Abstract
Aortic stenosis is one of the most frequent valvular diseases in developed countries, and its impact on public health resources and assistance is increasing. A substantial proportion of elderly people with severe aortic stenosis is not eligible to surgery because of the advanced age, frailty, and multiple co-morbidities. Transcatheter aortic valve implantation (TAVI) enables the treatment of very elderly patients at high or prohibitive surgical risk considered ineligible for surgery and with an acceptable life expectancy. However, a significant percentage of patients die or show no improvement in quality of life (QOL) in the follow-up. In the decision-making process, it is important to determine: (i) whether and how much frailty of the patient influences the risk of procedures; (ii) how the QOL and the individual patient’s survival are influenced by aortic valve disease or from other associated conditions; and (iii) whether a geriatric specialist intervention to evaluate and correct frailty or other diseases with their potential or already manifest disabilities can improve the outcome of surgery or TAVI. Consequently, in addition to risk stratification with conventional tools, a number of factors including multi-morbidity, disability, frailty, and cognitive function should be considered, in order to assess the expected benefit of both surgery and TAVI. The pre-operative optimization through a multidisciplinary approach with a Heart Team can counteract the multiple damage (cardiac, neurological, muscular, respiratory, and kidney) that can potentially aggravate the reduced physiological reserves characteristic of frailty. The systematic application in clinical practice of multidimensional assessment instruments of frailty and cognitive function in the screening and the adoption of specific care pathways should facilitate this task.
Keywords: Aortic stenosis, Elderly, TAVI, SAVR, Risk score, Frailty, Prognosis, Geriatric assessment
Revised by Roberto Antonicelli, Roberto Caporale, Donatella del Sindaco. Silvio Klugmann, Gennaro Santoro
Consensus Document Approval Faculty in appendix
Introduction
Aortic stenosis (AS) is one of the most common acquired valvular abnormalities in developed countries, with an increasing prevalence due to the ageing population.1–3 The prognosis of AS is relatively benign in the absence of symptoms; however, an incidence of sudden death between 1% and 3% must be taken into account. The onset of symptoms coincides with a dramatic reduction in life expectancy, with a median survival of 2–3 years in patients with angina or syncope and only 1–2 years in symptomatic patients with heart failure.3,4
Surgical aortic valve replacement (SAVR) remains the gold standard of care;5 however, at least 40% of potential patients are not candidate because of the prohibitive nature of their co-morbidities and consequent perioperative risk.6,7 Consistent with the epidemiological changes, in clinical practice, about three-quarters of patients with isolated SAVR receive a bioprosthesis.8 Advanced age alone cannot be considered an obstacle to surgery, but medical options are limited. Elderly patients who do not receive a SAVR have a higher risk of mortality compared with those treated surgically.9 Isolated SAVR can be performed in octogenarians with low post-operative mortality10 and result in significant improvement in quality of life (QOL), symptoms, and functional capacity.11 In addition, cost-effectiveness analyses have shown that SAVR is convenient also for very elderly patients.12
In the last years, transcatheter aortic valve implantation (TAVI) has emerged as a less invasive treatment strategy in high-risk patients, allowing the treatment of more complex, elderly patients, with severe symptomatic AS, previously considered ineligible for surgery.13–15 However, even today, a considerable percentage of these patients die or do not present a significant improvement in dyspnoea, fatigue, and functional impairment. This observation has raised a lively discussion on the need to identify and recognize the boundaries of indications for surgical and interventional procedures and, consequently, identify a possible futility in some patients.16 The decision-making process in this population is difficult because of co-morbidity, disability, frailty, and reduced life expectancy, and these factors, as well as traditional ones, should be considered in risk stratification. It is likely that TAVI will be used in an increasing number of AS patients, but its exact role alongside surgery will need to be defined in a judicious and evidence-based manner. The assessment by a multidisciplinary team is therefore essential to predict possible benefits and allow to make complex decisions with a clear communication to the patient. The decision that surgical treatment or with TAVI is useless/futile should include alternative routes to optimize the patient’s health state and to consider options for assistance to the terminal stages.17
Heterogeneity and complexity
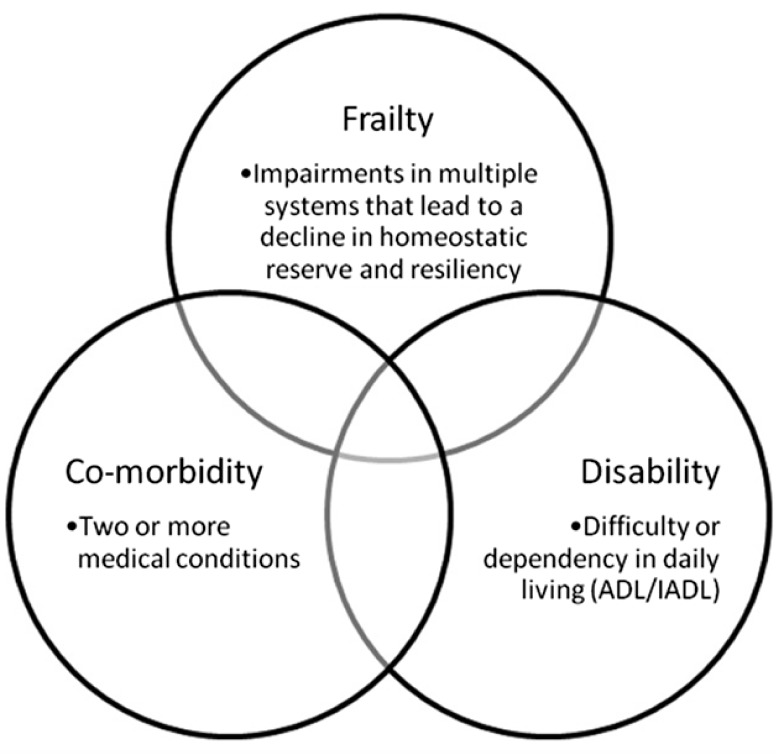
The peculiar feature of the elderly patient can be summarized in two words: phenotypic heterogeneity and complexity. In these two dimensions describe the effects of cardiovascular ageing, heart disease, lifestyle, and socio-environmental factors and three different entities: co-morbidity, disability, and frailty (Figure 1).18 Complexity considers not only the sum of all coexisting diseases and geriatric conditions but also their mutual interactions. From a conceptual point of view, therefore, the elderly person is in himself/herself a complex patient.
Figure 1.
Frailty, co-morbidity and disability. Modified from Afilalo et al.18 BADL: basic activities of daily living; IADL: instrumental ADL.
An accurate pre-operative/pre-procedural risk stratification in the elderly person should identify those who benefit from the intervention, exclude those who have a prohibitive risk, and identify those who need more intensive care in the post-operative period. In this sense, the ideal tool for risk stratification in the elderly person waiting for cardiac surgery should be reliable and targeted at easily obtainable variables. Any tool should be tested in a real-world ageing population with different degrees of frailty or disability, pointing out that the two concepts are interdependent but not synonymous. Pre-operative evaluation in the elderly patient should collect information on physical functional status, cognitive function, and non-cardiac co-morbidities of prognostic importance.19
Co-morbidity is defined as the simultaneous presence of two or more diseases in the same patient, an event that increases with age.20 About 16% of patients >65 years have co-morbidity with two or more diseases, a percentage that increases to 35% in octogenarians.21 At this age, chronic co-morbidities are associated with a higher risk of death, rehospitalization, disability, and reduced QOL beyond those related to individual disorders. Co-morbidity plays a central role in the implementation of evidence-based medicine in clinical geriatrics, because trials have often excluded older subjects with co-morbidities, raising questions about the transferability of the results obtained in the geriatric populations.22 Co-morbidity also becomes crucial in influencing the diagnostic–therapeutic process, because the onset of symptoms can be different from the usual and makes the interpretation of symptoms and signs of the index disease more difficult.
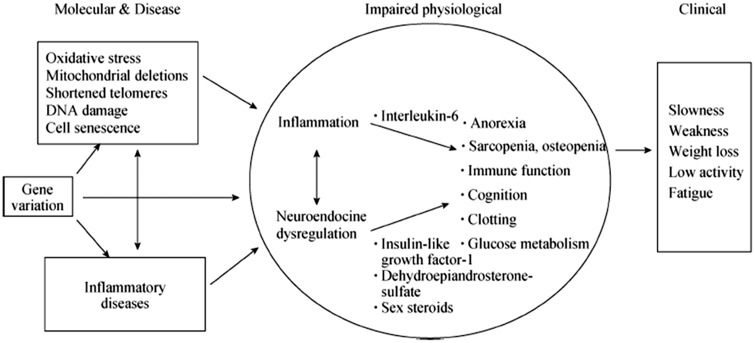
The elderly candidate to heart surgery is, regardless of his death risk, susceptible to frequent serious complications. These often result in a cascade of negative events that converge to lose autonomy in carrying out the activities of daily living, leading to significant health-care costs and decreased QOL. This phenotype of reduced homeostatic physiological reserves and greater vulnerability to stressful events is described as frailty.23 The complex pathophysiological substrate of frailty is described in Figure 2.24 Frailty has been defined ‘a condition or syndrome that results from a multi-systemic reduction in homeostatic reserve, to the extent that the physiological systems are close to the threshold of symptomatic clinical failure. Consequently the frail person is at increased risk of disability and death for a minimum external stress’.25 The concept of frailty (although the two conditions are frequently overlapping) is not synonymous of disability nor is equivalent to the concept of co-morbidity (Figure 1).
Figure 2.
Pathophysiology of fragility. Modified fromWalston et al.24
Given this definition, there are basically two conceptual models of frailty: the ‘frailty phenotype’26 and the ‘clinical frailty phenotype’.27 The first substantially recognizes a set of five domains such as unintentional weight loss, muscle strength measured by handgrip, self-reported fatigue, gait speed, and self-reported physical activity.26 The second model is based on a ‘deficit accumulation model of frailty’ and has been built from a list multiple items, both functional and clinical, exploring the physical, cognitive, and independence in activities of daily living, with a final score ranging from 0 to 7 for increasing frailty.27 Both models have been tested in the stratification of the elderly patient’s surgical risk but also showing some of the limitations related to the complexity of their clinical routine implementation.
The negative impact of frailty on prognosis of cardiovascular diseases has been demonstrated in a wide range of conditions, including stable cardiovascular disease,28 heart failure,29 ischaemic heart disease and acute coronary syndromes,30 cardiac surgery,31,32 and TAVI.33,34
Disability is defined by the level of dependence in performing basic and instrumental activities of daily living, i.e. basic activities of daily living (BADL)35 and instrumental activities of daily living (IADL).36
In the recent US TAVI guidelines,16 a specific section—Frailty and futulity versus Utility—was included, demonstrating that when deciding a therapeutic intervention in elderly subjects, the assessment of frailty and the risk of disability is a key variable for deciding the effectiveness and utility of the intervention itself.
Outcomes of surgical and interventional procedures in the elderly persons
Results of surgical aortic valve replacement
Overall, the outcomes of surgery for severe AS are excellent in patients with moderate-to-low surgical risk. A meta-analysis of 48 observational studies in 13 216 patients >80 years, undergoing isolated aortic valve surgery10 showed an immediate post-operative mortality of 6.7% (5.8% for patients treated from 2000 to 2006 and 7.5% in patients treated from 1982 to 1999). The post-operative stroke rate was 2.4%, dialysis of 2.6%, and pacemaker implant 4.6%. The 1-, 3-, 5-, and 10-year mortality rates after isolated SAVR were approximately 12.4%, 21.3%, 34.7% and 70.3%, respectively. These data confirm that advanced age, as the only risk factor, cannot be considered a contraindication to conventional isolated SAVR. In the Society of Thoracic Surgeons (STS) database, mortality at 30 days after isolated SAVR over 80 years was 3.7% in low-risk patients but rose to 10% and 17%, respectively, in patients with STS score ≥5 and ≥10.37 Octogenarians also report improvements in symptoms and ventricular function.38
Results of transcatheter aortic valve implantation in patients at high or prohibitive risk
On the basis of two main randomized trials and various observational studies, recent guidelines2 indicate TAVI as an option of choice in patients deemed inoperable because a prohibitive risk (with results far superior to medical therapy alone) and a reasonable alternative to SAVR in those at high risk (Table 1).13–15,39–47
Table 1.
Risk assessment combining STS risk estimate, frailty, major organ system dysfunction, and procedure-specific impediments4
| Low risk (must meet all criteria in this column) | Intermediate risk (any 1 criterion in this column) | High risk (any 1 criterion in this column) | Prohibitive risk (any 1 criterion in this column) | |
|---|---|---|---|---|
| STS PROMa | <4% AND | 4–8% OR | >8% OR | Predicted risk with surgery of death or major morbidity (all-cause) >50% at 1 year OR |
| Frailtyb | None AND | 1 Indice (lieve) OR | ≥2 Indices (moderate-severe) OR | |
| Major organ system compromise not to be improved postoperativelyc | None AND | 1 Organ system OR | No more than 2 organ system OR | ≥3 Organ system OR |
| Procedurespecific impedimentd | None | Possible | Possible | Severe |
aUse of the STS PROM to predict risk in a given institution with reasonable reliability is appropriate only if institutional outcomes are within 1 standard deviation of STS average observed/expected ratio for the procedure in question.
bSeven frailty indices: Katz activities of daily living (independence in feeding, bathing, dressing, transferring, toileting, and urinary continence) and independence in ambulation (no walking aid or assist required or 5 m walk in < 6 s). Other scoring systems can be applied to calculate no, mild-, or moderate-to-severe frailty.
cExamples of major organ system compromise: Cardiac—severe LV systolic or diastolic dysfunction or RV dysfunction, fixed pulmonary hypertension; CKD Stage 3 or worse; pulmonary dysfunction with FEV1 <50% or DLCO2 <50% of predicted; CNS dysfunction (dementia, Alzheimer’s disease, Parkinson’s disease, CVA with persistent physical limitation); GI dysfunction—Crohn’s disease, ulcerative colitis, nutritional impairment, or serum albumin <3.0; cancer—active malignancy; and liver—any history of cirrhosis, variceal bleeding, or elevated INR in the absence of VKA therapy.
dExamples include tracheostomy present, heavily calcified ascending aorta, chest malformation, arterial coronary graft adherent to posterior chest wall, and radiation damage.
CKD, chronic kidney disease; CNS, central nervous system; CVA, stroke; DLCO2, diffusion capacity for carbon dioxide; FEV1, forced expiratory volume in 1 s; GI, gastrointestinal; INR, international normalized ratio; LV, left ventricular; PROM, predicted risk of mortality; RV, right ventricular; STS, Society of Thoracic Surgeons; and VKA, vitamin K antagonist.
In the PARTNER trial (Cohort A), which included patients with high operative risk,44 patients undergoing SAVR had a higher incidence of bleeding and a lower incidence of vascular complications, compared with those undergoing TAVI at 5 years. The percentage of residual aortic moderate and severe insufficiency was 14% in patients undergoing TAVI and 1% of patients undergoing SAVR (P <0.0001) and, if moderate and severe, correlated with an increase in mortality.
The data of Registries confirm those of trials. In the GARY (Germany Aortic Valve Registry)48 hospital mortality was 5.2% and serious complications occurred in 5% in patients undergoing TAVI from 2011 to 2013. In the FRANCE TAVI Registry, enrolling 6827 patients undergoing TAVI in 2013 and 2014, the hospital mortality was 5.9%, the incidence of major bleeding was 9.3%, stroke 2.2%, need for pacemaker 15%, and severe aortic insufficiency 1.2%.49 The Italian OBSERVANT study analysed propensity-matched patients undergoing TAVI or SAVR. The mean logistic EuroSCORE 1 was 10.2 in SAVR group and 9.5 in the TAVI group. No significant differences were recorded in mortality (13.6% and 13.8%), MACE rate (17.6% vs. 18.2%), and in hospitalizations for cardiac causes.50
The recent STS–TVT Registry confirms that typical TAVI patients are highly symptomatic, frail elderly with multiple co-morbidities, a high STS predicted risk of mortality (STS PROM) score, advanced functional class, and a poor health status. From 2012 to 2014, in-hospital mortality decreased from 5.3% to 4.4%, vascular complications from 5.6% to 4.2%, while stroke rates remained stable at 2.2%.51 In this Registry, ∼16% of patients were aged >90 years. Although 30-day and 1-year mortality rates were statistically higher compared with younger patients, the absolute and relative differences were clinically modest. TAVI also improved QOL to the same degree in nonagenarians as in younger patients.52
Results of transcatheter aortic valve implantation in low-intermediate risk patients
NOTION study randomized low-risk patients >70 years to SAVR or TAVI (STS score 2.9% vs. 3.1%.53 At 1 year, the primary endpoint (death, stroke, or myocardial infarction) showed no significant differences. Patients undergoing surgery had a better functional outcome and those who underwent TAVI had a significantly higher incidence of moderate or severe peri- or intraprosthetic regurgitation (15.7% vs, 0.9%, P <0.001).53
In PARTNER 2 study,54 patients at intermediate risk (mean STS score 5.8%, mean age 81.6 years) randomized to TAVI with self-expandable SAPIEN XT prosthesis and SAVR. There was no significant difference in the primary endpoint of death from any cause or disabling stroke at 2 years between the two groups. Another randomized trial, SURTAVI, designed to evaluate the safety and efficacy of TAVI in intermediate surgical risk patients, will evaluate 2500 patients with STS score between 4 and 10. The primary endpoint is composed of mortality and disabling stroke at 24 months. Patients will be followed for 5 years (www.clinicaltrials.gov).
Procedural complications of transcatheter aortic valve implantation
Mortality is strongly conditioned by periprocedural complications, represented by stroke (1–5%), pacemaker implantation (7–40%), and vascular complications (up to 20%).55–57 The improvement of the femoral approach techniques has resulted in a reduction in major bleeding.56 In a recent meta-analysis57 of randomized trials and observational studies, the incidence of stroke was 2.9%, regardless the prosthesis used. That effect was not, however, different than in patients treated with SAVR.47
The main Achilles heel of TAVI compared with SAVR is represented by periprosthetic leak, that, if moderate to severe, is one of the major predictors of mortality.14,39,57–59 Numerous studies have shown that, for this complication, results are in favour of surgery.59 In the recent PARTNER 2, the incidence and severity of paravalvular regurgitation after TAVI were more frequent compared with SAVR, and TAVI patients with moderate-to-severe paravalvular regurgitation at 30 days had a higher mortality at 2 years than those without or with mild regurgitation.53 Proper sizing of the annulus, a better understanding of the procedure and optimization of the immediate result, may lead to a gradual reduction in incidence of this complication.
In the subgroup of inoperable, symptomatic patients at excessive risk for TAVI, valvuloplasty balloon can be a procedure of choice or bridge for further treatment.60 In this context, valvuloplasty could also be used to verify whether the patient’s frailty is related to valvular disease or not.
Clinical risk assessment
Clinical and imaging parameters
Risk stratification has assumed a key role in patient selection and recent guidelines from the European Society of Cardiology have stressed the importance of a multidisciplinary ‘Heart Team’ approach to help determine this risk.42 Appropriate selection is critical for the success of aortic procedures and must take into consideration several clinical and anatomical factors, also in asymptomatic patients, in which treatment remains controversial.61–64 Even patients with mild-to-moderate asymptomatic AS have an excess of mortality and cardiovascular events than the general population.65 Many parameters have been shown to have an important role in predicting adverse events and therefore considered useful for the prognostic stratification.
The echocardiographic parameters include speed of the transvalvular jet,65 average and maximum transvalvular gradients, changes of these parameters over time,66 degree of valve calcification, left ventricular hypertrophy, and excess mass compared with the predicted values (inappropriate mass)67 and increased left atrial systolic force. Main clinical parameters include coexistence of coronary artery disease, advanced age,66 diabetes mellitus,67 increased body mass index,68 worse functional capacity, cigarette smoking, and high arterial blood pressure.4 Finally, biological parameters include increased serum levels of brain-type natriuretic peptide measured at baseline conditions and/or C-reactive protein.4,69
A system to further improve the prognostic stratification of AS patients may also consist in the use of indexes alternative to the classical method of the equation of continuity, such as energy loss index,70 stroke work loss,71 and valvuloarterial impedance.72 A correct stratification must also include a quantification of aortic valve calcifications.
The assessment of systolic function and left ventricle (LV) geometry plays an important role in stratifying a patient’s prognosis. Ejection fraction remains normal for a long time even in the presence of a high chronic pressure overload, while myocardial contractility is reduced significantly.73–75 A reduced midwall shortening has been associated with the onset of symptoms and a worse prognosis.76 The use of recent technologies such as speckle tracking enables to analyse the reduced longitudinal myocardial deformation that identify the AS patients with the greatest risk to transit from the compensatory phase to the pathological remodelling77 and worst prognosis after valve replacement.78 In addition to subclinical deterioration of systolic function parameters, also the changes in LV geometry and hypertrophy are able prognosticators of poor outcome. The presence of an LV mass in excess with respect to the aforementioned theoretical values in the individual patient (inappropriate mass) identifies a subgroup of subjects at higher risk compared with subjects having the same degree of AS but appropriate LV mass.73 An intrinsic myocardial dysfunction in the presence of preserved ejection fraction and severe low flow/low gradient is of clinical and prognostic significance.77–79
Clinical and echocardiographic prognostic scores
A useful method for more accurate risk stratification in patients with AS is based on multiparametric scores.69,80,81 Monin et al.69 first proposed a score based on three variables: female sex, the maximum speed of the transvalvular aortic jet, and baseline BNP. The SEAS score,80 validated in mild-to-moderate AS, is based on seven parameters: age, sex, smoking, heart rate, serum bilirubin, serum levels of C-reactive protein, and LV mass. The ‘CAIMAN–ECHO score’,81 validated in moderate-to-severe AS, is based on three echocardiographic parameters: aortic valve calcium score assessed with conventional transthoracic echocardiography (TTE), maximum speed of transvalvular aortic flow, and inappropriate mass, measured as the ratio of measured and predicted mass.
Role of imaging in patient selection before transcatheter aortic valve implantation or surgical aortic valve replacement
Multimodality imaging plays an essential role in patient selection and procedural planning, performance, and follow-up. In each of these steps, optimal imaging can help to enhance successful outcome. Non-invasive imaging methods used for the selection of patients for TAVI are echocardiography,82 computed tomography (CT), and, less frequently, nuclear magnetic resonance.16,61 There is variability in the preferred imaging protocols in individual institutions, as a result of institutional and individual experience and equipment, and patient characteristics. The aim of the imaging is to: (i) confirm the severity of aortic stenosis; (ii) evaluate the anatomical suitability of revalving procedure and vascular accesses; and (iii) evaluate associated cardiovascular diseases.
Baseline TTE is usually adequate to confirm severity based on ACC/AHA/ESC criteria.61,83,84 A rigorous methodology that takes into account the possible technical difficulties and sources of error is however necessary. In the elderly people, the frequent coexistence of LV dysfunction can cause a discrepancy between low gradients and reduced area (AS with low flow and low gradient). In doubtful cases, low-dose dobutamine stress and anatomical evaluation using planimetric transoesophageal echocardiography (TEE) (preferably with three-dimensional reconstruction) can help differentiate a true AS by a pseudo-stenosis and to assess contractile reserve.85
The evaluation of the anatomic suitability for revalving procedure includes the study of the vascular iliofemoral axis accessibility and of the aortic valve. Multilayer CT is the method of choice for assessing the size of the vessel, inner diameter, tortuosity, degree and extent of calcification, presence of high risk of dissection of complex plaques. This information is required to express an opinion on the possibility of introducing and advancing the guides and catheters and to assess the risk of any vascular complications. In the case of apical access, TTE verify the absence of apical aneurysm with thrombosis that represents a contraindication to this approach.
The anatomy of aortic cusps must have certain characteristics that make it suitable for the proper anchoring of the bioprosthesis.84 The bicuspid valve86 is a relative contraindication to TAVI for the risk of spontaneous aortic dissection or of an incorrect opening of the bioprosthesis due to the elliptical orifice. A mildly calcified aortic valve is also a contraindication to TAVI.87 CT is the method of choice for the study of quantification and distribution of calcifications. Particular attention should be paid in the study of asymmetric distribution that could prevent perfect adhesion of the bioprosthesis and increase the risk of compressing the coronary ostia. The TTE and even more TEE can be used for the study of the number of cusps, their mobility, thickness, and calcification. The aortic rings are four (virtual basal ring, ventricular–arterial ring, crown-like ring, sino-tubular ring).88 The correct aortic valve size ring is crucial to choose the size of the prosthesis89 and prevent complications related to an inadequate size prosthesis implant. The measurement of virtual basal ring, corresponding to the implant of the cusps (nadir) is used for the implantation of the bioprosthesis, rather than the ventricular–arterial ring (located more distally within the aortic root). Multi-slice CT is the method currently considered as the gold standard. The extent of the area of the virtual basal ring, rather than the diameters or the perimeter, is used in many centres to choose the size of the prosthesis.90 Three-dimensional TEE, by multiplanar reconstruction of the rings, is a reliable alternative to CT.91 An outflow tract deformed by the hypertrophied septum similar to that of hypertrophic cardiomyopathy is a contraindication to TAVI. Aortic valve calcifications extending to the outflow tract are associated with a high risk of breaking the ring in case of a balloon-expandable bioprosthesis implant.92 The sigmoid septum, with a very acute angle between outflow and aortic vessel, can make the implantation of a self-expandable bioprosthesis and therefore prefer implantation of a balloon-expandable bioprosthesis difficult.93 A suitable distance between the aortic ring and coronary ostia is required, because compression of calcific aortic valve, during implantation of a balloon-expandable bioprosthesis can occlude the coronary ostium. The distance of the coronary ostia does not affect the self-expandable type. Computed tomography is the method of choice for this measure.94
A dilated ascending aorta is a relative contraindication to the system of self-expandable bioprosthesis, because the upper portion is positioned in the ascending aorta to direct the flow from the valve. Computed tomography and TTE or TEE provide equivalent information.
Left ventricular dysfunction is not a contraindication to TAVI also in view of the improvement observed in many patients undergoing this procedure. The TTE is the method of choice for evaluation of left ventricular function. Mitral regurgitation may be secondary to LV dysfunction or annulus dilation due to atriomegalia/atrial fibrillation, or recognize an organic cause, most frequently the annulus calcification and more rarely a myxoid degeneration or fibroelastica deficiency. The TTE/TEE are the methods of choice for the anatomical and functional assessment of mitral regurgitation.95,96Severe pulmonary hypertension and right ventricular dysfunction represent a contraindication for TAVI. The TTE/TEE are the methods of choice for the study of this associated pathology.
Conventional and new pre-procedural risk scores
Surgical risk stratification requires objective and reliable methods. Of the many risk scores published over the years, those most frequently used for the estimation of short-term mortality risk in adult cardiac surgery are basically three: the EuroSCORE II (online calculator http://www.euroscore.org/calc.html),97 the STS score (online calculator http://riskcalc.sts.org/stswebriskcalc/#/calculate version 2.81),98 and the ACEF score.99 While the EuroSCORE II and ACEF are models applicable for the estimation of the risk of both coronary artery bypass surgery to valvular procedures or combined interventions, STS score offer separate models for isolated valve surgery98 or in combination with myocardial revascularization. The use of these risk scores is recommended by the European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS)42 and the American Heart Association/American College of Cardiology (AHA/ACC)2 guidelines.
The EuroSCORE was developed over 15 years ago, mainly in candidates for bypass coronary artery, while only one-third of patients were candidate for valve surgery.100 Considering the year of publication, the EuroSCORE I is defined ‘out of date’ by the authors and should not be used in favour of the updated version, the EuroSCORE II in which some new variables have been introduced while others have been modified.97 The STS risk score, unlike the EuroSCORE, was developed to identify the risk of death or perioperative complications, taking into consideration the specific type of intervention, coronary surgery, mitral, aortic, or combined.
The main features of a risk score are the power of discrimination—the ability to differentiate between low- and high-risk patients101—and calibration—the ratio between observed and predicted mortality. Table 2 shows the characteristics of EuroSCORE, EuroSCORE II and STS score. A difference between these scores is that the calculation algorithms for EuroSCORE (I and II) and ACEF score are freely available but not periodically updated to the progressive change of patient’s clinical characteristics, while the algorithms of the STS risk scores are not freely available, but are regularly updated on the basis of the STS database. In SAVR, the performance of these algorithms is satisfactory for predicting the risk of category (high or low risk), with areas under the receiver-operating characteristics (ROC) curve ranging between 0.7 (fair discrimination) and 0.8 (good discrimination), even in octogenarians.102–105 These models tend to show more performance problems in calibration, in particular in the assessment of patients usually defined as ‘high’ and ‘very high’ risk.103 In high-risk patients, EuroSCORE, EuroSCORE II, and ACEF overestimate mortality, while STS score tends to underestimate the risk. With regard to the medium- and long-term mortality, these scores show little satisfactory performances both in discrimination (AUC between 0.6 and 0.7) and in calibration.106,107
Table 2.
Comparison of EuroSCORE, EuroSCORE II, and STS score (modified from Nashef et al.97 and O’Brien et al.98)
| EuroSCORE | EuroSCORE II | STS score | |
|---|---|---|---|
| Outcome | In-hospital mortality | 30-day Mortality | Mortalitày and post-operative complications |
| Surgery | Mainly CABG | Not specific | Specific for surgery |
| High risk threshold | >20% | >7% | >10% |
| Discrimination: ability to differentiate between low and high-risk patients (assessed using the ‘under the ROC curve area’ or the c-index) | AUC for valvular surgery = 0.72; | AUC = 0.81 | AUC |
| Acceptable | Acceptable | For lone valvular surgery = 0.80 | |
| For valvular surgery + CABG = 0.75 Acceptable | |||
| Calibration: report predicted/observed mortality | Greatly overestimated mortality in all categories of risk, especially in the high-risk group: suboptimal. Documented calibration loss in time because of the update to EuroSCORE II I | Low-risk group calibration: good | Low-risk group calibration: good |
| Overestimated mortality in high-risk group: suboptimal | Underestimated mortality in high-risk group: suboptimal | ||
| Discrimination in TAVI | Not acceptable | Not acceptable | Not acceptable |
| Calibration in TAVI | Not acceptable | Not acceptable | Not acceptable |
TAVI, transcatheter aortic valve implantation.
Several studies have recently addressed the issue of the applicability of these risk scores to TAVI candidates. Overall, the acceptable discrimination allows to estimate with good approximation risk category, but poor calibration performance prevents from using these scores to estimate the individual operative mortality, especially in the high-risk category.108 In several studies, an average area under the curve around 0.6–0.7 was observed,104,109 a lower performance that could be obtained with surgery.
EuroSCORE II and STS score performance was assessed in a meta-analysis of patients undergoing SAVR or TAVI. In the SAVR group, the difference between observed and expected mortality was not statistically significant with EuroSCORE II, while for the STS score the relationship was closer to 1. On the contrary, both the EuroSCORE II and the STS score have underestimated the risk of death in patients undergoing TAVI, confirming problems with calibration of these scores in this procedure.110
The proportion of patients considered at high surgical risk on the basis of a single score (EuroSCORE II >7%, logistic EuroSCORE >20% and STS score >10%) and the correlation between scores were assessed in patients considered at high surgical risk based on a multiparameter stratification and candidates for TAVI. About half of the patients did not reach the value of high-risk threshold on the basis of the only score. The correlation between logistic EuroSCORE, EuroSCORE II, and STS was modest.111
A recent analysis of the PARTNER trial suggests that performances for prediction of mortality with STS score and the logistic EurSCORE are even less satisfactory (AUCs ≤0.6) for both SAVR and TAVI in very elderly patients.112
In summary, traditional risk scores seem to be inadequate as the only way to identify older patients with severe symptomatic AS at high risk and candidates for TAVI. There is a need to develop and validate specific score for this population.
An Italian multicentre study enrolling patients undergoing TAVI identified a previous stroke, creatinine clearance, and pulmonary hypertension as independent predictors of death at 1 year and calculated a score that showed a better performance than the STS score.113
The TAVI2 score has been developed in a study of patients thought to be at high or prohibitive surgical risk.114 Variables independently associated with mortality at 1 year include: porcelain aorta, anaemia, low ejection fraction, recent myocardial infarction, male gender, mean aortic gradient >70 mmHg, age >85 years, creatinine clearance <30 mL/min. For each variable, a score was assigned and the sum identified the predicted risk of death at 1 year. The TAVI2 score showed improved power of discrimination and calibration compared with logistic EuroSCORE, EuroSCORE II, and the STS score.114 The OBSERVANT study (Efficacy And Effectiveness of AVR-TAVI Procedures For the Treatment Of Symptomatic Severe Aortic Stenosis) has proposed another 30 days death risk score in patients undergoing TAVI.115 Seven independent variables related to the risk of death at 30 days were identified and integrated into a risk score.
Multidimensional assessment
In recent years, the availability of new techniques has extended to an older and frail population effective AS treatments, however, currently used risk scores do not seem to be adequate in these patients.34,116–119 Therefore, it seems necessary to include other variables—i.e. co-morbidities and geriatric conditions—to improve risk stratification and compare the results of the procedures. Functional status is a multidimensional variable and also includes the ability of an individual to carry out daily activities within all functional domains. An overall assessment of functional status often requires multiple validated instruments, but on the other hand, a too complex battery may be little applicable and useful in clinical settings. The major open issues are the uncertainty of an operational definition of ‘frailty’ and what measurement tools to validate and use. However, there is no doubt that cardiac risk assessment of elderly people with heart disease should be performed, taking into account two fundamental points: (i) the instrument is valid, reproducible, and easy to use and (ii) is able to further stratify perioperative risk.
The European Valve Academic Research Consortium (VARC)120 described the importance of measuring clinical benefits such as functional status, but acknowledged the uncertainty that accompanied the choice of various measuring instruments and the overall lack of standardization.121 The Canadian Cardiovascular Society has also encouraged the use of an ‘objective assessment of function and neuro-cognitive Frailty’ to evaluate candidates for TAVI.122 Without providing an operational definition, the American Heart Association (AHA) recommended to include a functional assessment in the selection of elderly TAVI candidates.16
Different tools have been proposed to identify frailty.26,123,124 The frailtyindex26,123 includes five easily detectable characteristics: muscle strength (handgrip), gait speed, weight loss, exhaustion, and level of activity. The subject that has no deficiency in any item is considered ‘robust’, ‘pre–frail’ if it has a deficit in one or two, ‘frail’ if it is positive in three or more of the items.26 The predictive value of frailty is independent of co-morbidities and clinical disability. The identification of frailty in the elderly people should not be made only for prognosis but should lead to ‘pre-rehabilitation’ interventions125 that have proven effective in the prevention of functional decline and mortality.
It is still debated whether disability and cognitive impairment should be considered frailty domains or just modulating factors that catalyse the transition from frailty to manifest disability.126
The clinical evaluation of frailty as accumulation of deficits requires the evaluation of up to 70 symptoms, signs, morbidities, disability, and frailty, and for this reason, a simplified version was developed.127 However, disability, generally defined as difficulty or dependency in ADL or IADL, should be distinguished from frailty. Disability is more accurately conceptualized as a negative outcome associated with frailty or as an entirely separate entity. The International Academy Nutrition and Aging Frailty Task Force128 prefers the functional approach, stating that co-morbidity and disability must be separated from frailty.
A simple means for identification of frailty is the Short Physical Performance Battery (SPPB), which consists of three items129: reduced gait speed, weakness in standing up from a chair, and reduced balance in three positions. A score from 0 to 4 is assigned to each item, where a total score >5 of 12 indicates the presence of frailty. The SPPB predicts the risk of disability and death in the elderly general population130 and with heart failure.131 As an alternative to these composite scores, individual variables such as gait speed on 4–5 m distance and handgrip force have been proposed as a single valid, simple, and reliable marker of frailty.132,133
Several instruments and measures to assess the various components of functional status, QOL, disability, cognitive impairment, and various measures of frailty have been considered in pre-operative evaluation before TAVI.116–118
In PARTNER study,134 TAVI resulted in significant improvement in NYHA Class and QOL, without reporting results on disability.
Another study reported that elderly patients undergoing TAVI were significantly more likely to develop clinically significant cognitive decline than those who underwent SAVR, but it is difficult to determine whether the results can be attributed to old age, reduced level of education, co-morbidity, or to the different treatment.135
Therefore, recent studies have focused their interest on the concept of frailty and its importance in risk stratification before SAVR or TAVI.
Afilalo et al.31 have shown in elderly patients undergoing coronary and valvular surgery that a low gait speed on 5 m identified a subpopulation at high-risk mortality and morbidity. The speed of <0.83 m/s was chosen as the optimal threshold based on ROC curves. Importantly, gait speed had an incremental value when combined with the STS score in predicting incidence of mortality/morbidity. There was tendency towards interaction for female patients and those undergoing SAVR, both of which had a much higher relative risk in the presence of frailty.
Lee et al.136 and Sündermann et al.137,138 have demonstrated that pre-operative frailty is associated with post-operative 30-day and long-term mortality. These two studies differed in frailty scales used and in the results. Lee et al.,136 defined frailty as a dependency in walking, disability in ADLs, or dementia, a definition that includes more a concept of disability than frailty. Sündermann et al.137,138 defined frailty as an aggregate of 35 criteria reporting a prevalence of 50%.
In another study, Afilalo et al.32 observed a prevalence of 46% and 20% of frailty, using respectively, gait speed and Fried index, and a 5% prevalence of ADL disability; the measurement of gait speed alone was superior to the other scales in predicting outcomes.
Some studies have been conducted specifically on patients undergoing TAVI. Ewe et al.140 found that one-third of patients undergoing TAVI were frail according to Fried index and that frailty was among the most powerful predictors of death, myocardial infarction, stroke, or heart failure at 9 months. Frailty was not a significant predictor of outcome if defined only according to subjective judgement of the physician in the study by Rodés-Cabau et al. 39 Green et al.33 observed that frailty was predictive of 1-year mortality, but not at 30 days. There was a trend towards increased risk for major bleeding, vascular complications, and length of stay in frail patients. A gait speed of 0.50 m/s was selected as the optimal threshold, slower than the 0.65–0.85 m/s reported in other settings. The authors observed that >80% of their patients would be considered frail if they had used the traditional cut-off values. Only 19%–35% of patients were able to complete the gait speed test. This significant percentage of non-walkers, larger than usually reported for other cohorts, may reflect the heavy burden of morbidity and disability in patients undergoing TAVI. The impossibility to complete gait speed test was an indicator of advanced frailty or ADL disability.
In another study,34,139 where the vast majority of patients were able to complete the timed-up-and-go (TUG) test (which requires getting up from a chair and walk 3 m), 61% were able to do it in faster than 20 s. The frailty composite index used included TUG test, limitation of mobility, BADL and IADL disability, cognitive impairment, and nutritional assessment. Frailty was predictive of a three- to four-fold increase of functional decline in BADL at 6 months and higher incidence of cardiac and cerebral adverse events at 1 year. A trend for frailty and mortality was observed, which was stronger at 30 days compared with 1 year, although the number of events was small.
In all these studies, the overall approach to the evaluation of frailty aimed at predicting morbidity and mortality following TAVI involved the use of multidimensional assessment tools, including cognitive function, gait, nutritional status, and activities of daily living.34,139
The presence of disability is uncommon in the cardiac surgery population, partly because disabled patients are less likely to be considered for surgery. Therefore, the scales of disability for basic ADL are relatively insensitive to screen elderly patients in this context. Higher level disability scales such as the Nagi Scale145 are more sensitive and better predict outcomes. An interaction between frailty and disability was still observed, with the prognostic weight of frailty which decreases progressively in patients with more advanced stages of disability.32
An attempt to combine clinical risk scores and geriatric evaluation is reported in recent guidelines,16 where frailty indices proposed include gait speed and disability indicators such as those that contribute to the ADL score (Table 1). Frailty is summarily stratified as follows: not frail (able to carry out all ADL and to walk 5 m in <6 s), pre-frail (unable to perform one ADL or to walk 5 m >6 s), and moderately–severely frail (unable to perform >2 ADL). In addition to this risk classification, it would be appropriate to defer any kind of intervention in patients with reduced life expectancy.
In summary, it is not yet clear whether the standard of frailty assessment tools (frailty index) or surrogate measures, such as single physical performance test (gait speed, SPPB, and handgrip) are sufficiently valid if used alone, or if these measures should be combined with disability (inability to walk, low albumin, and ADL disability), a cognitive and nutritional screening or psychoemotional state to better discriminate the risk.126
In clinical practice, the feasibility of variables and speed of execution are fundamental as much as the reliability of the instruments themselves. Future studies should aim to develop more reliable and reproducible ways to identify frailty and validate its use to estimate risk and expected benefits.
Recent studies incorporate measures of frailty such as CoreValve,53 and Partner II.118 FRAILTY-AVR (NCT01845207) compared different frailty instruments to determine which is more predictive in high-risk patients with AS undergoing TAVI and SAVR.142 A European register (European CGA-TAVI registry) has been scheduled for the same purpose.143
Gait speed over 5 m has been added to the STS database. In this database, patients undergoing TAVI were classified in very slow walkers (<0.5 m/s), slow walkers (0.5–0.83 m/s), and normal walkers (>0.83 m/s), and 30-day all-cause mortality rates were 8.4%, 6.6%, and 5,4%, respectively (P <0.001). Each decrease of 0.2 m/s speed corresponded to an increase of 11% in 30-day mortality. Very slow walkers had 35% higher 30-day mortality compared with normal, longer hospital admissions and were less likely to be discharged home.144 Pending further results, it is reasonable to apply a decision algorithm based on an initial screening carried out with a gait speed test, followed by a more thorough multidimensional assessment in patients with indicative results of Frailty (Figure 3).147
Figure 3.
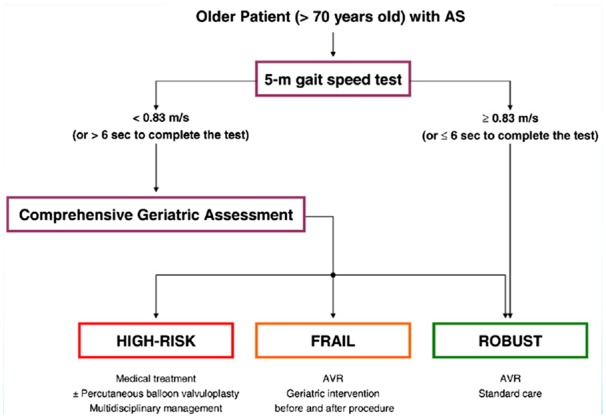
Decision-making algorithm for the initial screening of the frailty and the selection of elderly patients with SA. Modified from Lilamand et al.151
Conclusions
TAVI enables the treatment of very elderly patients with severe AS considered ineligible for surgery and with an acceptable life expectancy,42 with good results in terms of survival and symptoms. However, many patients still develop complications and die or show no improvement in QOL at follow-up. These issues raise important questions on the need to identify and recognize the possible futility of treatments in some patients approaching the final stages of life, where the clinical condition is too advanced and in which even a technically successful procedure is useless and does not improve health outcomes. Thus, in the decision-making process, it is important to determine: (i) whether and how much frailty affects the risk of the procedures; (ii) whether the QOL and the individual survival are influenced by aortic valve disease alone or from other factors; (iii) whether a geriatric specialist intervention to evaluate and correct co-morbid diseases, frailty, and disabilities can further improve outcomes.
Although futility may be invoked to justify the refusal to treat an individual patient, the threshold for the definition of futility itself is still not clear. In inoperable patients, therefore, a clear definition of the conditions that may adversely affect survival and QOL, despite the procedural success is essential to ensure that this therapy is properly used in patients who can benefit or not. These patients should receive targeted medical and palliative care.148
As TAVI patients are usually very elderly, with high risk score, advanced functional class, and frailty,144,51 to assess the expected benefit of TAVI, a number of variables should be considered in addition to the traditional risk scores. The evaluation of patient’s complexity can provide a valuable prognostic contribution and assist cardiologists and cardiac surgeons in the definition of the optimal treatment in the individual patient.17 Moreover, complexity therefore is not a sufficient reason to refuse a certain treatment but rather a means to choose a personalized and more patient-centred care.
At present time, the evaluation of the operative risk is carried out using standard indices that have not been calibrated to intercept all the risk factors of very elderly patients and do not estimate frailty with specific parameters. An attempt is reported in the recent guidelines,2 where a combination of frailty (reduced gait speed), disability indicators, and cognitive deficit are proposed. Probably the combination of traditional risk scores such as EuroSCORE II and STS score with indices of frailty and co-morbidity could better refine the assessment of the operative risk in SAVR, while specific risk scores should be used in TAVI.
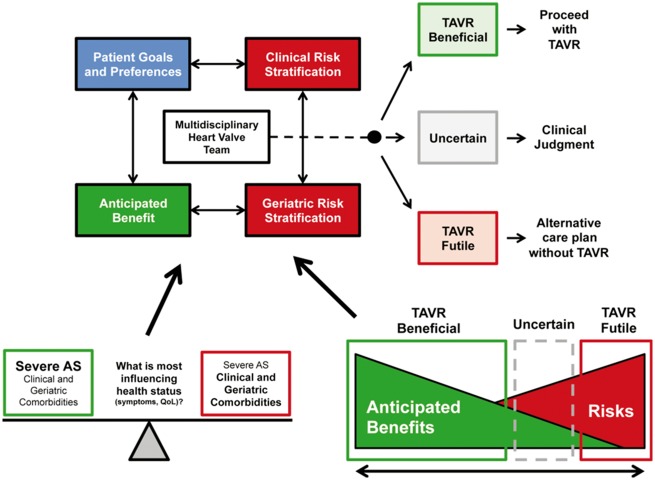
Moreover, because frailty is a physiological phenotype and may be reversible in some cases, it is premature to consider this a permanent feature of the patient. To the extent that AS may contribute to the decline in functional status, SAVR or TAVI may reduce or cancel the amount of frailty which depends on it. In this case, frailty can be a marker to identify the benefit of treatment. Conversely, if the individual is frail due to the decline of multiple organ systems, frailty may be a risk marker. Pre-operative optimization through a multidisciplinary approach with a Heart Team can counteract the multiple damage that can potentially aggravate the reduced physiological reserves characteristic of frailty (Figure 4).
Figure 4.
Algorithm decision by the multidisciplinary team of patients undergoing TAVI. Modified fromLindman et al.17
Finally, ensuring the best appropriateness and cost-effectiveness of care in times of economic restraints is vital for modern health systems.149 Technological progresses in new implants and techniques could lead to a reduction in costs and procedural complications, while the systematic application of a standardized multidimensional assessment150 (Table 3) should improve outcomes.
Table 3.
Pre-procedure screening recommendations. Modified from ref.150
| Laboratory indices | Full blood count, serum urea, creatinine and electrolytes, C-reactive protein, serum transaminases, serum albumin, coagulation profile, blood culture, sputum culture, mid-stream urine, glycosylated haemoglobin, human immunodeficiency virus, and hepatitis serology |
| Physical indices | Height, weight, and body mass index |
| Clinical data to calculate logistic EuroSCORE or STS score | Detailed clinical history, examination and current medication list, 12-lead electrocardiography, echocardiography (transthoracic/transoesophageal), coronary angiography, peripheral vascular screening (contrast angiography/multidetector computed tomography), pulmonary function testing, and right heart catheterization |
| Clinical parameters of co-morbid conditions | Pulmonary function tests, carotid, and vertebral and abdominal ultrasonography |
| Frailty and cognitive functiona | Grip strength, graded exercise testing, walk test, physical activity level, and mini-mental score |
| Confirmation of aortic stenosis severity and assessment of associated pathology | Echocardiography (transthoracic/transesophageal), exercise stress testing, and stress echocardiography |
| Procedural planning |
|
STS, Society of Thoracic Surgeons.
aFried frailty index.
Consensus Document Approval Faculty
Abrignani Maurizio Giuseppe, Alunni Gianfranco, Amico Antonio Francesco, Amodeo Vincenzo, Angeli Fabio, Aspromonte Nadia, Battistoni Ilaria, Bianca Innocenzo, Bisceglia Irma, Bongarzoni Amedeo, Cacciavillani Luisa, Calculli Giacinto, Caldarola Pasquale, Capecchi Alessandro, Caretta Giorgio, Carmina Maria Gabriella, Casazza Franco, Casolo Giancarlo, Cassin Matteo, Casu Gavino, Cemin Roberto, Chiarandaà Giacomo, Chiarella Francesco, Chiatto Mario, Cibinel Gian Alfonso, Clerico Aldo, Colivicchi Furio, De Luca Giovanni, De Maria Renata, Di Fusco Stefania Angela, Di Lenarda Andrea, Di Tano Giuseppe, Egman Sabrina, Enea Iolanda, Fattirolli Francesco, Ferraiuolo Giuseppe, Francese Giuseppina Maura, Gabrielli Domenico, Geraci Giovanna, Giardina Achille, Gregorio Giovanni, Iacoviello Massimo, Khoury Georgette, Ledda Antonietta, Luca Fabiana, Lukic Vjerica, Macera Francesca, Marini Marco, Maseri Attilio, Masson Serge, Maurea Nicola, Mazzanti Marco, Mennuni Mauro, Menotti Alberto, Menozzi Alberto, Mininni Nicola, Moreo Antonella, Moretti Luciano, Mortara Andrea, Mureddu Gian Francesco, Nardi Federico, Navazio Alessandro, Nicolosi Gian Luigi, Oliva Fabrizio, Parato Vito Maurizio, Parrini Iris, Pini Daniela, Pirelli Salvatore, Radini Donatella, Rao Carmelo Massimiliano, Rasetti Gerardo, Riccio Carmine, Roncon Loris, Rossini Roberta, Ruggieri Maria Pia, Rugolotto Matteo, Sanna Fabiola, Sauro Rosario, Scherillo Marino, Severi Silva, Sicuro Marco, Sisto Francesco, Tarantini Luigi, Uguccioni Massimo, Urbinati Stefano, Valente Serafina, Vatrano Marco, Vianello Gabriele, Vinci Eugenio, Zuin Guerrino.
Conflict of interest: none declared.
References
- 1. Iung B, Vahanian A.. Epidemiology of acquired valvular heart disease. Can J Cardiol 2014;30:962–970. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin IIIJP, Guyton RA, O'gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD.. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, American Society of Echocardiography, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. Circulation 2014;129:e521–ee64.24589853 [Google Scholar]
- 3. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M.. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 4. Stewart BF, Siscovick D, Lind BK.. Clinical factors associatedwith calcific aortic valve disease. J Am Coll Cardiol 1997;29:630–634. [DOI] [PubMed] [Google Scholar]
- 5. Lindman BR, Bonow RO, Otto CM.. Current management of calcific aortic stenosis. Circ Res 2013;113:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iung B, Cachier A, Baron G.. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005;26:2714–2720. [DOI] [PubMed] [Google Scholar]
- 7. Freed BH, Sugeng L, Furlong K, Mor-Avi V, Raman J, Jeevanandam V, Lang RM.. Reasons for nonadherence to guidelines for aortic valve replacement in patients with severe aortic stenosis and potential solutions. Am J Cardiol 2010;105:1339–1342. [DOI] [PubMed] [Google Scholar]
- 8. Furukawa H, Tanemoto K.. Current status and future perspectives of prosthetic valve selection for aortic valve replacement. Gen Thorac Cardiovasc Surg 2014;62:19–23. [DOI] [PubMed] [Google Scholar]
- 9. Kojodjojo P, Gohil N, Barker D, Youssefi P, Salukhe TV, Choong A.. Outcomes of elderly patients aged 80 and over with symptomatic, severe aortic stenosis: impact of patient’s choice of refusing aortic valve replacement on survival. QJM 2008;101:567–573. [DOI] [PubMed] [Google Scholar]
- 10. Vasques F, Messori A, Lucenteforte E, Biancari F.. Immediate and late out come of patients aged 80 years and older undergoing isolated aortic valve replacement: a systematic review and meta-analysis of 48 studies. Am Heart J 2012;163:477–485. [DOI] [PubMed] [Google Scholar]
- 11. Shan L, Saxena A, McMahon R, Wilson A, Newcomb A.. A systematic review on the quality of life benefits after aortic valve replacement in the elderly. J Thorac Cardiovasc Surg 2013;145:1173–1189. [DOI] [PubMed] [Google Scholar]
- 12. Wu Y, Jin R, Gao G, Grunkemeier GL, Starr A.. Cost-effectiveness of aortic valve replacement in the elderly: an introductory study. J Thorac Cardiovasc Surg 2007;133:608–613. [DOI] [PubMed] [Google Scholar]
- 13. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; PARTNER Trial Investigators. Transcatheter aortic valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 14. Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB; PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686–1695. [DOI] [PubMed] [Google Scholar]
- 15. Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696–1704. [DOI] [PubMed] [Google Scholar]
- 16. Holmes DR Jr, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH, Francis GS, Gardner TJ, Kappetein AP, Linderbaum JA, Mukherjee C, Mukherjee D, Otto CM, Ruiz CE, Sacco RL, Smith D, Thomas JD.. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59:1200–1254. [DOI] [PubMed] [Google Scholar]
- 17. Lindman BR, Alexander KP, O'gara PT, Afilalo J.. Futility, benefit, and transcatheter aortic valve replacement. J Am Coll Cardiol Intv 2014;7:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009;103:1616–1621. [DOI] [PubMed] [Google Scholar]
- 19. Baldasseroni S, Orso F, Pratesi A.. The complexity of risk stratification in older patient candidate to non-cardiac surgery. Monaldi Arch Chest Dis 2012;78:129–137. [DOI] [PubMed] [Google Scholar]
- 20. ISTAT. Istituto nazionale di statistica, Relazione sanitaria 2010. www.istat.it (14 July 2016).
- 21. Librero J, Peiró S, Ordiñana R.. Chronic comorbidity and outcomes of hospital care: length of stay, mortality, and readmission at 30 and 365 days. J Clin Epidemiol 1999;52:171–179. [DOI] [PubMed] [Google Scholar]
- 22. Cherubini A, Del Signore S, Ouslander J, Semla T, Michel JP. Fighting against age discrimination in clinical trials. J Am Geriatr Soc 2010;58:1791–1796. [DOI] [PubMed] [Google Scholar]
- 23. Partridge JSL, Harari D, Dhesi JK.. Frailty in the older surgical patient: a review. Age and Aging 2012;41:142–147. [DOI] [PubMed] [Google Scholar]
- 24. Walston J, Hadley EC, Ferrucci L.. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American geriatrics society/national institute on aging research conference on frailty in older adults. J Am Geriatr Soc 2006;54:991–1001. [DOI] [PubMed] [Google Scholar]
- 25. Campbell AJ, Buchner DM.. Unstable disability and the fluctuations of frailty. Age Ageing 1997;26:315–318. [DOI] [PubMed] [Google Scholar]
- 26. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J.. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 27. Rockwood K, Wolfson C, McDowell I.. The Canadian Study of Health and Aging: organizational lessons from a national, multicenter, epidemiologic study. Int Psychogeriatr 2001;13 Supp 1:233–237. [DOI] [PubMed] [Google Scholar]
- 28. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J.. Gait speed and survival in older adults. JAMA 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaudhry SI, McAvay G, Chen S.. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the Cardiovascular Health Study. J Am Coll Cardiol 2013;61:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ekerstad N, Swahn E, Janzon M.. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011;124:2397–2404. [DOI] [PubMed] [Google Scholar]
- 31. Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF.. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol 2010;56:1668–1676. [DOI] [PubMed] [Google Scholar]
- 32. Afilalo J, Mottillo S, Eisenberg MJ, Alexander KP, Noiseux N, Perrault LP, Morin JF, Langlois Y, Ohayon SM, Monette J, Boivin JF, Shahian DM, Bergman H.. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes 2012;5:222–228. [DOI] [PubMed] [Google Scholar]
- 33. Green P, Woglom AE, Genereux P.. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. J Am Coll Cardiol Intv 2012;5:974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stortecky S, Schoenenberger AW, Moser A.. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. J Am Coll Cardiol Intv 2012;5:489–496. [DOI] [PubMed] [Google Scholar]
- 35. Katz S, Ford AB.. studies of illness in the aged, the Index of ADL: a standardized measure of biological and psycosocial function. JAMA 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 36. Lawton MP, Brody EM.. Self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 37. Brennan M, Edwards FH, Zhao Y, O'brien SM, Douglas PS, Peterson ED.. Long-term survival after aortic valve replacement among high-risk elderly patients in the United States: insights from the society of thoracic surgeons adult cardiac surgery database, 1991 to 2007. Circulation 2012;126:1621–1629. [DOI] [PubMed] [Google Scholar]
- 38. Khounlaboud M, Donal E, Auffret V, Anselmi A, Ingels A, Flécher E, Verhoye JP, Daubert C, Le Breton H, Mabo P, Leguerrier A.. Comparison of preoperative and postoperative characteristics in octogenarians having isolated surgical aortic valve replacement before versus after introduction of transcatheter aortic valve implantation. Am J Cardiol 2015;116:933–937. [DOI] [PubMed] [Google Scholar]
- 39. Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochellie`Re R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E.. Trans- catheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080–1090. [DOI] [PubMed] [Google Scholar]
- 40. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ; PARTNER Trial Investigators. Trans- catheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 41. Généreux P, Head SJ, Wood DA, Kodali SK, Williams MR, Paradis JM, Spaziano M, Kappetein AP, Webb JG, Cribier A, Leon MB. Transcatheter aortic valve implantation 10–year anniversary: review of current evidence and clinical implications. Eur Heart J 2012;33:2388–2398. [DOI] [PubMed] [Google Scholar]
- 42. The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Authors/Task Force Members, Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baró N-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, SchäFers H, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M.. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 43. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK, et al. ; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 44. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Akin J, Davidson MJ, Svensson LG; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477–2484. [DOI] [PubMed] [Google Scholar]
- 45. Popma JJ, Adams DH, Reardon MJ.. Transcatheter aortic valve replacement using a self expanding bioprosthesis in patients with severe aortic stenosis in extreme risk for surgery. J Am Coll Cardiol 2014;63:1972–1981. [DOI] [PubMed] [Google Scholar]
- 46. Arnold SV, Reynolds MR, Wang K, Magnuson EA, Baron SJ, Chinnakondepalli KM, Reardon MJ, Tadros PN, Zorn GL, Maini B, Mumtaz MA, Brown JM, Kipperman RM, Adams DH, Popma JJ, Cohen DJ; CoreValve US Pivotal Trial Investigators. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the corevalve US Pivotal Trial. J Am Coll Cardiol Cardiovasc Interv 2015;8:1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reardon MJ, Adams DH, Kleiman NS, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Lee JS, Hermiller JB Jr, Chetcuti S, Heiser J, Merhi W, Zorn GL 3rd, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Maini B, Mumtaz M, Conte JV, Resar JR, Aharonian V, Pfeffer T, Oh JK, Qiao H, Popma JJ. 2-Year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol 2015;66:113–121. [DOI] [PubMed] [Google Scholar]
- 48. Walther T, Hamm CW, Schuler G, Berkowitsch A, Kötting J, Mangner N, Mudra H, Beckmann A, Cremer J, Welz A, Lange R, Kuck KH, Mohr FW, Möllmann H; GARY Executive Board. Perioperative results and complications in 15,964 transcatheter aortic valve replacements: prospective data from the GARY registry. J Am Coll Cardiol 2015;65:2173–2180. [DOI] [PubMed] [Google Scholar]
- 49. Auffret V, Bedossa M, Boulmier D, Verhoye JP, Ruggieri VG, Koning R, Laskar M, Van Belle É, Leprince P, Collet JP, Iung B, Lefèvre T, Eltchaninoff H, Gilard ML, Breton H.. From FRANCE 2 to FRANCE TAVI: are indications, technique and results of transcatheter aortic valve replacement the same? Presse Med 2015;44:752–760. [DOI] [PubMed] [Google Scholar]
- 50. Tamburino C, Barbanti M, D’errigo P, Ranucci M, Onorati F, Covello RD, Santini F, Rosato S, Santoro G, Fusco D, Grossi C, Seccareccia F.. 1-Year outcomes after transfemoral transcatheter or surgical aortic valve replacement results from the Italian OBSERVANT study. J Am Coll Cardiol 2015;66:804–812. [DOI] [PubMed] [Google Scholar]
- 51. Holmes DR Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, Peterson ED, Rumsfeld JS, Shahian DM, Thourani VH, Tuzcu EM, Vemulapalli S, Hewitt K, Michaels J, Fitzgerald S, Mack MJ; STS/ACC TVT Registry. Annual outcomes with transcatheter valve therapy: from the STS/ACC TVT Registry. J Am Coll Cardiol 2015;66:2813–2823. [DOI] [PubMed] [Google Scholar]
- 52. Arsalan M, Szerlip M, Vemulapalli S, Holper EM, Arnold SV, Li Z, DiMaio MJ, Rumsfeld JS, Brown DL, Mack MJ.. Should transcatheter aortic valve replacement be performed in nonagenarians? Insights from the STS/ACC TVT Registry. J Am Coll Cardiol 2016;67:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hørsted Thyregod HG, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Olsen P, Søndergaard L.. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis1-year results from the all-comers NOTION Randomized Clinical Trial. J Am Coll Cardiol 2015;65:2184–2194. [DOI] [PubMed] [Google Scholar]
- 54. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 55. Gurvitch R, Tay EL, Wijesinghe N, Ye J, Nietlispach F, Wood DA, Lichtenstein S, Cheung A, Webb JG. Stroke after transcatheter aortic valve replacement: incidence, risk factors, prognosis, and preventive strategies. Clin Cardiol 2014;12:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gurvitch R, Tay EL, Wijesinghe N, Ye J, Nietlispach F, Wood DA, Lichtenstein S, Cheung A, Webb JG.. Transcatheter aortic valve implantation: Lesson from the learning courve of the first 270 high-risk patients. Catheter Cardiovasc Inter 2011;78:977–984. [DOI] [PubMed] [Google Scholar]
- 57. Khatri PJ, Webb JG, Rodés-Cabau J, Fremes SE, Ruel M, Lau K, Guo H, Wijeysundera HC, Ko DT. Adverse effects associated with transcatheter aortic valve implantation: a metanalysis of contemporary studies. Ann Intern Med 2013;158:35–46. [DOI] [PubMed] [Google Scholar]
- 58. Sinning JM, Hammerstingl C, Vasa-Nicotera M.. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol 2012;59:1134–1141. [DOI] [PubMed] [Google Scholar]
- 59. Makkar Miller DC, Blackstone EH, Mack MJ.. Transcatheter (TAVI) versus surgical (AVR) aortic valve replacement: occurrence, hazard, risk factors, and consequences of neurologic events in the PARTNER trial. J Thorac Cardiovasc Surg 2012;143:832–843. [DOI] [PubMed] [Google Scholar]
- 60. Ussia GP, Capodanno D, Barbanti M.. Balloon aortic valvuloplasty for severe aortic stenosis as a bridge to high-risk transcatheter aortic valve implantation. J Invasive Cardiol 2010;22:161–166. [PubMed] [Google Scholar]
- 61. The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012). Eur J Cardiothorac Surg 2012;42:S1–S44. [DOI] [PubMed] [Google Scholar]
- 62. Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS. 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 focused update incorporated into ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2008;118:523–661. [DOI] [PubMed] [Google Scholar]
- 63. Otto CM. Aortic stenosis: even mild disease is significant. Eur Heart J 2004;25:185–187. [DOI] [PubMed] [Google Scholar]
- 64. Cioffi G, Tomasi C, Rossi A, Nistri S, Tarantini L, Faden G, Mazzone C, Di Lenarda A, Ettori F, Stefenelli C, Faggiano P.. Does treatment assignment influence the prognosis of patients with symptomatic severe aortic stenosis? Cardiovasc Ultrasound 2015;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J 2004;25:199–205. [DOI] [PubMed] [Google Scholar]
- 66. Nistri S, Faggiano P, Olivotto I.. Hemodynamic progression and outcome of asymptomatic aortic stenosis in primary care. Am J Cardiol 2012;109:718–723. [DOI] [PubMed] [Google Scholar]
- 67. Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, de Simone G.. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011;97:301–307. [DOI] [PubMed] [Google Scholar]
- 68. Kamalesh M, Ng C, El Masry H.. Does diabetes accelerate progression of calcific aortic stenosis?. Eur J Echocardiogr 2009;10:723–725. [DOI] [PubMed] [Google Scholar]
- 69. Monin JL, Lancellotti P, Monchi M.. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation 2009;120:69–75. [DOI] [PubMed] [Google Scholar]
- 70. Bahlmann E, Gerdts E, Cramariuc D, Gohlke-Baerwolf C, Nienaber CA, Wachtell K, Seifert R, Chambers JB, Kuck KH, Ray S.. Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation 2013;127:1149–1156. [DOI] [PubMed] [Google Scholar]
- 71. Tobin JR Jr, Rahimtoola SH, Blundell PE.. Percentage of left ventricular stroke work loss. A simple hemodynamic concept for estimation of severity in valvular aortic stenosis. Circulation 1967;35:868–879. [DOI] [PubMed] [Google Scholar]
- 72. Zito C, Salvia J, Cusmà-Piccione M, Antonini-Canterin F, Lentini S, Oreto G, Di Bella G, Montericcio V, Carerj S.. Prognostic significance of valvuloarterial impedance and left ventricular longitudinal function in asymptomatic severe aortic stenosis involving three-cuspid valves. Am J Cardiol 2011;108:1463–1469. [DOI] [PubMed] [Google Scholar]
- 73. Cramariuc D, Cioffi G, Rieck AE, Devereux RB, Staal EM, Ray S, Wachtell K, Gerdts E.. Low-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS Substudy. J Am Coll Cardiol Cardiovasc Imaging 2009;2:390–399. [DOI] [PubMed] [Google Scholar]
- 74. Bartko PE, Heinze G, Graf S, Clavel MA, Khorsand A, Bergler-Klein J, Burwash IG, Dumesnil JG, Sénéchal M, Baumgartner H, Rosenhek R, Pibarot P, Mundigler G.. Two-dimensional strain for the assessment of left ventricular function in low flow—low gradient aortic stenosis, relationship to hemodynamics and outcome: a substudy of the multicenter TOPAS Study. Circ Cardiovasc Imaging 2013;6:268–276. [DOI] [PubMed] [Google Scholar]
- 75. Kearney LG, Lu K, Ord M, Patel SK, Profitis K, Matalanis G, Burrell LM, Srivastava PM.. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging 2012;13:827–833. [DOI] [PubMed] [Google Scholar]
- 76. Cioffi G, Mazzone C, Barbati G.. Value of combined circumferential and longitudinal left ventricular systolic dysfunction to predict adverse outcome in patients with asymptomatic aortic stenosis. Echocardiography 2015;32:1064–1072. [DOI] [PubMed] [Google Scholar]
- 77. Dahl JS, Videbæk L, Poulsen MK, Rudbæk TR, Pellikka PA, Møller JE.. Global strain in severe aortic valve stenosis: relation to clinical outcome after aortic valve replacement. Circ Cardiovasc Imaging 2012;5:613–620. [DOI] [PubMed] [Google Scholar]
- 78. Adda J, Mielot C, Giorgi R, Cransac F, Zirphile X, Donal E, Sportouch-Dukhan C, Réant P, Laffitte S, Cade S, Le Dolley Y, Thuny F, Touboul N, Lavoute C, Avierinos JF, Lancellotti P, Habib G.. Low-flow, low-gradient severe aortic stenosis despite normal ejection fraction is associated with severe left ventricular dysfunction as assessed by speckle-tracking echocardiography: a multicenter study. Circ Cardiovasc Imaging 2012;5:27–35. [DOI] [PubMed] [Google Scholar]
- 79. Rossi A, Nistri S, Cioffi G, Tomasi C, Faden G, Fiorina C, Dini FL, Ghio S, Temporelli PL, Tarantini L, Faggiano P.. Severe aortic valve stenosis with normal left ventricular function and low vs high pressure gradient: Different hemodynamic profiles but similar clinical presentation, comorbidity and outcome. Int J Cardiol 2013;167:2326–2328. [DOI] [PubMed] [Google Scholar]
- 80. Holme I, Pedersen TR, Boman K, Egstrup K, Gerdts E, Kesäniemi YA, Malbecq W, Ray S, Rossebø AB, Wachtell K, Willenheimer R, Gohlke-Bärwolf C.. A risk score for predicting mortality in patients with asymptomatic mild to moderate aortic stenosis. Heart 2012;98:377–383. [DOI] [PubMed] [Google Scholar]
- 81. Cioffi G, Mazzone C, Faggiano P, Tarantini L, Di Lenarda A, Russo TE, Selmi A, Stefenelli C, Furlanello F.. Prognostic stratification by conventional echocardiography of patients with aortic stenosis: the "CAIMAN-ECHO Score". Echocardiography 2012; doi: 10.1111/echo.12065. [DOI] [PubMed] [Google Scholar]
- 82. Zamorano JL, Badano LP, Bruce C, Chan KL, Goncalves A, Hahn RT, Keane MG, La Canna G, Monaghan MJ, Nihoyannopoulos P, Silvestry FE, Vanoverschelde JL, Gillam LD.. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. J Am Soc Echocardiogr 2011;24:937–965. [DOI] [PubMed] [Google Scholar]
- 83. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M.. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 2009;10:1–25. [DOI] [PubMed] [Google Scholar]
- 84. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodès-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB.. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document. Eur Heart J 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 85. Monin JL, Quere JP, Monchi M, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Tribouilloy C, Gueret P.. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003;108:319–324. [DOI] [PubMed] [Google Scholar]
- 86. Himbert D, Pontnau F, Messika-Zeitoun D, Descoutures F, Detaint D, Cueff C, Sordi M, Laissy JP, Alkhoder S, Brochet E, Iung B, Depoix JP, Nataf P, Vahanian A.. Feasibility and outcomes of transcatheter aortic valve implantation in high-risk patients with stenotic bicuspid aortic valves. Am J Cardiol 2012;110:877–883. [DOI] [PubMed] [Google Scholar]
- 87. Jilaihawi H, Kashif M, Fontana G, Furugen A, Shiota T, Friede G, Makhija R, Doctor N, Leon MB, Makkar RR.. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol 2012;59:1275–1286. [DOI] [PubMed] [Google Scholar]
- 88. Piazza N, De Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH.. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv 2008;1:74–81. [DOI] [PubMed] [Google Scholar]
- 89. Messika-Zeitoun D, Serfaty JM, Brochet E, Ducrocq G, Lepage L, Detaint D, Hyafil F, Himbert D, Pasi N, Laissy JP, Iung B, Vahanian A.. Multimodal assessment of the aortic annulus diameter: implications for transcatheter aortic valve implantation. J Am Coll Cardiol 2010;55:186–194. [DOI] [PubMed] [Google Scholar]
- 90. Willson AB, Webb JG, LaBounty TM, Achenbach S, Moss R, Wheeler M, Thompson C, Min JK, Gurvitch R, Norgaard BL, Hague CJ, Toggweiler S, Binder R, Freeman M, Poulter R, Poulsen S, Wood DA, Leipsic J.. Three-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement. A multicenter retrospective analysis. J Am Coll Cardiol 2012;59:1287–1294. [DOI] [PubMed] [Google Scholar]
- 91. Jilaihawi H, Doctor N, Kashif M, Chakravarty T, Rafique A, Makar M, Furugen A, Nakamura M, Mirocha J, Gheorghiu M, Stegic J, Okuyama K, Sullivan DJ, Siegel R, Min JK, Gurudevan SV, Fontana GP, Cheng W, Friede G, Shiota T, Makkar RR.. Aortic annular sizing for transcatheter aortic valve replacement using crosssectional 3-dimensional transesophageal echocardiography. J Am Coll Cardiol 2013;61:908–916. [DOI] [PubMed] [Google Scholar]
- 92. Barbanti M, Yang TH, Rodes Cabau J, Tamburino C, Wood DA, Jilaihawi H, Blanke P, Makkar RR, Latib A, Colombo A, Tarantini G, Raju R, Binder RK, Nguyen G, Freeman M, Ribeiro HB, Kapadia S, Min J, Feuchtner G, Gurtvich R, Alqoofi F, Pelletier M, Ussia GP, Napodano M, De Brito FS Jr, Kodali S, Norgaard BL, Hansson NC, Pache G, Canovas SJ, Zhang H, Leon MB, Webb JG, Leipsic J.. Anatomical and procedural features ssociated with aortic root rupture during balloon expandable transcatheter aortic valve replacement. Circulation 2013;128:244–253. [DOI] [PubMed] [Google Scholar]
- 93. Al-Lamee R, Godino C, Colombo A.. Transcatheter aortic valve implantation: current principles of patient and technique selection and future perspectives. Circ Cardiovasc Interv 2011;4:387–395. [DOI] [PubMed] [Google Scholar]
- 94. Binder RK, Webb JG, Willson AB, Urena M, Hansson NC, Norgaard BL, Pibarot P, Barbanti M, Larose E, Freeman M, Dumont E, Thompson C, Wheeler M, Moss RR, Yang TH, Pasian S, Hague CJ, Nguyen G, Raju R, Toggweiler S, Min JK, Wood DA, Rodes-Cabau J, Leipsic J.. The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: a prospective, multicenter, controlled trial. J Am Coll Cardiol 2013;62:431–438. [DOI] [PubMed] [Google Scholar]
- 95. Saikrishnan N, Kumar G, Sawaya FJ, Lerakis S, Yoganathan AP.. Accurate assessment of aortic stenosis. A review of diagnostic modalities and Hemodynamics. Circulation 2014;129:244–253. [DOI] [PubMed] [Google Scholar]
- 96. O’Sullivan CJ, Stortecky S, Buellesfeld L, Wenaweser P, Windecker S.. Preinterventional screening of the TAVI patient: how to choose the suitable patient and the best procedure. Clin Res Cardiol 2014;103:259–274. [DOI] [PubMed] [Google Scholar]
- 97. Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R.. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9–1314. [DOI] [PubMed] [Google Scholar]
- 98. O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP.. Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2–isolated valve surgery. Ann Thorac Surg 2009;88(1 Suppl):S23–S42. [DOI] [PubMed] [Google Scholar]
- 99. Ranucci M, Castelvecchio S, Menicanti L, Frigiola A, Pelissero G.. Risk of assessing mortality risk in elective cardiac operations: age, creatinine, ejection fraction, and the law of parsimony. Circulation 2009;119:3053–3061. [DOI] [PubMed] [Google Scholar]
- 100. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U.. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–744. [DOI] [PubMed] [Google Scholar]
- 101. Hanley JA, McNeil BJ.. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 102. Basraon J, Chandrashekhar YS, John R, Agnihotri A, Kelly R, Ward H, Adabag S.. Comparison of risk scores to estimate perioperative mortality in aortic valve replacement surgery. Ann Thorac Surg 2011;92:535–540. [DOI] [PubMed] [Google Scholar]
- 103. Barili F, Pacini D, Capo A, Ardemagni E, Pellicciari G, Zanobini M, Grossi C, Shahin KM, Alamanni F, Di Bartolomeo R, Parolari A.. Reliability of new scores in predicting perioperative mortality after isolated aortic valve surgery: a comparison with the society of thoracic surgeons score and logistic EuroSCORE. Ann Thorac Surg 2013;95:1539–1544. [DOI] [PubMed] [Google Scholar]
- 104. Wendt D, Thielmann M, Kahlert P, Kastner S, Price V, Al-Rashid F, Patsalis P, Erbel R, Jakob H.. Comparison between different risk scoring algorithms on isolated conventional or transcatheter aortic valve replacement. Ann Thorac Surg 2014;97:796–802. [DOI] [PubMed] [Google Scholar]
- 105. Vanhuyse F, Maureira P, Folliguet T, Villemot JP.. Predictive value of five risk scores to predict outcomes after aortic valve replacement in octogenarians. J Heart Valve Dis 2013;22:517–523. [PubMed] [Google Scholar]
- 106. Barili F, Pacini D, D’ovidio M, Ventura M, Alamanni F, Di Bartolomeo R, Grossi C, Davoli M, Fusco D, Perucci C, Parolari A.. Reliability of modern scores to predict long-term mortality after isolated aortic valve operations. Ann Thorac Surg 2016;101:599–605. [DOI] [PubMed] [Google Scholar]
- 107. Osnabrugge RL, Speir AM, Head SJ, Fonner CE, Fonner E, Kappetein AP, Rich JB.. Performance of EuroSCORE II in a large US database: implications for transcatheter aortic valve implantation. Eur J Cardiothorac Surg 2014;46: 400–408. [DOI] [PubMed] [Google Scholar]
- 108. Rosenhek R, Iung B, Tornos P, Antunes MJ, Prendergast BD, Otto CM, Kappetein AP, Stepinska J, Kaden JJ, Naber CK, Acarturk E, Gohlke-Barwolf C.. (2012) ESC working group on valvular heart disease position paper: assessing the risk of interventions in patients with valvular heart disease. Eur Heart J 2012;33:822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Durand E, Borz B, Godin M, Tron C, Litzler PY, Bessou JP, Dacher JN, Bauer F, Cribier A, Eltchaninoff H.. Performance analysis of EuroSCORE II compared to the original logistic EuroSCORE and STS scores for predicting 30-day mortality after transcatheter aortic valve replacement. Am J Cardiol 2013;111:891–897. [DOI] [PubMed] [Google Scholar]
- 110. Takagi H, Niwa M, Mizuno Y, Yamamoto H, Goto SN, Umemoto T; All Literature Investigation Of Cardiovascular Evidence Group; A meta-analysis comparing observed 30-dayall-cause mortality with the Society of Thoracic Surgeons Predicted Risk of Mortality in contemporary studies using Valve Academic Research Consortium definitions. Int J Cardiol 2013;168:1598–1602. [DOI] [PubMed] [Google Scholar]
- 111. Arangalagea D, Cimadevilla C, Alkhoderc S, Chiampana A, Himberta D, Brocheta E, Iunga B, Natafc P, Depoixd JP, Vahaniana A, Messika-Zeitouna D.. Agreement between the new EuroSCORE II, the Logistic EuroSCORE and the Society of Thoracic Surgeons score: implications for transcatheter aortic valve implantation. Archives of Cardiovascular Disease 2014;107:353–360. [DOI] [PubMed] [Google Scholar]
- 112. Beohar N, Whisenant B, Kirtane AJ, Leon MB, Tuzcu EM, Makkar R, Svensson LG, Miller DC, Smith CR, Pichard AD, Herrmann HC, Thourani VH, Szeto WY, Lim S, Fischbein M, Fearon WF, O'neill W, Xu K, Dewey T, Mack M.. The relative performance characteristics of the logistic European System for Cardiac Operative Risk Evaluation score and the Society of Thoracic Surgeons score in the Placement of Aortic Transcatheter Valves trial. J Thorac Cardiovasc Surg 2014;148:2830–2837. [DOI] [PubMed] [Google Scholar]
- 113. D'Ascenzo F, Capodanno D, Tarantini G, Nijhoff F, Ciuca C, Rossi ML, Brambilla N, Barbanti M, Napodano M, Stella P, Saia F, Ferrante G, Tamburino C, Gasparetto V, Agostoni P, Marzocchi A, Presbitero P, Bedogni F, Cerrato E, Omedè P, Conrotto F, Salizzoni S, Biondi Zoccai G, Marra S, Rinaldi M, Gaita F, D'Amico M, Moretti C.. Usefulness and validation of the survival posT TAVI score for survival after transcatheter aortic valve implantation for aortic stenosis. Am J Cardiol 2014;114:1867–1874. [DOI] [PubMed] [Google Scholar]
- 114. Debonnaire P, Fusini L, Wolterbeek R, Kamperidis V, van Rosendael P, van der Kley F, Katsanos S, Joyce E, Tamborini G, Muratori M, Gripari P, Bax JJ, Ajmone Marsan N, Pepi M, Delgado V.. Value of the ‘TAVI2-SCORe’ versus surgical risk scores for prediction of one year mortality in 511 patients who underwent transcatheter aortic valve implantation. Am J Cardiol 2015;115:234–242. [DOI] [PubMed] [Google Scholar]
- 115. Capodanno D, Barbanti M, Tamburino C, D’errigo P, Ranucci M, Santoro G, Santini F, Onorati F, Grossi C, Covello RD, Capranzano P, Rosato S, Seccareccia F; on behalf of the OBSERVANT Research Group. A Simple Risk Tool (the OBSERVANT Score) for prediction of 30-day mortality after transcatheter aortic valve replacement. Am J Cardiol 2014;113:1851e–1858. [DOI] [PubMed] [Google Scholar]
- 116. Chen J. Frailty and cardiovascular disease: potential role of gait speed in surgical risk. stratification in older adults. Geriatr Cardiol 2015;12:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bagnall NM, Faiz O, Darzi A, Athanasiou T.. What is the utility of preoperative frailty assessment for risk stratification in cardiac surgery? Interact Cardiovasc Thorac Surg 2013;17:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sepehri A, Beggs T, Hassan A, Rigatto C, Shaw-Daigle C, Tangri N, Arora RC.. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg 2014;148:3110–3117. [DOI] [PubMed] [Google Scholar]
- 119. Leontyev S, Walther T, Borger MA, Lehmann S, Funkat AK, Rastan A, Kempfert J, Falk V, Mohr FW. Aortic valve replacement in octogenarians: utility of risk stratification with EuroSCORE. Ann Thorac Surg 2009;87:1440–1445. [DOI] [PubMed] [Google Scholar]
- 120. Kappetein AP, Head SJ, Genereux P, Piazza N, Van Mieghem NM, Blackstone EH, Leon MB.. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 Consensus Document. J Thorac Cardiovasc Surg 2013;145:6–23. [DOI] [PubMed] [Google Scholar]
- 121. Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Serruys PW.. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the valve academic research consortium. Eur Heart J 2011;32:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Webb JG. Mid-term follow-up after transcatheter aortic valve implantation. Eur Heart J 2012;33:947–948. [DOI] [PubMed] [Google Scholar]
- 123. Fried LP. Untangling the concepts of disability, frailty, and comorbility: implication for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–263. [DOI] [PubMed] [Google Scholar]
- 124. Rockwood K, Mitnitski A.. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011;27:17–26. [DOI] [PubMed] [Google Scholar]
- 125. Perera S. Ability to improve physical performance predicts long-term survival. Gerontology 2005;45:331. [Google Scholar]
- 126. Afilalo J, Alexander KP, Mack M, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE.. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014;63:747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rockwood K, Song X, MacKnight C.. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B.. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008;12:29–37. [DOI] [PubMed] [Google Scholar]
- 129. Guralnik JM1, Ferrucci L, Simonsick EM, Salive ME, Wallace RB.. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Di Bari M, Virgillo A, Matteuzzi D, Inzitari M, Mazzaglia G, Pozzi C, Geppetti P, Masotti G, Marchionni N, Pini R. Predictive validity of measures of comorbidity in older community dwellers: the Insufficienza Cardiaca negli Anziani Residenti a Dicomano Study. J Am Geriatr Soc 2006;54:210–216. [DOI] [PubMed] [Google Scholar]
- 131. Chiarantini D, Volpato S, Sioulis F.. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail 2010;16:390–395. [DOI] [PubMed] [Google Scholar]
- 132. Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009;13:881–889. [DOI] [PubMed] [Google Scholar]
- 133. Ling CHY, Taekema D, de Craen AJM, Gussekloo J, Westendorp RGJ, Maier AB.. Handgrip strength and mortality in the oldest old population: the Leiden 85-Plus Study. CMAJ 2010;182:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lefèvre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schächinger V, De Bruyne B, Eltchaninoff H, Thielmann M, Himbert D, Romano M, Serruys P, Wimmer-Greinecker G; PARTNER EU Investigator Group. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J 2011;32:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Knipp SC, Kahlert P, Jokisch D, Schlamann M, Wendt D, Weimar C, Jakob H, Thielmann M. Cognitive function after transapical aortic valve implantation: a single-centre study with 3-month follow-up. Interact Cardiovasc Thorac Surg 2013;16:116–122. PMID: 23148084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lee DH, Buth KJ, Martin B-J, Yip AM, Hirsch GM.. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 2010;121:973–978. [DOI] [PubMed] [Google Scholar]
- 137. Sündermann S, Dademasch A, Praetorius J.. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg 2011;39:33–37. [DOI] [PubMed] [Google Scholar]
- 138. Sündermann S, Dademasch A, Rastan A.. One-year follow-up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interact Cardiovasc Thorac Surg 2011;13:119–123. [DOI] [PubMed] [Google Scholar]
- 139. Schoenenberger AW, Stortecky S, Neumann S.. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J 2012;34:684–692. [DOI] [PubMed] [Google Scholar]
- 140. Ewe SH, Ajmone Marsan N, Pepi M.. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J 2010;160:1113–1120. [DOI] [PubMed] [Google Scholar]
- 141.Safety and Efficacy Study of the Medtronic CoreValve® System in the Treatment of Severe, Symptomatic Aortic Stenosis in Intermediate Risk Subjects Who Need Aortic Valve Replacement (SURTAVI). https://clinicaltrials.gov/ct2/show/NCT01586910 (14 July 2016).
- 142.Frailty Assessment Before Cardiac Surgery & Transcatheter Interventions. http://clinicaltrials.gov/show/NCT01845207.
- 143. Schoenenberger AW, Werner NP, Bramlage P, Martinez-Selles M, Maggi S, Bauernschmitt R, Thoenes M, Kurucova J, Michel JP, Ungar A.. Comprehensive geriatric assessment in patients undergoing transcatheter aortic valve implantation–rationale and design of the European CGA-TAVI registry. Eur. Geriatr Med 2014;5: 8–13. [Google Scholar]
- 144. Alfredsson J, Stebbins A, Brennan JM, Matsouaka R, Afilalo J, Peterson ED, Vemulapalli S, Rumsfeld JS, Shahian D, Mack MJ, Alexander KP.. Gait speed predicts 30-day mortality after transcatheter aortic valve replacement: results from the society of thoracic surgeons/American college of cardiology transcatheter valve therapy registry. Circulation 2016;133:1351–1359. [DOI] [PubMed] [Google Scholar]
- 145. Nagi SZ. An epidemiology of disability among adults in the united states. Milbank Mem Fund Q Health Soc 1976;54:439–467. [PubMed] [Google Scholar]
- 146. Katz NM, Chase GA.. Risks of cardiac operations for elderly patients: Reduction of the age factor. Ann Thorac Surg 1997;63:1309–1314. [DOI] [PubMed] [Google Scholar]
- 147. Lilamand M, Dumonteil N, Nourhashémi F, Hanon O, Marcheix B, Toulza O, Elmalem S, Abellan van Kan G, Raynaud-Simon A, Vellas B, Afilalo J, Cesari M.. Gait speed and comprehensive geriatric assessment: Two keys to improve the management of older persons with aortic stenosis. Int J Cardiol 2014;173: 580–582. [DOI] [PubMed] [Google Scholar]
- 148. McDermid RC, Bagshaw SM.. Prolonging life and delaying death: the role of physicians in the context of limited intensive care resources. Philos Ethics Humanit Med 2009;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Reynolds MR, Lei Y, Wang K, Chinnakondepalli K, Vilain KA, Magnuson EA, Galper BZ, Meduri CU, Arnold SV, Baron SJ, Reardon MJ, Adams DH, Popma JJ, Cohen DJ; CoreValve US High Risk Pivotal Trial Investigators. Cost-effectiveness of transcatheter aortic valve replacement with a self-expanding prosthesis versus surgical aortic valve replacement. J Am Coll Cardiol 2016;67:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mylotte D, Martucci G, Piazza N.. Patient selection for transcatheter aortic valve implantation: An interventional cardiology perspective. Ann Cardiothorac Surg 2012;1:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]