Abstract
Telemedicine applied to heart failure patients is a tool for recording and providing remote transmission, storage and interpretation of cardiovascular parameters and/or useful diagnostic images to allow for intensive home monitoring of patients with advanced heart failure, or during the vulnerable post-acute phase, to improve patient’s prognosis and quality of life.
Recently, several meta-analyses have shown that telemedicine-supported care pathways are not only effective but also economically advantageous. Benefits seem to be substantial, with a 30–35% reduction in mortality and 15–20% decrease in hospitalizations. Patients implanted with cardiac devices can also benefit from an integrated remote clinical management since all modern devices can transmit technical and diagnostic data. However, telemedicine may provide benefits to heart failure patients only as part of a shared and integrated multi-disciplinary and multi-professional ‘chronic care model’. Moreover, the future development of remote telemonitoring programs in Italy will require the primary use of products certified as medical devices, validated organizational solutions as well as legislative and administrative adoption of new care methods and the widespread growth of clinical care competence to remotely manage the complexity of chronicity.
Through this consensus document, Italian Cardiology reaffirms its willingness to contribute promoting a new phase of qualitative assessment, standardization of processes and testing of telemedicine-based care models in heart failure.
By recognizing the relevance of telemedicine for the care of non-hospitalized patients with heart failure, its strategic importance for the design of innovative models of care, and the many challenges and opportunities it raises, ANMCO and SIC through this document report a consensus on the main directions for its widespread and sustainable clinical implementation
Keywords: e-Health, Telemedicine, Remote telemonitoring, Heart failure, Implantable devices
Revised by: Carlo Campana, Mario Chiatto, Giuseppe Di Tano, Francesco Fedele, Domenico Gabrielli, Achille Giardina, Federico Nardi, Massimo Zecchin
Consensus Document Approval Faculty in appendix
Background and objectives of the document
Telemedicine (TM), and in general terms e-Health, identifies the exchange of digital, social, and health information with the purpose of supporting and optimizing the remote care process, allowing the patient to stay at home in conditions of safety and well-being1 and/or supporting the best adherence to therapeutic or preventive measures. A glossary of terms and definition used in the field of TM is reported in Table 1.
Table 1.
Glossary of terms and definitions used in the field of telemedicine
| e-Health | Electronic health is a general definition used to describe most aspects of healthcare delivery or management that is enabled by information technology or communications. |
| Telemedicine | Provision of patient care and consultation over a distance, using telecommunications technology. Basically, telemedicine considers the use of medical information, also known as Electronic Health Records, exchanged via electronic communications improving the patient’s health status. |
| Telehealth | Similar to telemedicine, refers to ‘remote clinical care enabled by technology supported information sharing and communication between patient and clinical staff’. It comprises remote health care delivery or monitoring between a health care professional and a patient outside of clinical settings, in their home or assisted living residence and integration of electronic transfer of physiological data via mobile phones, wearable electronic devices, or implantable electronic devices. |
| mHealth | Mobile health: all the mobile technologies (including phone and smart phone, tablet, digital device, with or without wearable sensors), used to deliver healthcare anytime and anywhere in the medical field. Typical mHealth services architectures use the Internet and Web services to provide an authentic pervasive interaction among doctors and patients. |
| Telemonitoring | Remote data collection from a patient through a device (ICD, pacemaker, ECG, blood pressure, glycaemia…) to measure his/her vital parameters and symptoms at home on a daily base. |
| Remote control | The device interrogation is made periodically to the patient’s home, manually by the patient or automatically by the monitoring system at predefined intervals. |
| Remote monitoring | Remote monitoring of patients’ physiological signals is one of the common applications in telemedicine. There is a continuous monitoring of the device, integrated by unplanned data transmissions, in case of alarm. It can be performed in either real-time or store-and-forward and checked by the health staff. |
| Teleconsultation | Second opinion consultation by specialist. |
| Tele + specialty (i.e. Telecardiology) | Application of telemedicine to a specific branch of medicine. |
| Telesurveillance = telemanagement = telesupport | All these words indicate surveillance over a distance with the use of mobile wireless devices. |
When applied to cardiovascular diseases, telemonitoring (TLM) include the recording, remote transmission (intermittent or continuous), storage and interpretation of cardiovascular parameters [such as ECG, heart rate (HR), blood pressure (BP), oxygen saturation (SatO2)] and, more recently, of diagnostic imaging (as echocardiography, computerized tomography, magnetic resonance, nuclear medicine, coronary angiography).
Heart failure (HF) is a growing health problem, with major impact on healthcare costs, mainly due to frequent hospitalizations. The most recent HF Guidelines2 have emphasized the role of daily monitoring of body weight and vital signs. Home TLM, integrated with educational interventions, can provide optimized home-based clinical management of HF patients by reducing hospitalizations and emergency department access, while improving outcomes and quality of life.3 Currently available devices show adequate levels of efficiency and safety and allow for easy implementation of home-based TM programs and high-quality patient care.
Recently, several meta-analyses have shown that disease management programs for HF patients supported by TLM are not only effective but also economically advantageous. Benefits are substantial, with a 30–35% reduction in mortality and 15–20% decrease in hospitalizations.4–7 The use of the TLM allows for early identification of symptoms or signs of HF and prompt intervention. In particular, both clinical parameters (weight, BP, HR, SatO2) and the ECG signal, together with some descriptive indices of health status (score of fatigue, dyspnoea and peripheral congestion) are monitored at the patient’s home. From the health centre, educational messages and recommendations on adherence can be sent to patients. All information is being collected in an electronic database equipped with privacy-preserving features and protocols.
Tailor-made alarm thresholds automatically activate alarm signals which trigger specific interventions. Even patients treated with a cardiac implantable device (ID) may benefit from monitoring, since all modern IDs can transmit technical and diagnostic data remotely, allowing an integrated clinical management. However, it should be clearly stressed that TM can be beneficial only when integrated with a disease management multi-disciplinary program involving HF centres’ professionals and knowledgeable general practitioners (GPs), according to shared protocols and decision-making flow charts. It is also vital that patients enrolled in the TM program receive appropriate information about the disease and specific education on self-care and self-monitoring.
The real challenge that accompanies the development of e-Health is, above all, cultural, since the successful development of information and communication technology (ICT) in health care lies in the ability to maintain a fair balance between ‘high tech’ and ‘high touch’ perspectives, taking into account the multidimensional, person-centred aspects of HF management.8
Also, there is a need to assess the actual clinical and organizational innovations offered by technological development, avoiding investments with no added values or, worse, the flourishing of a fragmented application development without clearly defined health goals.
Telemedicine in the management of heart failure patients to improve communication and continuity of care
Heart failure and continuity of care
Data from international registries clearly show that a large proportion of acute hospitalisation for HF could be avoided by improving the follow-up of patients at home after discharge.9
The new techniques for the remote control of vital signs and health status can help to prevent deterioration in the patient’s overall health due to hypertension, brady or tachyarrhythmias, or comorbidities such as renal failure, anaemia, infections, and low compliance with pharmacological and non-pharmacological therapies.
TM and all e-Health technologies identify any digital exchange of health information to support and optimize the care process when a distance separates patients and healthcare providers (Table 1); the final goal being to optimize home care and citizens’ quality of life. Home-based TM models should consider not only the clinical needs of patients but also their individual and social well-being, based on characteristics of frailty, multiple comorbidities, high-clinical complexity, and reduced mobility, through an integrated approach.
Devices should ideally transmit different signals from a single home control unit, with low impact for the patient and his/her family, to a secure remote control without the need for any telephone contact between care recipient and operators. A dedicated call centre may handle management of transmitted signals, verification of data quality, alarm control, and system malfunctioning.
The most common devices used for HF patients’ TLM are the pulse oximeter, the scale, the BP/HR monitor, the ECG (one or more leads), the detector of motion and fall, and the personal alarm services. Environmental sensors including temperature, smoke, humidity, water, and gas detectors may also be part of the integrated monitoring system. All data are sent to a web portal which may be accessed by healthcare providers, patients or caregivers, according to strict security protocols. Patient data (sociodemographic information, contact persons in case of emergencies, type of disease, clinical status, comorbidities, and current treatment) should be collected in a separate folder, and graphical representation of measures should be provided for an easier monitoring of alarms. Furthermore, the web portal may be integrated by questionnaires to be filled out by patients, to communicate symptoms and signs of instability.
TLM in HF can be of great support to guarantee patient’s continuity of care between hospitals and the GP or community structures. It allows:
to monitor weight and fluid balance and adjust diuretic therapy to avoid over-diuresis and hypotension;
to control HR, BP, and arrhythmias;
to better titrate therapy after hospital discharge;
to enhance educational interventions to promote empowerment and self-management.
However, from a practical point of view, the synergy between some factors is critical for an effective TLM.10 First of all, vital parameters thresholds should be carefully set, transmitted efficiently and with proper frequency, based on the clinical characteristics of each patient, presence/absence of a caregiver and severity of the disease. Information received by qualified personnel needs to be translated into specific recommendations to correct the abnormalities. This requires experienced staff with good knowledge of patient’s history. Finally, it is important to check that patients have correctly implemented the new recommendations, or changes in therapy. At the end of the process, confirmation is needed that the abnormality has been solved or, alternatively, that further interventions are needed. This last part of the process is the core of TLM because it translates a simple monitoring action into effective clinical management, which may reduce rehospitalizations.
Care models
Transition from a simple technological device (e.g. phone) to a more sophisticated monitoring of different signals is accompanied by higher costs and fewer chances to demonstrate a substantial benefit in terms of cost-effectiveness. Many reviews and meta-analyses have been published in recent years3,5,11–15 in the attempt to demonstrate that TM may reduce cardiac events after hospitalization for acute HF. A recent review by Inglis et al.12 has confirmed that both simple telephone support and vital signs’ monitoring affect readmission for HF, but only TM reduced all-cause mortality. However, meta-analyses have been largely criticized because they are often small, and include single-centre, non-randomized studies. When looking at the most recently published multi-centre studies, data appear to be less convincing.
The largest multi-centre study was published at the end of 2010 by Chaudhry et al.16 They enrolled 1653 patients discharged within 30 days after an episode of acute HF. Mean age was 61 years, 42% had New York Heart Association (NYHA) functional class I–II and 30% had preserved or mildly reduced left ventricular ejection fraction (LVEF >40%). The group randomized to TM was instructed to make daily toll-free calls to the system. During each call, patients were asked a series of questions and they entered responses using the telephone keypad. Information was reviewed every weekday by site co-ordinators. After a follow-up of 6 months, no significant differences were observed for all primary and secondary endpoints between the TM and the control group with a high incidence of cardiac events (nearly 50% at 6 months). A critical aspect of this study16 is the low adherence of patients to the program (14% of patients had not performed any phone calls and only 55% of those patients were still transmitting data at the end of the 6 months’ follow-up). This experience is very different from that of the Dial study17 where clinical information was collected by an expert nursing staff simply via phone showing good adherence of patients and a significant reduction of hospitalizations for HF.
The second important study was published by Koehler et al.18 in 2011. This German multi-centre study enrolled ambulatory patients with stable disease. Their mean age was 67 years, mean LVEF 27%, NYHA functional class II in 50% of the patients, and more than 90% were optimally treated with RAS inhibitors and beta-blockers. The TLM system was technologically advanced, with direct Bluetooth transmission of vital sign parameters to the core lab and a parallel system for handling emergency calls. Again, during a 24-months’ follow-up, death, and hospitalization for HF were similar in the TM and control groups. In this stable and well-treated population of HF patients, only 10% experienced a cardiac event, as compared to the 50% observed in the study by Chaudhry et al.15 which enrolled patients admitted for acute HF. This negative message about effectiveness of TM to prevent cardiac events from these two studies both in post-acute and chronic HF, confirm data from other previous European trials.19–21
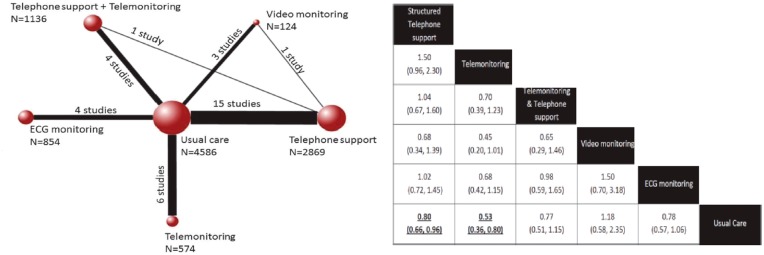
However, in a more recent meta-analysis,15 remote monitoring systems have been again analysed in various randomized trials. As in the past, both ordinary telephone support and TLM were associated with significant clinical benefits in terms of re-hospitalization and mortality (Figure 1).
Figure 1.
Left: Comparisons among interventions included in the analyses of all-cause mortality (30 studies, n = 10 193). Each node represents an intervention and the size of each node indicates the number of included patients. The solid lines connecting the nodes together indicate the existence of this comparison in the literature and the thickness of the lines represents the number of studies that included a particular comparison. Right: The effect of different forms of telemedicine on all-cause mortality. The effect of structured telephone support and telemonitoring was significant in comparison with usual care (bottom line, in bold). Adapted from Kotb et al.15
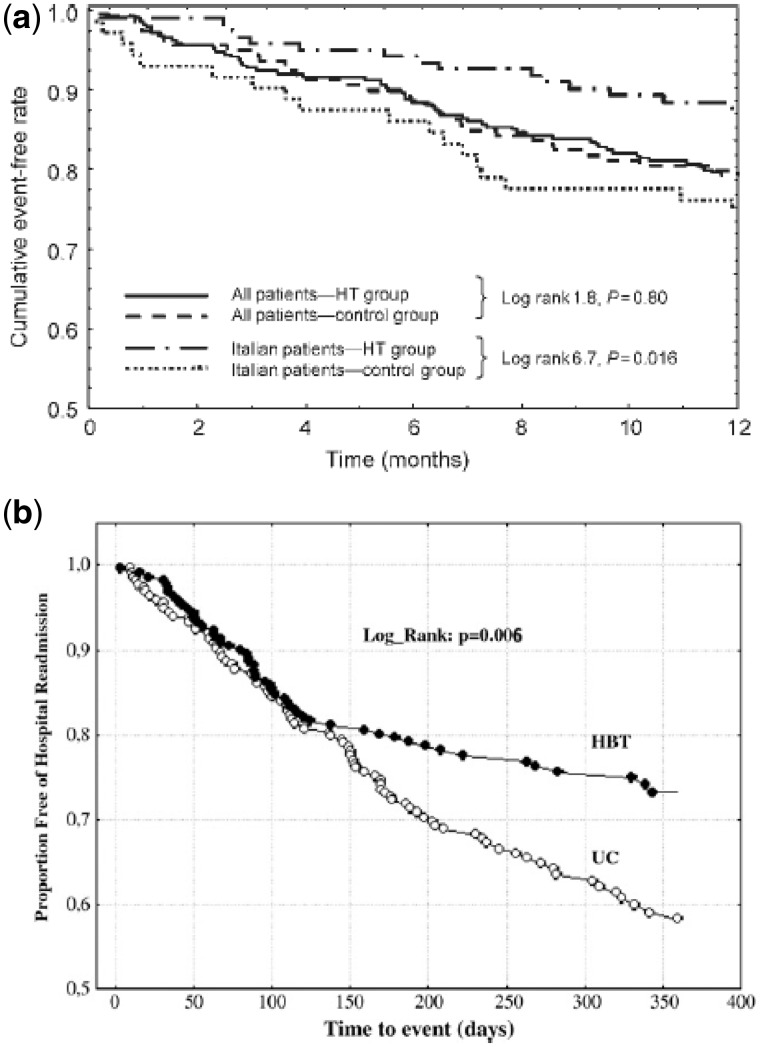
Several critical aspects can help understand the reasons why many studies have failed to demonstrate a clinical benefit of TM in HF care (Table 2). None of the studies actually detailed which interventions had been suggested by the core lab to address initial signs of decompensation, or what kind of interventions were implemented to manage the patients’ out-of-range clinical parameters. TM requires very experienced staff, who knows the needs and details of their patient’s disease, any comorbidities and social issues. Moreover, even when the team has extensive expertise in HF and knowledge of their patients, they may be not be sufficiently prepared to rapidly and efficiently manage a large flow of TLM data. Enrolling centres are often involved in multi-centre studies because of their experience with HF, but they have not a structured organisation to handle large volumes of clinical data and promptly manage relevant information. The HHH study20 provided a good example of such a concept. In preceding pilot study, Italian centres contributed to testing and ameliorating the TLM system subsequently utilized in the trial. This acquired expertise was translated into a significant reduction of the primary end-point not observed in the other HHH European centres (Figure 2A).
Table 2.
Possible explanations about failure of large multi-centre trials to demonstrate any benefit from home telemonitoring in heart failure patients
|
HF, heart failure; TM, telemedicine.
Figure 2.
(A) Kaplan–Meier curves for cumulative events (death + heart failure hospitalization) in the two groups (TLM vs. control group), considering all population and only patients enrolled in Italy.13 Modified from Mortara et al.20 (B) Kaplan–Meier curves for HF hospitalizations in patients randomized to telemonitoring (HBT—home-based telemanagement) in comparison to control group UC=Usual Care. Modified from Giordano et al.22
As far as the German study17 is concerned, if patients in usual care are followed by a dedicated out-patient HF clinic, it is difficult to obtain substantially different results.
In conclusion, methodological approaches to TLM, and procedures for the management of patients and their diseases are manifold. The core issue pertains to the service organization and the customization of interventions aimed at providing more clinical benefits. This is particularly important for the management of HF where the ability to send different information and to activate teleconsultations by healthcare personnel (nurses, specialist physician, or GP) may lead to a more efficient care with the integrated involvement of patients and families.
The Italian experience
The Italian experience is summarized in Table 3. The earliest experiences of TM in HF patients in Italy dates back to the work carried out by the ‘S. Maugeri’ Foundation23,25. In 2004, Scalvini et al. described a pilot study (‘Gussago Model’) where HF patients discharged from the hospital received a portable monitor capable of transmitting their ECG every 1–2 weeks. The intervention also included a structured interview about their health status and adherence to the pharmacologic treatment plan administered by an experienced nurse. Patients were instructed to contact the nurse at any time in case of need with the possibility of immediate access to either their GP or a cardiologist. Hospitalizations in the TM group decreased from 1.8 to 0.2 per patient per year (P < 0.001). In the same year, Capomolla et al.23 from the Montescano Center published the results of a different model, later implemented in the multi-centre European study HHH.26 In their experience, two specialized nurses trained the patients before discharge from the hospital, collected the vital signs automatically transmitted by a TLM system every 24–48 h and kept closed contacts with the patients requesting, when necessary, the intervention of a cardiologist according to a predefined protocol. At the end of the 10 months’ observation, the intervention group experienced a significantly lower incidence of events.23
Table 3.
The Italian experience in TM programs
| Authors | Type of the study | Device | Intervention | Results |
|---|---|---|---|---|
|
|
Testing the Montescano model:
|
|
|
| Giordano et al.24 |
|
One-lead ECG transmission once a week | Evaluation of a billing system for home telesurveillance in 38 hospitals (Lombardy Region, Italy) |
|
| Scalvini et al.25 |
|
One-lead ECG, transmission every 1–2 weeks |
|
|
| Capomolla et al.23 |
|
Interactive voice response |
|
133 patients: 66 assigned to intervention, 67 to control group followed up for 11 months (median value). After 10 months, intervention group experienced lower incidence of events (death, all cause hospitalization, heart transplantation, emergency ward access) 24% vs. 76%, P < 0.01 |
| Giordano et al.22 |
|
One-lead ECG transmission once a week |
|
|
| Antonicelli et al.27 |
|
Phone contact once a week for symptoms and adherence to treatment | Testing the effects of TLM on mortality, rate of hospitalizations, compliance and quality of life |
|
| Villani et al.28 |
|
|
Testing clinical efficacy and costs |
|
TLM, telemonitoring; NYHA, New York Heart Association; CV, cardiovascular; OR, odds ratio; CI, confidence interval; HF, heart failure.
Following these initial experiences, these models were tested on larger populations. Giordano et al.22 tested the ‘Gussago model’ on 460 HF patients, randomly assigned to either a conventional treatment or a TLM guided follow-up. The intervention registered a significantly lower incidence of hospitalizations for cardiovascular causes (Figure 2B).22 The Montescano model was also tested in a large international trial (the HHH-Home or Hospital in Hf).20,21,23–26 Patients were randomized to a TM intervention or usual care. The intervention group was further randomized to either receive a simple phone contact or to a phone contact plus an automatic transmission of vital parameters. At the end of the study, no difference emerged among the different strategies adopted in terms of HF hospitalizations or deaths.20 However, a significant heterogeneity of results was noted among different countries, with a significant reduction of events in Italy (Figure 2A).20
In the same years, several other experiences were conducted in Italy. Among others, the study from Antonicelli et al.27 observed a significantly lower incidence of HF hospitalizations in patients randomized to a TM intervention as compared to usual care.
In 2014, Villani et al.28 showed the results of the ICAROS Project on 80 HF patients randomized to either a conventional follow-up or a TLM system. The latter included vital signs’ transmission and an ECG to a remote centre and a questionnaire designed to measure depression and anxiety. At the end of the observation period, patients assigned to the intervention showed better adherence to therapy, improved functional class, and lower levels of anxiety and depression. The cumulative end point was significantly decreased although an increased number of unwanted ambulatory visits was observed.
In 2006, Lombardia Region established a specific billing system for the domiciliary telesurveillance of chronic HF. This led to the accreditation of 38 hospitals offering this kind of assistance and the definition of a specific voice of reimbursement to apply for a TM program of 6 months after a hospitalization for HF. Giordano et al.24 published the preliminary data of this regional implementation on 602 patients who completed the program, showing a significant improvement in their NYHA functional class, left ventricular function, six-minute walk test, and quality of life. About 50% of patients assigned to the intervention were classified as responders. In this latter group, a significantly lower incidence of events was observed. These results suggest the importance of improving the organization of such models. In particular, effective identification of candidates best suitable for this kind of intervention is of paramount importance in order to increase the likelihood of positive outcomes.
Other instruments available for remote assistance: mobile health
A fast growing branch of TM is mobile Health (mHealth), using mobile devices (mobile phone, smartphone, tablet, handheld, wearable sensors) and popular communication technologies (SMS, e-mail) for remote health status monitoring.29 Thanks to their advanced technology, smartphones can now be fully considered real pocket computers capable of collecting information. Other devices can be worn, applied to the skin, even ingested, to quantify daily activities or physiological changes (type of exercise, heart, sleep, medication intake).
The interesting aspect of these devices is that knowledge of the acquired data allows the patient to have an increasingly active role in the management of their chronic disease.30 These innovations are increasingly well accepted by patients31 and may gradually reach also those users who are not familiar with technology (elderly, low-income groups, or people with low education levels).32–34 This has led to the creation of at least 100 000 ‘apps’ related to the health care area. In the near future, mobile technology may become a useful resource in the field of health care, providing an excellent cost/effectiveness ratio.35,36
The future perspective is to enhance the ‘Personal Health Record’, an electronic personal health information record, complying with interoperability standards recognized at least at national level, that can be sustained by various sources, while remaining under individual control, and be shared wherever appropriate.
However, there are several critical issues still to be solved, such as security, privacy and legal implications of physician’s responsibility in the event of incorrect diagnosis based on inaccurate data supplied by patients.8 In fact, only very few applications have been scientifically validated and certified as medical devices and even those best studied have often proved deficient in a comparison with a randomized control group.37–40 A meta-analysis including data from 13 studies, with a total of 3337 HF patients, has shown that in the TM group, the outcome was better in patients with increased frequency of contacts and in those benefiting from a greater rapidity of intervention and therapy management.41 Among these studies, only the MOBITEL study used the phone as a console for transmitting and receiving data to the monitoring centre.42 The aim of the study was to assess the impact of TLM on cardiovascular mortality and HF readmissions after discharge from an acute hospitalization by sending an e-mail alert in case of abnormal values. This study randomized 120 patients (85 males), with an average age of 66 years. It demonstrated that TM is able to reduce the frequency and the length of hospitalizations, as long as facilitation of daily recording and transmission of data is provided, especially for the elderly. In another study,43 the effectiveness of using mHealth was evaluated alone, or associated with the intervention of a caregiver, in symptomatic HF patients (NYHA II–III) with LVEF <40%. In the control group, patients received an automated call every week for 12 months, which included questions on general status, HF symptoms and self-management of the disease, to which they responded by typing answers. Based on these results, personalized advice on how to continue self-management was provided and, in the case of significant clinical changes, the system automatically sent a warning fax to the cardiologist. Compared to this group, patients randomized to mHealth with caregivers also received an email with personalized recommendations including advice to caregivers for providing support in the continuation of care. Caregiver’s involvement was associated with improvement in dyspnoea and adherence to drug therapy.43
In summary, even well-conducted studies have several limitations, such as being typically single-centre, being based on information provided by patients rather than objective (as in the case of wearable devices), and excluding several potential participants due to language issues, lack of equipment, or confidence in the use of the phone. Generally, these studies have focused on selected patients from specialized centres and they have hardly ever evaluated long-term adherence, behavioural changes as well as cost-effectiveness in relation to clinical outcomes. Thus far, mHealth seems to have been tested on relatively young HF patients whereas, in the future, it will be necessary to expand this technology also to real-world patients (i.e. elderly), providing for a gradual implementation of educational programs, focused on the use of these new technologies; this may provide patients with a strong stimulus in improving adherence to treatment measures.
The remote control of patients with devices
Remote monitoring in patients with ID is an essential part of clinical management, but it is made increasingly difficult by the number and complexity of ID and patients. For several years, in fact, all the major companies of devices have introduced remote monitoring systems,44 based on ‘patient units’ capable of interrogating the device and downloading both programmed parameters and diagnostic data. The information is transmitted to a central database where the data are decrypted and placed on a secure web portal that can be accessed only by the components of the staff assigned to the care of each patient. Initially, the systems were based on a manual interrogation of the device from the patient with subsequent data transmission to the central server through analogue telephone connections. Currently, available systems are based on automatic transmissions, fully independent of both physician and patient. A clear distinction must be made between ‘remote control’ and ‘remote monitoring’. In the former case, the device interrogation is made periodically to the patient’s home, manually by the patient or automatically by the monitoring system at pre-defined intervals. In the latter case, there is a continuous monitoring of the ID, integrated by unplanned data transmissions, in case of alarm.
The introduction of remote monitoring in clinical practice has required the implementation of new organizational models, which coordinate the activities of the professionals involved in the diagnosis and treatment process, such as electrophysiologists, nurses, technicians, bioengineers, suppliers of services, clinical cardiologists, and HF specialists. These models need to ensure a clear definition of roles and responsibilities, traceability of actions, a content of resource consumption, the appreciation and acceptance on the part of the patient, the integration with traditional and out-of-hospital care pathways. Tested in pilot studies45 and in a large registry that enrolled 1650 patients,46,47 a new model based on the concept of ‘Primary Nursing’ was implemented by national and international guidelines,48,49 The model is essentially based on a close working relationship between experienced nurse, to whom each patient is assigned, and a doctor in charge, with a shared list of tasks and responsibilities. The operating protocol contents include a strict definition of workflows, an early reaction to the events, the traceability of operations, continuity of care, the maintenance of human and interpersonal relationships with patients. In particular, the nurse’s duties include contact with the patient, educational interventions, monitoring of adherence to therapy and disease activity as a whole, inclusion of data in the web portal, systematic screening data with identification of critical issues, revision of transmission and alarms, clinical discussion of critical cases with the physician. Physician’s tasks include submission of informed consent from the patient, analysis of critical transmissions submitted by the nurse, clinical evaluation of the patient and relevant treatment decisions, as well as communication with GPs and other specialists.
In addition to providing the necessary therapies for ventricular tachyarrhythmias and pacing for cardiac resynchronization, modern devices provide several diagnostic information that can be useful in monitoring and early recognition of the progression of the disease and HF deterioration. The available diagnostics include measuring HR variability, daily activities, percentage of right ventricular pacing (single- and dual-chamber devices), and effective cardiac resynchronization (biventricular devices), thoracic impedance, and pressure measurements from haemodynamic sensors. The periodic intracavitary electrogram also allows to check the effective capture of the left ventricular pacing. The HR variability has also been shown in numerous studies to be predictive of cardiovascular mortality and HF hospitalization.50–52
Another important aspect of HF patient management is the monitoring of arrhythmias, notably of atrial fibrillation. In patients with automatic monitoring defibrillator,53 the recognition of atrial fibrillation occurred 34.5 days in advance; in another population of only pacemaker with daily monitoring,54 the incidence of hospitalizations for atrial arrhythmias and stroke was 7.3% in the control group and 2.4% in the group with remote monitoring, with an incidence of stroke of 3.3% and 0.8%, respectively, due to the earlier start of anticoagulation therapy following the anticipated recognition of arrhythmia in the device group. Remote monitoring of the devices also showed a reduction in inappropriate shocks,55,56 with possible positive effects in HF patients. Recent Consensus Document does include the use of remote monitoring devices as Class 1A recommendation, both to reduce inappropriate shocks, and for early detection of atrial fibrillation.49
From the HF point of view, an abrupt reduction of thoracic impedance is correlated to pulmonary congestion and can induce an alarm in case of exceeding the predefined threshold. Continuous monitoring of diagnostic information stored on the device may allow the early detection of increasing filling pressures with activation of sympathetic system in asymptomatic patients. The goal being to modify the strategy of clinical management from a ‘reactive’ type, where the therapy is delivered in response to worsening of symptoms or overt clinical congestion to a ‘pro-active’ type in which the therapeutic changes are decided when the patient is still asymptomatic, typically 2–3 weeks in advance of the acute event.
The expected results of this strategy are the prevention of HF hospitalizations and progression of the disease, in addition to an improvement of quality of life. The algorithms based on the thoracic impedance alone have demonstrated good sensitivity in the early detection of HF, on average over 2 weeks in advance, but a modest specificity.57–60 In fact, although many non-randomized studies59,61 have highlighted the benefits of using this sensor, it can be easily influenced by other factors not related to pulmonary congestion (infections, anaemia, pleural effusions, etc.), potentially causing false positive alarms and even, as recently reported, an increase in hospitalizations.60 For this reason, the degree of recommendation of the guidelines49 is currently relatively low (IIb). Finally, we recently introduced advanced technologies based on direct measurement of intracardiac and vascular pressures. Among these, a wireless implantable haemodynamic sensor, recently approved by the FDA, allows continuous monitoring of pulmonary artery pressure and the ability for the physician to review the data remotely. In the CHAMPION study62 on 550 patients, the group randomized to the treatment guided by CardioMEMS showed a significant 37% reduction in HF hospitalizations in comparison to the control group.
An improvement in the ability to predict the risk of events can provide even better results from a multi-parametric evaluation. The study PARTNERS HF, involving about 700 ICD patients, has shown that a dynamic multi-parametric score, including the impedance associated with the measures of HR, HR variability, number of shocks etc., was able to identify subjects with about 5.5 higher risk of HF hospitalization.20 This study demonstrated the effectiveness of the poliparametric approach, with respect to the measurement of individual parameters, and the strengths of a TM system carrying out automatic measurement of parameters. Finally, the study EVOLVO63 has shown that it is possible to reduce unplanned visits and access to the emergency department for medical reasons or reasons relating to the device. There is growing evidence that remote monitoring can increase patient survival. In fact, the analysis of large database involving over 200 000 patients (ALTITUDE64 and MERLIN65) showed that remote monitoring was associated with a reduction in risk of death by 50% as compared to the control group. A very significant finding came from randomized IN-TIME66 of 664 patients with mild-to-moderate HF and implant of bicameral or biventricular ICD. Enrolled patients were randomized in two parallel groups: the remote arm benefited from a multi-parametric monitoring with data transmission, in addition to standard care; the standard arm included the classic monitoring of device data through periodic planned hospital visits. The authors reported a worsening of the composite score in 63 patients out of 333 (18.9%) of the remote arm and 90 of 331 (27.2%) of the standard arm (P = 0.013) [odds ratio 0.63, 95% confidence interval (CI) 0.43–0.90]. This clinical benefit associated with the remote monitoring was associated with a significantly lower mortality at 1 year (3.0% vs. 8.2%; HR 0.36 95% CI 0.17–0.74; P = 0.004).
On the other hand, in a recent meta-analysis of 11 studies, TLM of cardiac implantable electronic devices was associated with a marked reduction in planned hospital visits and overall costs, without compromising survival or markedly increasing unplanned hospital visits, ER visits, or hospitalizations. Since remote monitoring is essentially a diagnostic adjunct rather than an intervention, this may explain the lack of effect on patient outcome.67 Similar results were obtained in two other recent studies.68,69
Further studies are under way to identify a multi-parametric score that combines high sensitivity and specificity in predicting episodes of acute exacerbation of HF.70
Technological development is rapidly evolving (ventilation sensors per minute,71,72 accelerometric endocardial sensor related to myocardial contractility (dP/dt) for the optimization of biventricular pacing,73 detection of ST alterations ST, automatic transmission to the device of weight and BP through dedicated tools). Apart from the technical validation phase, for all these tools a clinical validation is necessary to wait for the data and their advantages in the panel of parameters already available to prevent incidents of HF.
Patient’s empowerment
Over the past few years, healthcare institutions have promoted the use of TM to support the growth of patient’s empowerment.74 The final objective of TM programs is not limited to the improvement of prognosis, reduction of re-hospitalizations and educational intervention, but it also aims at promoting patient’s self-care through a gradual educational process.
Within chronic care programs, TM becomes a way to promote information and synergies with operating healthcare staff and caregivers, to improve patient’s knowledge on his/her disease, to increase self-management abilities as well as personal accountability towards one’s own health and wellbeing.75–77 Firstly, each patient develops generic competences on the use of devices and collection of clinical parameters, secondly he/she acquires specific knowledge on monitoring and early recognition of signs and symptoms of congestion, self-management and adherence to therapy, according to shared algorithms generated by decisions support system software.
The ICT also contributes to redefine the relationships between hospital and territory within the framework of a chronic care model. Each patient develops self-efficacy and self-esteem so as to improve adherence and reduce needs and costs related to social and health care.78 The use of home devices for monitoring clinical and environmental parameters as well as the web-based support for the interactive educational interventions, the administration of questionnaires for customer satisfaction or the availability of people supporting the patient on-line, determine a dynamic and flexible relationship between the patient and the new communication technologies spurring the growth of techno-social nets79 to overcome the fragmentation of the old healthcare structure.
Technical and regulatory affairs
The ageing population, the prevalence of multiple chronic conditions and the co-occurrence of two or more chronic diseases, have steadily increased. Thus, ICT could improve the quality and cost-effectiveness of healthcare systems, supporting self-management, increasing adherence and information about disease progression, and collecting biological data.80 However, although there is an important need to invest in ICT, a decrease in expenditure is being recorded in the welfare system. The result is an unequal access to health care and to the citizens rights. The investment reduction on ICT concerns:
A personal health record is an electronic application used by patients to maintain and manage their health information in a private, secure, and confidential environment.
E-prescribing or electronic prescribing is a technology framework that allows physicians and other medical practitioners to write and send prescriptions to a participating pharmacy electronically instead of using handwritten prescriptions.
The use of ICT to provide and support healthcare when distance separates participants; TM could be used to reorganize health care in chronic diseases.
In Italy, two further issues have emerged concerning ICT investments. Notably:
Digital healthcare is still excessively fragmented, with no clear common policies shared among central government and the regions;
Health management of chronic disease requires in-depth reorganization of home care, primary care, and rehabilitation services to overcome fragmentation of healthcare provision.
Regulatory framework
ICT is a cornerstone of efficient and effective services. Despite the significant spread of broadband networks and the development of new TLM applications, access to this development is not universal, and many countries do not benefit as they might from advances in ICT and TM. TM and ICT policy makers need to work together to ensure that national ICT policy meets the interest of the health sector including the use of TM. Healthcare professionals, hospitals, and laboratories are using more and more ICT applications to communicate health data for treatment, diagnosis, consultation and other purposes.81 It is important to underline that TM is not a new medical act, and is not intended to completely replace all traditional methods of care delivery, such as face-to-face consultations. Rather, it represents an innovative way of providing healthcare services, which can complete and potentially increase the quality and efficiency of traditional healthcare delivers.
Unfortunately, in Italy, directives on TM come from many different ‘Tables’: Ministry of Health, Ministry of Finance and Economy, Digital Italy Agency (Agid) etc. Several regions have also developed different directives on data protection, medical devices, and reimbursement. This situation determines further barriers to the real-life implementation of TM. However, the absence of a TM legal and regulatory framework at national level remains the main problem. For this reason, both in the year 200982 and more recently83 a position paper on TM was published by the Italian Society of Cardiology to better define operational standards starting from the Italian best practice on TM. In 2014, the national strategic guidelines on TM were published by the ‘State-Regions Conference’.84 Other important documents in this field are ‘Italian Ultra Wide Band Strategy’85 and the ‘Strategy for Digital Growth 2014-2010’,86 both published in November 2015 with the purpose of transforming ICT investments in engines of development. Actually, Italian ministerial guidelines identify criteria for accreditation to deliver TM, notably for reimbursement by the National Health System.
Reimbursement
One of the most significant challenges to widespread TM adoption is reimbursement. National guidelines must support reimbursement for appropriately structured TM communications, whether synchronous or asynchronous and whether solely text-based or with voice, video, or device feeds. As a matter of fact, all these forms of communication may be a clinically appropriate service, comparable to a face-to-face meeting.
The roadmap that will hopefully turn projects into «routine» services, passes through an intermediate step which may be defined «experimental services». From 2002 to 2010, regional social-health plans expected a gradual shift from hospital to home through the deployment of some experimental projects supported by TM.87
New healthcare networks (NRS)88 is a home-based telesurveillance program for chronic patients (Congestive Heart Failure and Chronic Obstructive Pulmonary Disease) living in Lombardy region. NRS is a framework in which patients’ data of chronic disease home-management are collected from all hospitals involved, centrally managed and evaluated. The service run over a 6-months’ period, with the possibility to be prolonged for additional 6 months. This is an example of multi-disciplinary approach, characterized by structured telephone support and TLM, consisting of scheduled calls performed by a nurse and occasional calls made by patients. During calls, the nurse carried out a scheduled standardized interview on the patient’s general clinical condition, drugs therapy and compliance. During the calls the nurse reinforced the initial educational intervention implementing strategies to improve patient’s adherence. Occasional calls could be performed 24/24 h by patients in case of symptoms or signs of possible decompensation/worsening. During the period of service implementation, all the hospitals involved in the network sent the records of their patients to the Lombardy Region every 3 months, according to a specific information flow. For this procedure a reimbursement was granted following upon detailed assessment by healthcare authorities. In the Lombardy Region, reimbursement for TM services started in 200689 with two types of services on patients with chronic HF and on patients’ home-rehabilitation after a cardiac artery by-pass graft following the indication provided by the Cerebro-Cardiovascular Plan (2005).90 Subsequently, palliative care for oncological patients, telesurvellaince for chronic obstructive pulmonary disease91 patients, and multi-specialty second opinion for GPs92 started.
Health technology assessment and the systems
Technological innovation has yielded truly remarkable advances in health care during the last five decades. In recent years, breakthroughs in a variety of areas have improved health care delivery and patient outcomes; the proliferation of health care technology and its expanding use have contributed to burgeoning healthcare costs, and the former has been cited as ‘culprit’ for the latter. However, this relationship is variable, complex, and in a constant progress. In this era of increasing cost pressure, restructuring of healthcare delivery and payment, and heightened consumer demand, technology remains the substance of healthcare. Culprit or not, technology can be managed in ways that improve patient access and health outcomes, while continuing to encourage useful innovation. Health technology assessment (HTA) is a multi-disciplinary process that summarizes information about medical, social, economic and ethical issues related to the use of health technology in a systematic, transparent, unbiased and robust manner. The overall aim of HTA is to support decision-making by clinicians and patients providing a structured framework for assessing the effectiveness and contribution to quality of care of TM applications.
At the same time, the framework or model should be based on the users’ (e.g. the medical profession, payers, health authorities) need for information in order to make decisions on whether or not to use new TM applications. The HTA should also be based on a review of the scientific literature on methodologies for assessment of TM.
Privacy
Up to a few decades ago, the treatment of health personal data was based on a fiduciary relationship between the patient and his/her GP mainly managed by paper records or mnemonic modalities.92 The introduction of the ICT has deeply changed the approach to health personal data underlying the necessity of a procedure to assist the patient in the treatment of his/her personal data.93 The creation of software able to elaborate clinical data or electronic files able to pick up a great amount of data and nets able to manage data in real-time, offers both scientific and medical advantages allowing to elaborate and compare clinical information in a few seconds. Nevertheless, such development, by increasing the number of subjects with ‘visible’ health personal data, the speed of data transmission and the quantity of electronical information recorded, has determined an exponential increase of the risk of misuse both in terms of confidentiality and of data protection. In other words, although we acknowledge the role of new technologies in the medical field, their use should never neglect the patient, who is the actual recipient of care.
ICT has transformed medical information, both related to the clinical diagnosis and care, in a collection of health data, documented, interpreted, and finally archived. Hence, accrued sensitivity by the European and National legislators to protect such data has arisen.93 The protection of patient’s information is currently the objective of a relevant part of the legal system, as well as of important directives by the Italian Data Protection Authority. Their activity has contributed to put into practice the principles of necessity, proportionality, indispensability, and adequacy of data treatment in the electronic health records. Such activity is sustained by a strong knowledge of ethical and deontological matters. The patient’s consent for processing his/her data is similar to the one requested for clinical procedures. Processing of health data as sensitive data was disciplined in 1996 by the law N. 675,94 to protect patient’s life and personal safety with the right to privacy, as confirmed by the Data Protection Code. This latter affirms the centrality of the consent as anticipated by the European directive 95/46/CE.95–97 Moreover, the European Union is adopting new rules to protect citizens’ privacy, with completely different characteristics in comparison with those currently in force. This new complex of laws will be applied in each state member.
Storing information
TM generates a great amount of data/information automatically, and these data have to be available for clinicians, GPs etc. Hence, it is important to have a directive which contains several important principles that require compliance from stakeholders who process personal health data.98 If national healthcare systems or other e-health stakeholders create health grids, electronic national records, or information systems to be used for treatment, quality review or research purposes, they have to comply with the principles of the Data Protection Directive.
It is important to respect general principles, like protecting individuals in regards to the processing and free movement of personal data, applying automatic processing of personal data, prohibiting processing of personal health data unless required for purposes of preventive medicine, medical diagnosis, provision of care or treatment, or management of healthcare services, or where that information is processed under national law or rule related to a professional or patient subject to professional confidentiality.
There are further principles relevant to TM:
personal data used in TM must be processed fairly and lawfully;
data must be collected for specified, explicit and legitimate purposes;
data must be adequate, relevant and not excessive in relation to the purposes for which they are collected;
data should be identifiable for no longer than is necessary or for as long as required for further processing;
data owners should be informed regarding the processing of their data.
Future perspectives
All recent epidemiologic studies show two major aspects: firstly, patients suffering from HF are increasing; secondly, there is a huge amount of costs related to hospitalizations. Up today, there is a poor application of recent findings on TM disease management that could help better coordinate patients’ treatment. In this context, TM inserted in the ‘ICT-assisted Chronic Care Model’ may be a turning point for HF treatment.99 A big part of the healthcare world is still opposed to ICT, even if new technologies, in particular the wireless ones, have thus far proved effective in improving quality and efficiency of care while at the same time increasing the possibility of satisfying patients’ needs.
In the future, new models of telesurveillance may benefit from the following:
Implementation of an ‘ICT-assisted’ management for chronic diseases. In order to be efficient, any model needs to be integrated within an organized care system; so, TM has to be an addition, not an alternative, to the classic model of integrated care, so as to facilitate, intensify and personalize disease management. TM makes easier to perform standard interventions through integrated communication systems and TLM. Some of these activities are difficult to implement (e.g. education of the patient, relatives and caregivers, close monitoring of the patient follow-up, best therapeutic choices, and identification of a true case manager to satisfy the needs of a patient with a chronic disease and several comorbidities). It is very important to choose the right patient and the right TM program, based upon patient’s age, severity of the disease and HF stage (with particular focus on the post-discharge transition phase), associated comorbidities, patient’s self-care and cognitive abilities, family context and presence of a caregiver.
Close cooperation between specialists and GPs. This is the key point of the system and must be implemented both in terms of sharing medical information and collaboration between specialists of the same field (i.e. cardiologists) with different specificities (haemodynamists, electrophysiologists’ etc.), and specialists in different fields (diabetologists, neurologists etc.). The absence of this collaboration excludes the possibility of inserting the patient in the chronic care model.
Sharing patient’s information. With TM, a medical practice or hospital system can immediately expand access to medical specialists. This makes it easy for primary care doctors to consult medical specialist, and for patients to see a specific specialist, no matter their location. Also, small hospitals without specialists on-staff could outsource evaluation of examinations via TLM. Interoperability (software, networks, communication devices, computers and other types of information technology components) is essential to interact with one another and exchange information so that predictable results can be achieved.
Patient case manager. Case managers try to find the best, most efficient, economically feasible ways to meet patient’s needs with the available resources. In this new era of patient safety, the case manager, as an advocate and facilitator of care, has a pivotal role on the front line of healthcare delivery. Effective communication and collaboration between disciplines are the keys to the promotion of patient’s safety. Across the healthcare continuum and within hospitals in particular, patients are routinely transferred from one service to another, from one level of care to another. As patients are stabilized and transitioned through the hospital system, home case managers could be the reference for a multi-disciplinary home management with the use of TM. The coordination functions performed by a case manager include helping patients to navigate healthcare systems and connecting them with community resources. Case managers can also perform clinical functions, including disease-oriented assessment and monitoring, medication adjustment, health education, and self-care instructions. Such clinical functions are often the defining aspects of other chronic illness management interventions.
Clinical decision support system (CDS). It is a health information technology system designed to provide physicians and other health professionals with clinical decision support, that means assistance with clinical decision-making tasks. CDS may provide support to clinicians at various stages of the care process, from preventive care through diagnosis and treatment to monitoring and follow-up. The most common use of CDS is for addressing clinical needs, such as ensuring accurate diagnoses, screening in a timely manner for preventable diseases, or averting adverse drug events. However, CDS can also potentially lower costs, improve efficiency, and reduce patient inconvenience. In fact, CDS can sometimes address all the three areas simultaneously for example, by alerting clinicians to potentially duplicative tests. The CDS may offer suggestions, but the clinician must filter and review the information and decide whether or what action to perform.
Care customization. From the patient’s point of view, care customization has always been an important aspect of quality in healthcare. As the traditional ‘doctor-patient relationship’ implies, customization is at the core of the conception of care for healthcare professionals. As in other sectors, a customized service is perceived not only as better quality but also as more attractive, thus allowing a premium to be charged. Two relatively new concepts, patient-centred care (organizing patient management to meet the needs of the individual patient) and personalized medicine (tailoring therapy to the patient’s biological characteristics, and in particular to his/her genetic profile) have generated a great deal of interest and investment and have been developed independently. We see a significant potential for these concepts to be integrated and introduced into day-to-day patient management, up to now we know of no attempts, either conceptually or practically to do so. There is a need to understand both how they might be integrated and whether the underlying economics could make it feasible.
The Internet of You. The concept of the Internet of Things entails the use of electronic devices that capture or monitor data and are connected to a private or public cloud, enabling them to automatically trigger certain events. Internet-connected devices have been introduced to patients in various forms. Data come from monitors, electrocardiograms, temperature monitors, or blood glucose levels, tracking health information that are vital for some patients. Many of these measures require follow-up interaction with a healthcare professional. This creates an opening for smarter devices to deliver more valuable data, lessening the need for direct patient-physician interaction.
The ‘ICT-assisted Chronic Care Model’ could be the best way to care for chronic patients in the near future.98
Conclusions
The next years will be inevitably characterized by a number of technological developments that will further influence our ways to communicate and interact with the patients. However, for the time being, technological development is still mostly market-driven.
There is an absolute need for a close collaboration between institutions and scientific societies to manage this development, to define and apply certified standards of quality and process, and to evaluate common solutions both at national and regional levels to be applied to elderly and chronic patients.
If we fail to do so, we run the risk of being quickly swept away by a large amount of information, of unverifiable quality and scarce utility, which may determine a series of non-sustainable interventions. A first step towards the management of the system is to provide something like ‘identity cards’ of the high number of solutions provided and certification on the use of products as medical devices. The following step will be to assess, apply and improve ICT-based solutions on the field as part of a renewed organizational, multi-disciplinary and multi-professional model of integration between hospital-territory named ‘Chronic Care Model’.
The active role that Italy has played in the development of these systems over the past few years seems to suggest that this is the right path to follow to avoid useless hospitalizations for HF patients. An important organizational effort should be undertaken firstly adapting and updating the existing rules, and secondly growing competence to manage the complexity of chronicity.
Conflict of interest: none declared.
Consensus Document Approval Faculty
Abrignani Maurizio Giuseppe, Amico Antonio Francesco, Amodeo, Vincenzo, Angeli Fabio, Audo Andrea, Azzarito Michele, Battistoni Ilaria, Bianca Innocenzo, Bisceglia Irma, Bongarzoni, Amedeo, Bonvicini Marco, Cacciavillani Luisa, Calculli Giacinto, Caldarola Pasquale, Capecchi Alessandro, Caporale Roberto, Caretta Giorgio, Carmina Maria Gabriella, Casazza Franco, Cassin, Matteo, Casu Gavino, Cemin Roberto, Chiarandà Giacomo, Chiarella Francesco, Cibinel Gian Alfonso, Ciccone Marco Matteo, Cicini Maria Paola, Clerico Aldo, Colivicchi Furio, D’Agostino, Carlo, De Luca Leonardo, De Luca Giovanni, De Maria Renata, Del Sindaco Donatella, Di Fusco Stefania Angela, Egidy Assenza, Gabriele, Egman Sabrina, Enea Iolanda, Fattirolli Francesco, Francese Giuseppina Maura, Geraci Giovanna, Greco Cesare, Gregorio Giovanni, Iacoviello Massimo, Khoury Georgette, Ledda, Antonietta, Lucà Fabiana, Macera Francesca, Marini Marco, Mascia Franco, Masson Serge, Maurea Nicola, Mazzanti Marco, Mennuni Mauro, Menotti Alberto, Menozzi Alberto, Mininni Nicola, Molon Giulio, Moreo Antonella, Moretti Luciano, Mureddu, Gian Francesco, Murrone Adriano, Musumeci Giuseppe, Navazio, Alessandro, Nicolosi Gian Luigi, Oliva Fabrizio, Oreglia Jacopo, Parato, Vito Maurizio, Parrini Iris, Patanè Leonardo, Pini Daniela, Pino, Paolo Giuseppe, Pirelli Salvatore, Procaccini Vincenza, Pugliese, Francesco Rocco, Pulignano Giovanni, Radini Donatella, Rao Carmelo, Massimiliano, Rasetti Gerardo, Riccio Carmine, Roncon Loris, Rossini Roberta, Ruggieri Maria Pia, Rugolotto Matteo, Sanna, Fabiola, Sauro Rosario, Scherillo Marino, Severi Silva, Sicuro Marco, Silvestri Paolo, Sisto Francesco, Tarantini Luigi, Themistoclakis, Sakis, Uguccioni Massimo, Urbinati Stefano, Valente Serafina, Vatrano Marco, Vianello Gabriele, Vinci Eugenio, Zuin Guerrino.
References
- 1.Communication from the Commission to the European Parliament, the Council, The European Economic and Social Committee and the Committee of the Regions. eHealth Action Plan 2012-2020. Innovative healthcare for the 21st century. Available at: http://eur_lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2004:0356:FIN:EN:PDF (April 8, 2017).
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology. Eur Heart J 2016;37:2129–2200.27206819 [Google Scholar]
- 3. Polisena J, Tran K, Cimon K, Hutton B, McGill S, Palmer K, Scott RE. Home telemonitoring for congestive heart failure: a systematic review and meta-analysis. J Telemed Telecare 2010;16:68–76. [DOI] [PubMed] [Google Scholar]
- 4. Klersy C, De Silvestri A, Gabutti G, Regoli F, Auricchio A.. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol 2009;54:1683–1694. [DOI] [PubMed] [Google Scholar]
- 5. Clark RA, Inglis SC, McAlister FA, Cleland JG, Stewart S.. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ 2007;334:942.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaudhry SI, Phillips CO, Stewart SS, Riegel B, Mattera JA, Jerant AF, Krumholz HM. Telemonitoring for patients with chronic heart failure: a systematic review. J Card Fail 2007;13:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mortara A, Oliva F, Di Lenarda A.. Prospettive della telemedicina e del monitoraggio mediante dispositivi nel paziente con scompenso cardiaco cronico: luci e ombre. G Ital Cardiol 2010;11(Suppl 2):33S–37S. [PubMed] [Google Scholar]
- 8. Buccoliero L. e-Health 2.0. Tecnologie per il Patient Empowerment. Mondo Digitale, dicembre 2010:3–17. [Google Scholar]
- 9. Billot L, Corcoran K, McDonald A, Powell-Davies G, Feyer AM.. Impact evaluation of a system-wide chronic disease management program on health service utilisation: a propensity-matched cohort study. PLoS Med 2016;13:e1002035.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai AS, Stevenson LW.. Connecting the circle from home to heart-failure disease management. N Eng J Med 2010;363:2364–2367. [DOI] [PubMed] [Google Scholar]
- 11. Clarke M, Shah A, Sharma U.. Systematic review of studies on telemonitoring of patients with congestive heart failure: A meta-analysis. J Telemed Telecare 2011;17:7–14. [DOI] [PubMed] [Google Scholar]
- 12. Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JG. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. Eur J Heart Fail 2011;13:1028–1040. [DOI] [PubMed] [Google Scholar]
- 13. Seto E. Cost comparison between telemonitoring and usual care of heart failure: a systematic review. Telemed e-Health 2008;14:679–686. [DOI] [PubMed] [Google Scholar]
- 14. Pandor A, Gomersall T, Stevens JW, Al-Mohammad A, Bakhai A, Cleland JG, Cowie MR, Wong R. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta-analysis. Heart 2013;99:1717–1726. [DOI] [PubMed] [Google Scholar]
- 15. Kotb A, Cameron C, Hsieh S, Wells G.. Comparative effectiveness of different forms of TM for individuals with heart failure (HF): a systematic review and network meta-analysis. PLoS ONE 2015;10:e0118681.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. New Engl J Med 2010;363:2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrante D, Varini S, Macchia A, Soifer S, Badra R, Nul D, Grancelli H, Doval H. GENICA investigators. long-term results after a telephone intervention in chronic heart failure: DIAL (Randomized Trial of Phone Intervention in Chronic Heart Failure) follow-up. J Am Coll Cardiol 2010;56:372–378. [DOI] [PubMed] [Google Scholar]
- 18. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, B�M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD; Telemedical Interventional Monitoring in Heart Failure Investigators. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The telemedical interventional monitoring in heart failure study. Circulation 2011;123:1873–1880. [DOI] [PubMed] [Google Scholar]
- 19. Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH. TEN-HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-european network-home-care Management system (TEN-HMS) study. J Am Coll Cardiol 2005;45:1654–1664. [DOI] [PubMed] [Google Scholar]
- 20. Mortara A, Pinna GD, Johnson P, Maestri R, Capomolla S, La Rovere MT, Ponikowski P, Tavazzi L, Sleight P; HHH Investigators. Home telemonitoring in heart failure patients: the HHH study (Home or Hospital in Heart failure). Eur J Heart Fail 2009;11:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar S, Porter CB, Pavri BB, O'Connor CM; PARTNERS Study Investigators. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and review Trending information and evaluate correlation to symptoms in Patients With heart failure) study. J Am Coll Cardiol 2010;55:1803–1810. [DOI] [PubMed] [Google Scholar]
- 22. Giordano A, Scalvini S, Zanelli E, Corrà U, Longobardi GL, Ricci VA, Baiardi P, Glisenti F. Multicenter randomised trial on home-based telemanagement to prevent hospital readmission of patients with chronic heart failure. Int J Cardiol 2009;131:192–199. [DOI] [PubMed] [Google Scholar]
- 23. Capomolla S, Pinna G, La Rovere MT, Maestri R, Ceresa M, Ferrari M, Febo O, Caporotondi A, Guazzotti G, Lenta F, Baldin S, Mortara A, Cobelli F. Heart failure case disease management program: a pilot study of home telemonitoring versus usual care. Eur Heart J Suppl 2004;6:F91–F98. [Google Scholar]
- 24. Giordano A, Scalvini S, Paganoni AM, Baraldo S, Frigerio M, Vittori C, Borghi G, Marzegalli M, Agostoni O. Home-based telesurveillance program in chronic heart failure: effects on clinical status and implications for 1-year prognosis. Telemed J E Health 2013;19:605–612. [DOI] [PubMed] [Google Scholar]
- 25. Scalvini S, Zanelli E, Volterrani M, Martinelli G, Baratti D, Buscaya O, Baiardi P, Glisenti F, Giordano A. A pilot study of nurse-led, home-based telecardiology for patients with chronic heart failure. J Telemed Telecare 2004;10:117. [DOI] [PubMed] [Google Scholar]
- 26. Pinna GD, Maestri R, Andrews D, Witkowski T, Capomolla S, Scanferlato JL, Gobbi E, Ferrari M, Ponikowski P, Sleight P, Mortara A, Johnson P. Home telemonitoring of vital signs and cardiorespiratory signals in heart failure patients: system architecture and feasibility of the HHH model. Int J Cardiol 2007;120:371–379. [DOI] [PubMed] [Google Scholar]
- 27. Antonicelli R, Testarmata P, Spazzafumo L, Gagliardi C, Bilo G, Valentini M, Olivieri F, Parati G. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare 2008;14:300–305. [DOI] [PubMed] [Google Scholar]
- 28. Villani A, Malfatto G, Compare A, Della Rosa F, Bellardita L, Branzi G, Molinari E, Parati G. Clinical and psychological telemonitoring and telecare of high risk heart failure patients. J Telemed Telecare 2014;20:468–475. [DOI] [PubMed] [Google Scholar]
- 29.Libro verde sulla SanitÁ Mobile (mHealth). Available at: http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=1775 (April 8, 2017).
- 30. Demiris G, Afrin LB, Speedie S, Courtney KL, Sondhi M, Vimarlund V, Lovis C, Goossen W, Lynch C. Patient-centered applications: use of information technology to promote disease management and wellness: a white paper by the AMIA Knowledge in Motion Working Group. J Am Med Inform Assoc 2008;15:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brennan PF, Strombom I.. Improving health care by understanding patient preferences: the role of computer technology. J Am Med Inform Assoc 1998;5:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leena K, Tomi L, Arja RR.. Intensity of mobile phone use and health compromising behaviours: how is information and communication technology connected to health-related lifestyle in adolescence? J Adolesc 2005;28:35–47. [DOI] [PubMed] [Google Scholar]
- 33. Smith A. Mobile access 2010. Available at: http://www.pewinternet.org/files/old-media//Files/Reports/2010/PIP_Mobile_Access_2010.pdf (28 October 2014).
- 34.Pew Research Center. Internet, Science & Tech. The Demographics of Device Ownership. 2015. Available at: http://www.pewinternet.org/2015/10/29/the-demographics-of-device-ownership/ (April 8, 2017).
- 35.Rock HealthDigital Health Funding 2015 Midyear Review. Rock Health website.http://rockhealth.com/2-1b-digital-health-funding -first-half-2015-keeping-pace-2014/ (21 August 2015).
- 36.mHealth App Developer Economics 2016. The current status and trends of the mHealth app market. Available at: http://research2guidance.com/r2g/r2g-mHealth-App-Developer-Economics-2016.pdf (April 8, 2017).
- 37.Burke LE, Ma J, Azar KM, Bennett GG, Peterson ED, Zheng Y, Riley W, Stephens J, Shah SH, Suffoletto B, Turan TN, Spring B, Steinberger J, Quinn CC. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation 2015;132:1157–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, Jan S, Graves N, de Keizer L, Barry T, Bompoint S, Stepien S, Whittaker R, Rodgers A, Thiagalingam A. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA 2015;314:1255–1263. [DOI] [PubMed] [Google Scholar]
- 39. Patrick K, Raab F, Adams MA, Dillon L, Zabinski M, Rock CL, Griswold WG, Norman GJ. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res 2009;11:e1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whittaker R, Borland R, Bullen C, Lin RB, McRobbie H, Rodgers A.. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2009;4:CD006611.. [DOI] [PubMed] [Google Scholar]
- 41. Nakamura N, Koga T, Iseki H.. A meta-analysis of remote patient monitoring for chronic heart failure patients. J Telemed Telecare 2014;20:11–17. [DOI] [PubMed] [Google Scholar]
- 42. Scherr D, Kastner P, Kollmann A, Hallas A, Auer J, Krappinger H, Schuchlenz H, Stark G, Grander W, Jakl G, Schreier G, Fruhwald FM; MOBITEL Investigators. Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial. J Med Internet Res 2009;11:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Piette JD. A Mobile health intervention supporting heart failure patients and their informal caregivers: a randomized comparative effectiveness trial. J Med Internet Res 2015;17:e142.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dubner S, Auricchio A, Steinberg JS, Vardas P, Stone P, Brugada J, Piotrowicz R, Hayes DL, Kirchhof P, Breithardt G, Zareba W, Schuger C, Aktas MK, Chudzik M, Mittal S, Varma N. ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs). Europace 2012;14:278–293. [DOI] [PubMed] [Google Scholar]
- 45. Ricci RP, Morichelli L, Santini M.. Home monitoring remote control of pacemaker and ICD patients in clinical practice. Impact on medical management and health care resource utilization. Europace 2008;10:164–170. [DOI] [PubMed] [Google Scholar]
- 46. Ricci RP, Morichelli L, D’onofrio A, Calò L, Vaccari D, Zanotto G, Curnis A, Buja G, Rovai N, Gargaro A. Effectiveness of remote monitoring of CIEDs in detection and treatment of clinical and device-related cardiovascular events in daily practice: the HomeGuide Registry. Europace 2013;15:970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ricci RP, Morichelli L, D'onofrio A, Calò L, Vaccari D, Zanotto G, Curnis A, Buja G, Rovai N, Gargaro A. Manpower and outpatient clinic workload for remote monitoring of patients with cardiac implantable electronic devices: data from the HomeGuide Registry. J Cardiovasc Electrophysiol 2014;25:1216–1223. [DOI] [PubMed] [Google Scholar]
- 48. Ricci RP, Calcagnini G, Castro A, Giada F, Igidbashan D, Landolina M, Melissano D, Perego GB, Toselli T. Documento di consenso sul monitoraggio remoto dei dispositivi impiantabili: tecnologie disponibili, indicazioni, modelli organizzativi, accettabilità, responsabilità ed aspetti economici. G Ital Cardiol 2011;12:450–467. [DOI] [PubMed] [Google Scholar]
- 49. Slotwiner D, Varma N, Akar JG, Annas G, Beardsall M, Fogel RI, Galizio NO, Glotzer TV, Leahy RA, Love CJ, McLean RC, Mittal S, Morichelli L, Patton KK, Raitt MH, Ricci RP, Rickard J, Schoenfeld MH, Serwer GA, Shea J, Varosy P, Verma A, Yu CM. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015;12:e69–e100. [DOI] [PubMed] [Google Scholar]
- 50. Adamson PB, Smith AL, Abraham WT, Kleckner KJ, Stadler RW, Shih A, Rhodes MM; InSync III Model 8042 and Attain OTW Lead Model 4193 Clinical Trial Investigators. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation 2004;110:2389–2394. [DOI] [PubMed] [Google Scholar]
- 51. Gilliam FRIII, Kaplan AJ, Black J, Chase KJ, Mullin CM. Changes in heart rate variability, quality of life, and activity in cardiac resynchronization therapy patients: results of the HF-HRV registry. Pacing Clin Electrophysiol 2007;30:56–64. [DOI] [PubMed] [Google Scholar]
- 52. Gasparini M, Lunati M, Santini M, Tritto M, Curnis A, Bocchiardo M, Vincenti A, Pistis G, Valsecchi S, Denaro A; INSYNC/INSYNC ICD ITALIAN Registry Investigators. Long-term survival in patients treated with cardiac resynchronization therapy: a 3-year follow-up study from the InSync/InSync ICD Italian Registry. Pacing Clin Electrophysiol 2006; 29(Suppl 2):S2–S10. [DOI] [PubMed] [Google Scholar]
- 53. Varma N, Epstein A, Irimpen A, Schweikert R, Shah J, Love CJ.. Efficacy and safety of automatic remote monitoring for ICD follow-up: The TRUST trial. Circulation 2010;122:325–332. [DOI] [PubMed] [Google Scholar]
- 54. Mabo P, Victor F, Bazin P, Ahres S, Babuty D, Da Costa A, Binet D, Daubert JC; COMPAS Trial Investigators. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial). Eur Heart J 2012;33:1105–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guedon-Moreau L, Lacroix D, Sadoul N, Clémenty J, Kouakam C, Hermida JS, Aliot E, Boursier M, Bizeau O, Kacet S; ECOST trial Investigators. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J 2012;34:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guedon-Moreau L, Kouakam C, Klug D, Marquié C, Brigadeau F, Boulé S, Blangy H, Lacroix D, Clémenty J, Sadoul N, Kacet S. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial. J Cardiovasc Electrophysiol 2014;25:763–770. [DOI] [PubMed] [Google Scholar]
- 57. Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, Christensen J, Stadler RW, Lau CP. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005;112:841–848. [DOI] [PubMed] [Google Scholar]
- 58. Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse B, Yu CM, Cowie MR. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE-HF trial. Eur Heart J 2011;32:2266–2273. [DOI] [PubMed] [Google Scholar]
- 59. Catanzariti D, Lunati M, Landolina M, Zanotto G, Lonardi G, Iacopino S, Oliva F, Perego GB, Varbaro A, Denaro A, Valsecchi S, Vergara G; Italian Clinical Service Optivol-CRT Group. Italian Clinical Service Optivol-CRT Group. Monitoring intrathoracic impedance with an implantable defibrillator reduces hospitalizations in patients with heart failure. Pacing Clin Electrophysiol 2009;32:363–370. [DOI] [PubMed] [Google Scholar]
- 60. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Aguilera RM, Lunati M, Yu CM, Gerritse B, Borggrefe M; DOT-HF Investigators. DOT-HF Investigators. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011;124:1719–1726. [DOI] [PubMed] [Google Scholar]
- 61. Maines M, Catanzariti D, Cemin C, Vaccarini C, Vergara G.. Usefulness of intrathoracic fluids accumulation monitoring with an implantable biventricular defibrillator in reducing hospitalizations in patients with heart failure: a case-control study. J Interv Card Electrophysiol 2007;19:201–207. [DOI] [PubMed] [Google Scholar]
- 62. Abraham WT, Adamson PB, Bourge RC, Aranda JM Jr, Bourge RC, Smith A, Stevenson LW, Bauman JG, Yadav JS. CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 63. Landolina M, Perego GB, Lunati M, Curnis A, Guenzati G, Vicentini A, Parati G, Borghi G, Zanaboni P, Valsecchi S, Marzegalli M. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012;125:2985–2992. [DOI] [PubMed] [Google Scholar]
- 64. Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, Meyer TE, Jones PW, Boehmer JP. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359–2367. [DOI] [PubMed] [Google Scholar]
- 65. Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S.. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol 2015;65:2601–2610. [DOI] [PubMed] [Google Scholar]
- 66. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Søgaard P. IN-TIME study group . Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583–590. [DOI] [PubMed] [Google Scholar]
- 67.Klersy C, Boriani G, De Silvestri A, Mairesse GH, Braunschweig F, Scotti V, Balduini A, Cowie MR, Leyva F. Effect of telemonitoring of cardiac implantable electronic devices on healthcare utilization: a meta-analysis of randomized controlled trials in patients with heart failure. Eur J Heart Fail 2016; 18: 195–204. [DOI] [PubMed] [Google Scholar]
- 68. Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, Clementy N, Amori V, Mangoni di S Stefano L, Burri H. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail 2017;19:416–425. [DOI] [PubMed] [Google Scholar]
- 69.Cowie M. REM-HF - Remote monitoring: an evaluation of implantable devices for management of heart failure patients Presentation 1223, ESC Congress, Rome 2016
- 70. Padeletti L, Botto GL, Curnis A, De Ruvo E, D'Onofrio A, Gronda E, Ricci RP, Vado A, Zanotto G, Zecchin M, Antoniou X, Gargaro A. Selection of potential predictors of worsening heart failure: rational and design of the SELENE HF study. J Cardiovasc Med 2015;16:782–789. [DOI] [PubMed] [Google Scholar]
- 71. Bonnet JL, Geroux L, Cazeau S; for the French Talent DR Pacemaker Investigators. Evaluation of a dual sensor rate responsive pacing system based on a new concept. Pacing Clin Electrophysiol 1998;21:2198–2203. [DOI] [PubMed] [Google Scholar]
- 72. Defaye P, de la Cruz I, Marti-Almor J, Villuendas R, Bru P, Sénéchal J, Tamisier R, Pépin JL. A pacemaker transthoracic impedance sensor with an advanced algorithm to identify severe sleep apnea: The DREAM European study. Heart Rhythm 2014;11:842–848. [DOI] [PubMed] [Google Scholar]
- 73. Brugada J, Brachmann J, Delnoy PP, Padeletti L, Reynolds D, Ritter P, Borri-Brunetto A, Singh JP. Automatic optimization of cardiac resynchronization therapy using SonR-rationale and design of the clinical trial of the SonRtip lead and automatic AV-VV optimization algorithm in the paradym RF SonR CRT-D (RESPOND CRT) trial. Am Heart J 2014;167:429–436. [DOI] [PubMed] [Google Scholar]
- 74.European Commission (2011). Digital Agenda for Europe. Available at: http://ec.europa.eu/europe2020/pdf/digital-agenda-communication-en.pdf (April 8, 2017).
- 75. Stellato K, Humar F, Montesi C. et al. Integrated outpatient care in advanced heart failure: the beehive person-centered model. Int J Pers Cent Med 2014;4:23–30. [Google Scholar]
- 76. Riley JP, Gabe JPN, Cowie MR, Radini D, Antonione R, Sinagra G, Di Lenarda A. Does telemonitoring in heart failure empower patients for self care? A qualitative study. J Clin Nurs 2012;22:2444–2455. [DOI] [PubMed] [Google Scholar]
- 77. Suter P, Suter WN, Johnston D.. Theory-based telehealth and patient empowerment. Pop Health Man 2011;14:87–92. [DOI] [PubMed] [Google Scholar]
- 78. Anderson RM, Funnell MM.. Patient empowerment: myths and misconceptions. Patient Educ Couns 2010;79:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Anderson RM, Funnell MM, Aikens JE, Krein SL, Fitzgerald JT, Nwankwo R, Tannas CL, Tang TS. Evaluating the efficacy of an empowerment-based self-management consultant intervention: results of a two-year randomized controlled trial. Patient Educ 2009;1:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Osservatorio ICT in Sanità: Innovazione Digitale in Sanità: l’ICT non basta! Politecnico di Milano. Maggio: Dipartimento di Ingegneria Gestionale; 2014. 1–169. [Google Scholar]
- 81. Wootton R. Recent advances in Telemedicine. Br Med J 2001;323:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fedele F, Scalvini S.. Modelli di telecardiologia attualmente in uso. G Ital Cardiol 2009;10(Suppl 1):31S–33S. [Google Scholar]
- 83. Brunetti ND, Scalvini S, Acquistapace F, Parati G, Volterrani M, Fedele F, Molinari G. Telemedicine for cardiovascular disease continuum: a position paper from the Italian Society of Cardiology Working Group on Telecardiology and Informatics. Int J Cardiol 2015;184:452–458. [DOI] [PubMed] [Google Scholar]
- 84. Linee guida Telemedicina. Ministero della Salute Available at: www.salute.gov.it/imgs/C_17_pubblicazioni_2129_allegato.pdf (April 8, 2017).
- 85.Presidenza del Consiglio dei Ministri “Strategia italiana per la banda ultralarga”. Available at: www.agid.gov.it/sites/default/files/documenti_indirizzo/strategia_bul_nov._2014.pdf (April 8, 2017).
- 86.Presidenza del Consiglio dei Ministri “Strategia per la Crescita Digitale 2014–2020”. http://www.agid.gov.it/sites/default/files/documenti_indirizzo/crescita_digitale_nov_2014.pdf (April 8, 2017).
- 87.Social Services and Health Care Plan 2002-2004. Available at: www.cittametropolitana.mi.it/export/sites/default/affari_sociali/Normativa/ an_2002_psr.pdf. (April 8, 2017).
- 88.Social Services and Health Care Plan 2007-2009 Available at: http://www.promozionesalute.regione.lombardia.it/cs/Satellite?c=Redazionale_P&childpagename=DG_Sanita%2FDetail&cid=1213282167680&packedargs=NoSlotForSitePlan%3Dtrue%26menu-to-render%3D1213286247836&pagename=DG_SANWrapper (April 8, 2017)
- 89.D.g.r. 14 dicembre 2005 - n. 8/1375. Determinazioni in ordine alla gestione del Servizio Socio Sanitario regionale per l esercizio 2006. Available at: http://www.bollettino.regione.lombardia.it/cs/Satellite?c=Page&childpagename=Regione%2FMILayout&cid=1213382515758&p=1213382515758&pagename=RGNWrapper (April 8, 2017).
- 90.Piano Cardio Cerebrovascolare d.g.r. n. VII/20592 del 11.02.2005. Available at: https://normativasan.servizirl.it/port/GetNormativaFile?fileName=571_DGR2005_20592.pdf (April 8, 2017).
- 91.Nuove Reti Sanitarie: Determinazioni i merito alle strutture che hanno presentato richiesta per l attuazione del modello di ospedalizzazione domiciliare cure palliative oncologiche ai sensi della D.G.R. N.VIII/7180 del 24.4.2008. Available at: http://www.fedcp.org/normative-regionali/DGR%20VIII-7933.pdf (April 8, 2017).
- 92.D.g.r. n. IX/409 del 05.08.2010. Attivazione di un modello di telesorveglianza domiciliare per pazienti con broncopneumopatia cronica ostruttiva nell'ambito delle nuove reti sanitarie e di servizi di teleconsulto specialistici in aree disagiate e montane”. Available at: http://www.epicentro.iss.it/igea/raccolta/Allegati/lombardia/SERVIZI_TELEMEDICINA_BPCO_2010_dgr_409_del_5_8_10.PDF
- 93.Chiara Rabbito. Le buone pratiche per una corretta gestione della privacy in telemedicina. I Quaderni di Eleonora (14). Ed. Gli Amici di Eleonora Onlus. Settembre 2012. Available at: http://www.gliamicidieleonora.it/sites/www.gliamicidieleonora.it/files/Quaderno%2014_Rabbito-1.pdf (April 8, 2017). [Google Scholar]
- 94.Legge n. 675 del 31 dicembre 1996. Tutela delle persone e di altri soggetti rispetto al trattamento dei dati personali. Available at: http://garanteprivacy.it/web/guest/home/docweb/-/docweb-display/docweb/28335 (April 8, 2017).
- 95.Direttiva 95/46/CE del Parlamento Europeo e del Consiglio relativa alla tutela delle persone fisiche con riguardo al trattamento dei dati personali, nonché alla libera circolazione di tali dati. Available at: http://eur-lex.europa.eu/legal-content/it/TXT/?uri=celex%3A31995L0046 (April 8, 2017).
- 96.Decreto legislativo 30 giugno 2003, n. 196 Codice in materia di protezione dei dati personali. AvAILABLE AT: http://www.garanteprivacy.it/web/guest/home/docweb/-/docweb-display/docweb/1311248 (April 8, 2017).
- 97.Convenzione sulla protezione delle persone rispetto al trattamento automatizzato di dati a carattere personale STCE n° 108 Available at: https://www.coe.int/it/web/conventions/full-list/-/conventions/ treaty/108?_coeconventions_WAR_coeconventionsportlet_ languageId=en_GB (April 8, 2017).
- 98.Decreto Legge 9 febbraio 2012, n. 5. Disposizioni urgenti in materia di semplificazione e di sviluppo. Available at: http://www.gazzettaufficiale.it/gunewsletter/dettaglio.jsp?service=1&datagu=2012-02-09&task=dettaglio&numgu=33&redaz=012G0019&tmstp=1329227796113 (April 8, 2017).
- 99. Scalvini S, Giordano A.. Heart failure: optimal postdischarge management of chronic HF. Nat Rev Cardiol 2012;10:9–10. [DOI] [PubMed] [Google Scholar]