atrial appendage; RCT, randomized controlled trial.
Results
The incidence of stroke was significantly reduced in patients who underwent LAAO. Mortality was significantly lower in the LAAO group. No significant differences were identified between the two groups with regards to periprocedural bleeding requiring reoperation.
The Left Atrial Appendage Occlusion Study (LAAOS)38 is the first randomized trial to assess the feasibility, safety, and efficacy of LAAO during elective coronary surgery using surgical sutures or automatic staplers. The study, conducted in Germany during 2001–2002, considers 52 patients who underwent LAAO vs. 25 non-LAAO patients. Occlusion was found to be effective in 66% of patients and it was even more so when the surgeon performed a complete amputation of the LAA rather than simply suturing it; the studies analysed reveal the uncertainty concerning the efficacy of surgical suturing in LAAO.
Echocardiogram assessment
Imaging plays a fundamental role in both the pre- and the intraprocedural phase, and in the follow-up.44 The LAA can occasionally be viewed by transthoracic echocardiography, but the transoesophageal method (TEE), sometimes with three-dimensional assessment, is always necessary.
TEE plays a fundamental role in the pre-procedural assessment, to confirm that the LAA is free from thrombi; any thrombi must be carefully considered to ensure that the procedure is safe and free from complications. It also allows the acquisition of additional information concerning the size, shape and position of the LAA. At the end of the surgical procedure, TEE is fundamental for defining the positioning of the device and the achievement of LAAO, by checking for any leaks. During follow-up, TEE is the most accurate assessment method for the description of any leaks or thrombosis.
TEE is the gold standard for confirming the failure of LAAO; in this case the patient continues to be exposed to the risk of thromboembolism, with an increased risk of stroke.45,46 In fact, incomplete occlusion of the LAA has been reported in up to over 50% of cases, with thrombi recorded in up to more than 25% of these patients.46 Furthermore, thromboembolic events have been reported in up to 22% of these cases.47
A variety of surgical techniques are currently used in LAAO: surgical removal, surgical suture, and more recently the application of specific devices (Tiger Paw and Atriclip).
Surgical removal of the LAA generally involves tangential clamping as close as possible to the orifice. It is then removed and the orifice is sealed with a double surgical suture. Since the LAA is an extremely fragile structure, this technique may lead to tearing at its orifice, with bleeding and difficulties in achieving haemostasis. This technique is therefore not widely used, although in the literature no increase in morbidity arising from the surgical operation is described.38
Occlusion of the LAA by means of surgical suture is currently the most commonly used technique. Especially during mitral valve surgery, it is a simple, quick procedure with no complications. The surgical suture can be created with a tobacco-pouch shape around the orifice, or by means of two seams at its point of origin. During the last few years, the data in the literature indicate that the use of dedicated devices has been introduced. The devices currently available remain epicardial and therefore are not fitted into the cavity, and they potentially have a low incidence of thrombosis, infections and embolization.43
The Tiger Paw System II (Maquet Cardiovascular, Mahwah, NJ, USA) is currently not available on the market because of a suspicion that it may apply traction to the atrial tissues. The Atriclip LAA Occlusion System device (Atricure, Inc, West Chester, OH, USA) allows easy, safe LAAO. It has been approved by the Food and Drug Administration since 2009 and consists of a convenient handle with the device at one end; the latter comprises a self-clamping clip consisting of two parallel crossbars in titanium with etinol, with a polyester cladding; during the operation the system can be positioned several times prior to deployment, and it comes in different sizes for selection in relation to the dimensions of the orifice of the LAA. It is a simple, safe procedure with minimal complications and allows complete occlusion of the LAA. It is an epicardial and therefore not an intracavitary device, usable for all the various shapes and sizes of LAA, and it allows anatomical and electric LAAO with short procedural times. This device is currently used in a large number of patients who undergo mini-invasive surgery or mini-invasive surgical treatment for AF.
Indications for surgical occlusion of the left atrial appendage
The ESC guidelines maintain that there is no conclusive evidence concerning the efficacy of LAAO or the best techniques for its performance and provide a recommendation of IIB, evidence level C, only for patients who are to undergo cardiac surgery.17 Nor have the recent AHA/ACC guidelines16 on the management of AF arrived at a clear consensus on this procedure, and they confirm this recommendation with the same level of evidence.
The panel agrees with these recommendations and maintains that every candidate must be discussed by the Heart Team. Potential candidates may be:
patients with high thromboembolic risk with permanent, persistent or paroxystic AF undergoing concomitant cardiac surgery, possibly in association with surgical ablation of the AF and
patients with high-thromboembolic risk undergoing mini-invasive surgery (mini-thoracotomy) or a sternotomy surgical procedure for AF.
Requirements for staff and centres
In-depth knowledge of cardiac anatomy, especially of the left atrium, the LAA, and the surrounding structures, is essential for staff performing LAAO. Staff must have in-depth knowledge of the anatomical variables of the LAA in terms of size, angles and mobility. The procedure’s success and safety depend to a large extent on the knowledge and experience of every member of the team, including the echocardiographer.
Specific, properly organized training is necessary for every staff member, including the echocardiographer. The training must include basic principles and study of the characteristics of the device and the procedure. It must comprise a short course on anatomy, clinical procedures and device implantation techniques and the analysis of critical factors such as patient and device selection and the possible complications and their prevention and management, as well as a practical part and the use of simulators on virtual cases. The nurses and technicians on the team must also receive training. During the first few procedures, a surgeon with documented experience in use of the device must be present.44
Percutaneous occlusion of the left atrial appendage
Left atrial appendage occlusion devices currently available
There are currently four endocardial devices with CE marking available for percutaneous occlusion of the LAA: the Watchman (Boston Scientific, Maple Grove, MN, USA), the Amplatzer Cardiac Plug and Amulet (St. Jude Medical, Minneapolis, MN, USA), the WaveCrest (Coherex Medical, Salt Lake City, UT, USA) and the Lariat (SentreHEART, Inc., Palo Alto, CA, USA), which has a combined endocardial/epicardial percutaneous access.
The Watchman is a self-expandable device with a basket-like structure in nitinol clad with a polyethylene membrane covering the exposed surface in the left atrium; it has hooks around its external perimeter which anchor it to the walls of the LAA. The device is available in various sizes (21, 24, 27, 30, and 33 mm) and is deployed with the aid of a delivery catheter of 14 Fr which may have one or two bends at its distal end for easier approach to the LAA. The device’s length is equal to its diameter (Figure 1).
Figure 1.
Watchman Device (Boston Scientific).
As of today, clinical studies conducted with the Watchman medical device have included more than 2400 patients with more than 6000 patient years of follow-up. The most important clinical studies supporting the efficacy and safety of this device are the Watchman Left Atrial System for Embolic Protection in Patients With Atrial Fibrillation (PROTECT AF)48–50 and Prospective Randomized Evaluation of the Watchman LAA Closure Device In Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL)51 randomized studies. In the PROTECT AF randomized study, Holmes et al.48 compared implantation of the Watchman with conventional pharmacological treatment with warfarin in 707 patients with non-valvular AF and CHADS2 score ≥ 1. The device was successfully implanted in 90.9% of the patients who underwent the procedure and the administration of warfarin was stopped in 87% of them 45 days later. The primary endpoint for efficacy was defined as the composite of stroke, cardiovascular or unexplained death, and systemic embolism. The rate of events recorded for effectiveness was 3.0 per 100 patient years in the Watchman group compared to 4.9 per 100 patient years in the warfarin group; this 38% reduction is equivalent to a non-inferiority of the Watchman in relation to warfarin. However, ischaemic stroke was more frequent in the group treated with the Watchman than in the control group due to five pre-procedural events linked to gaseous embolism. Excluding these events, the incidence of ischaemic stroke was not substantially different between the two groups (1.3%/year in the successfully implanted group compared to 1.6%/year in the control group). The primary endpoint for safety, on the other hand, was defined as a composite of major bleeding and complications correlated to the procedure. The rate of these adverse events was 7.4 per 100 patient years in the Watchman group compared to 4.4 per 100 patient years in the warfarin group. The complications which occurred in the patients treated with the Watchman were: cardiac tamponade (5%), major haemorrhages (3.5%), pericardial effusions (1.7%), periprocedural stroke (1%) mainly arising from gaseous embolism, embolization of the device (0.6%), which in most cases (67%) made surgical removal necessary, and the induction of arrhythmias (0.2%). In total, 2.2% of attempts to implant the device led to cardiac surgery to deal with complications correlated to it. Most of these events occurred in the periprocedural period (55% of adverse events relate to the day of implantation) and pericardial effusion was the most common event (7.1% of the first 3 patients at each centre and 4.4% of subsequent patients); none of these events caused permanent disability or death. In fact, it should be emphasized that the adverse events correlated to the procedure decreased as the staff’s experience grew. In particular, pericardial effusions, which accounted for about 50% of adverse events recorded in the PROTECT AF study, decreased significantly in the subsequent CAP registry as the operators’ experience increased (5 vs. 2.2%, P = 0.019), as did periprocedural strokes (0.9 vs. 0%; P = 0.039).48,49
The PREVAIL study enrolled 407 patients with the aim of comparing the Watchman device with warfarin patients with AF at high risk of thromboembolism (average CHADS2 score of 2.6) and eligible for long-term therapy with warfarin. The implantation success rate was 95.1%, a higher value than reported in the previous PROTECT AF randomized study (90.9%). In this study, LAAO with the Watchman proved to be not inferior to warfarin in the prevention of ischaemic stroke or peripheral embolism 1 week after the procedure. Although statistical non-inferiority was not achieved for the total efficacy of the procedure, including periprocedural events, it should be kept in mind that the incidence of these events was low and similar in the two arms of the study.
With regards to adverse events correlated to the procedure (within 7 days), an event was reported in 2.2% of implanted patients. Major complications correlated to the device or the procedure within 7 days of implantation occurred in 4.4% of patients compared to the 8.7% of the PROTECT AF study, meaning a 49% reduction. Pericardial effusion occurred in 1.9% of patients compared to 4.0% in PROTECT AF, and thus with a reduction of 52%. It is important to underline that the results of the PREVAIL study revealed very low-complication rates amongst both clinicians who were performing their first implantations and more skilled clinicians. The data relating to the use of this device in patients with contraindications for OAT are rather limited. Specifically, in the ASAP study52 LAAO was performed in 150 patients: the rate of cerebral ischaemic events observed was 2.3%/year, meaning a risk reduction calculated as 77% in a population of patients with an expected rate of events per year of 7.3%.
A meta-study including 2406 patients recruited to PROTECT AF, PREVAIL, and the respective registries has recently been published.53 Compared to warfarin therapy, percutaneous occlusion of the LAA was associated with a significant reduction of haemorrhagic stroke [0.15 vs. 0.96 events/100 patient years, hazard ratio (HR) 0.22, P = 0.004], cardiovascular or undefined mortality (1.1 vs. 2.3 events/patient year, HR 0.48, P = 0.006) and non-procedural bleeding (6 vs. 11.3 events/100 patient years, HR 0.51, P = 0.006).53
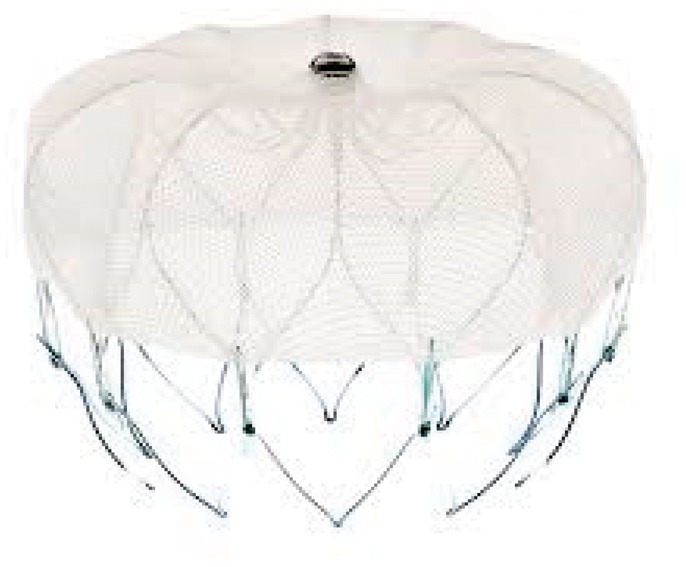
The Amplatzer Cardiac Plug (ACP) is a self-expanding nitinol device, clad completely in polyester, in three parts: a nitinol cylinder, called the lobe, at the tip, used to anchor the device inside the LAA, a larger disc below it which covers and blocks the orifice of the LAA, and a very short wire in the middle connecting the lobe and disc (Figure 2).
Figure 2.

Amplatzer Cardiac Plug (St. Jude Medical).
There are anchor hooks around the edge of the lobe. The lobe sizes available are 16, 18, 20, 22, 24, 26, 28, and 30 mm, while the disc is 4 mm larger for the sizes from 16 to 22 m and 6 mm larger for the 24 to 30 mm sizes. The lobe’s length is much less than its diameter, making it preferable for particularly shallow LAAs. The only data available for the ACP are provided by feasibility reports covering only small patient populations. A retrospective registry study on a population of 137 patients in which an attempt at occluding the LAA with ACP was performed54 show that the procedure was successful in 96% of cases; major intraprocedural complications were observed in 7% of cases (3 ictus, 2 device embolizations, and 5 cardiac tamponades). The percentage rose if minor complications, including four negligible pericardial effusions and transient myocardial ischaemia caused by gaseous embolism, were also considered. In another study conducted on 20 patients, the ACP was successfully implanted in 95% of cases with 2 periprocedural complications (one transient myocardial ischaemia caused by gaseous embolism and one oesophageal lesion due to the TEE). In this study, no adverse events such as stroke or death were observed during a follow-up of 12.7 ± 3.1 months, compared to a theoretical risk of ischaemic events of 5.3%.55
The first Italian registry study, including a population of 134 patients with high-cardioembolic risk (average CHA2DS2-VASc 4.3 ± 1.3) with contraindication to the administration of anticoagulant therapy) was published in 2014. The authors report a high-procedural success rate (95.5%) and a low rate of major complications (2.2%). In the follow-up, a reduction in cerebral ischaemic events of 85.5% compared to the risk predicted by the scores was described.56 Recently, Tzikas et al.57 published the data of a multicentric registry study which describes, on a total of 1047 patients, a procedural success rate of 97.3%, and an incidence of procedural complications of 5%, with a 59% reduction in embolic events and a 60% decrease in haemorrhagic events calculated in relation to the CHA2DS2-VASc and HAS-BLED scores.
Another registry study, performed on the Iberian Peninsula, collected the data of 167 patients with contraindication for OAT and indicated a procedural success rate of 94.6% with an incidence of procedure-related complications of 5.38%. The incidence of embolic events was lower than that predicted by the CHA2DS2-VASc score (2.4 vs. 8.3%), and the incidence of haemorrhagic events was also below the prediction provided by the HAS-BLED score (3.1 vs. 6.6%).58
The latest version of this device is the Amulet, which has the same three-part structure as the ACP but a wider range of sizes (16, 18, 20, 22, 25, 28, 31, and 34 mm), allowing the treatment of a wider spectrum of anatomies, a longer lobe with more anchor hooks, a longer central wire and a screw fitting for the disc, which has been retracted to create a uniform surface (Figure 3).
Figure 3.
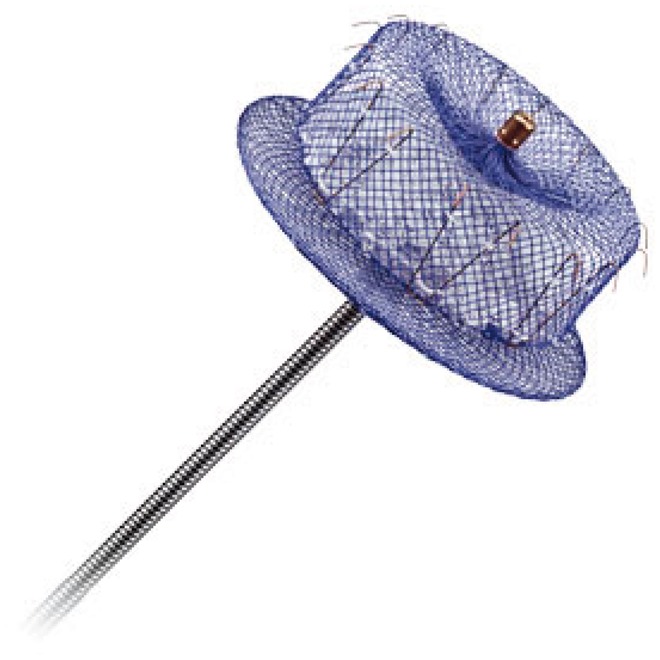
Amulet Device (St. Jude Medical).
The WaveCrest, not currently available on the market, is a self-expanding device in nitinol with a polytetrafluoroethylene coating on the surface which remains exposed in the left atrium; it has anchor hooks around its circumference, which engage with the walls of the left atrium appendage. This device is designed to be positioned close to the orifice of the left atrium appendage, with no need to penetrate deep down with the deployment system (Figure 4).
Figure 4.
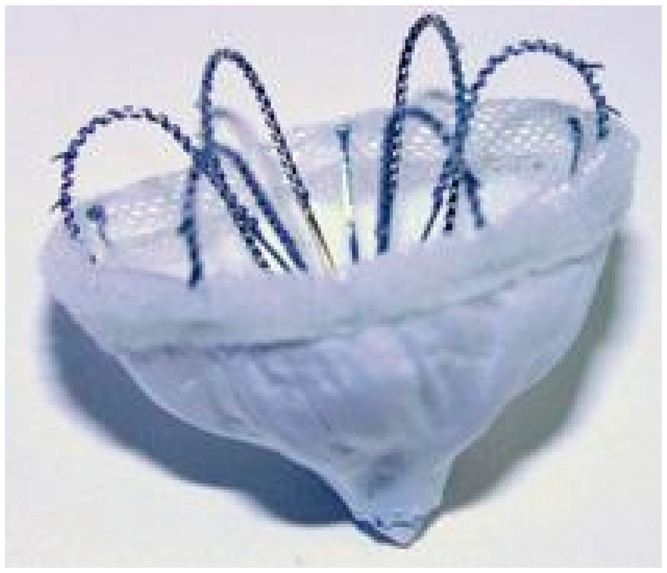
WaveCrest Device (Coherex Medical).
At present, data from feasibility and safety trials59 are available, but there are no major clinical studies.
The Lariat, not currently available in Italy, is an occlusion system with a combined approach consisting of three components: a balloon, a guide wire with magnetic tip and a loop suture wire which will be closed on the orifice of the LAA (Figure 5).
Figure 5.
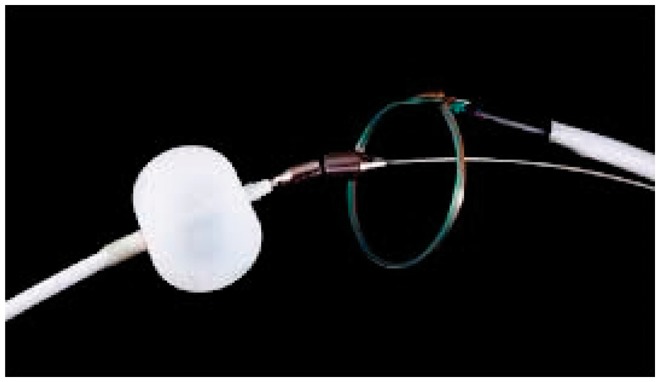
Lariat (SentreHEART) system with its three components.
The system requires the collimation of two magnets at the tips of the first two components (used with percutaneous and epicardial approach), which form a single body that serves as guide for the third component, the lariat, positioned with epicardial approach to occlude the orifice of the LAA. The benefit of this procedure is that once it has been performed nothing prosthetic is left inside the left atrium, and therefore no anticoagulant or antiplatelet therapy is required. However, the only studies performed on this device so far are feasibility studies on small populations of patients.
The results of a study on LAAO on 89 patients using this device revealed that the LAA was completely occluded in 91% of cases with 3 acute complications (2 during pericardial access and 1 during transseptal catheterization). After the procedure 2 cases of pericarditis, 1 delayed pericardial effusion, 2 sudden deaths from undetermined causes, and 2 strokes considered not to be of cardioembolic origin were observed.60
Devices currently under development
The Watchman FLX device, (Boston Scientific) a development on the Watchman device already described, will apparently soon be available. It differs from the previous version in shorter length, the presence of a fluoroscopic marker on the distal tip, the possibility of recapture and redeployment, a larger range of sizes (20, 24, 27, 31, and 35 mm) allowing treatment of a wider range of anatomies, a larger number of perimeter anchors arranged in two rows and retraction of the device's screw connection, to create a flat surface on the atrial side (Figure 6).
Figure 6.

Watchman FLX Device (Boston Scientific).
The Transcatheter Patch (Custom Medical Devices, Athens, Greece) is a device consisting of a polyurethane patch with an adhesive band at the distal end which is carried into the LAA by a balloon; the proximal side of the patch is tightened with a loop, and is then released once the balloon has been deflated and the insertion catheter has been withdrawn (Figure 7).
Figure 7.

Lariat (SentreHEART) system with its three components.
This device is currently undergoing clinical assessment.
Lifetech (LambreTM, Lifetech Scientific Corp., Shenzhen, China) is a self-expanding nitinol device consisting of three parts: a disc and an umbrella, connected by a very short central component. This device is undergoing preclinical assessment.
Cost-effectiveness studies
A number of cost-effectiveness studies have been performed in different social and health-care contexts. An analysis of the German health system, which compared costs and outcomes in terms of mortality of the LAAO procedure compared to warfarin and dabigatran, reveals long-term medical and financial benefits in favour of LAAO. In fact, after 8 years LAAO is less expensive than dabigatran (€15,061 vs. €16,184) and at 10 years it only costs 10% more than warfarin (€16,736 vs. €15,168). The assumption is therefore that LAAO may be financially beneficial for the health system over the long term.61
Another study, based on costs and the years of quality-adjusted life (QALY) gained by percutaneous occlusion of the LAA compared to warfarin and dabigatran therapy, reveals that the cost per QALY of medical therapy is $46 560, while for this device this value is $41 565 over a lifetime period.62
From the point of view of the Italian National Health Service, the comparison between LAAO and treatment with a single antiplatelet drug or no drug in patients with contraindications for OAT is in favour of occlusion of the LAA with a benefit which comes into effect at 3 years, due to the increase in the cost of stroke-related disability in patients treated with antiplatelet medication.63
A comparison between the costs of treatment with the Watchman and with the NOAC drugs by the United Kingdom Department of Health also found in favour of LAAO in patients with high risk of embolism with contraindications for OAT: an investigation of the cost of 10 patients reveals that the cost of the device (€49 300) is recouped within 3 years, considering that the cost per annum of the untreated patients would amount to €14 600 (1 stroke prevented per year for every 10 patients).64
The cost-effectiveness studies therefore reveal the potential benefit of LAAO compared to anticoagulant therapy in the long term. This benefit apparently also occurs in the short-medium term for populations with high risk of embolism and haemorrhage.
Indications for left atrial appendage occlusion
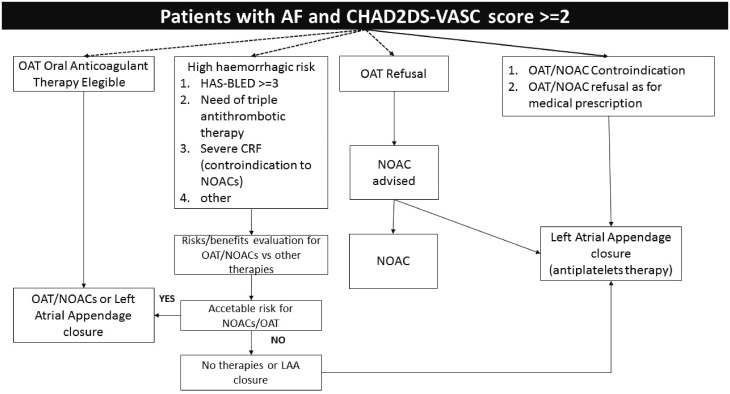
According to the current indications for percutaneous LAAO, which reflect the ESC guidelines,17 the procedure is indicated (recommendation class IIb, level of evidence B) in patients with non-valvular AF with high-thromboembolic risk (CHA2DS2-VASc score ≥ 2) with long-term contraindication for OAT (e.g. history of intracranial bleeding, life-threatening bleeding, coagulation diseases).
The recent EHRA/EAPCI44 consensus suggests that the spectrum of patients who could benefit from this technique should be extended (Table 4). Moreover, this therapy could also be considered in the following clinical situations:
Table 4.
Left atrial appendage occlusion: possible clinical situations
|
NOACs, new oral anticoagulants; OAT, oral anticoagulant therapy.
patients with non-valvular AF with high-thromboembolic risk and high-haemorrhagic risk (HAS-BLED ≥ 3);
patients requiring triple antithrombotic therapy indefinitely;
patients with tumours with increased risk of haemorrhage, underestimated by the HAS-BLED score;
patients in whom OAT is ineffective in providing protection against cerebral ischaemic events probably correlated to thromboembolisms originating from the LAA;
patients with kidney failure or undergoing dialysis, bearing in mind that all NOACs are contraindicated with creatinine clearance < 15 mL/min and that in these patients warfarin could increase tissue calcification and the degree of atherosclerosis;
patients with major bleeding of the urogenital or gastrointestinal system, or any other districts, such as the ocular area;
frail patients (the very old, dementia, neurodegenerative diseases, malnutrition, etc.);
patients with difficulty in managing oral therapies (e.g. mental illnesses, vision impairment); and
patients who, after being suitably informed about the OAT/NOACs therapy, refuse it and demand a ‘definitive’ therapy. In this context, it should be underlined that the Watchman has had approval by the US regulatory authority as a valid alternative to warfarin in patients who refuse or prefer not to take OAT.
The decision must therefore be made in the context of the doctor–patient relationship, after appropriate information about all the benefits and drawbacks related to the percutaneous LAAO procedure or the alternative OAT/NOACs therapies (Figure 8). It should however be underlined that at present there is no evidence on this question and additional clinical studies are required to assess the usefulness of LAAO in these clinical situations before the indications for this type of treatment are extended to the aforesaid groups of patients.
Figure 8.
Algorithm for stroke prevention in patients with atrial fibrillation. CRF, chronic renal failure; NOACs, new oral anticoagulants, OAT, oral anticoagulant therapy. Adapted from Meier et al.44
Screening of patients who are possible candidates for left atrial appendage occlusion
Screening must be carried out in the context of discussion and choice of a strategy in agreement with the patient, giving due consideration to personal preferences and health expectations65 and avoiding superfluous tests or investigations.
Screening must aim to establish the individual risk/benefit profile and in particular the ratio between embolic and haemorrhagic risk and its progression over time, and include the anatomical characteristics of the LAA in order to allow planning of the most important procedural aspects.
As has been shown to be the case in cardiac surgery, with large benefits for outcomes,66 screening must be disciplinary and preferably use local protocols which clearly identify the role of each player within the team, and ensure strong interaction between its members and the patient.67 Assessment of the patient’s preferences and health and quality of life factors must include clear, appropriate methods for their formal expression.68
Table 5 summarizes the main factors to be considered in this phase of the diagnostic and therapeutic process. From the anatomical point of view, in-depth echocardiogram examination is required. An anatomical assessment of the LAA with computed tomography or magnetic resonance can be useful if echocardiography is not definitive.
Table 5.
Screening factors
|
CT, computed tomography; MR, magnetic resonance; NYHA, New York Heart Association.
Echocardiogram assessment and echocardiogram anatomy of the left atrial appendage
TEE is the method of choice for the anatomical and functional assessment of the LAA. Three-dimensional echocardiography may be useful although not essential for a correct assessment.
When assessing thrombotic risk, it is important to consider both anatomical and functional aspects in order to assign a specific LAA-related risk within the overall cardiac and systemic thrombotic risk. As already described, the LAA often has several lobes and its contractility is the main determinant of its blood outflow.69 In the context of AF, it has been shown that a LAA outflow rate > 40 cm/s is correlated, after cardioversion of the arrhythmia, with a reduction in spontaneous echo contrast.70 A high level of spontaneous echo contrast correlates with endo-LAA flow rates < 40 cm/s and the formation of sludge in the LAA (a pre-thrombotic condition).
The main data on the linear average measurements relating to this anatomical structure are derived from autopsy investigations, which have also revealed a correlation with age and gender. More recently, a study of LAA volumes has been conducted using multilayer computer tomography, reaching the conclusion that there are no gender-related differences although there are differences linked to age-band (larger at 60–70 years than at 40–59 years).71,72 TEE allows calculation of the main planimetric parameters (transverse and longitudinal dimensions and area) which allow correct planning of the occlusion procedure (Table 6).
Table 6.
Main echocardiographic parameters to be obtained
|
LAA, left atrial appendage.
‘Step by step’ procedure
There are now a large number of publications which offer a detailed description of the LAAO procedure.45 Below, we list some suggestions to be considered useful for standardising the procedure as far as possible, in order to render outcomes reproducible and reduce complications.
Choice of anaesthesia
Although the procedure can be performed both under general anaesthesia and in analgosedation, the former option appears to be advisable. On the one hand, the need for transoesophageal monitoring requires prolonged collaboration from patients, who often have difficulty in tolerating the presence of the oesophageal probe. On the other hand, general anaesthesia allows the patient's complete immobility to be guaranteed, preventing small, involuntary movements which may increase the risk of mechanical complications, especially during the transseptal puncture or during manipulation of the deployment system inside the LAA. Although the possibility of performing the procedure under local anaesthetic with intracardiac echocardiographic guidance is intriguing, sufficient data are not available for this approach to be recommended. In fact, although transseptal puncture under intracardiac echocardiography is feasible, it currently appears difficult to establish the size of the device and assess its positioning without the use of TEE.
Choice of vascular access
The access of choice is the right femoral vein, which simplifies the manipulation of the materials and gives more safer transseptal puncture; the literature describes performance of the procedure through a puncture in the left femoral vein,73 but this access should only be used in patients where the right access is impracticable (severely twisted vessel, previous vascular access, large arteriovenous fistula, etc.). Here again, in-depth knowledge of the materials and specific experience in the management of the possible vascular complications are important.
Transseptal puncture
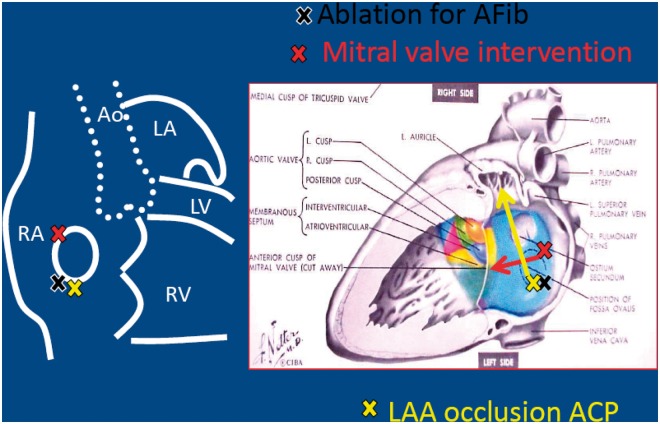
This is one of the most delicate phases of the procedure. Although for a skilled electrophysiologist or haemodynamics specialist performance of the transseptal puncture with fluoroscopic monitoring only (with or without aids such as a coronary catheter) is not a specific problem, the use of echocardiographic guidance for the puncture is strongly recommended in order to obtain a better angle of approach to the LAA. A puncture low down to the rear exposes the LAA with the ideal angle for existing deployment systems (Figure 9). Passage of the interatrial septum through the patency of the foramen ovale offers an angle which is less than ideal, which may render the subsequent procedure more problematical and less safe.
Figure 9.
View of the fossa ovalis and the preferred points for the puncture for LAA occlusion. ACP, Amplatzer Cardiac Plug; AF, atrial fibrillation; Ao, aorta; LA, left atrium; LAA, left atrial appendage; LV, left ventricle; RA, right atrium; RV, right ventricle.
Intraprocedural antithrombotic therapy
Although the literature describes cases of LAAO performed during therapy with warfarin in the therapeutic range, in order to minimize the risks all anticoagulant therapy should have been suspended at least 48 h prior to the procedure, and the INR value should be < 2. Once the transseptal puncture has been performed, antithrombotic therapy must be administered: non fractionated heparin (dosage 70–100 U/kg to obtain an activated coagulation time of 250–300 s) is the first choice. In our opinion, the dwell time in the left atrium should be minimized in order to reduce the risk of embolism. In the event that the TEE reveals thrombosis on the deployment system, we advise manual aspiration of the thrombus through the catheter, associated with the administration of heparin; if this strategy fails, the procedure should be interrupted.
Insertion of the deployment system into the left atrium
There are two possible approaches:
the deployment system delivery catheter is exchanged over a stiff guidewire which is placed in the left upper pulmonary vein and then manoeuvred, containing a pigtail catheter through which the angiography of the LAA will also be performed. If this approach is chosen when using the ACP-Amulet system, a longer pigtail catheter (125 cm) will be needed. It should be emphasized that with this procedure the system can be manoeuvred with greater safety and the risk of iatrogenic damage when the delivery catheter is moved into the LAA is reduced and
the alternative method is to use the pigtail for the angiography and then exchange it for the deployment system delivery catheter on a rigid guide positioned in the LAA (this ‘saves’ one exchange but increases the risks of tearing of the wall of the LAA).
Choice of device
A number of devices have been developed and placed on the market. However, in clinical practice the devices currently available are the Watchman (Boston Scientific) and the New Amulet (St. Jude). The Lariat device is used for combined endocardial-epicardial ligature of the LAA and will not be discussed here. The scientific findings supporting the use of the devices were discussed in the previous section; here, we will simply detail the devices’ specific technical features. It should be underlined that both the devices available on the market are completely repositionable and recapturable in the event of imperfect deployment or if the chosen size is not suitable.
The Watchman device is inserted by means of a preformed deployment system (single or double bend) of 14 Fr. For optimal sealing, it is recommended that the device chosen should be oversized by 10–20% (it is available in sizes from 21 to 33 mm). A higher device oversizing percentage appears to offer better leak reduction but could be more risky. The new generation of the device, which will probably be on the market from 2016, will provide better sealing even without oversizing, thanks to an increase in the number of anchor hooks and a shallower profile. For the current generation of the device, the depth of the LAA must be similar to the diameter to allow safe deployment. In the Panel’s experience this characteristic, which is apparently the device’s biggest drawback, has never prevented safe implantation, probably because several studies74 have shown that as AF persists, LAA increase in size, generally with the same rate of expansion in their diameter and depth. However, if in doubt it is important to use the radiopaque markers provided on the deployment system which easily reveal the minimum device deployment depth.
The New Amulet occlusion device (the evolution of the Amplatzer ACP system, now more or less replaced on the market by the New Amulet) has an unusual structure which enables it to adapt to virtually any LAA morphology. This device is able to occlude all LAAs between 11 and 31 mm and is inserted in a deployment system with 9–14 Fr delivery catheters (depending on the size of the device). It should be remembered that while the delivery catheters of the ACP were available in diameters 9, 10, and 13 Fr, those of the Amulet are in sizes 12 or 14 Fr.
Preparation of the system and device
After the device and size have been chosen, the implantation system and device must be prepared. Experience has shown that through flushing of the catheter and device is essential to minimize the risk of air embolization during implantation.
Implantation of the device
Once the deployment system is in an appropriate positon, it is withdrawn, enabling the device to open. In this phase, small movements are sometimes required to adjust the device’s positon, turning, pushing, or pulling to correct the angle of access to the LAA, which is not always optimal in spite of careful planning of the transseptal puncture. Once the device is open, a combined check is performed by angiography (the electrical projections are the right cranial and right caudal arteries) and echocardiography, to check stability (shape and compression) and efficacy (presence of leaks). This is followed by a tug test on the deployment wire.
Deployment of the device
Once the result is considered satisfactory, the device can be deployed by turning the deployment wire anticlockwise. At the end of the procedure, an additional echocardiographic check is required to ensure that the device is correctly positioned and that there are no pericardial effusions (which may occur at any phase of the implantation process). The system is then removed and local haemostasis is performed (manual compression or figure-of-8 suture). The decision as to whether to administer protamine sulphate will depend on the assessment of its clinical utility as appropriate to the individual patient.
Management of antithrombotic therapy after the procedure
After the procedure, antithrombotic therapy is recommended to prevent the formation of thrombi on the device until its complete endothelialization. The current management protocol is the outcome of the PROTECT AF,48 PREVAIL,51 and ASAP52 studies based on the Watchman device and experience gained from use of the Amplatzer PFO Occluder device.
For the Watchman device, the antithrombotic protocol of the PROTECT AF trial involved the suspension of warfarin 45 days after the procedure and its replacement with dual antiplatelet therapy if the TEE revealed the absence of thrombi or a residual leak < 5 mm; the second antiplatelet medication was suspended after 6 months if the follow-up TEE did not show significant periprosthetic leaks around the device. The PREVAIL trial and the ASAP study revealed the feasibility of DAPT as an alternative to therapy with warfarin after implantation of the Watchman device.
Observational studies assessed DAPT for 1 month after implantation of the ACP device and single antiplatelet therapy for the next 3–6 months, in accordance with the antithrombotic therapy protocol acquired from experience with the Amplatzer PFO Occluder.75
In all these various therapeutic approaches, single antiplatelet therapy is indicated quoad vitam.
DAPT with aspirin and clopidogrel can be suggested with the execution of TEE at 3 months or even earlier in the event of significant post-procedural leaks (≥5 mm). If there are no complications of any kind, the second antiplatelet therapy could be suspended after this period and an echocardiogram (by the transoesophageal approach) repeated after about 1 year. However, widely varying protocols are found in the records of the largest centres for patients with high and very high risk of haemorrhage, with the use of low molecular weight heparin therapy for a period ranging from a few days to some weeks, the use of a single antiplatelet therapy or, in specific cases, no antithrombotic therapy.
The incomplete occlusion of the LAA is another topic open to discussion. In theory, this could create pockets, which would be a potential source of cardioembolism. However, small residual leaks (<5 mm) are considered irrelevant and may close spontaneously; their presence has not been shown to imply an increased risk of thromboembolism compared to patients with complete LAAO76 although their clinical significance requires assessment in large randomized prospective studies with longer term follow-up.
Recommended organisational and operating standards
The expansion of the LAAO procedure within interventionist cardiology has led both national77 and international48,67 scientific societies to put forward recommendations and propose operating standards for the centres intending to adopt it. Some requirements should be considered essential; those listed below are the requirements to be considered fundamental for centres and staff.
General requirements
Within the hospital, the LAAO procedure should include joint decision-making within the Heart Team, including the interventionist cardiologist (haemodynamic specialist or electrophysiologist), the clinical cardiologist, the echocardiographer, the anaesthetist, and the cardiac surgeon, to ensure optimal coordination of the procedure. Since patient selection is the first fundamental step, other specialists (neurologists, nephrologists, internists, geriatrists, and haematologists) may be involved in indicating LAAO as the most appropriate antithrombotic strategy for a given patient. Therefore, the formation of a multidisciplinary team should be a prerequisite for the choice of the ideal candidate, the success of the procedure and post-procedural management. The presence/availability of a cardiac surgery unit on site is considered as a requirement in the North American Heart Society document67 and is also recommended by the recent EHRA/EAPCI guidelines44 which consider access to a cardiac surgery theatre within 60 min. adequate. The joint document issued by the Società Italiana di Cardiologia Interventistica/Associazione Italiana di Aritmologia e Cardiostimolazione (GISE/AIAC) requires the presence of a cardiac surgery unit on-site.77
Staff training and preparation
Interventionist cardiologists or electrophysiologists intending to perform the LAAO procedure must have in-depth knowledge of the anatomy of the heart, and of the morphological characteristics of the atrium and the LAA in particular. All scientific societies underline the need for the professional performing the procedure to have specific expertise in transseptal puncture and how to perform it in relation to implementation of LAAO devices. With regards to the transseptal puncture, the position of the national and international electrophysiology societies [AIAC/Heart Rhythm Society (HRS)] is that the professional should have performed at least 50 transseptal punctures and be skilled in manoeuvring catheters/inserters in the left atrium. The professional must be familiar not only with the anatomical position of the LAA and how to reach it but also the relationships between this structure and the surrounding anatomical formations. He or she must also have sufficient experience in the interpretation of radiological images (from angiography, tomography and magnetic resonance) and echocardiogram images of the LAA to obtain all the information needed for the correct choice of device. In particular, the scientific societies emphasize the need for consultation between the professional performing the procedure and the echocardiographer, a key factor for success.
Professional performing the procedure must also have experience in pericardial puncture (or at least an interventionist cardiologist with this technical skill must be present) in order to deal with the eventuality of pericardial tamponade during manoeuvres in the left atrium or positioning of the device in the LAA.
Training of the first professional in the skills needed to perform the procedure is the fundamental first step in becoming autonomous. Training is provided by a proctor who is able to supply the trainee with explanations of all the phases of the procedure for successful LAAO. In detail, the recommendations of all the various scientific societies agree that every professional must attend theoretical lessons together with technical support through familiarization with the procedure and the instruments required, involving virtual sessions using simulators and/or practical sessions, in animal laboratories where possible.
It is advisable for those who are learning the procedure to attend implantation operations in order to learn the various steps with precision. The proctor must be present at the first implantation performed in every centre. Although there is no minimum number required to allow a professional to acquire complete independence, it is reasonable to assume that at least 15 implants must be performed in each centre before a satisfactory degree of autonomy and independence is gained.
One key factor for the achievement of procedural success, strongly recommended in the documents of the various scientific societies, is the creation of a well-organized operating team including the echocardiographer, the anaesthetist, and the nursing staff, who must also be familiar with the characteristics of the various devices and have proven experience in interventionist cardiology/electrophysiology procedures.
Requirements for the centre and operating room
Since most procedures are performed under general anaesthetic, the operating room must have the instrumentation required for anaesthesiology and have sufficient physical space not only for the anaesthetist but also for the echocardiographer, who plays a fundamental role in the procedure. The international scientific societies require the operating room staff, including nursing and technical staff, to have experience in interventionist procedures and be able to understand every step of the procedure, such as, for example, how to respond quickly to emergencies.
Staff's familiarity with the materials used is an additional factor in facilitating all steps of the procedure. Although not specifically recommended by the various scientific societies, it would be preferable for the nursing members of the team also to be involved in the technical training, attending LAAO sessions performed by the proctor and getting to know the materials by means of virtual sessions.
All operating room staff involved in the procedure must have a clearly defined role to play, in order to prevent overlaps during the procedure and delays in responding to emergencies.
Procedural recommendations and minimum skill levels
In view of the large number of procedures already performed and the potential additional increase in the near future, it appears appropriate for the national and scientific societies to draw up a position which can act as a consensus guideline. Therefore, on the one hand, the existing literature has been combined to extract indications for the selection and preparation of patients for the procedure. On the other hand, considering the opinions of staff skilled in the procedure, it appears useful to analyse the most purely technical aspects of percutaneous LAAO in order to standardize the various steps of the method, increasing its success in acute cases and reducing complications.
Requirements for the facility and staff
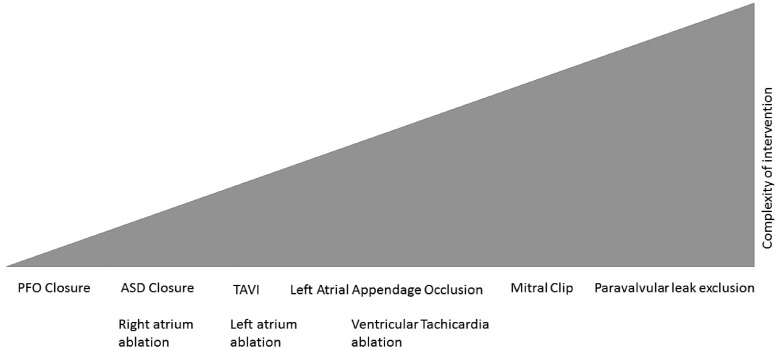
As already stated, percutaneous LAAO is a procedure which requires a multidisciplinary approach and can be performed either by an electrophysiologist or by a haemodynamic specialist: professionals from both these disciplines can combine their specific skills. However, the general opinion is that this procedure is technically complex and is a considerable challenge within interventionist-structural cardiology, due to the large number of skills required (transseptal puncture, navigation in left atrium and LAA, use of contrast medium, catheters and specific materials) (Figure 10).
Figure 10.
Complexity ratio between the procedures used in interventionist cardiology. ASD, atrial septal defect; PFO, patent foramen ovale; TAVI, transcatheter aortic valve implantation.
Therefore, this working group believes that a facility wishing to start a percutaneous LAAO programme must have a multidisciplinary team allowing optimal assessment of the patient for the procedure. Particular attention will be focused on cardiac imaging techniques, in order to assess feasibility and plan the procedure most effectively: the presence of a specialist echocardiographer, both to monitor the positioning of the device and to recognize acute complications, is fundamental (Table 7).
Table 7.
Intraprocedural complications which can be identified by echocardiography.
|
Although it is not a sine qua non, radiological imaging plays a major role: high definition multidetector computed tomography and cardiac magnetic resonance are able to identify any thrombotic formations and study the anatomy of the LAA most effectively.78 However, it must be underlined that these techniques are not the ideal support during the procedure, although they are useful, especially during the initial phases.
It thus appears advisable to perform the entire procedure under the guidance of echocardiography and fluoroscopy, as already emphasized in the PROTECT AF study. In all cases, according to the data in the literature and the experience of many professionals, it does not seem to be more risky than other interventionist procedures such as transcatheter ablation of AF, with which it shares the risk of mechanical complications (cardiac tamponade and damage to the structures adjoining the left atrium). It is important to point out that the incidence of what is to all effects the most serious complication, cardiac tamponade, has fallen over the years as operators’ experience has increased and the technical characteristics of devices have improved: the rate has decreased from the about 5% of PROTECT AF48 to a minimum of 0.8–1.3% in the latest ‘real world’ registry studies.51,58 Analysing the data available to us from the PROTECT AF48 and PREVAIL51 studies, which together total 1271 patients, we find 40 (3.1%) pericardial effusions; the two studies report that 10 (0.8%) of these were surgically drained within 7 days after deployment of the device. If we compare the data of the two studies, PROTECT AF and PREVAIL, there is a significant reduction in surgically drained pericardial effusions (P = 0.027), with a fall from 1.6% to 0.4%.
Considering the complexity of the procedure and the potential complications, with current knowledge and the data now available, the need for a cardiac surgery team on-site has been the subject of debate, as reflected by the different positons taken in the international documents. Although the majority of the Panel believed that the presence of a cardiac surgery team on-site was not an indispensable requirement and that access to a cardiac surgery room within 60 min. was sufficient a unanimous position on this point was not achieved, even after lengthy discussion. One essential prerequisite (relevant for both interventionist cardiologists and electrophysiologists) is skill in pericardiocentesis.79
Management of complications
Pericardial effusion and cardiac tamponade
Pericardial effusion, in all its clinical forms (from asymptomatic effusion to cardiac tamponade) is one of the most serious complications of the percutaneous LAAO procedure. The risk factors include the transseptal puncture, the manipulation of rigid structures like the delivery catheters and the devices themselves inside the left atrium, and the intrinsic fragility of the atrial wall.80
In the PROTECT AF study, conducted on the Watchman device, the incidence of pericardial effusion within 7 days of implantation was 4.4%, with pericardiocentesis required in 3.3% of patients.48
It should be underlined that this complication was observed more frequently at the start of the learning curve. In the registry of the PAC system, which contains the results achieved by skilled staff, the rate of pericardial effusion fell to 2.2%74 and the same values were also reported in the subsequent PREVAIL trial.51 The recent study performed by Tzikas et al.57 on the results achieved with the ACP device in more than 20 European and Canadian centres revealed a rate of pericardial effusion requiring drainage of 1.2%. In a multicentric study analysing the data on the use of the Lariat device, Price et al.81 report an incidence of pericardial effusion requiring drainage of 10.4%.
The procedural strategies to reduce the incidence of pericardial effusion include:
performance of the device implantation procedure under TEE guidance and pressure monitoring to ensure safe transseptal puncture in the correct position;
use of a pigtail catheter inside the access sheath or a loop wire in the LAA to facilitate safe, trauma-free movement inside it;
the use of slow, careful movements during manipulation of the catheter and occlusion device inside the left atrium; and
performance of the tug test after implantation of the device under echocardiographic and fluoroscopic guidance, with injection of small amounts of contrast medium.
With regards to the therapeutic management of pericardial effusion, in the case of cardiac tamponade with hypotension a swift, aggressive approach is required, with pericardiocentesis and suspension of anticoagulation therapy. Early diagnosis of a pericardial effusion by means of TEE/intracardiac echography can often prevent the onset of cardiac tamponade, leading to serious deterioration of the haemodynamic condition.
Pericardiocentesis can be performed by means of a subxiphoid or subcostal transcutaneous puncture with echocardiographic guidance, or through a small cutaneous incision of about 3–5 cm underneath the xiphoid appendix (subxiphoid window), which allows the pericardial cavity to be accessed directly through its incision, followed by insertion of a pericardial drain.
In the event of recurrent tamponade or perforation of the left atrial wall, conventional surgery is required to review the pericardial cavity, suturing the breach in the atrial wall in an off-pump, beating heart procedure (if possible). If the lesion requires luxation of the heart for better surgical exposure, and tolerance of this procedure is poor, extracorporeal circulation may be necessary.
In selected cases, a thoracoscopic approach may initially be indicated, with fitting of an epicardial device (e.g. AtriClip) to close the breach in the LAA, if the lesion does not involve its orifice. This technique has not yet been described in the literature, and where it is not indicated the thoracoscopic approach can be transformed into minithoracotomy, which allows easier control of the anatomical structures and control of the haemostasis.
Although most cases of pericardial effusion occur early, subacute forms and delayed effusions are still described in the literature after the implantation of percutaneous LAAO devices. Therefore, monitoring of heart rate and arterial pressure are recommended for at least 48 h after implantation of the device, with performance of a transthoracic echocardiogram before discharge to ensure that no pericardial effusion has occurred and that the device is correctly positioned. Subacute effusions may be due to rubbing or irritation caused by the device’s anchors against the wall of the atrium, in which lesions may eventually occur in view of its thinness. In these cases, if the pericardial effusion is of significant extent or produces instrumental signs of haemodynamic impairment, pericardiocentesis, or in some cases conventional surgical exploration, must be performed.
Delayed, haemodynamically insignificant effusions can probably be ascribed to post-procedural inflammation. They are normally treated with non-steroid anti-inflammatories such as aspirin, ibuprofen and diclofenac, or more rarely with steroid therapy.
Gaseous embolism
Gaseous embolism is often a clinically silent event, although in rare cases it may cause TIA, acute coronary ischaemia, hypotension and/or cardiac arrest.48 Gaseous embolisms may reach the left atrium due to an accidental injection of air or may be the consequence of gas bubbles generated by a gradient between atmospheric and intracardiac pressure due to a deep intake of breath.82
Most of the management of gaseous embolisms consists of support. Hyperbaric oxygen has shown clinical benefits in 80% of cases of cerebral gaseous embolism, although no controlled studies have yet been performed. Gaseous embolism in the coronary circulation most often becomes evident in the right coronary due to the anatomical position of its orifice, and is often resolved within a few minutes. However, it may generate an ST depression, hypotension and ventricular arrhythmias, through to cardiogenic shock. Aspiration of the air with a specific device or the injection of contrast medium into the coronary artery may be useful in these cases.
Embolization of the device
The implantation procedure may be complicated by immediate or delayed formation of embolisms around the perimeter of the device itself. The careful selection of patients with favourable LAA morphology and use of the appropriate size of device are crucial for preventing this complication. Conversely, negative predictors are:
large LAA orifice size,
use of undersized devices,
short LAA, and
unusual anatomical variants.
Embolized devices in the left atrium or left ventricle can be captured and then driven forwards towards the mitral and aortic valves by means of endocatheters, allowing them to be recovered in the descending aorta. The recovery of devices from inside the heart itself is difficult and dangerous, while recovery in the aorta with a loop or bioptome catheter is a safer, more reproducible method. In the rare cases of the embolization of larger-sized devices in the direction of the iliac-femoral axis, recovery through open surgery is necessary.
Conclusions
OAT is the strategy of first choice for the prevention of thromboembolic events in AF patients with medium/high risk (CHA2DS2-VASc ≥1). However, many of these patients are difficult to treat, especially due to bleeding, a dangerous adverse event related to the therapy, which limits or contraindicates its use. The observation that most thrombi are generated by the LAA has led to the consideration of its surgical or percutaneous occlusion as an alternative; during the last few years, the Watchman percutaneous occlusion device has proved to be not inferior to anticoagulant therapy for the prevention of thromboembolic events, with the added benefit of a lower rate of haemorrhagic events; other, new devices have recently been introduced or are under development.
The procedure for the percutaneous occlusion of the LAA requires considerable technical skill and theoretical knowledge, as well as suitable training under the supervision of expert colleagues. Anyone wishing to perform it will require the cooperation of skilled echocardiographers, familiar with the transoesophageal technique, to obtain the support of suitable imaging; he or she must also be capable of managing any complications, meaning, in particular, experience in the execution of pericardiocentesis, since cardiac tamponade is the most common and most dangerous complication.
The presence of a cardiac surgery unit on-site does not appear to be indispensable although it is reasonable to ensure access to a cardiac surgery room within 60 min. if necessary.
At present, the main indication for LAAO is the relative or absolute contraindication of anticoagulant therapy in patients with AF. These indications derive from observational or registry studies made comparing devices with the use of warfarin. Fur the future, the indication for LAAO in association with the AF ablation procedure AF83–85 and MitraClip implantation, and the data obtained from comparison with the NOACs, remain to be assessed.
Large populations of very high-risk patients have still not been studied, including those who are unable to take any type of anticoagulant or antiplatelet therapy, in order to establish the impact of these factors on the prevention of complications and mortality.
Acknowledgments
The authors wish to thank Dr. Pierluigi Merella (Cardiologia, Ospedale San Francesco, Nuoro) and Dr. Simonetta Genovesi (Dipartimento di Medicina e Chirurgia, Università degli Studi Milano-Bicocca, Clinica Nefrologica, A.O. San Gerardo, Monza) for their useful comments during drafting of the document.
Conflict of interest: none declared.
Annex
Consensus Document Approval Faculty
Abrignani Maurizio Giuseppe, Alunni Gianfranco, Amico Antonio Francesco, Amodeo Vincenzo, Angeli Fabio, Aspromonte Nadia, Azzarito Michele, Battistoni Ilaria, Bianca Innocenzo, Bisceglia Irma, Bongarzoni Amedeo, Bonvicini Marco, Cacciavillani Luisa, Calculli Giacinto, Caldarola Pasquale, Capecchi Alessandro, Caporale Roberto, Caretta Giorgio, Carmina Maria Gabriella, Casazza Franco, Cassin Matteo, Cemin Roberto, Chiarandà Giacomo, Chiarella Francesco, Chiatto Mario, Cibinel Gian Alfonso, Ciccone Marco Matteo, Cicini Maria Paola, Clerico Aldo, Colivicchi Furio, D’Agostino Carlo, De Luca Leonardo, De Luca Giovanni, De Maria Renata, Del Sindaco Donatella, Di Fusco Stefania Angela, Di Lenarda Andrea, Di Tano Giuseppe, Egidy Assenza Gabriele, Egman Sabrina, Enea Iolanda, Fattirolli Francesco, Francese Giuseppina Maura, Gabrielli Domenico, Geraci Giovanna, Giardina Achille, Greco Cesare, Gregorio Giovanni, Iacoviello Massimo, Khoury Georgette, Ledda Antonietta, Lucà Fabiana, Macera Francesca, Marini Marco, Mascia Franco, Masson Serge, Maurea Nicola, Mazzanti Marco, Mennuni Mauro, Menotti Alberto, Menozzi Alberto, Mininni Nicola, Moreo Antonella, Moretti Luciano, Mortara Andrea, Mureddu Gian Francesco, Murrone Adriano, Nardi Federico, Navazio Alessandro, Nicolosi Pier Luigi, Oliva Fabrizio, Parato Vito Maurizio, Parrini Iris, Patanè Leonardo, Pini Daniela, Pino Paolo Giuseppe, Pirelli Salvatore, Procaccini Vincenza, Pugliese Francesco Rocco, Pulignano Giovanni, Radini Donatella, Rao Carmelo Massimiliano, Rasetti Gerardo, Riccio Carmine, Roncon Loris, Rossini Roberta, Ruggieri Maria Pia, Rugolotto Matteo, Sanna Fabiola, Sauro Rosario, Scalvini Simonetta, Scherillo Marino, Severi Silva, Sicuro Marco, Silvestri Paolo, Sisto Francesco, Tarantini Luigi, Uguccioni Massimo, Urbinati Stefano, Valente Serafina, Vatrano Marco, Vianello Gabriele, Vinci Eugenio, Zuin Guerrino.
References
- 1. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS.. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ.. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 3. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC.. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 4. Lip GY, Tse HF.. Management of atrial fibrillation. Lancet 2007;370:604–618. [DOI] [PubMed] [Google Scholar]
- 5. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449–1457. [PubMed] [Google Scholar]
- 6. Penado S, Cano M, Acha O, Hernandez JL, Riancho JA.. Atrial fibrillation as a risk factor for stroke recurrence. Am J Med 2003;114:206–210. [DOI] [PubMed] [Google Scholar]
- 7. Hart RG, Pearce LA, Aguilar MI.. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 8. Steger C, Pratter A, Martinek-Bregel M, Avanzini M, Valentin A, Slany J, Stollberger C.. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur Heart J 2004;25:1734–1740. [DOI] [PubMed] [Google Scholar]
- 9. Blackshear JL, Odell JA.. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755–759. [DOI] [PubMed] [Google Scholar]
- 10. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY.. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123:638–645. [DOI] [PubMed] [Google Scholar]
- 11. Gladstone DJ, Bui E, Fang J, Laupacis A, Lindsay MP, Tu JV, Silver FL, Kapral MK.. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke 2009;40:235–240. [DOI] [PubMed] [Google Scholar]
- 12. Lip GY, Frison L, Halperin JL, Lane DA.. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011;57:173–180. [DOI] [PubMed] [Google Scholar]
- 13. Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY.. Performance of the HEMORR(2)HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol 2012;60:861–867. [DOI] [PubMed] [Google Scholar]
- 14. Olesen JB, Lip GY, Lindhardsen J, Lane DA, Ahlehoff O, Hansen ML, Raunso J, Tolstrup JS, Hansen PR, Gislason GH, Torp-Pedersen C.. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a “real world” nationwide cohort study. Thromb Haemost 2011;106:739–749. [DOI] [PubMed] [Google Scholar]
- 15. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW.. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 16. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P.. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 17. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM.. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a metaanalysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 18. Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE.. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med 1999;131:927–934. [DOI] [PubMed] [Google Scholar]
- 19. Stafford RS, Singer DE.. Recent national patterns of warfarin use in atrial fibrillation. Circulation 1998;97:1231–1233. [DOI] [PubMed] [Google Scholar]
- 20. Gallagher AM, Rietbrock S, Plumb J, van Staa TP.. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost 2008;6:1500–1506. [DOI] [PubMed] [Google Scholar]
- 21. Connolly SJ, Eikelboom JW, Ng J, Hirsh J, Yusuf S, Pogue J, de Caterina R, Hohnloser S, Hart RG.. Net clinical benefit of adding clopidogrel to aspirin therapy in patients with atrial fibrillation for whom vitamin K antagonists are unsuitable. Ann Intern Med 2011;155:579–586. [DOI] [PubMed] [Google Scholar]
- 22. Olesen JB, Lip GY, Kamper AL, Hommel K, Kober L, Lane DA, Lindhardsen J, Gislason GH, Torp-Pedersen C.. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012;367:625–635. [DOI] [PubMed] [Google Scholar]
- 23. Seliger SL, Gillen DL, Longstreth WT, Kestenbaum B, Stehman-Breen CO.. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int 2003;64:603–609. [DOI] [PubMed] [Google Scholar]
- 24. Boccardo P, Remuzzi G, Galbusera M.. Platelet dysfunction in renal failure. Semin Thromb Hemost 2004;30:579–589. [DOI] [PubMed] [Google Scholar]
- 25. Schurgers LJ., Vitamin K.. warfarin and calcification in experimental chronic kidney disease. Kidney Int 2013;83:782–784. [DOI] [PubMed] [Google Scholar]
- 26. Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, Humphries KH, Tu JV, Behlouli H, Guo H, Pilote L.. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 2014;129:1196–1203. [DOI] [PubMed] [Google Scholar]
- 27. Lopes RD, Pieper KS, Horton JR, Al-Khatib SM, Newby LK, Mehta RH, Van de Werf F, Armstrong PW, Mahaffey KW, Harrington RA, Ohman EM, White HD, Wallentin L, Granger CB.. Short and long-term outcomes following atrial fibrillation in patients with acute coronary syndromes with or without ST-segment elevation. Heart 2008;94:867–873. [DOI] [PubMed] [Google Scholar]
- 28. Su P, McCarthy KP, Ho SY.. Occluding the left atrial appendage: anatomical considerations. Heart 2008;94:1166–1170. [DOI] [PubMed] [Google Scholar]
- 29. Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, Horton R, Sanchez JE, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Gallinghouse GJ, Burkhardt JD, Cesarani F, Scaglione M, Natale A, Gaita F.. Does the left atrial appendance morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012;60:531–538. [DOI] [PubMed] [Google Scholar]
- 30. Madden JL. Resection of the left auricular appendix. JAMA 1948;140:769–772. [DOI] [PubMed] [Google Scholar]
- 31. Bailey CP, Olsen AK, Keown KK, Nichols HT, Jamison WL.. Commissurotomy for mitral stenosis: technique for prevention of cerebral complication. JAMA 1952;149:1085–1091. [DOI] [PubMed] [Google Scholar]
- 32. Belcher JR, Somerville W.. Systematic embolism and left auricular thrombosis in relation to mitral valvotomy. BMJ 1955;2:1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jordan RA, Scheifley CH, Edwards JE.. Mural thrombosis and arterial embolism in mitral stenosis. Circulation 1951;3:363–367. [DOI] [PubMed] [Google Scholar]
- 34. Halseth WL, Elliot DP, Walker EL, Smith EA.. Open mitral commissurotomy. A modern reevaluation. J Thorac Cardiovasc Surg 1980;80:842–848. [PubMed] [Google Scholar]
- 35. Cox JL. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg 1991;101:584–592. [PubMed] [Google Scholar]
- 36. Tsai YC, Phan K, Munkholm-Larsen S, Tian DH, La Meir M, Tan TD.. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: a meta-analysis. Eur J Cardiothorac Surg 2015;47:847–854. [DOI] [PubMed] [Google Scholar]
- 37. Nagpal AD, Torracca L, Fumero A, Denti P, Cioni M, Alfieri O.. Concurrent prophylactic left atrial appendage exclusion: results from a randomized controlled trial pilot study. Eur J Cardiothorac Surg 2009;36:553–557. [DOI] [PubMed] [Google Scholar]
- 38. Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, Hohnloser SH, Cybulsky I, Abouzahr L, Sawchuck C, Carroll S, Morillo C, Kleine P, Chu V, Lonn E, Connolly SJ.. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005;150:288–293. [DOI] [PubMed] [Google Scholar]
- 39. Lee CH, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW.. Left atrial appendage resection versus preservation during the surgical ablation of atrial fibrillation. Ann Thorac Surg 2014;97:124–132. [DOI] [PubMed] [Google Scholar]
- 40. Kim R, Baumgartner N, Clements J.. Routine left atrial appendage ligation during cardiac surgery may prevent postoperative atrial fibrillation-related cerebrovascular accident. J Thorac Cardiovasc Surg 2013;145:582–589. [DOI] [PubMed] [Google Scholar]
- 41. Zapolanski A, Johnson CK, Dardashti O, O'Keefe RM, Rioux N, Ferrari G, Shaw RE, Brizzio ME, Grau JB.. Epicardial surgical ligation of the left atrial appendage is safe, reproducible, and effective by transesophageal echocardiographic follow-up. Innovations (Phila) 2013;8:371–375. [DOI] [PubMed] [Google Scholar]
- 42. Garcia-Fernandez MA, Pérez-David E, Quiles J, Peralta J, García-Rojas I, Bermejo J, Moreno M, Silva J.. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis—a transesophageal echocardiographic study. J Am Coll Cardiol 2003;42:1253–1258. [DOI] [PubMed] [Google Scholar]
- 43. Whitlock RP, Vincent J, Blackall MH, Hirsh J, Fremes S, Novick R, Devereaux PJ, Teoh K, Lamy A, Connolly SJ, Yusuf S, Carrier M, Healey JS.. Left Atrial Appendage Occlusion Study II (LAAOS II). Can J Cardiol 2013;29:1443–1447. [DOI] [PubMed] [Google Scholar]
- 44. Meier B, Blaauw Y, Khattab AA, Lewalter T, Sievert H, Tondo C, Glikson M.. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace 2014;16:1397–1416. [DOI] [PubMed] [Google Scholar]
- 45. Alli O, Holmes D Jr, Left atrial appendage occlusion. Heart 2015;101:834–841. [DOI] [PubMed] [Google Scholar]
- 46. Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL.. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008;52:924–929. [DOI] [PubMed] [Google Scholar]
- 47. Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I.. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol 2000;36:468–471. [DOI] [PubMed] [Google Scholar]
- 48. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomized non-inferiority trial. Lancet 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 49. Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, Halperin JL, Holmes D; PROTECT AF Investigators. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) Trial. Circulation 2013;127:720–729. [DOI] [PubMed] [Google Scholar]
- 50. Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, Huber K, Whisenant B, Kar S, Swarup V, Gordon N, Holmes D; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014;312:1988–1998. [DOI] [PubMed] [Google Scholar]
- 51. Holmes DR, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY.. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 52. Reddy VY, Möbius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, Sick P, Sievert H.. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013;61:2551–2556. [DOI] [PubMed] [Google Scholar]
- 53. Holmes DR, Doshi SK, Kar S, Price MJ, Sanchez JM, Sievert H, Valderrabano M, Reddy VY.. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol 2015;65:2614–2623. [DOI] [PubMed] [Google Scholar]
- 54. Park JW, Bethencourt A, Sievert H, Santoro G, Meier B, Walsh K, Lopez-Minguez JR, Meerkin D, Valdés M, Ormerod O, Leithäuser B.. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv 2011;77:700–706. [DOI] [PubMed] [Google Scholar]
- 55. Lam YY, Yip GW, Yu CM, Chan WW, Cheng BC, Yan BP, Clugston R, Yong G, Gattorna T, Paul V.. Left atrial appendage closure with Amplatzer cardiac plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter Cardiovasc Interv 2012;79:794–800. [DOI] [PubMed] [Google Scholar]
- 56. Santoro G, Meucci F, Stolcova M, Rezzaghi M, Mori F, Palmieri C, Paradossi U, Pastormerlo LE, Rosso G, Berti S.. Percutaneous left atrial appendage occlusion in patients with non-valvular atrial fibrillation: implantation and up to four years follow-up of the Amplatzer Cardiac Plug. EuroIntervention 2016;11:1188–1194. [DOI] [PubMed] [Google Scholar]
- 57. Tzikas A, Shakir S, Gafoor S, Omran H, Berti S, Santoro G, Kefer J, Landmesser U, Nielsen-Kudsk JE, Cruz-Gonzalez I, Sievert H, Tichelbäcker T, Kanagaratnam P, Nietlispach F, Aminian A, Kasch F, Freixa X, Danna P, Rezzaghi M, Vermeersch P, Stock F, Stolcova M, Costa M, Ibrahim R, Schillinger W, Meier B, Park JW.. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the Amplatzer Cardiac Plug. EuroIntervention 2016;11:1170–1179. [DOI] [PubMed] [Google Scholar]
- 58. Lopez-Minguez JR, Asensio JM, Gragera JE, Costa M, González IC, de Carlos FG, Díaz JA, Martín Yuste V, González RM, Domínguez-Franco A, Buendía AB, Garibi JH, Hernández FH, Ribeiro VG.. Two-year clinical outcome from the Iberian registry patients after left atrial appendage closure. Heart 2015;101:877–883. [DOI] [PubMed] [Google Scholar]
- 59. Cheng Y, McGregor J, Sommer R.. Safety and biocompatibility of the Coherex WaveCrestTM Left Atrial Appendage Occluder in a 30-day canine study [abstract]. J Am Coll Cardiol 2012;60:108–118. [Google Scholar]
- 60. Bartus K, Han FT, Bednarek J, Myc J, Kapelak B, Sadowski J, Lelakowski J, Bartus S, Yakubov SJ, Lee RJ.. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108–118. [DOI] [PubMed] [Google Scholar]
- 61. Amorosi L, Armstrong S, Da Deppo L, Garfield S, Stein K.. The budget impact of left atrial appendage closure compared with adjusted-dose warfarin and dabigatran etexilate for stroke prevention in atrial fibrillation. Europace 2014;16:1131–1136. [DOI] [PubMed] [Google Scholar]
- 62. Singh SM, Micieli A, Wijeysundera HC.. Economic evaluation of percutaneous left atrial appendage occlusion, dabigatran, and warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. Circulation 2013;127:2414–2423. [DOI] [PubMed] [Google Scholar]
- 63. Jommi C, Iorio A, Crippa L, Gelera A, Senatore G.. Analisi di impatto sul budget di WatchmanTM, dispositivo per la prevenzione tromboembolica nei pazienti con fibrillazione atriale. Pharmacoecon Ital Res Articles 2013;5:45–63. [Google Scholar]
- 64.Department of Health, UK. A review of emerging cardiac technologies: their potential impact on cardiac services over the next 10 years. 2016.
- 65. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS.. Evidence based medicine: what it is and what it isn’t. BMJ 1996;312:71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Neily J, Mills PD, Young-Xu Y, Carney BT, West P, Berger DH, Mazzia LM, Paull DE, Bagian JP.. Association between implementation of a medical team training program and surgical mortality. JAMA 2010;304:1693–1700. [DOI] [PubMed] [Google Scholar]
- 67. Masoudi FA, Drozda JP Jr, Gainsley P, Slotwiner DJ, Turi ZG.. 2015 ACC/HRS/SCAI left atrial appendage occlusion device societal overview. Heart Rhythm 2015;12:e122–e136. [DOI] [PubMed] [Google Scholar]
- 68. Charon R, Wyer P; NEBM Working Group. Narrative evidence based medicine. Lancet 2008;371:296–297. [DOI] [PubMed] [Google Scholar]
- 69. Ernst G, Stöllberger C, Abzieher F, Veit-Dirscherl W, Bonner E, Bibus B, Schneider B, Slany J.. Morphology of the left atrial appendage. Anat Rec 1995;242:553–561. [DOI] [PubMed] [Google Scholar]
- 70. Antonielli E, Pizzuti A, Pálinkás A, Tanga M, Gruber N, Michelassi C, Varga A, Bonzano A, Gandolfo N, Halmai L, Bassignana A, Imran MB, Delnevo F, Csanády M, Picano E.. Clinical value of left appendage flow for prediction of long-term sinus rhythm maintenance in patients with non valvular atrial fibrillation. J Am Coll Cardiol 2002;39:1443–1449. [DOI] [PubMed] [Google Scholar]
- 71. Veinot JP, Harrity PJ, Gentile F, Khandheria BK, Bailey KR, Eickholt JT, Seward JB, Tajik AJ, Edwards WD.. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation 1997;96:3112–3115. [DOI] [PubMed] [Google Scholar]
- 72. Nucifora G, Faletra FF, Regoli F, Pasotti E, Pedrazzini G, Moccetti T, Auricchio A.. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: implications for catheter-based left atrial appendage closure. Circ Cardiovasc Imaging 2011;4:514–523. [DOI] [PubMed] [Google Scholar]
- 73. Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S.. Safety of percutaneous left atrial appendage closure: results from the watchman left atrial appendage system for embolic protection in patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation 2011;123:417–424. [DOI] [PubMed] [Google Scholar]
- 74. Guérios EE, Schmid M, Gloekler S, Khattab AA, Wenaweser PM, Windecker S, Meier B.. Left atrial appendage closure with the Amplatzer cardiac plug in patients with atrial fibrillation. Arq Bras Cardiol 2012;98:528–536. [DOI] [PubMed] [Google Scholar]
- 75. Viles-Gonzalez JF, Kar S, Douglas P, Dukkipati S, Feldman T, Horton R, Holmes D, Reddy VY.. The clinical impact of incomplete left atrial appendage closure with the Watchman device in patients with atrial fibrillation: a PROTECT AF (percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation) substudy. J Am Coll Cardiol 2012;59:923–929. [DOI] [PubMed] [Google Scholar]
- 76. Berti S, Themistoclakis S, Santoro G, De Ponti R, Danna P, Zecchin M, Bedogni F, Padeletti L; Societa Italiana di Cardiologia Invasive; Associazione Italiana di Aritmologia e Cardiostimolazione. Documento di posizione GISE/AIAC sui requisiti di processo diagnostico ed interventistico riferiti al trattamento della chiusura percutanea dell’auricola sinistra in pazienti affetti da fibrillazione atriale non valvolare. G Ital Cardiol 2014;15:508–519. [DOI] [PubMed] [Google Scholar]
- 77. Regazzoli D, Ancona F, Trevisi N, Guarracini F, Radinovic A, Oppizzi M, Agricola E, Marzi A, Sora NC, Della Bella P, Mazzone P.. Left atrial appendage: physiology, pathology, and role as a therapeutic target. Biomed Res Int 2015;2015:205013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Molon G, Canali G, Casu G, Mazzone P, Barbato G, Ramondo A, Saccà S, Scaglione M, Senatore G, Solimene F, Grassi G, Nardi S, Luise R.. G Ital Cardiol 2015;16:188–189. [DOI] [PubMed] [Google Scholar]
- 79. Bayard YL, Omran H, Neuzil P, Thuesen L, Pichler M, Rowland E, Ramondo A, Ruzyllo W, Budts W, Montalescot G, Brugada P, Serruys PW, Vahanian A, Piéchaud JF, Bartorelli A, Marco J, Probst P, Kuck KH, Ostermayer SH, Büscheck F, Fischer E, Leetz M, Sievert H.. PLAATO (percutaneous left atrial appendage transcatheter occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention 2010;6:220–226. [PubMed] [Google Scholar]
- 80. Price MJ, Gibson DN, Yakubov SJ, Schultz JC, Di Biase L, Natale A, Burkhardt JD, Pershad A, Byrne TJ, Gidney B, Aragon JR, Goldstein J, Moulton K, Patel T, Knight B, Lin AC, Valderrábano M.. Early safety and efficacy of percutaneous left atrial appendage suture ligation. Results from the US Transcatheter LAA Ligation Consortium. J Am Coll Cardiol 2014;64:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Franzen OW, Klemm H, Hamann F, Koschyk D, von Kodolitsch Y, Weil J, Meinertz T, Baldus S.. Mechanisms underlying air aspiration in patients undergoing left atrial catheterization. Catheter Cardiovasc Interv 2008;71:553–558. [DOI] [PubMed] [Google Scholar]
- 82. Calvo N, Salterain N, Arguedas H, Macias A, Esteban A, García de Yébenes M, Gavira JJ, Barba J, García-Bolao I.. Combined catheter ablation and left atrial appendage closure as a hybrid procedure for the treatment of atrial fibrillation. Europace 2015;17:1533–1540. [DOI] [PubMed] [Google Scholar]
- 83. Fassini G, Conti S, Moltrasio M, Maltagliati A, Tundo F, Riva S, Dello Russo A, Casella M, Majocchi B, Zucchetti M, Russo E, Marino V, Pepi M, Tondo C.. Concomitant cryoisolation of pulmonary veins and mechanical closure of left atrial appendage in patients with atrial fibrillation. Europace 2016;18:1705–1710. [DOI] [PubMed] [Google Scholar]
- 84. Swaans MJ, Post MC, Rensing BJ, Boersma LV.. Ablation for atrial fibrillation in combination with left atrial appendage closure: first results of a feasibility study. J Am Heart Assoc 2012;1:e002212. [DOI] [PMC free article] [PubMed] [Google Scholar]