Abstract
Chest pain is a common general practice presentation that requires careful diagnostic assessment because of its diverse and potentially serious causes. However, the evaluation of acute chest pain remains challenging, despite many new insights over the past two decades. The percentage of patients presenting to the emergency departments because of acute chest pain appears to be increasing. Nowadays, there are two essential chest pain-related issues: (i) the missed diagnoses of acute coronary syndromes with a poor short-term prognosis; and (ii) the increasing percentage of hospitalizations of low-risk cases. It is well known that hospitalization of a low-risk chest pain patient can lead to unnecessary tests and procedures, with an increasing trend of complications and burden of costs. Therefore, the significantly reduced financial resources of healthcare systems induce physicians and administrators to improve the efficiency of care protocols for patients with acute chest pain. Despite the efforts of the Scientific Societies in producing statements on this topic, in Italy there is still a significant difference between emergency physicians and cardiologists in managing patients with chest pain. For this reason, the aim of the present consensus document is double: first, to review the evidence-based efficacy and utility of various diagnostic tools, and, second, to delineate the critical pathways (describing key steps) that need to be implemented in order to standardize the management of chest pain patients, making a correct diagnosis and treatment as uniform as possible across the entire country.
Keywords: Chest pain, Acute coronary syndromes, Differential diagnosis, Emergency department
Revised by: Luisa Cacciavillani, Aldo Clerico, Emilio Di Lorenzo, Luigi Raffaele Elia, Alberto Menozzi, Adriano Murrone, Marco Sicuro, Massimo Uguccioni
Consensus Document Approval Faculty in Appendix
Introduction
‘Chest pain’ is the symptom causing 5–9% of admissions to hospital emergency departments (ED) in the western countries.1 The most frequent cause of chest pain is acute coronary syndrome (ACS), at rates of up to 45%.2 In about 2% of cases, ACS is not correctly detected and patients are mistakenly discharged,3 at a cost estimated to be higher than 6 billion dollars,4,5 with missed diagnoses of ACS triggering increases in legal and medical expenses.6
Issues associated with the management of these patients are two-fold, prompting the need: (i) on one hand, to avoid missed diagnoses of ACS, which has a high-mortality rate; and (ii) on the other, to avoid extensive hospitalization of cases at low risk.
The preparation of this consensus paper was prompted by the evolution and innovative implementation of diagnostic tools available in this setting of patients, following publication of the previous ANMCO-SIMEU Italian consensus document in 2009.7
The mentioned evolution is reflected in: (i) the introduction of high-sensitivity troponins and new biomarkers; (ii) the adoption of the newer ESC guidelines on ACS; (iii) the development of non-invasive imaging tests (at rest and with stress); and (iv) the need to introduce the concept of the ‘Chest Pain Team’.
Technically, a chest pain is any pain occurring, anteriorly, between the base of the nose and the umbilicus and, posteriorly, between the occiput and the 12th vertebra, which has no traumatic or other clearly identifiable underlying cause.7
Among patients entering ED with chest pain, ACS is diagnosed and confirmed in 10–20% of cases.8
When a patient is admitted to ED because of acute chest pain, the true goal is to confirm or exclude an ACS [ST elevation myocardial infarction (STEMI), non ST elevation myocardial infarction (NSTEMI) unstable angina (UA)].
Among the causes of chest pain, there are life threatening conditions such as acute aortic syndrome (AAS), pulmonary embolism (PE), and hypertensive pneumothorax.
Aortic dissection and other AASs (aortic ulcer, intramural haematoma) are the causes of chest pain associated with the highest rate of mortality. PE may present with chest pain, dyspnoea, syncope, haemoptysis, cardiac arrest, or a combination of all these symptoms, which may be non-specific, hence the recommendation to use clinical predictive scores such as the Wells Score for PE.9
Table 1 shows a checklist of the causes of chest pain that must always be taken into consideration in a differential diagnosis with ACS.10
Table 1.
Modified from ACCA clinical decision-making toolkit10
| Causes of chest pain/discomfort not related to ACS |
| Cardiovascular |
| Acute pericarditis, pericardial effusion |
| Acute myocarditis |
| Severe hypertensive crisis |
| Stress induced cardiomyopathy (Takotsubo-like syndrome) |
| Hypertrophic cardiomyopathy, aortic stenosis |
| Acute left ventricular failure |
| Acute aortic syndrome (dissection, aortic ulcer, intramural haematoma) |
| Pulmonary embolism, pulmonary infarction, severe pulmonary hypertension |
| Cardiac contusion |
| Acute bio or mechanical prosthetic valve failure/malfunction |
| Non-cardiovascular |
| Oesophageal spasm, oesophagitis, gastroesophageal reflux disease |
| Peptic ulcer, acute cholecystitis-pancreatitis |
| Pneumonitis, bronchitis, asthma attack |
| Pleurisy, pleural effusion, pneumothorax |
| Chest injury |
| Costochondritis, rib fracture |
| Damage to cervical/thoracic vertebrae or discs |
| Herpes zoster |
| Anxiety, depression |
Diagnostic tools
Clinical history (anamnesis) and physical examination
A missed diagnosis of ACS can occur because uncorrected ECG interpretation, young age of the patient, inexperience of the physician, atypical symptoms presentation, missing ECG at first medical contact (FCM).11
In the absence of ECG abnormalities, the initial anamnestic evaluation also serves to sort the patients into different groups having a lesser or greater likelihood of ACS.
As reported in a meta-analysis of Fanaroff et al.,2 the characteristics of chest pain/discomfort and the associated symptoms can be of used to obtain a probability stratification, but have no significant diagnostic power [LR + (positive likelihood ratio) < 3 for all assessed characteristics and for all associated symptoms]. Diaphoresis indicates a slightly higher probability of ACS (LR + 1.3–1.4). The presence of symptoms that derive benefit from taking sub-lingual nitrates or from resting is not a predictor of ACS.12
The presentation of symptoms is more atypical in elderly, where dyspnoea tends to dominate over the pain symptom, and also in women and in diabetics.
There are certain scores that integrate anamnesis with ECG and with the results of the first troponin test, introduced initially to assess the prognosis for known ACS: the PURSUIT score,13 the TIMI risk score,14 the HEART score,15 and the GRACE score.16
The physical examination of patients with chest pain/discomfort and suspected ACS is very often normal or almost normal, and if there are signs of haemodynamic instability, or of heart failure, these indicate a particularly severe prognosis. Particular physical signs to look for are those indicating a disease not of myocardial ischaemic origin: pericarditis (pleuritic pain worsening by change in position, pericardial friction rub), PE (sudden onset of dyspnoea and pain, signs of right heart failure), aortic dissection (sudden onset pain often radiating to back, alterations of peripheral pulses) and those suggesting a suspicion of non-cardiac disorders: pneumothorax (abrupt onset of dyspnoea and pain), pneumonia (pleuritic pain, dyspnoea), musculoskeletal (intense fleeting pain), peptic ulcer, and pancreatitis (intense epigastric or substernal pain) diseases.17,18
| Anamnesis and physical examination. Executive summary | |
|---|---|
| 1. | Recommended procedure: careful anamnestic assessment of symptoms at presentation, previous illnesses and risk factors, which can be integrated with an assessment of the likelihood of disease.Recommended procedure: calculation and use of risk/likelihood scores (GRACE, TIMI or HEART) associating anamnestic data with data relative to ECG and to initial troponin dosage. |
| 2. | Recommended procedure: baseline and serial assessment of vital parameters; search for signs possibly indicating life threatening severe diseases. |
The electrocardiogram
When a patient has chest pain, 12-lead ECG should be performed within 10 min of the FMC. ECG can turn out to be normal in 1/5 of patients with chest pain caused by ACS.19 In terms of electrocardiographic diagnosis, patients affected by ACS can be divided into two categories:
Patients with a persistent elevation of the ST segment (or new onset of left bundle branch block)20 can be classified as STEMI–ACS;
Patients with ECG abnormalities other than elevation of the ST segment can be classified as NSTEMI–ACS.21 ACS–NSTEMI can also progress in the absence of any ECG abnormalities.
In these cases, only increased biochemical markers of myocardial necrosis will allow a distinction between NSTEMI and UA.
The number of leads involved, the presence and magnitude of ST depression or a transient elevation are all indicative of the extent of the ischaemia and correlated likewise with the prognosis.21
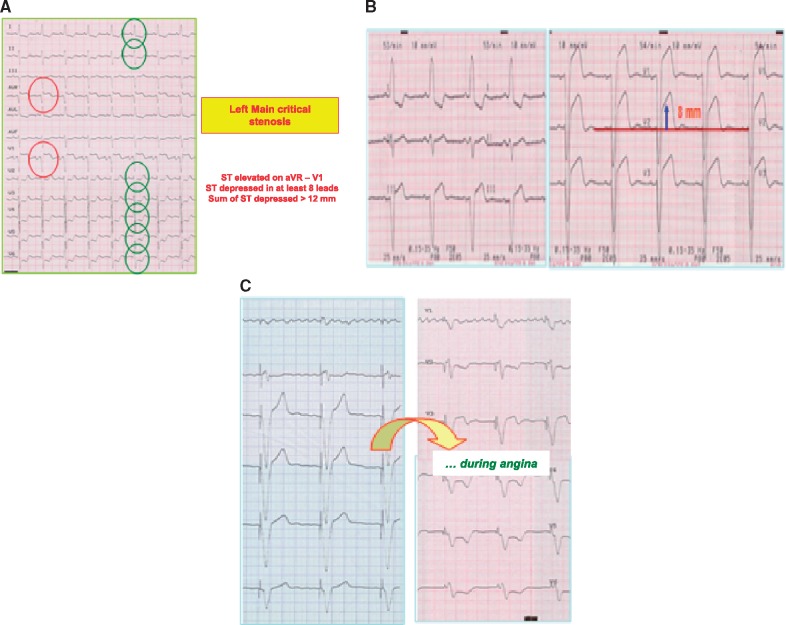
Registry studies show that in 18.5% of cases, the ECG appears normal or doubtful on initial observation, and there is a minimal percentage of patients, with undiagnosed ACS as the result of an ECG having been incorrectly interpreted or badly performed.19 Accordingly, where there is a high likelihood of ACS on the basis of anamnesis, the patient should be monitored continuously or serial 12-lead ECG should be taken. A 12-lead ECG must necessarily be taken in case of symptom recurrence or where symptoms worsen.22 ESC guidelines21 stress the importance of continuous 12-lead monitoring; for certain types of patients, moreover, comparison with previous ECGs is fundamentally important. In the case of left bundle branch block, indications of ischaemic damage will be given by a marked elevation of the ST segment beyond 5 mm in the right precordial leads or using the Sgarbossa criteria23: (i) ST elevation more than 1 mm and in the same direction (concordant) with the QRS complex, 5 points; (ii) ST depression more than 1 mm in leads V1–V3, 3 points; (iii) ST elevation more than 5 mm and in the opposite direction (discordant) with the QRS, 2 points. A score of 3 points is required to diagnose an acute myocardial infarction. Similarly, significant ST/T changes accompanying chest pain during right ventricular pacing ECG may be indicative of acute myocardial ischaemia (Figure 1).
Figure 1.
Unusual ECG abnormalities during acute myocardial ischaemia. (A) Critical stenosis in the left main of the left coronary artery; (B) marked elevation of ST segment >5mm in leads V1–V2 with left bundle branch block and during chest pain; (C) significant abnormalities during chest pain under pace-maker rhythm.
When there is transmural necrosis of the antero-septal wall in a patient with right bundle branch block, there will be a fall of the R wave in leads V1–V4 with appearance of the Q wave and simultaneous elevation of the ST segment.
If, during an acute episode of chest pain, previously inverted T waves should return to be positive (pseudonormalization), the suspicion of transmural myocardial ischaemia is confirmed.
The need to comparison with previous ECG traces should encourage the use of electronic systems for electrocardiographic archiving. The launch of a national electrocardiography database (https://www.bancadelcuore.it/) by ANMCO is emblematic of the response to this need. With this project, the subject is provided with an electronic card called BancomHeart, allowing remote access to 12-lead electrocardiograms archive stored over time, by way of computer, tablet, and smartphone. An essential characteristic of these databases must be their ease of consultation, hence the possibility of using them via web tools.
For some clinical conditions, the 12-lead ECG can simulate transmural ischaemic myocardial lesion: acute pericarditis, ventricular pre-excitation, Brugada syndrome, early repolarization, electrolytic disorders.
If the 12-lead ECG results normal/non diagnostic, or not ‘definitely ischaemic’, it is better to have the interpretation confirmed as soon as possible by an expert.
Special care must be taken to ensure that the ECG is performed correctly. IEC Standards state that acquisition of the diagnostic ECG signal must be accomplished with a passband of 0.05–150 Hz for the diagnosis of ischaemia, infarction, etc. Many other diseases are detectable provided that measures are taken to ensure the quality of the electrical signal, which otherwise could occasion huge diagnostic errors, invalidity and possible legal consequences, as well as considerable ancillary costs. It is also essential that ergometric stress, Holter, telemetry and monitor traces are always ensured compliant with requirements for 12-lead diagnostic ECG, using unreconstructed signals, with a passband of at least 0.05 up to 150 Hz. The diagnostic electrocardiography signal must be acquired from 10 electrodes, using 12, simultaneous ECG leads: 6 peripheral, and 6 unipolar precordial.24,25
| 12-leads ECG—executive summary |
|---|
| 1. A 12-lead ECG must be performed on a patient with chest pain within 10’ after the FMC, and the results interpreted immediately by an expert. |
| 2. Always perform V3R–4R in the case of inferior STEMI (to detect a possible lesion in the free wall of the right ventricle) and V7–V9 in the case of STEMI of the inferior and lateral wall, or of suspected posterior STEMI (to detect extension to the posterior myocardial tissue). |
| 3. A quick comparison should be made with previous traces, if possible using electronic archives that will allow fast retrieval and consultation of previous ECGs. This comparison is indispensable for patients with bundle branch block, pacing or previous myocardial infarction. |
| 4. For cases under observation in ED, continuous 12-lead ECG monitoring is recommended. Alternatively, serial 12-lead ECGs must be performed. |
| 5. It is important that a 12-lead trace be taken in the event of a recurrence or worsening of symptoms. |
Biomarkers
Many biomarkers are related to diagnostic and prognostic processes associated with acute myocardial infarction. Diagnostic biomarkers for necrosis are creatine-kinase, troponin, Heart-Type Fatty Acid Binding Protein and Copeptin an Arginine Vasopressor Activation. Other biomarkers are related to prognostic process: brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) with biomechamical stress, high sensitive C-reactive protein (Hs-CRP) with inflammation, myeloperoxidase with neutrophilic activation, and endothelin with endothelial activation.26
Cardiac troponin
Abnormal levels of cardiac troponin (cTn), type I (cTn-I) or type T (cTn-T) are considered the standard diagnostic indicator of acute myocardial infarction (AMI).27,28 The third universal definition of myocardial infarction29 considers the raised level of troponin, with a typical curve, as one of the elements indispensable to the diagnosis of AMI.
The elevation of troponin T or I occurs 2–4 h following the onset of symptoms.
Laboratory tests of high-analytical sensitivity are used to measure troponin I e T; these employ immunometric methods capable of measuring the 99th percentile of the reference population with an error equal to or less than 10%, as recommended by national guidelines.30
With the ‘new’ troponins, the negative predictive power of a single test is up to 95%, becoming 100% with 2 tests.31 ESC 2015 guidelines on ACS–NSTEMI21 recommend the use of high-sensitivity cardiac troponin (hs-cTn).
The kinetic pattern of hs-cTn is different to that of conventional cTn and consequently the timing of the measurements must be reviewed. Positivity with hs-cTn occurs much earlier than with conventional cTn, and there is evidence32 to show that with this marker, it is possible to rule out AMI within 3 h after registration and triage, with a sensitivity and negative predictive values of around 100%.
An increase of cTn indicates only a cardiomyocyte damage and it can occur even in clinical situations other than ACS (Table 2). Being of lower specificity, these markers can cause a high and growing number of cases involving ‘false positives’, impacting adversely in terms of unsuitability and waste of resources.
Table 2.
Elevation of troponin due to causes other than myocardial ischaemia
| Cardiovascular |
| Tachyarrhythmias |
| Acute cardiac insufficiency |
| Hypertensive crises |
| Myocarditis—pericarditis |
| Aortic dissection |
| Infiltration/accumulation diseases |
| Pulmonary embolism |
| Acute neurological episodes (stroke or subarachnoid haemorrhage) |
| Cardiac/thoracic contusion/trauma |
| Cardiac procedures: electrical cardioversion, ablation, endomyocardial biopsy |
| Systemic |
| Respiratory distress/bronchial pneumonia |
| Dehydration/cachexia |
| Systemic diseases (fever/infection/shock/burns) |
| Hypo and hyper thyroidism |
| Post-operative disorders |
| Severe anaemia/gastrointestinal bleeding |
| Use of cardiotoxic drugs |
| Kidney failure |
| Prolonged endurance sports |
| Rhabdomyolysis |
| Analytical |
| Poor analytical platform performance |
| Calibration errors/dilution problems |
| Limitations relating to sample collection: heterophile antibodies |
| Interfering substances (fibrin) |
Algorithms in using cTn
0–3 h Algorithm
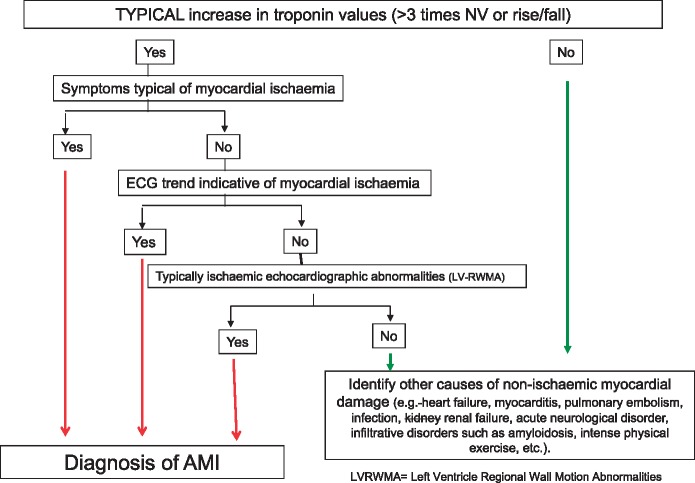
The 0–3 h algorithm (Figure 2) is currently the standard of reference for hs-cTn markers, and confirmed as such by the 2015 ESC Guidelines on ACS–NSTEMI.21
Figure 2.
Interpretation of elevated troponin values.
0–1 h Algorithm
An alternative algorithm validated and proposed more recently, of shorter duration with a 0/1 hour assessment, is based on a lower and more sensitive cut-off compared to that of the 99th percentile (6 ng/L).21 This latter algorithm is based on the fact that hs-cTn is a continuous variable, and as its values increase, so does the probability of AMI. Moreover, this algorithm is based on the assumption that early absolute variations in levels at 1 h can be used as surrogates for variations at 3 and 6 h, guaranteeing an incremental diagnostic value of the protocol. This protocol has proved useful especially in rule-out procedures, ensuring reliable early discharge of low-risk patients with negative troponin.
0–6 h Algorithm
To rule out acute infarction of the myocardium, the cTn assay, conducted utilizing methods of the latest generation, though not high sensitivity, should be performed at time 0 and after 6 h (considering measurement at 3 h as well, however, so as not to miss the peak of the curve). If all the values observed are ≤99th percentile and the variation in concentration is <50% (below the 99th percentile), the patient can be discharged.
If the onset of the symptom was >6 h and the patient is asymptomatic, with a GRACE score of <140, and a differential diagnosis can be ruled out, discharge can be allowed possibly after a provocative test, or the test can be performed within a few days following discharge, as will be explained in due course.
Even using hs-cTn, but with tests not directly validated for the 0–1 h and 0–3 h algorithms, it is advisable to consider application of the 0–6 h algorithm.
Novel biomarkers
Among the new biomarkers, copeptin appears to have the most scientific evidence in medical literature.33–37 It represents the C-terminal portion of the pro-hormone of vasopressin secreted from the pituitary gland, and is able to quantify the level of endogenous stress characterizing many clinical conditions, including AMI. Owing to the rapid rise in the level of endogenous stress at the onset of ACS, copeptin immediately becomes positive even as the symptoms are first experienced, hence at the first blood test. A single copeptin assay is therefore sufficient, and this should always be employed in combination with troponin (dual-marker strategy). A patient dischargeable after the first blood test will therefore be one who is troponin-negative and copeptin-negative (<10 pmol/L). This combination has a high negative predictive power in respect of AMI (close to 100%) and avoids the need for further troponin tests, thus speeding up management timescales considerably.
There are future developments in this filed. There is an increasing interest in circulating microRNAs, non-coding small ribonucleic acid protein, as potential novel biomarkers for AMI. MicroRNAs increase their plasma levels shortly after the onset of a coronary event and certain isoforms appear exclusively after an AMI. Circulating miRNA seems to improve the diagnostic potential but at the moment the clinical data are very preliminar.38
| Biomarkers: executive summary | |
|---|---|
| 1. | The use of high-sensitivity cardiac troponin (hs-cTn) is recommended. |
| 2. | The recommended algorithm is the 0–3 h type, using specifically validated hs-cTn. |
| 3. | Whilst troponin is the diagnostic standard for AMI, it must be remembered that levels can be high in conditions other than ACS and an isolated increase does not allow the diagnosis of AMI, given that troponin is considered only to be a marker of myocardial damage. |
| 4. | Stable values or inconsistent variations of troponin in the absence of dynamic variations in its plasma concentration (rise/fall with generation of a kinetic curve) do not constitute a marker of ACS. |
| 5. | Practical cooperation with the blood analysis laboratory is essential, as also is knowledge of the diagnostic method (assay) utilized. |
| 6. | Severe kidney failure causes cTn to increase (more so cTn-T than cTn-I). A higher cut-off value based on estimated glomerular filtration rate (eGFR) levels is needed for a more accurate diagnosis of AMI–NSTEMI in patients suffering from end-stage kidney failure.39 |
Exercise test
A maximal exercise test resulting negative for inducible ischaemia can help to avoid needless hospitalizations and allow patient discharge directly from Short-Stay Intensive Observation Unit.40 In certain cases, the exercise test could also be scheduled within a few days following discharge.
The ergometric test (treadmill or bicycle) should be conducted once the patient has been asymptomatic for at least 12 h and showing no signs of heart failure for at least 48 h. Should the provocative test prove negative, the patient can be discharged directly and considered at low risk.41
The exercise ECG does not reveal high specificity or sensitivity. False responses can be produced in the case of bundle branch blocks or pacing, and reduced specificity of the findings is expected in case of former myocardial infarction. Nonetheless, one study documents a high-negative predictive value (99%) and high confidence in a population with a disease prevalence of 5%.42 On the other hand, the appearance of angina or abnormalities of the ST segment during a low-work load would indicate a high likelihood of critical coronary disease.43
Non-invasive imaging tests
Chest X-ray
The standard chest X-ray is diagnostic test performed frequently in a critical area on patients presenting with chest pain, especially to rule out pulmonary and vascular disorders.44
ESC guidelines on aortic dissection include the chest X-ray among the diagnostic tests.45
Rest echocardiography
Performing echocardiography quickly in ED was strongly recommended in the ANMCO-SIMEU position paper of 2009,4 especially in cases with a combination of ongoing chest pain, non-diagnostic 12-lead ECG, and haemodynamic instability.46 The latest 2015 ESC guidelines on ACS–NSTEMI21 indicate echocardiography as having an essential role in the diagnosis of acute myocardial ischaemia, as it is able to detect regional wall motion abnormalities of the left ventricle (hypokinesia, akinesia), especially when symptoms are ongoing, and when the differential diagnosis of chest pain are non-coronary cardiovascular (aortic dissection, myocarditis-pericarditis, valve prosthetic dysfunction, etc.).47
Accordingly, an ultrasound scan, exploring not the ‘organ’ (heart, kidney, gall bladder, etc.) but the ‘problem’ (chest pain, haemodynamic instability, dyspnoea, etc.), is frequently performed by a cardiologist, or even by an emergency physician or resuscitator, using echocardiography to investigate not only chest pain but also dyspnoea, transient ischaemic attack (TIA)/stroke to identify cardioembolic sources, low-blood pressure, syncope, etc.48,49
There is still strong debate surrounding the echocardiographic diagnosis of acute myocardial infarction: studies on patients suffering chest pain, observed at a cardiologists-run Chest Pain Unit, showed the usefulness of serial echocardiographic scan of regional left ventricular wall motion in the final diagnosis of acute myocardial infarction, when 12-lead ECG and troponin were still negative. Many other publications insist on the limitations and scant effectiveness of the method in this patient setting.50,51
Stress echocardiography
Stress echocardiography is a technique used for the diagnosis, risk stratification, and prognosis of patients with known or suspected coronary disease employing various stressors that include inotropic drugs (dobutamine) or vasodilators (dipyridamole, adenosine) and also non-pharmacological stressors. Physical exercise is the most physiological stressor and consequently the most utilized and most recommended in literature.52 Assessment is based on abnormalities in regional wall motion of the left ventricle during stress, compared to baseline, and the test is sometimes associated with the assessment of coronary flow reserve (CFR) on the anterior descending artery with adenosine.53
Numerous studies showed that a negative stress echo (using physical or pharmacological stressors) assumes a high negative predictive power and is associated with favourable outcome, as well as demonstrating a higher prognostic value than the ECG ergometric test.54,55
This operator-dependent technique also requires a suitable level of experience and skill which, as suggested by the major European and American scientific societies (European Association of CardioVascular Imaging, American Society of Echocardiography) is obtainable by performing and reporting >100 examinations per year.56
Contrast echocardiography and strain imaging
Contrast echocardiography, in a setting of patients with chest pain, serves a dual purpose, allowing: (i) enhancement of the endocardial border during ventricular opacification, significantly improving the analysis of regional wall motion; and (ii) analysis of myocardial perfusion.57
The use of a contrast, which has the effect of enhancing the endocardial border, also increases the sensitivity of the stress echo, improving the assessment of all segments of the left ventricle wall, especially in patients having poor acoustic windows.
For the first time, the 2015 ESC Guidelines on ACS–NSTEMI21 also mention Strain Imaging derived from 2D Speckle-Tracking echocardiography as a possible tool in detecting abnormalities of regional and global systolic function in patients with chest pain.
Multislice coronary computed tomography
Coronary computed tomography (CT) using scanners with at least 64 rows of detectors will allow the identification of haemodynamically significant stenosis (>50%), in addition to an already high negative predictive value (higher than 90%) as well as a clear improvement in terms of positive predictive value and specificity.58 This technique should be reserved for an extremely restricted population with a low to intermediate pre-test likelihood, quantifiable at between 20% and 70%.59 This group also includes patients with chest pain not immediately identifiable using the more common diagnostic tools (ECG, biomarkers, blood gas analysis, chest X-ray), or who might be candidates for a triple rule-out (ACS, AAS, and PE) using a single diagnostic test, albeit this approach remains controversial.60 ROMICAT studies61,62 comparing coronary CT with standard assessment procedure in the management of patients with chest pain, found that CT improved clinical decision making, reducing hospitalization time, but was also associated with an increase in downstream testing, exposure to radiation and higher care costs. Consequently, as of today there are no certainties on the real usefulness of coronary CT in decisional algorithms for acute chest pain.
Cardiac magnetic resonance imaging
As of today there are no proofs to support the accuracy and the impact of magnetic resonance imaging (MRI) on the management of patients with acute chest pain.
The use of cardiac MRI on patients presenting with chest pain is referable almost exclusively to pharmacological stress testing with dobutamine.63,64
In images obtained during the course of a pharmacological stress test, any myocardial areas with low CFR will show a signal enhancement delayed and attenuated in relation to the surrounding myocardium (hypoperfusion area). A factor of fundamental importance in the interpretation of a myocardial perfusion scan during pharmacological stress is comparison with perfusion images obtained at rest and with delayed-enhancement images.
The capture of diagnostic quality coronary angio-MRI images from the single patient tends not to be constant, and generally will allow the identification of any significant stenoses (>50%) only along the proximal and medial segments of the large epicardial coronary vessels.
A meta-analysis,65 conducted on 37 studies of cardiac MRI involving 2191 patients with known or suspected coronary disease, reported sensitivity, and specificity values of 83% and 86% respectively, in the case of kinematic stress, and of 91% and 81% respectively, in the case of perfusion stress tests. On the basis of these findings, the most recent ESC guidelines on myocardial revascularization indicate recommendation level IA for stress cardiac MRI in the case of symptomatic patients with intermediate pre-test likelihood of obstructive coronary disease, and attribute recommendation class IIB to coronary angio-MRI for the assessment of symptomatic patients examined for obstructive coronary disease.66
Nuclear medicine tests
Myocardial tomoscintigraphy (SPECT) is used widely for assessing the perfusion of myocardial tissue, the extent of myocardial damage and its impact on cardiac function. In the PROMISE study,67 nuclear tests were the ones most utilized in functional testing arm: 67% as against 22% for stress echo tests and 10% for ergometric tests.
The severity of the patient's illness can influence the result of the test: the number of vessels affected influences results. In effect, sensitivity increases with the rise in the number of vessels affected, from around 85% to 95% if all the 3 main branches are involved. Similarly, the extent of the stenosis is critical in determining positivity. If the stenosis is severe, the test is positive in over 70% of cases. The vessels found most often to be stenotic are the anterior descending and the right coronary artery, on visual inspection, whereas the semi-quantitative method functions better for the right and the circumflex. Indicators of triple vessel disease, in addition to diffuse deficits in the areas involved, include pulmonary capture of the radioactive drug and transient enlargement of the left ventricular lumen. The test should be restricted to those patients presenting with chest pain indicative of coronary disease, but without significant abnormalities on their ECG trace or without biomarker-related abnormalities, making it possible to identify patients at high risk, i.e. those with reversible perfusion defects rather than those who do not have perfusion defects or those who have irreversible perfusion defects as from former necrosis.67,68
Comparison between anatomical and functional testing and appropriateness in the use of imaging tests for patients with chest pain
The PROMISE study69 was the first to draw a real comparison between anatomical tests (multislice coronary CT) and functional tests (nuclear, stress-echo, ergometric) conducted on patients with chest pain and suspected coronary disease. The primary hypothesis of the study was that cardiac events (combination of death from whatever cause, myocardial infarction, hospitalization for unstable angina, and major peri-procedural complications) at 2 years into follow-up would be fewer in the CT group than in the functional testing group. The study, which enrolled 5000 patients for each arm, showed no difference in predetermined outcomes, legitimating the use of functional tests for the initial diagnosis of symptomatic patients for suspected coronary disease. In short, the study rehabilitated the clinical procedure that had always been practised (and suggested by guidelines) before multislice coronary imaging was made possible by the groundbreaking advent of coronary CT. It is reasonable to concur with Gaibazzi70 in saying that functional tests will never attain the diagnostic accuracy of multislice coronary CT if compared with the gold standard of ‘coronarography’, likewise anatomical, but their capacity for the stratification of patients according to the presence and extent of reversible ischaemia places them notionally on a higher level of usefulness than an anatomical test such as coronary CT.
The paper entitled ‘2015 ACR/ACC/AHA/AATS/ACEP/ASNC/NASCI/SAEM/SCCT/SCMR/SCPC/SNMMI/STR/STS Appropriate Utilization of Cardiovascular Imaging in Emergency Department Patients With Chest Pain’71 provides indications for correct and appropriate use of imaging tests in a setting of patients with chest pain.
It assesses the appropriateness of tests on the basis of four diagnostic entry points: (i) suspected ACS; (ii) suspected PE; (iii) suspected AAS; and (iv) patients for whom it is not possible to establish a probable diagnosis. The grading of tests adopted in the paper is A = appropriate, M = may be appropriate, R = rarely appropriate. Starting from this statement, in this document we report a modified table summarizing the appropriateness of the different non-invasive ultrasound test in the daily management of chest pain patients (Table 3).
Table 3.
Indications for correct and appropriate use of imaging tests in a setting of patients with chest pain
| Appropriateness in non-invasive ultrasound procedures | ACR/ACC/AHA/AATS/ ACEP/ASNC/NASCI/ SAEM/SCCT/SCMR/SCPC/ SNMMI/STR/STS 2015 | ASE 2013 (pericardium) 2015 (aorta) | ESC 2014 (Aorta PE) 2015 (ACS) | ANMCO-SIMEU 2016 |
|---|---|---|---|---|
| STEMI–ACS (HUS) | R | A | A | |
| ACS—certain (HUS) | R/M | A | A | |
| ACS—probable (HUS) | M | A | A | |
| ACS—possible (HUS) | R/M | A | A | |
| PE low probability and DD—(CUS) | R | R | R | |
| PE no low probability or DD + (CUS) | M/A | M/A | A | |
| PE no low probability or DD + (HUS) | M/A | A | ||
| AAS possible/probable (HUS) | M | M | A | A |
| Pericarditis possible/probable (HUS) | A | A | A | A |
| Pnx possible/probable (TUS) | A | |||
| Not classified chest pain | A |
ACS, acute coronary syndrome; HUS, heart ultra-sounds; CUS, compressive ultra-sounds; PE, Pulmonary Embolism; Pnx, pneumothorax; AAS, acute aortic syndrome; TUS, thorax ultra-sounds; DD, D-dimer; A, appropriate; M, intermediate appropriateness; R, rarely appropriate; O, not indicated.
Proposal of in-hospital diagnostic pathway
The diagnostic pathway for chest pain patients proposed in this document has the following goals:
to identify patients with a high probability of ACS (STEMI, NSTEMI, unstable angina) with the aim of ensuring timely reperfusion of STEMI patients and initiating the appropriate specialist procedure for those with NSTEMI and unstable angina;
to identify other diseases of non-coronary origin requiring emergency or urgent treatment procedures;
to assess the likelihood of ACS in patients with chest pain having no clear cause and with non-diagnostic or normal 12-lead ECG on initial assessment.
The diagnostic pathway proposed in this consensus document will be divided into single steps.
Step 1: triage
The following actions are recommended at triage (by nurses):
| Steps | Actions | Timing |
|---|---|---|
| 1. Assessment on the door | Assessment of symptoms type | Immediate |
| 2. Targeted collection of clinical/ anamnestic data |
|
Within 10’ |
| 3. Perform 12-lead ECG | Consider performing V3R-4R and V7-9; acquisition of report. | Within 10’, or immediately if patient is in pain |
| 4. Brief physical examination to assess vital parameters | Fill in report indicating vital parameters | |
| 5. Assign priority colour code72 | For method of assignment, see text | After steps 1, 2, and 3 |
| 6. Re-assessment |
|
|
Step 2: ED—emergency room (ER)
In the ER, the following actions should be performed.
| Actions | Description | Indications |
|---|---|---|
| Venous access | Cannulation of antecubital vein | Always |
| 12-lead ECG | Interpret, report, repeat or perform for first time if not performed during triage period | Always |
| Anamnesis | Collection of complete anamnestic data, expanding on details recorded at triage73 | Always |
| Physical examination | Assess ‘vital parameters’ sheet filled in during triage, perform a complete physical examination, drafting structured second report if appropriate | Always |
| Take blood for troponin | The troponin (T or I) utilized should preferably be high sensitivity (hs-cTn)74 | See Figure 3 |
| Other blood-chemical tests | Complete blood count, coagulation, creatinine, plasma electrolytes, others according to clinical suspicion | Always |
| Blood gas analysis | Arterial blood sample | If respiratory insufficiency or suspicion of pleuro-pulmonary disease or PE |
| Bedside ultrasound scan | Protocol as per guidelines | According to cases, not least to rule out causes other than ACS |
| Request radiology exams | Chest X-ray, chest angio CT, multislice coronary CT | According to clinical suspicion |
| Activate consultations | Cardiac consultation and/or transthoracic-transoesophageal echocardiogram | According to cases, during the diagnostic orientation process |
Step 3: diagnostic orientation and in-hospital observation protocol
The main decision-making crossroads coincide with: (i) 12-lead ECG acquiring; and (ii) cardiac troponin dosage.
The 12-lead ECG must answer the following questions:
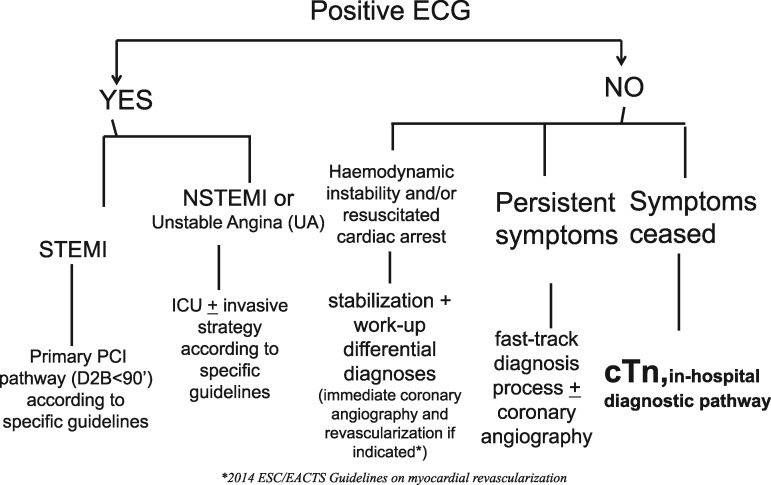
are there signs of acute transmural ischaemia (ACS–STEMI) detectable in the 12-lead ECG? If so, initiate a fast track cardiac care pathway (referral to the cathlab for primary PCI or to the reference STEMI network) in order to ensure reperfusion in the shortest time possible (door-to-balloon time, ideally <90 min); refer to specific ESC guidelines20 (Figure 4);
are there signs of acute non transmural ischaemia (ACS–NSTEMI) detectable in the 12-lead ECG? If so, initiate a cardiac care pathway with transfer to Intensive Cardiac Care Unit and/or cathlab; refer to specific ESC guidelines21 (Figure 4);
if the answer to both of the above questions is no, but there are signs of haemodynamic instability (resuscitated cardiac arrest, cardiogenic shock scenario, acute pulmonary oedema, low blood pressure, etc.) assess differential diagnosis with other acute and life threatening diseases (by way of echocardiogram and/or CT scan or other diagnostic procedures)75,76 (Figure 4)
if the answer to all the above questions is no, but pain is persistent and ongoing at the time of observation, a more specific fast track diagnosis pathway must be put in hand (echocardiogram and/or CT scan and/or urgent coronary angiography if indicated.
Figure 4.
Decision-making on the basis of 12-lead ECG.
If the answer to all the above questions is no and there are no signs of haemodynamic instability, a specific diagnostic pathway should be initiated, which will include a troponin assay (Figures 4and5).
Figure 5.
High sensitivity troponin algorithm 0 h/3 h.
Whilst an increase in troponin plasma level may be indicative of myocytes damage, it does not provide any information about the underlying mechanism responsible for such damage.
Small amounts of myocardial damage associated with necrosis can occur in patients with heart failure, kidney failure, myocarditis, arrhythmias, PE, or even during the course of percutaneous coronary or uncomplicated surgical procedures. In these cases, there is no question of infarction, but only of myocardial damage. Accordingly, to have a diagnosis of AMI there must be (even if this alone is not sufficient) a very high initial value of troponin or at least a three-fold increase at the second determination, or, better still, a typical troponin curve showing a rise followed by a fall, and evidence of at least one value higher than the values indicating normality, associated with symptoms or ECG abnormalities suggesting ischaemia, or with positive imaging tests or angiographic data.
A ‘routine’ approach and an unreasoned assessment of troponin plasma levels will result inevitably in:
inappropriate recourse to cardiological consultations;
inappropriate hospitalizations, often with occupation of intensive care beds;
erroneous diagnostic pathway of the patient, who is labelled wrongly as suffering from acute coronary syndrome, with the real risk that other acute pathological conditions could be underestimated or left undiagnosed.
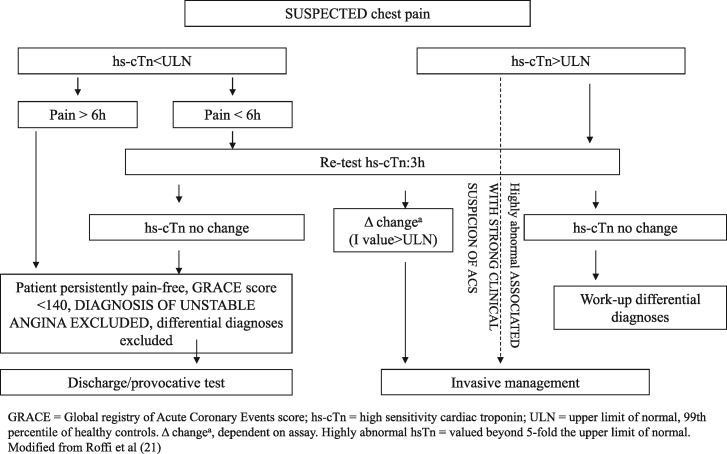
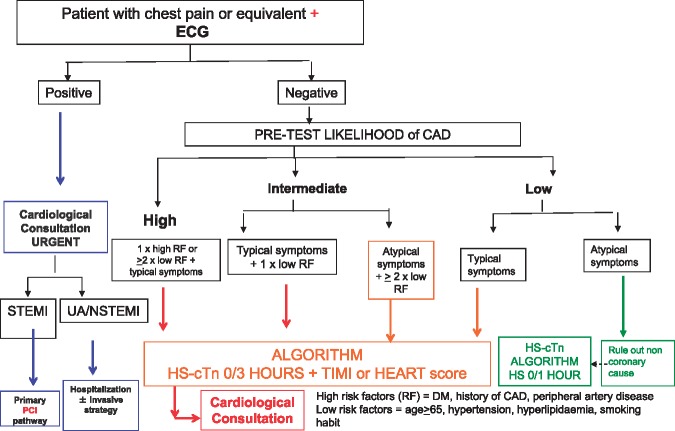
Figure 3 illustrates the decision-making process with regards to selection of the patient who should undergo a troponin assay. The flow chart delineates, first, the assessment of pre-test likelihood of coronary disease (high, intermediate, and low) and thus identifies a subgroup of patients on which the test is not to be performed (low likelihood, atypical symptoms). For this category of patients, if anything, the 0–1 h algorithm, indicated in the 2015 ESC guidelines on ACS–NSTEMI, could be applicable.21 For the remaining categories of patients, the 0–3 h algorithm with hs-cTn must be integrated with clinical/anamnestic prediction tools such as HEART score or TIMI score.
Figure 3.
Selection of patient undergoing cardiac troponin assay in ED.
Values of cTn must be determined on the basis of the 0–3h algorithm (Figure 5) in accordance with ESC guidelines. If the high sensitivity test is not available, the 0–6 h algorithm can be used. During this period, the patient must be kept under observation by monitoring the 12-lead ECG continuously, or alternatively, by taking serial traces (every hour). It is important that an ECG trace is acquired immediately in case of recurrence/worsening of symptoms. The administration of ASA is advisable.
Where values are above the normality range, the first step is to establish whether or not the increase can be considered typical of ACS (at least a three-fold increase, or a rise/fall type pattern). The delta change depends on the used assay.
If the cTn increase is typical (Figure 2), in a patient with a high clinical likelihood of ACS, or an ECG trend appearing positive for ischaemia, or alternatively, an echocardiographic assessment showing positive for left ventricle regional wall motion abnormalities, then a diagnosis of AMI can be given. The inclusion of rest echocardiography in the decision-making pathway is a true novelty in this document.
If the increased troponin level is not typical, other causes of myocardial damage must be considered (heart failure, myocarditis, PE, infection, kidney failure, acute neurological disorder, infiltrative disorders such as amyloidosis, intense physical exercise, etc.).
If a diagnosis of AMI is formulated, the next step in ED (Pathway A, Figure 6) is to look for possible factors that might have produced a discrepancy between the demand and supply of O2 (anaemia, hypoxaemia, tachyarrhythmia, etc.). If any one of these factors is found to be present, the diagnosis will be AMI type 2.28 In this case, the possibility of hospitalization in Cardiology ward can be considered especially for a patient with haemodynamic instability or very elevated values of troponin, or persistent ischaemia, or where an extensive ischaemic area is identified (by ECG or echocardiogram). Alternatively, and in the absence of the above criteria, the patient could be hospitalized in an Emergency or Internal Medicine Unit, where steps will be taken promptly to correct the precipitating factors. If factors responsible for the discrepancy between the demand and supply of O2 are not identified, the patient can be diagnosed with myocardial infarction type 1 and must be admitted to a ICU and/or referred to the Cath-lab.
Figure 6.
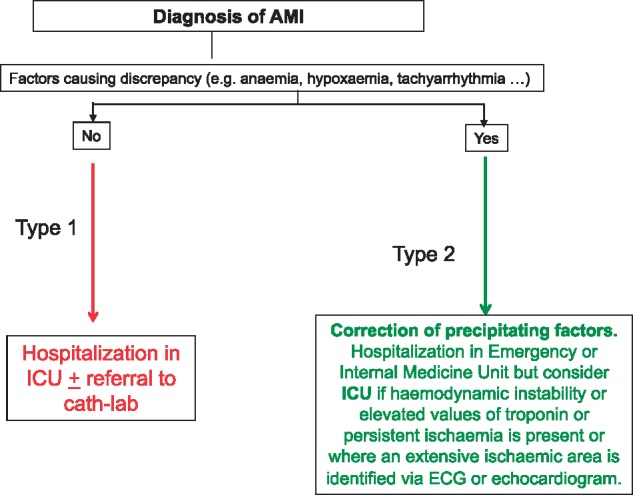
In-hospital pathway through ED (A).
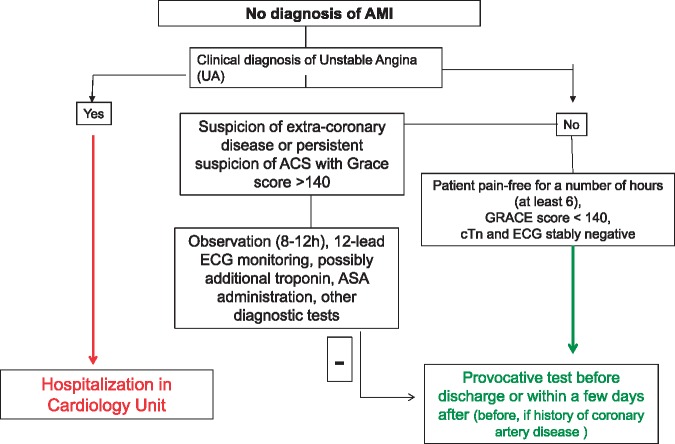
If no diagnosis of myocardial infarction is made (Pathway B, Figure 7), and both 12-lead ECG and troponin assays give negative results, the next step in ED is to establish whether or not a clinical diagnosis of unstable angina may be possible. If the answer is yes, the patient should be hospitalized in Cardiology ward. If the answer is no and the patient has had no symptoms for a number of hours (at least 6), given a GRACE score of <140 with cTn and ECG both stable, a provocative test can be performed either immediately or after discharge (within a few days), depending on the case.
Figure 7.
In-hospital pathway through ED (B).
If the answer is no but there remains a strong suspicion of non-coronary disease or ACS continues to be suspected or the GRACE score is >140, the patient can be observed for a period (8–12 h depending on local logistics) in Short Stay Intensive Observation, Chest Pain Unit or ED. Steps that must be taken during this stage of the pathway include 12-lead ECG monitoring, additional troponin tests if opportune, the administration of aspirin if opportune, and any other diagnostic test (including chest X-ray) that could be of use in formulating a definitive diagnosis. This stage likewise can be concluded with a provocative test before discharge or thereafter (within a few days), depending on the case, favouring a test before discharge if the patient has a history of coronary artery disease.
The management of patients with chest pain in an ED setting needs a close integration and cooperation between emergency physicians and cardiologists, together with nursing staff, especially triage nurses. A prerequisite for such integration is that the organization
should hinge on a shared awareness of the diagnostic pathway. This is the basis for proposing the creation of a Chest Pain Team—comprising an emergency physician, a cardiologist and a triage nurse—in every hospital facility.
Conclusions
Although the many advances and innovations of recent years, the assessment of patients with chest pain continues to be a daily challenge. Given the economic impact and the risk of complications induced by inappropriate tests and examinations carried out on a patient with chest pain, better pathways must be studied, not least in order to avoid pointless examinations and unnecessary hospitalizations.
This document embraces and applies the new proposals offered by medical literature in matters of diagnostic testing and clinical/anamnestic assessment, in seeking to formulate a rule-in/rule-out process for ACS that is both modern and efficient. Above all, the introduction of hs-cTn assays has allowed healthcare professionals to identify a higher number of ACS cases, improving the sensitivity of rule-in/rule-out tests in particular, but has led at the same time to overestimations in diagnosis, and very often to unnecessary hospitalizations, accompanied by a significant waste of economic resources.
The role of this document is to provide operators in the field with guidance on making the right choices, so that the results of different diagnostic tests can be balanced and integrated correctly.
The organization of ED and cardiology departments around a shared diagnostic pathway is the first step in ensuring efficient management of patients with chest pain.
The main purpose of the document is to promote a nationwide standardization of diagnosis and care protocols for patients with chest pain, with the aim of improving outcomes.
Conflict of interest: none declared.
Consensus Document Approval Faculty
Abrignani Maurizio Giuseppe, Alunni Gianfranco, Amico Antonio, Francesco, Amodeo Vincenzo, Angeli Fabio, Aspromonte Nadia, Audo Andrea, Azzarito Michele, Battistoni Ilaria, Bianca Innocenzo, Bisceglia Irma, Bongarzoni Amedeo, Bonvicini Marco, Calculli Giacinto, Caldarola Pasquale, Capecchi Alessandro, Caporale Roberto,Caretta Giorgio, Carmina Maria Gabriella, Casazza Franco, Casolo Giancarlo, Casu Gavino, Cemin Roberto, Giacomo Chiarandà, Chiarella Francesco, Chiatto Mario, Ciccone Marco Matteo, Cicini Maria Paola, Colivicchi Furio, D’Agostino Carlo, De Luca Giovanni, De Maria Renata, Del Sindaco Donatella, Di Fusco Stefania Angela, Egidy Assenza Gabriele, Egman Sabrina, Enea Iolanda, Fattirolli Francesco, Favilli Silvia, Ferraiuolo Giuseppe, Francese Giuseppina Maura, Gabrielli Domenico, Geraci Giovanna, Giardina Achille, Greco Cesare, Gregorio Giovanni, Iacoviello Massimo, Khoury Georgette, Ledda Antonietta, Luca Fabiana, Lukic Vjerica, Macera Francesca, Marini Marco, Masson Serge, Maurea Nicola, Mazzanti Marco, Mennuni Mauro, Menotti Alberto, Mininni Nicola, Moreo Antonella, Moretti Luciano, Mortara Andrea, Mureddu Gian Francesco, Musumeci Giuseppe, Navazio Alessandro, Nicolosi Gian Luigi, Oliva Fabrizio, Parrini Iris, Patanè Leonardo, Pini Daniela, Pino Paolo Giuseppe, Pirelli Salvatore, Procaccini Vincenza, Pugliese Francesco Rocco, Pulignano Giovanni, Radini Donatella, Rao Carmelo Massimiliano, Riccio Carmine, Roncon Loris, Rugolotto Matteo, Sanna Fabiola, Sauro Rosario, Scherillo Marino, Severi Silva, Silvestri Paolo, Sisto Francesco, Tarantini Luigi, Urbinati Stefano, Vatrano Marco, Vianello Gabriele, Vinci Eugenio.
References
- 1. Pitts SR, Niska RW, XuS J, Burt CW.. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008;7:1–38. [PubMed] [Google Scholar]
- 2. Fanaroff AC, Rymer JA, Goldstein SA, Simel DL, Newby LK.. Does this patient with chest pain have acute coronary syndrome?: the rational clinical examination systematic review. JAMA 2015;314:1955–1965. [DOI] [PubMed] [Google Scholar]
- 3. Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL, Selker HP.. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163–1170. [DOI] [PubMed] [Google Scholar]
- 4. Pittet V, Burnand B, Yersin B, Carron PN.. Trends of pre-hospital emergency medical services activity over 10 years: a population-based registry analysis. BMC Health Serv Res 2014;14:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foy AJ, Filippone L.. Chest pain evaluation in the emergency department. Med Clin N Am 2015; 99:835–847. [DOI] [PubMed] [Google Scholar]
- 6. McCarthy BD, Beshansky JR, D'Agostino RB, Selker HP. Missed diagnosis of acute myocardial infarction in the emergency department: results from a multicenter study. Ann Emerg Med 1993;22:579–582. [DOI] [PubMed] [Google Scholar]
- 7. Filippo O, Nicola B, Casagranda I, Cassin M, Cavazza M, Grifoni S, Lenzi T, Lorenzoni R, Sbrojavacca R, Tanzi P, Vergara G; Commissione Congiunta ANMCO-SIMEU. [Chest pain evaluation project] [Italian]. G Ital Cardiol (Rome) 2009;10:46–63. Erratum appears in: G Ital Cardiol (Rome) 2009;10:351. [PubMed] [Google Scholar]
- 8. Lindsell CJ, Anantharaman V, Diercks D, Han JH, Hoekstra JW, Hollander JE, Kirk JD, Lim SH, Peacock WF, Tiffany B, Wilke EK, Gibler WB, Pollack CV Jr; EMCREG-International i*trACS Investigators. The Internet Tracking Registry of Acute Coronary Syndromes (i*trACS): a multicenter registry of patients with suspicion of acute coronary syndromes reported using the standardized reporting guidelines for emergency department chest pain studies. Ann Emerg Med 2006;48:666–677, 677.e1-9. [DOI] [PubMed] [Google Scholar]
- 9. Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, Forgie M, Kovacs G, Ward J, Kovacs MJ.. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med 2001;135:98–107. [DOI] [PubMed] [Google Scholar]
- 10. Bueno H. The ACCA Clinical Decision Making Toolkit, 2nd ed.2015. https://www.escardio.org/Education/Practice-Tools/ACCA-Toolkit.
- 11. Kontos MC, Diercks DB, Kirk JD.. Emergency department and office-based evaluation of patients with chest pain. Mayo Clin Proc 2010;85:284–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diercks DB, Boghos E, Guzman H, Amsterdam EA, Kirk JD.. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med 2005;45:581–585. [DOI] [PubMed] [Google Scholar]
- 13. Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, Akkerhuis KM, Harrington RA, Deckers JW, Armstrong PW, Lincoff AM, Califf RM, Topol EJ, Simoons ML.. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation 2000;101:2557–2567. [DOI] [PubMed] [Google Scholar]
- 14. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E.. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 15. Backus BE, Six AJ, Kelder JC, Mast TP, van den Akker F, Mast EG, Monnink SH, van Tooren RM, Doevendans PA.. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol 2010;9:164–169. [DOI] [PubMed] [Google Scholar]
- 16. Ramsay G, Podogrodzka M, McClure C, Fox KAA.. Risk prediction in patients presenting with suspected cardiac pain: the GRACE and TIMI risk scores versus clinical evaluation. Q J Med 2007;100:11–18. [DOI] [PubMed] [Google Scholar]
- 17. Rubini Gimenez M, Reiter M, Twerenbold R, Reichlin T, Wildi K, Haaf P, Wicki K, Zellweger C, Hoeller R, Moehring B, Sou SM, Mueller M, Denhaerynck K, Meller B, Stallone F, Henseler S, Bassetti S, Geigy N, Osswald S, Mueller C.. Sex-specific chest pain characteristics in the early diagnosis of acute myocardial infarction. JAMA Intern Med 2014;174:241–249. [DOI] [PubMed] [Google Scholar]
- 18. Gräni C, Senn O, Bischof M, Cippà PE, Hauffe T, Zimmerli L, Battegay E, Franzen D.. Diagnostic performance of reproducible chest wall tenderness to rule out acute coronary syndrome in acute chest pain: a prospective diagnostic study. BMJ Open 2015;5:e007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rouan GW, Lee TH.. Clinical characteristics and out come of acute myocardial infarction in patients with initially normal or non-specific electrocardiograms (a report from the Multicenter Chest Pain Study). Am J Cardiol 1989; 64:1087–1092. [DOI] [PubMed] [Google Scholar]
- 20. Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D.. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 21. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J; Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 22. Akkerhuis KM, Klootwijk PA, Lindeboom W.. Recurrent ischaemia during continuous multilead ST-segment monitoring identifies patients with acute coronary syndromes at high risk of adverse cardiac events; meta-analysis of three studies involving 995 patients. Eur Heart J 2001;22:1997–2006. [DOI] [PubMed] [Google Scholar]
- 23. Sgarbossa EB, Pinski SL, Barbagelata A, Underwood DA, Gates KB, Topol EJ, Califf RM, Wagner GS.. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med 1996;334:481–487. Erratum appears in: N Engl J Med 1996;334:931. [DOI] [PubMed] [Google Scholar]
- 24. Kligfield P, Gettes LS, Bailey JJ, Childers R, Deal BJ, Hancock EW, van Herpen G, Kors JA, Macfarlane P, Mirvis DM, Pahlm O, Rautaharju P, Wagner GS; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society, Josephson M, Mason JW, Okin P, Surawicz B, Wellens H.. Recommendations for the standardization and interpretation of the electrocardiogram: part I: The electrocardiogram and its technology: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2007;115:1306–1324. [DOI] [PubMed] [Google Scholar]
- 25. Gulizia MM, Casolo G, Zuin G, Morichelli L, Calcagnini G, Ventimiglia V, Censi F, Caldarola P, Russo G, Leogrande L, Gensini GF; ANMCO/AIIC/SIT. [ANMCO/AIIC/SIT Consensus document: Definition, precision and appropriateness of the electrocardiographic signal of electrocardiographic recorders, ergometry systems, Holter systems, telemetry and bedside monitors]. G Ital Cardiol (Rome) 2016;17:393–415. [DOI] [PubMed] [Google Scholar]
- 26. Chan D, Ng LL.. Biomarkers in acute myocardial infarction. BMC Med 2010;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mair J. High-sensitive cardiac troponins in eveyday clinical practice. World J Cardiol 2014;6:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, Blankenberg S, Huber K, Plebani M, Biasucci LM, Tubaro M, Collinson P, Venge P, Hasin Y, Galvani M, Koenig W, Hamm C, Alpert JS, Katus H, Jaffe AS; Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 2012;33:2252–2257. [DOI] [PubMed] [Google Scholar]
- 29. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; ESC Committee for Practice Guidelines (CPG). Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 30. Casagranda I, Cavazza M, Clerico A, Galvani M, Ottani F, Zaninotto M, Biasucci LM, Cervellin G, Lenzi T, Lippi G, Plebani M, Tubaro M.. Proposal for the use in emergency departments of cardiac troponins measured with the latest generation methods in patients with suspected acute coronary syndrome without persistent ST-segment elevation. Clin Chem Lab Med 2013;51:1727–1737. [DOI] [PubMed] [Google Scholar]
- 31. Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Fröhlich M, Sinning CR, Eleftheriadis MS, Wild PS, Schnabel RB, Lubos E, Jachmann N, Genth-Zotz S, Post F, Nicaud V, Tiret L, Lackner KJ, Münzel TF, Blankenberg S.. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med 2009;361:868–877. [DOI] [PubMed] [Google Scholar]
- 32. Casagranda I, Cavazza M, Clerico A. et al. Proposta per l’utilizzo in PS delle proponine cardiache misurate con metodi di ultima generazione in pazienti con sospetta di sindrome coronaria acuta senza sopraslivellamento del tratto ST. Ligand Assay 2012;17:350–360. [Google Scholar]
- 33. Reichlin T, Hochholzer W, Stelzig C, Laule K, Freidank H, Morgenthaler NG, Bergmann A, Potocki M, Noveanu M, Breidthardt T, Christ A, Boldanova T, Merki R, Schaub N, Bingisser R, Christ M, Mueller C.. Incremental value of copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol 2009;54:60–68. [DOI] [PubMed] [Google Scholar]
- 34. Keller T, Tzikas S, Zeller T, Czyz E, Lillpopp L, Ojeda FM, Roth A, Bickel C, Baldus S, Sinning CR, Wild PS, Lubos E, Peetz D, Kunde J, Hartmann O, Bergmann A, Post F, Lackner KJ, Genth-Zotz S, Nicaud V, Tiret L, Münzel TF, Blankenberg S.. Copeptin improves early diagnosis of acute myocardial infarction. J Am Coll Cardiol 2010;55:2096–2106. [DOI] [PubMed] [Google Scholar]
- 35. Lipinski MJ, Escárcega RO, D'Ascenzo F, Magalhães MA, Baker NC, Torguson R, Chen F, Epstein SE, Miró O, Llorens P, Giannitsis E, Lotze U, Lefebvre S, Sebbane M, Cristol JP, Chenevier-Gobeaux C, Meune C, Eggers KM, Charpentier S, Twerenbold R, Mueller C, Biondi-Zoccai G, Waksman R.. A systematic review and collaborative meta-analysis to determine the incremental value of copeptin for rapid rule-out of acute myocardial infarction. Am J Cardiol 2014;113:1581–1591. [DOI] [PubMed] [Google Scholar]
- 36. Möckel M, Searle J, Hamm C, Slagman A, Blankenberg S, Huber K, Katus H, Liebetrau C, Müller C, Muller R, Peitsmeyer P, von Recum J, Tajsic M, Vollert JO, Giannitsis E.. Early discharge using single cardiac troponin and copeptin testing in patients with suspected acute coronary syndrome (ACS): a randomized, controlled clinical process study. Eur Heart J 2015;36:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maisel A, Mueller C, Neath SX, Christenson RH, Morgenthaler NG, McCord J, Nowak RM, Vilke G, Daniels LB, Hollander JE, Apple FS, Cannon C, Nagurney JT, Schreiber D, deFilippi C, Hogan C, Diercks DB, Stein JC, Headden G, Limkakeng AT Jr, Anand I, Wu AH, Papassotiriou J, Hartmann O, Ebmeyer S, Clopton P, Jaffe AS, Peacock WF.. Copeptin helps in the early detection of patients with acute myocardial infarction: primary results of the CHOPIN trial (Copeptin Helps in the early detection of patients with acute myocardial INfarction). J Am Coll Cardiol 2013;62:150–160. [DOI] [PubMed] [Google Scholar]
- 38. Rayner K, Dimmeler S, Calin GA, Thum T, Raizman JE, Diamandis EP.. Novel biomarkers for acute myocardial infarction: is microRNA the new kid on the block? Clin Chem 2014;60:812–817. [DOI] [PubMed] [Google Scholar]
- 39. Huang H, Zhu S, Wang W, Yi H, Du X, Nie X, He Y, Song H, Miao Q, Wang L, Li G.. Diagnosis of acute myocardial infarction in patients with renal insufficiency using high-sensitivity troponin T. Clin Chem Lab Med 2015;53:723–730. [DOI] [PubMed] [Google Scholar]
- 40. Severi S, Orsini E, Marraccini P, Michelassi C, L’abbate A.. The basal electrocardiogram and the exercise stress test in assessing prognosis in patients admitted with unstable angina. Eur Heart J 1988;9:441–446. [DOI] [PubMed] [Google Scholar]
- 41. Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O'Reilly MG, Winters WL, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 2002;40:1531–1540. Erratum appears in: J Am Coll Cardiol 2006;48:1731. [DOI] [PubMed] [Google Scholar]
- 42. Nyman I, Wallentin L.. Risk stratification by early exercise testing after an episode of unstable coronary artery disease. The RISC Study Group. Int J Cardil 1993;39:131–142. [DOI] [PubMed] [Google Scholar]
- 43. Erhardt L, Herlitz J, Bossaert L, Halinen M, Keltai M, Koster R, Marcassa C, Quinn T, van Weert H; Task Force on the management of chest pain. Task force on the management of chest pain. Eur Heart J 2002;23:1153–1176. [DOI] [PubMed] [Google Scholar]
- 44. Gordon J, Miller G, Pan Y.. Ordering chest X-rays in Australian general practice. Aust Fam Physician 2015;44:537–539. [PubMed] [Google Scholar]
- 45. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–2926. Erratum appears in: Eur Heart J 2015;36:2779. [DOI] [PubMed] [Google Scholar]
- 46. Parato VM, Mehta A, Delfino D, Amabili S, Partemi M, Grossi P, Nardini E.. Resting echocardiography for the early detection of acute coronary syndromes in chest pain unit patients. Echocardiography 2010;27:597–602. [DOI] [PubMed] [Google Scholar]
- 47. Trambaiolo P. Utilizzo dei test di imaging non invasivi nella valutazione del dolore toracico. Il loro utilizzo garantisce l’accuratezza diagnostica. G Ital Cardiol 2014;15:405–407. [DOI] [PubMed] [Google Scholar]
- 48. American College of Cardiology Foundation Appropriate Use Criteria Task Force; American Society of Echocardiography; American Heart Association; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Critical Care Medicine; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance, Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Ward RP, Weiner RB.. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol 2011;57:1126–1166. [DOI] [PubMed] [Google Scholar]
- 49. Lancellotti P, Price S, Edvardsen T. et al. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2015;4:3–5. [DOI] [PubMed] [Google Scholar]
- 50. Kansal M, Kessler C, Frazin L.. Hand held echocardiogram does not aid in triaging chest pain patients from the emergency department. Echocardiography 2009;26:625–629. [DOI] [PubMed] [Google Scholar]
- 51. Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice Guidelines, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL.. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. Erratum appears in: Eur Heart J 2014;35:2260–2261. [DOI] [PubMed] [Google Scholar]
- 52. Shah BN, Balaji G, Alhajiri A, Ramzy IS, Ahmadvazir S, Senior R.. Incremental diagnostic and prognostic value of contemporary stress echocardiography in a chest pain unit: mortality and morbidity outcomes from a real-world setting. Circ Cardiovasc Imaging 2013;6:202–209. [DOI] [PubMed] [Google Scholar]
- 53. Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL.. Stress echocardiography expert consensus statement—executive summary: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur Heart J 2009;30:278–289. [DOI] [PubMed] [Google Scholar]
- 54. Gaibazzi N, Reverberi C, Badano L.. Usefulness of contrast stress echocardiography or exercise-electrocardiography to predict long-term acute coronary syndromes in patients presenting with chest pain without electrocardiographic abnormalities or 12-hour troponin elevation. Am J Cardiol 2011;107:161–167. [DOI] [PubMed] [Google Scholar]
- 55. Sicari R, Pasanisi E, Venneri L, Laudi P, Cortigiani L, Picano E.. Stress echocardiography results predict mortality. A large scale multicentre prospective international study. J Am Coll Cardiol 2003; 41:589–595. [DOI] [PubMed] [Google Scholar]
- 56. Pellikka PA, Nogueh SF, Elhendy AA, Kuehl CA, Sowade SG.. American Society of Echocardiography recommendation for performance, interpretation and application of stress echocardiography. J Am Soc Echocardiogr 2007;20:1021–1041. [DOI] [PubMed] [Google Scholar]
- 57. Gaibazzi N, Squeri A, Reverberi C, Molinaro S, Lorenzoni V, Sartorio D, Senior R. Contrast stress-echocardiography predicts cardiac events in patients with suspected acute coronary syndrome but nondiagnostic electrocardiogram and normal 12-hour troponin. J Am Soc Echocardiogr 2011; 24:1333–1341. [DOI] [PubMed] [Google Scholar]
- 58. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, Rubin GD. ; American College of Cardiology Foundation Appropriate Use Criteria Task Force; Society of Cardiovascular Computed Tomography; American College of Radiology; American Heart Association; American Society of Echocardiography; American Society of Nuclear Cardiology; North American Society for Cardiovascular Imaging; Society for Cardiovascular Angiography and Interventions; Society for Cardiovascular Magnetic Resonance, Kramer CM, Berman D, Brown A, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser J, McGann C, Rosenberg A, Schwartz R, Shelton M, Smetana GW, Smith SC Jr. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 2010; 56: 1864–1894. [DOI] [PubMed] [Google Scholar]
- 59. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, Rubin GD; American College of Cardiology Foundation Appropriate Use Criteria Task Force; Society of Cardiovascular Computed Tomography; American College of Radiology; American Heart Association; American Society of Echocardiography; American Society of Nuclear Cardiology; North American Society for Cardiovascular Imaging; Society for Cardiovascular Angiography and Interventions; Society for Cardiovascular Magnetic Resonance, Kramer CM, Berman D, Brown A, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser J, McGann C, Rosenberg A, Schwartz R, Shelton M, Smetana GW, Smith SC Jr. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010 Nov 23;56:1864-1894. [DOI] [PubMed] [Google Scholar]
- 60. Samad Z, Hakeem A, Mahmood SS, Pieper K, Patel MR, Simel DL, Douglas PS.. A meta-analysis and systematic review of computed tomography angiography as a diagnostic triage tool for patients with chest pain presenting to the emergency department. J Nucl Cardiol 2012;19:364–376. [DOI] [PubMed] [Google Scholar]
- 61. Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE; ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ayaram D, Bellolio MF, Murad MH, Laack TA, Sadosty AT, Erwin PJ, Hollander JE, Montori VM, Stiell IG, Hess EP.. Triple rule-out computed tomographic angiography for chest pain: a diagnostic systematic review and meta-analysis. Acad Emerg Med 2013;20:861–871. [DOI] [PubMed] [Google Scholar]
- 63. Nikolaou K, Alkadhi H, Bamberg F, Leschka S, Wintersperger BJ.. MRI and CT in the diagnosis of coronary artery disease: indications and applications. Insights Imaging 2011;2:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ, Patel M, Pohost GM, Stillman AE, White RD, Woodard PK.. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;121:2462–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pennell DJ. Contemporary reviews in cardiovascular medicine cardiovascular magnetic resonance. Circulation 2010;121:692–705. [DOI] [PubMed] [Google Scholar]
- 66. Authors/Task Force Members, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A.. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 67. Lim SH, Anantharaman V, Sundram F, Chan ES, Ang ES, Yo SL, Jacob E, Goh A, Tan SB, Chua T.. Stress myocardial perfusion imaging for the evaluation and triage of chest pain in the emergency department: a randomized controlled trial. J Nucl Cardiol 2013;20:1002–1012. [DOI] [PubMed] [Google Scholar]
- 68. Nabi F, Chang SM, Xu J, Gigliotti E, Mahmarian JJ.. Assessing risk in acute chest pain: the value of stress myocardial perfusion imaging in patients admitted through the emergency department. J Nucl Cardiol 2012;19:233–243. [DOI] [PubMed] [Google Scholar]
- 69. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL; PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gaibazzi N, Cademartiri F, Maffei E.. Lo studio PROMISE. G Ital Cardiol 2015;16:462–468. [DOI] [PubMed] [Google Scholar]
- 71. Rybicki FJ, Udelson JE, Peacock WF, Goldhaber SZ, Isselbacher EM, Kazerooni E, Kontos MC, Litt H, Woodard PK.. 2015 ACR/ACC/AHA/AATS/ACEP/ASNC/NASCI/SAEM/SCCT/SCMR/SCPC/SNMMI/STR/STS Appropriate Utilization of Cardiovascular Imaging in Emergency Department Patients With Chest Pain: A Joint Document of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Appropriate Use Criteria Task Force. J Am Coll Cardiol 2016;67:853–879. [DOI] [PubMed] [Google Scholar]
- 72. Leite L, Baptista R, Leitão J, Cochicho J, Breda F, Elvas L, Fonseca I, Carvalho A, Costa JN.. Chest pain in the emergency department: risk stratification with Manchester triage system and HEART score. BMC Cardiovasc Disord 2015;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Han JH, Lindsell CJ, Storrow AB, Luber S, Hoekstra JW, Hollander JE, Peacock WF, Pollack CV, Gibler WB; EMCREG i*trACS Investigators. The role of cardiac risk factor burden in diagnosing acute coronary syndromes in the emergency department setting. Ann Emerg Med 2007;49:145–152, 152.e1. [DOI] [PubMed] [Google Scholar]
- 74. Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C.. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858–867. [DOI] [PubMed] [Google Scholar]
- 75. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266–369. [DOI] [PubMed] [Google Scholar]
- 76. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069, 3069a-3069k. Erratum appears in: Eur Heart J 2015;36:2666. Eur Heart J 2015;36:2642.25173341 [Google Scholar]