Abstract
The purpose of this multicenter, prospective, randomized, placebo-controlled study was to evaluate and compare the efficacy of two cognitive rehabilitation interventions (Memory and Attention Adaptation Training (MAAT) and Attention Builders Training (ABT)), with and without pharmacological enhancement (ie, with methylphenidate (MPH) or placebo), for treating persistent cognitive problems after traumatic brain injury (TBI). Adults with a history of TBI at least 4 months before study enrollment with either objective cognitive deficits or subjective cognitive complaints were randomized to receive MPH or placebo and MAAT or ABT, yielding four treatment combinations: MAAT/MPH (N=17), ABT/MPH (N=19), MAAT/placebo (N=17), and ABT/placebo (N=18). Assessments were conducted pre-treatment (baseline) and after 6 weeks of treatment (post treatment). Outcome measures included scores on neuropsychological measures and subjective rating scales. Statistical analyses used linear regression models to predict post-treatment scores for each outcome variable by treatment type, adjusting for relevant covariates. Statistically significant (P<0.05) treatment-related improvements in cognitive functioning were found for word-list learning (MAAT/placebo>ABT/placebo), nonverbal learning (MAAT/MPH>MAAT/placebo and MAAT/MPH>ABT/MPH), and auditory working memory and divided attention (MAAT/MPH>ABT/MPH). These results suggest that combined treatment with metacognitive rehabilitation (MAAT) and pharmacotherapy (MPH) can improve aspects of attention, episodic and working memory, and executive functioning after TBI.
Introduction
Functionally important cognitive impairments are common in the late period following traumatic brain injury (TBI). Working and episodic memory, attention, speed of information processing, and aspects of executive functioning are among the most common post-traumatic cognitive impairments, and are typical targets of rehabilitative efforts (Cappa et al, 2005; Cicerone et al, 2011). Treatment of cognitive complaints and deficits in individuals with TBI has taken the form of either cognitive rehabilitation or pharmacological augmentation.
The two principal approaches to cognitive rehabilitation after TBI include remediation and compensatory training. Remediation (cognitive retraining) approaches are based on the theory that cognitive abilities can be improved by activating particular aspects of the cognitive process through graded mental exercise (Sohlberg et al, 2003; Tate, 1997). The techniques employed in a remediation approach often focus on repetitive practice regimens within a specific cognitive domain. Work using the attention process training (APT) intervention has shown significant improvements on measures of objective and subjective attentional and executive functioning after TBI (Palmese and Raskin, 2000; Sohlberg et al, 2000). Similarly, treatments targeting ‘working attention’ have been shown to improve performance on objective measures of attention as well as reduce self-reported attentional difficulties in daily functioning (Cicerone, 2002).
Compensatory training approaches focus on adapting to and compensating for cognitive impairment by capitalizing on remaining cognitive strengths and functional abilities. Many such approaches incorporate ‘metacognitive training’ using cognitive domain-specific compensatory techniques and evidence-based remediation techniques (where available) in order to develop strategies to improve performance and generalization of skills to daily tasks. Such metacognitive approaches can include training in cognitive strategy use, self-monitoring, self-regulation, and therapist feedback (Cicerone et al, 2011). Systematic reviews and meta-analyses have suggested that the evidence for the efficacy of such interventions is sufficiently strong to rise to the level of a practice guideline for treatment of cognitive deficits after TBI, and that inclusion of metacognitive training strategies is likely to be more effective than direct attention training alone (Cicerone et al, 2011).
Pharmacotherapy of cognitive complaints and deficits generally targets major neurotransmitter systems modulating the function of brain regions underlying the relevant cognitive process (eg, attention, working or episodic memory). Both human and animal studies suggest that the neural circuitry and neurochemistry of different components of memory and attention overlap significantly, and involve broad networks of brain regions including bilateral prefrontal and parietal cortices, cingulate gyrus, basal ganglia, hippocampus and related mesial temporal structures, and regions of the brain stem and midbrain forming the reticular activating system (Arciniegas et al, 2013). Cerebral catecholamines, as well as catecholaminergic-acetylcholinergic interactions, modulate the function of several of these large-scale distributed networks, which subserve attention, episodic and working memory, and executive control of these cognitive functions, and in which the dorsolateral and inferolateral prefrontal cortices are critical nodes (Arciniegas, 2013).
Methylphenidate (MPH) augments cerebral dopaminergic and adrenergic systems. Evidence suggests that MPH improves performance on measures of attention, memory, verbal fluency, processing speed, motor performance, and arousal, and is also associated with improvements in subjective (self or informant) assessment of cognitive functioning (Gualtieri and Evans, 1988; Johansson et al, 2015; Kaelin et al, 1996; McAllister et al, 2015; Plenger et al, 1996; Whyte et al, 1997; Whyte et al, 2004; Whyte et al, 2002; Willmott and Ponsford, 2009). Indeed, the Defense and Veterans Brain Injury Center’s Neurobehavioral Guidelines Working Group evidence-based review of pharmacotherapies for post-traumatic cognitive impairments recommended MPH at the Guideline level for attention and processing speed impairments, and at the Option level for general cognitive deficits and memory impairments (Warden et al, 2006). More recent meta-analyses also concluded that the evidence supports MPH as a treatment for attention impairments, including working memory impairments, after TBI (Huang et al, 2016; Wheaton et al, 2011). MPH therefore appears to be a promising agent for remediation of persistent cognitive deficits after TBI.
The available literature suggests that domain-specific evidence-based cognitive rehabilitation and symptom-targeted pharmacotherapy can improve cognitive problems after TBI. Preliminary reports suggest that pharmacological facilitation of brain injury rehabilitation may improve cognitive outcomes (Berthier et al, 2009; Bragoni et al, 2000); however, few studies examine the efficacy of pharmacologically facilitated cognitive rehabilitation, and none have focused specifically on this approach to the treatment of cognitive impairments in the late period following TBI. This study implemented a double-blind, placebo-controlled trial of the effectiveness of two forms of cognitive rehabilitation (remediation and compensatory training) and MPH, alone and in combination, to treat cognitive symptoms after TBI. We hypothesized a synergistic effect, such that participants receiving MPH and a compensatory training intervention would show greater improvement than those receiving other treatment combinations. On the basis of the available literature, we focused on outcome measures testing attention-related functions, including working memory and processing speed. As downstream effects of improved attention can also result in better performance in other cognitive domains, we also examined episodic memory and executive function.
Materials and methods
Design and Participants
Overview
This double-blind, placebo-controlled, 2 × 2 factorial design tested the efficacy of pharmacotherapy, cognitive rehabilitation, and combination therapies for persistent cognitive symptoms and/or deficits after TBI in a 6-week trial (ClinicalTrials.gov Identifier NCT00453921). Participants gave written informed consent approved by the Institutional Review Boards of Dartmouth-Hitchcock Medical Center, Indiana University School of Medicine, and University of Colorado School of Medicine.
Inclusion criteria
Inclusion criteria were: (1) age 18–65 at study entry; (2) TBI of at least mild severity (Kay et al, 1993) at least 4 months before study entry; and (3) self-report of cognitive deficits as a result of the injury of sufficient severity to interfere with social and/or occupational functioning as measured by a score at least one standard deviation above the normative mean on the multiple abilities self-report questionnaire (MASQ) (Ahles et al, 2008; Seidenberg et al, 1994), and/or objective evidence of cognitive deficits represented by either (a) score two or more standard deviations below age-adjusted normative data or estimates of premorbid function (Barona et al, 1984) on one or more tests of attention and/or memory or (b) score one or more standard deviations below either age-adjusted normative data or estimates of premorbid function on two or more tests of attention and/or memory administered as part of the screening cognitive battery.
Exclusion criteria
Exclusion criteria were: (1) history of other neurological or systemic medical disorder that was unstable, likely to need repeated medication adjustments, or likely to affect cognitive function or be a contraindication to use of MPH; (2) current DSM-IV (APA, 2000) Axis I diagnosis of psychiatric illness, other than mild-moderate depression or anxiety, screened for using the Mini-International Neuropsychiatric Interview (M.I.N.I. English Version 6.0.0, modified based on the version for attention-deficit/hyperactivity disorder (ADHD) studies) (Sheehan et al, 1998), Beck Depression Inventory-II (Beck et al, 1996), State-Trait Anxiety Inventory (Spielberger, 1983), and PTSD Checklist-Stressor Specific (PCL-S) (Weathers et al, 1993); (3) currently pregnant or lactating; (4) pre-injury diagnosis of learning disability or ADHD; (5) lack of English fluency sufficient to complete study measures; or (6) standard score below 70 on the Wide Range Achievement Test-4 Word Reading subtest (Wilkinson and Robertson, 2006), to ensure reading ability sufficient to participate in cognitive rehabilitation.
Study protocol
Eligible participants underwent a pre-treatment baseline assessment, then were randomized to receive MPH or placebo and one of two cognitive rehabilitation interventions, a metacognitive intervention, Memory and Attention Adaptation Training (MAAT, designed by R.J.F.), or Attention Builders Training (ABT), a manualized repetitive practice intervention with no active cognitive-behavioral component. This resulted in four treatment combinations: MAAT/MPH, ABT/MPH, MAAT/placebo, and ABT/placebo. Phone contacts (weeks 1, 3, and 5) assessed for side effects and monitored compliance. At in-person study visits (weeks 2 and 4) participants also engaged in their assigned therapy (MAAT or ABT). After 6 weeks of treatment, participants completed post-treatment outcome measures (see Supplementary Figure 1 for study flow).
Cognitive assessment
The test battery emphasized cognitive domains most commonly reported as impaired after TBI, including memory, attention-related and executive functions, and processing speed, and included measures of general cognitive ability for sample characterization. Cognitive eligibility screening included the California Verbal Learning Test-II (Delis et al, 2000), Delis–Kaplan Executive Function System (Delis et al, 2001) Trail Making, Verbal Fluency, and Color-Word Interference subtests, Paced Auditory Serial Addition Test (Gronwall, 1977), Wechsler Adult Intelligence Scale-III (The Psychological Corporation, 1997) Letter Number-Sequencing Test, and Gordon Diagnostic System Continuous Performance Test (Gordon et al, 1996). Assessments at the pre-treatment visit included the Vocabulary and Block Design subtests of the Wechsler Abbreviated Scale of Intelligence (The Psychological Corporation, 1999), Wechsler Adult Intelligence Scale-III Digit Symbol-Coding and Digit Span subtests, Delis–Kaplan Executive Function System Sorting Test, Craft Story Memory Test (Craft et al, 1996), Brown Location Test (Brown et al, 2007), Grooved Pegboard Test (Lafayette Instrument, 1989), Finger Tapping Test (Reitan and Wolfson, 1993), and Thumb-Finger Sequencing Test (Saykin et al, 1995). The complete neuropsychological battery and the MASQ were repeated at post-treatment, with alternate test forms used where possible.
Interventions
Memory and Attention Adaptation Training (MAAT)
MAAT is a brief cognitive-behavioral therapy aimed at enhancing skills for self-managing and coping with cognitive failures in daily life. Initially developed for use in patients experiencing post-concussive symptoms (Ferguson and Mittenberg, 1996), MAAT was later adapted for treatment of cognitive symptoms after breast cancer chemotherapy, where preliminary data showed good feasibility and acceptability and beneficial treatment outcomes (Ferguson et al, 2007; Ferguson et al, 2012). The version of MAAT used in this study consisted of four biweekly 50 min individual office visits focused on cognitive-behavioral strategy use. Briefer telephone contacts between visits were intended to reinforce use of new behaviors or modify the strategy to enhance effectiveness.
MAAT includes four cognitive-behavioral components: (1) Education regarding ‘normal’ cognitive failures, as well as potential effects of TBI on cognitive function; (2) self-awareness training to identify ‘at-risk’ situations where cognitive failures are likely to occur; (3) self-regulation training emphasizing applied relaxation techniques and stress management; and (4) cognitive compensatory strategy training. Each participant received a MAAT workbook with detailed descriptions of compensatory strategies, educational material, and guides on how to apply compensatory strategies. Clinicians followed a detailed manual to enhance treatment fidelity. R.J.F. trained and supervised therapists in conducting the MAAT treatment protocol, reviewed recordings of treatment visits, and provided corrective feedback as necessary.
Attention Builders Training (ABT)
ABT was modeled after Berg et al, (1991). Participants were provided information about cognitive remediation techniques related to the use of repetitive cognitive tasks to build skills through ‘mental exercise’ (Tate, 1997), akin to the direct attention training approaches discussed above. Visit timing and duration were identical for ABT and MAAT. ABT also included an educational component discussing common cognitive symptoms after TBI, but did not emphasize behavioral compensatory and functional reorganization strategies, nor was instruction provided on how to implement the exercises in daily living situations.
At the initial therapy visit, participants were given an ABT manual and a packet consisting of a variety of tasks, which were carefully explained and practiced to ensure understanding. A repetitive practice regimen was assigned. Tasks were not tailored to the participant’s cognitive complaints/deficits, there was no ‘coaching’ on the tasks, and no compensatory strategy training was provided. On subsequent visits, the home-practice regimen was reviewed, refresher drills and practice sessions were performed, and new assignments were given. Phone contacts entailed evaluation of the home-practice regimen by the therapist and empathetic listening without any instructions.
Methylphenidate
Participants weighing <100 kg received MPH 0.1 mg/kg of body weight twice daily (BID) for 2–4 days, then 0.2 mg/kg BID for 2–4 days (default choice for both intervals was 2 days; study physicians could extend the titration interval to 4 days at their discretion), then 0.3 mg/kg BID for the remainder of the study. Doses were rounded to the nearest 2.5 mg increment. Those weighing ⩾100 kg received doses of 10 mg BID, then 15 mg BID, then 30 mg BID for the same intervals. Therefore, dosing did not exceed 60 mg/day total. For an average 70 kg adult, this translates into about 20 mg BID. This target dose was based on studies demonstrating efficacy in improving cognitive function in TBI populations (Plenger et al, 1996; Whyte et al, 1997; Whyte et al, 2002). Participants with modest side effects who wished to continue in the protocol were allowed dose reduction at physician discretion, but needed to be able to tolerate a dose of at least 10 mg/day to remain in the study.
Placebo
Placebo was provided in capsules indistinguishable from the MPH capsules in taste and appearance, with BID dosing and similar titration increases (ie, an increase in number of capsules of placebo).
Randomization and masking
Group assignment occurred through block randomization. Group allocation was concealed. Therapists were blind to outcome data, and staff conducting outcome assessments were blind to participant randomization. Medications were prepared and distributed by site-specific pharmacies which maintained the allocation code and ensured blinding of study medications.
Adverse events and treatment compliance were monitored via phone contacts and at in-person visits, and by inventorying study capsules at in-person visits.
Statistical analysis
Demographic and clinical covariates were assessed for group differences using ANOVA or χ2-tests as appropriate. Primary outcome variables included measures of episodic memory (California Verbal Learning Test-II initial encoding over trials 1–5 number correct: CVLT), attention (Continuous Performance Test Distractibility trial reaction time: CPT_RT), divided attention and working memory (Paced Auditory Serial Addition Test Trial 3 (1.6’ pacing) number correct: PASAT), processing speed (Wechsler Adult Intelligence Scale-III Digit Symbol-Coding number correct: DSC; Delis–Kaplan Executive Function System Trail Making Test, trial 2 completion time: DTR2), and self-reported cognitive function (MASQ total score: MASQ). Secondary outcome measures included additional measures of episodic memory (Craft Stories immediate recall total score: CRAFT; Brown Location Test initial encoding over trials 1–5 number correct: BLT), and executive function (D-KEFS Sorting Test-Free Sorting Description total score: SORT).
All analyses were conducted using the R statistical software package (v3.1.2), as follows. Main effects of MAAT, MPH, and their interaction were examined via nonparametric Kruskal–Wallis test for changes between baseline and post-treatment measurements. Linear regression models were fit for post-treatment scores for each outcome variable for the primary factors of interest, cognitive rehabilitation (MAAT vs ABT) and pharmacotherapy (MPH vs placebo), together with their interaction, adjusting for baseline performance, study site, days since injury (log transformed), and treatment adherence (percent of prescribed doses taken). Post-treatment scores adjusted for covariates are referred to as ‘predicted’ scores. For each treatment combination the estimated means were obtained at the average value of the adjustment variables. It was hypothesized that the MAAT/MPH group would show greater cognitive improvement than the other groups. A P-value <0.05 was considered to indicate statistical significance. Standardized (adjusted) coefficients and effect sizes (unadjusted) are reported. Standardized coefficients are estimated from the regression model used to predict post-treatment scores. To create comparable adjusted coefficients among outcome variables, both outcome and adjustment variables are standardized to have the mean equal to zero and standard deviation equal to one. Effect sizes (Cohen’s d) are estimated by the ratio of the difference between the post-treatment and baseline scores for each treatment combination and the pooled standard error estimate of the differences.
Results
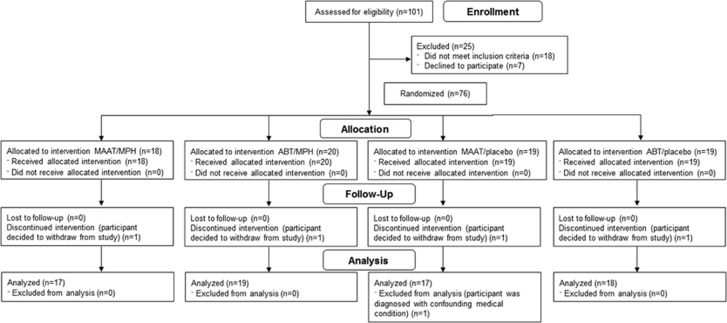
One hundred and one individuals consented to participate and were screened for eligibility; 76 were randomized to the four treatment arms (see CONSORT flow diagram (Figure 1)). One participant who completed the study was subsequently diagnosed with a confounding medical condition, and so was excluded from all analyses. Demographics and injury severity by treatment group are shown in Table 1. Time since injury for the 75 participants included ranged from 5 months to 35 years. Forty-nine participants (65%) were eligible for the study based on both subjective and objective symptom entry criteria. Thirteen participants (17%) were eligible based on objective cognitive deficits, but did not have subjective cognitive complaints based on the MASQ threshold, and 13 participants (17%) were eligible based on MASQ score, but did not have objective cognitive deficits per the entry thresholds. Entry eligibility criteria met did not significantly differ by treatment group (P=0.44).
Figure 1.
CONSORT diagram of study subject flow. CONSORT flow diagram showing disposition of patients assessed for study eligibility. ABT, Attention Builders Training; MAAT, Memory and Attention Adaptation Training; MPH, methylphenidate.
Table 1. Demographic and Injury Characteristics.
| MAAT/MPH Mean (SD) or N (%) | ABT/MPH Mean (SD) or N (%) | MAAT/placebo Mean (SD) or N (%) | ABT/placebo Mean (SD) or N (%) | P | |
|---|---|---|---|---|---|
| Number enrolled | 18 | 20 | 18 | 19 | |
| Age | 43.1 (12.3) | 43.0 (15.0) | 37.2 (12.0) | 37.3 (14.2) | 0.33 |
| Education (years) | 15.1 (2.4) | 15.4 (2.4) | 14.7 (2.1) | 14.1 (2.1) | 0.30 |
| Years post injury | 9.0 (8.5) | 7.6 (10.3) | 5.4 (7.5) | 8.2 (10.1) | 0.68 |
| Race | 0.52 | ||||
| American Indian/ Alaska Native | 0 (0%) | 0 (0%) | 1 (5.6%) | 0 (0%) | |
| African American | 1 (5.6%) | 1 (5.0%) | 0 (0%) | 0 (0%) | |
| White | 17 (94.4%) | 19 (95.0%) | 17 (94.4%) | 19 (100%) | |
| Not Hispanic or Latino | 17 (94.4%) | 20 (100%) | 18 (100%) | 18 (94.7%) | 0.55 |
| Right-handed | 17 (94.4%) | 15 (75.0%) | 16 (88.9%) | 18 (94.7%) | 0.19 |
| Male | 8 (44.4%) | 14 (70.0%) | 14 (77.8%) | 13 (68.4%) | 0.17 |
| TBI severity | 0.42 | ||||
| Mild | 9 (50.0%) | 10 (50.0%) | 10 (55.6%) | 7 (36.8%) | |
| Complicated Mild | 4 (22.2%) | 2 (10.0%) | 1 (5.6%) | 1 (5.3%) | |
| Moderate | 2 (11.1%) | 3 (15.0%) | 2 (11.1%) | 1 (5.3%) | |
| Severe | 3 (16.7%) | 5 (25.0%) | 5 (27.8%) | 10 (52.6%) |
Abbreviations: ABT, Attention Builders Training; ANOVA, analysis of variance; MAAT, Memory and Attention Adaptation Training; MPH, methylphenidate.
P-values are from ANOVA or χ2-tests as appropriate.
Treatment groups did not significantly differ for age, education, race, ethnicity, handedness, sex, time since injury, or injury severity (all P>0.05). Four participants (one from each study arm) enrolled in the study and began treatment but chose not complete the trial, and so were not included in outcome analyses. This resulted in a final sample of 71 participants for analysis: MAAT/MPH (N=17), ABT/MPH (N=19), MAAT/placebo (N=17), and ABT/placebo (N=18). Medication adherence and pre-treatment cognitive performance data are presented in Supplementary Table 1. Subjective and objective cognitive performance showed no significant differences between groups pre-treatment (all P>0.05), though the ABT/MPH group showed somewhat lower PASAT performance than the other groups (P=0.09).
Medication adherence and mean total daily dose of study medication did not differ significantly between treatment groups (both P>0.05). Vital signs, including blood pressure and heart rate, were checked at in-person visits and compared with pre-treatment measurements, and no clinically meaningful changes were noted. The two most common side effects, reported in >25% of the overall sample, were headache (47.1%) and insomnia (27.9%); frequency did not differ between patients receiving placebo and MPH (both P>0.05). Four symptoms were significantly more common in patients receiving MPH: nervousness, appetite loss, palpitations (all P<0.05), and abdominal discomfort (P<0.01). Side effects led to dose reduction for nine participants (including one who received placebo); dose reduction was significantly more common in patients receiving MPH (P<0.05). No participant withdrew from the study due to adverse events, and no serious adverse events were reported.
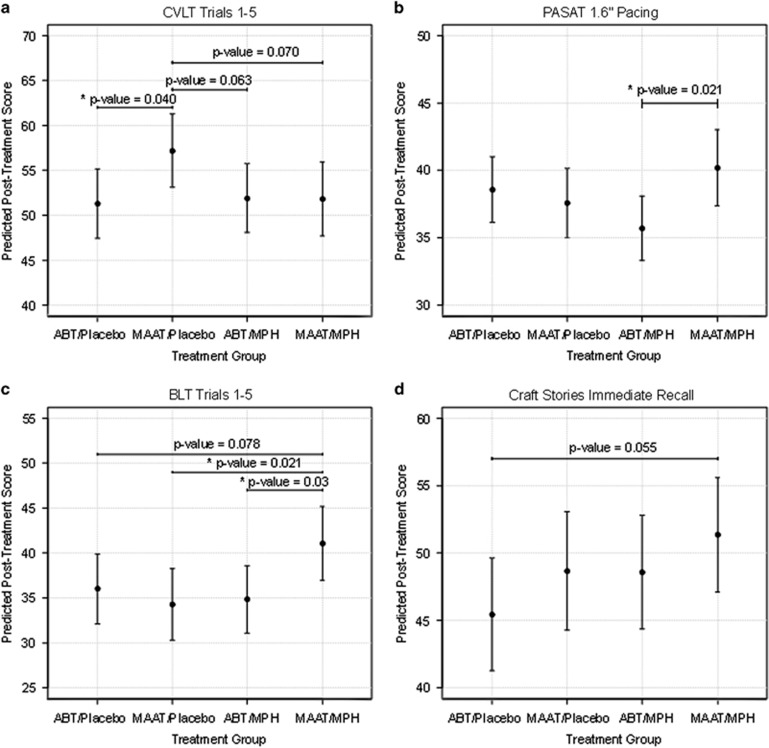
As described under Statistical Analysis above, outcome variables were examined post-treatment for each treatment condition relative to the other three conditions, adjusting for pre-treatment performance, study site, time since injury, and treatment adherence. Predicted scores for all outcome variables are shown in Table 2, and between-group treatment-related comparison data are presented in Table 3. Raw scores for post-treatment cognitive performance can be found in Supplementary Table 2. For the primary outcome variables, at post treatment the MAAT/Placebo group showed significantly higher CVLT performance relative to the ABT/Placebo group (P=0.040, Figure 2a). For PASAT, the MAAT/MPH group showed significantly higher performance than the ABT/MPH group (P=0.021, Figure 2b). For the secondary outcome variables, the MAAT/MPH group showed significantly higher BLT performance (Figure 2c) than both the MAAT/placebo (P=0.021) and ABT/MPH (P=0.030) groups. No significant differences were seen for CRAFT, CPT_RT, DSC, DTR2, MASQ, or SORT.
Table 2. Predicted Post-Treatment Cognitive Performance Scores.
| MAAT/MPH | ABT/MPH | MAAT/Placebo | ABT/Placebo | |
|---|---|---|---|---|
| Sample size (N) | 17 | 19 | 17 | 18 |
| CVLT-II total score trials 1–5 | 51.8 (2.1) | 51.9 (1.9) | 57.2 (2.0) | 51.3 (1.9) |
| CPT Distractibility Trial reaction time (ms) | 394.7 (17.3)a | 416.6 (15.9)a | 413.0 (17.3)a | 426.4 (15.3) |
| PASAT 1.6” Pacing number correct | 40.2 (1.4)b | 35.7 (1.2)a | 37.6 (1.3)c | 38.6 (1.2)c |
| WAIS-III Digit Symbol-Coding raw score | 71.7 (2.5) | 73.5 (2.4) | 71.2 (2.5) | 75.7 (2.4) |
| D-KEFS Trail Making Number Sequencing Trial time (s) | 33.4 (2.3) | 30.8 (2.2) | 32.5 (2.3) | 30.5 (2.2) |
| MASQ total score | 120.5 (4.8) | 114.1 (4.5) | 118.1 (4.8) | 116.4 (4.5) |
| Craft Stories immediate recall total score | 51.4 (2.1) | 48.6 (2.1)c | 48.7 (2.2)a | 45.4 (2.1)a |
| BLT total score trials 1-5 | 41.1 (2.1) | 34.9 (1.9) | 34.3 (2.0) | 36.0 (1.9) |
| D-KEFS Sorting Test-free sorting description score | 38.2 (2.1) | 40.0 (2.0)a | 37.9 (2.2)a | 38.7 (2.0)a |
Abbreviations: ABT, Attention Builders Training; BLT, Brown Location Test; CPT, Gordon Diagnostic System Continuous Performance Test; CVLT-II, California Verbal Learning Test-II; D-KEFS, Delis–Kaplan Executive Function System; MAAT, Memory and Attention Adaptation Training; MASQ, Multiple Abilities Self-report Questionnaire; MPH, methylphenidate; PASAT, Paced Auditory Serial Addition Test; WAIS-III, Wechsler Adult Intelligence Scale-III.
Data were missing for one participant.
Data were missing for three participants.
Data were missing for two participants.
Values are Predicted Score (SE).
Table 3. Between-Group Treatment-Related Comparison Data.
| Adjusted R2 | P | Standardized coefficient | Cohen’s d effect size | |
|---|---|---|---|---|
| CVLT | 0.548 | |||
| MAAT/Placebo-ABT/Placebo | 0.040 | 0.491 | 0.792 | |
| MAAT/Placebo-ABT/MPH | 0.063 | 0.439 | 0.752 | |
| MAAT/Placebo-MAAT/MPH | 0.070 | 0.449 | 0.828 | |
| PASAT | 0.814 | |||
| MAAT/MPH-ABT/MPH | 0.021 | 0.392 | 0.301 | |
| CRAFT | 0.496 | |||
| MAAT/MPH-ABT/Placebo | 0.055 | 0.511 | 0.789 | |
| BLT | 0.517 | |||
| MAAT/MPH-ABT/Placebo | 0.078 | 0.447 | 0.719 | |
| MAAT/MPH-MAAT/Placebo | 0.021 | 0.603 | 0.745 | |
| MAAT/MPH-ABT/MPH | 0.030 | 0.550 | 0.680 |
Abbreviations: ABT, Attention Builders Training; MAAT, Memory and Attention Adaptation Training; MPH, methylphenidate; PASAT, Paced Auditory Serial Addition Test.
Figure 2.
Treatment group comparisons. Outcome variables showing between-group differences from pre- to post-treatment for: (a) CVLT, (b) PASAT, (c) BLT and (d) CRAFT. Predicted post-treatment scores are post-treatment performance adjusted for pre-treatment performance, study site, time since injury and treatment adherence. ABT, Attention Builders Training; BLT, Brown Location Test; CVLT, California Verbal Learning Test-II; MAAT, Memory and Attention Adaptation Training; MPH, methylphenidate; PASAT, Paced Auditory Serial Addition Test.
In addition to these significant results, moderate to large effect sizes (Table 3) were noted for some primary and secondary outcome variables where statistical tests demonstrated P-values which did not rise to the a priori threshold of p<0.05. Tests yielding P<0.10 were examined to determine those additional measures which might have risen to statistical significance with a larger sample size. For the primary outcome variables, the MAAT/Placebo group showed higher CVLT performance (Figure 2a) relative to the ABT/MPH (P=0.063) and MAAT/MPH (P=0.070) groups. For the secondary outcome variables, the MAAT/MPH group showed higher performance than the ABT/placebo group on BLT (P=0.078, Figure 2c) and CRAFT (P=0.055, Figure 2d).
Discussion
Persistent cognitive changes are among the most problematic symptoms after TBI, with potential negative effects on quality of life across multiple domains. Consistent with findings from prior studies, the current results suggest that cognitive rehabilitation (MAAT) and pharmacotherapy (MPH) may improve cognitive performance in individuals with chronic cognitive impairments and/or complaints after TBI. To our knowledge, this is the first study to examine the utility of cognitive rehabilitation and pharmacotherapy in combination; our results suggest that combining pharmacotherapy and cognitive rehabilitation treatments may achieve greater improvement in cognitive functioning after TBI than either treatment alone.
For two of the outcomes examined (PASAT and BLT), patients who received a compensatory training intervention and MPH (the MAAT/MPH group) showed significantly stronger performance than other treatment groups, demonstrating a benefit for combined treatment in terms of performance on objective measures of learning, working memory, and divided attention. This finding is particularly important given that these domains are among those most commonly affected after TBI. On a measure of verbal learning (CVLT), the MAAT/placebo group showed significantly stronger performance than the ABT/placebo group. This finding offers evidence that a metacognitive (compensatory training) cognitive rehabilitation intervention is more beneficial for verbal encoding deficits than is a repetitive practice (remediation) treatment approach.
The present observations also suggest a possible synergistic effect of catecholaminergic augmentation and a metacognitive rehabilitation approach. Westbrook and Braver (2016) summarize the role of dopamine in multiple aspects of cognition, including motivation, learning, working memory, and decision-making, and propose that dopamine’s role in regulating working memory has direct relevance for cognitive effort, due to involvement of the dopamine system in incentive (reward) processing. MPH works directly on the dopaminergic system by inhibiting dopamine reuptake. The metacognitive approach of MAAT emphasizes improving behavioral aspects of executive functioning. As metacognition is sensitive to dopaminergic modulation (Joensson et al, 2015), and metacognitive tasks also increase prefrontal dopamine (Westbrook and Braver, 2016), this may explain the relatively greater improvements seen in patients receiving both MPH and MAAT relative to metacognitive rehabilitation without MPH or repetitive practice treatment (ie, ABT) with MPH.
Among the strengths of the study are its design, the representativeness of the sample of individuals with persistent post-traumatic cognitive impairments residing in the community, and demonstration of treatment feasibility and acceptability. This double-blind, placebo-controlled study incorporated comparison conditions for both its pharmacotherapeutic (ie, MPH vs placebo) and cognitive rehabilitation (ie, MAAT vs ABT) components. Our data suggest that the active cognitive-behavioral component of MAAT was more effective in generating gains in cognition than the repetitive practice approach of ABT, although the concurrent administration of placebo with both limits inferences that can be drawn about the relative intrinsic efficacies of these very different approaches to cognitive rehabilitation.
Participants were representative of individuals with TBI residing in three geographically distinct areas of the country (Northern New England, Midwest, Rocky Mountain Region). Inclusion criteria were relatively broad, and potential participants were only excluded for conditions likely to confound outcome measures or to present a potential safety risk. In addition, while the majority (59%) of participants experienced mild or complicated mild TBI, generally consistent with the preponderance of mild TBI in terms of overall incidence of injury severity, the study sample included good representation of moderate (11%) and severe (31%) injury as well. These factors increase the generalizability of the findings.
We also demonstrated acceptable tolerability, safety, and treatment adherence (>90% on average, as noted above), with minimal attrition (5%) over the course of the trial. This suggests that the types of pharmacologically facilitated cognitive rehabilitation employed in this study are sufficiently acceptable to those with persistent post-traumatic cognitive impairments to merit further study in larger-scale efficacy and effectiveness trials.
The principal limitation of the study is the small sample size. As has been noted in related literature, despite the need for interventional studies to address cognitive problems after TBI, recruitment for this and similar studies is extremely challenging (McAllister et al, 2015). The initial target for this study was to recruit 50 patients into each treatment group, for a total of 200 participants. As reflected in the CONSORT diagram (Figure 1), 101 individuals were screened in person for this study. That number represents only ~10% of the total pool of individuals screened, the majority of whom were found to be ineligible after an initial phone screen. Of those who completed in-person screening, some otherwise eligible individuals were unwilling to consider taking MPH, or unable to make the commitment required to participate in cognitive therapy.
In the context of small sample size, the population heterogeneity mentioned above as a strength with regard to representativeness could also be considered a weakness in terms of factors which may influence effectiveness of treatment, such as injury severity, injury to treatment interval, and other such metrics. The sample also had very limited racial/ethnic diversity. We therefore are unable to comment on whether there are subgroups for whom individual treatments may be more effective than others, and whether or not duration of time since injury significantly impacts on improvement after intervention.
Challenges in identifying and recruiting participants to treatment trials are unfortunately common in the field, making it extremely difficult to conduct definitive, appropriately powered trials to determine the efficacy of a given intervention. This study was initially designed as a single-center trial, but was expanded to be a multicenter study given the recruitment challenges. This was an early phase study with a targeted directional hypothesis, and we wanted to discover possible sensitive candidate outcomes. Therefore, no adjustments were made for multiple comparisons, and differences with an associated P-value <0.05 were considered statistically significant. Additional studies replicating the present findings are needed, and such studies will likely also need to be multicenter trials in order to accrue sufficient numbers of participants. However, the presence of significant results and medium to large effect sizes in the current modest sample showing positive effects of combined cognitive rehabilitation and pharmacotherapy treatment for cognitive changes after TBI is highly encouraging, and suggests that additional findings may have risen to statistical significance had the study been powered as initially intended.
In summary, we demonstrated that combined treatment with a manualized cognitive rehabilitation approach and MPH resulted in modest but statistically significant improvements in cognitive functioning on measures of verbal and nonverbal learning, working memory, and divided attention in adults with persistent cognitive symptoms after TBI. While additional research is needed to replicate these promising initial findings, the current results provide support for multimodality treatment approaches to improve cognitive functioning even months to years after TBI.
Funding and disclosure
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (R01 HD047242). The authors declare no conflict of interest. DBA receives research support from the National Institute on Disability, Independent Living, and Rehabilitation Research (H133A120020, H133A130047) and Department of Veterans Affairs (CX000239) and receives compensation from American Psychiatric Association Publishing.
Acknowledgments
We thank our study participants for their time and effort. We also thank the other study staff, coordinators, MAAT/ABT therapists, and physicians who contributed to the completion of this study, including Jessica Bailey, Rachel Dawson Burrows, Kimberly Campbell, Anna Cassel, Marcia Davis, Richard Ferrell, Angel Garcia, Andrew Goddard, Brenda Haynes, Mary Hynes, Mary Jo Manley, Jody Newman, Heather Pixley, and Kaloyan Tanev.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS et al (2008). Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat 110: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA (2000). Diagnostic and Statistical Manual of MentalDisorders, 4th edn, Text Revision. American Psychiatric Association: Washington, D.C.
- Arciniegas DB (2013) Executive function. In: Arciniegas DB, Anderson CA, Filley CM (eds). Behavioral Neurology and Neuropsychiatry. Cambridge University Press: Cambridge. pp 225–249. [Google Scholar]
- Arciniegas DB, Wortzel HS, Frey KL (2013). Rehabilitation and pharmacotherapy of cognitive impairments. In: Arciniegas DB, Anderson CA, Filley CM (eds). Behavioral Neurology and Neuropsychiatry. Cambridge University Press: Cambridge. pp 511–542. [Google Scholar]
- Barona A, Reynolds CR, Chastain R (1984). A demographically based index of pre-morbid intelligence for the WAIS-R. J Consult Clin Psychol 52: 885–887. [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996) Beck Depression Inventory-II (BDI-II). The Psychological Corporation: San Antonio, TX, USA. [Google Scholar]
- Berg I, Konning-Haanstra M, Deelman B (1991). Long term effects of memory rehabilitation. A controlled study. Neuropsychol Rehabil 1: 97–111. [Google Scholar]
- Berthier ML, Green C, Lara JP, Higueras C, Barbancho MA, Davila G et al (2009). Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Ann Neurol 65: 577–585. [DOI] [PubMed] [Google Scholar]
- Bragoni M, Altieri M, Di Piero V, Padovani A, Mostardini C, Lenzi GL (2000). Bromocriptine and speech therapy in non-fluent chronic aphasia after stroke. Neurol Sci 21: 19–22. [DOI] [PubMed] [Google Scholar]
- Brown FC, Roth RM, Saykin AJ, Beverly-Gibson G (2007). A new measure of visual location learning and memory: development and psychometric properties for the Brown Location Test (BLT). Clin Neuropsychol 21: 811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappa SF, Benke T, Clarke S, Rossi B, Stemmer B, van Heugten CM (2005). EFNS guidelines on cognitive rehabilitation: report of an EFNS task force. Eur J Neurol 12: 665–680. [DOI] [PubMed] [Google Scholar]
- Cicerone KD (2002). Remediation of ‘working attention’ in mild traumatic brain injury. Brain Inj 16: 185–195. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M et al (2011). Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil 92: 519–530. [DOI] [PubMed] [Google Scholar]
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y et al (1996). Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol Aging 17: 123–130. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH (2001) Delis-Kaplan Executive Function System. The Psychological Corporation: San Antonio, TX, USA. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA (2000). California Verbal Learning Test-Second Edition: Adult Version Manual. The Psychological Corporation: San Antonio, TX, USA.
- Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF et al (2007). Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology 16: 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Rocque MA, Furstenberg CT, Horrigan S, Ahles TA et al (2012). Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology 21: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, Mittenberg W (1996) Cognitive behavioral treatment of postconcussion syndrome. In: Van Hasselt VB, Herson M (eds). Sourcebook of Psychological Treatment Manuals for Adult Disorders. Plenum: New York, NY, USA. [Google Scholar]
- Gordon M, McClure FD, Aylward GP (1996) Gordon Diagnostic System: Instruction manual and interpretive guide. Gordon Systems, Inc.: DeWitt, NY, USA. [Google Scholar]
- Gronwall D (1977). Paced auditory serial addition task: a measure of recovery from concussion. Percept Mot Skills 44: 367–373. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Evans RW (1988). Stimulant treatment for the neurobehavioural sequelae of traumatic brain injury. Brain Inj 2: 273–290. [DOI] [PubMed] [Google Scholar]
- Huang CH, Huang CC, Sun CK, Lin GH, Hou WH (2016). Methylphenidate on cognitive improvement in patients with traumatic brain injury: a meta-analysis. Curr Neuropharmacol 14: 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensson M, Thomsen KR, Andersen LM, Gross J, Mouridsen K, Sandberg K et al (2015). Making sense: dopamine activates conscious self-monitoring through medial prefrontal cortex. Hum Brain Mapp 36: 1866–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Wentzel AP, Andréll P, Mannheimer C, Rönnbäck L (2015). Methylphenidate reduces mental fatigue and improves processing speed in persons suffered a traumatic brain injury. Brain Inj 29: 758–765. [DOI] [PubMed] [Google Scholar]
- Kaelin DL, Cifu DX, Matthies B (1996). Methylphenidate effect on attention deficit in the acutely brain-injured adult. Arch Phys Med Rehabil 77: 6–9. [DOI] [PubMed] [Google Scholar]
- Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K et al (1993). Definition of mild traumatic brain injury. J Head Trauma Rehabil 8: 86–87. [Google Scholar]
- Lafayette Instrument (1989) Grooved Pegboard: Instruction/Owner's Manual. Lafayette Instrument: Lafayette, IN, USA. [Google Scholar]
- McAllister TW, Zafonte R, Jain S, Flashman LA, George MS, Grant GA et al (2015). Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology 41: 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmese CA, Raskin SA (2000). The rehabilitation of attention in individuals with mild traumatic brain injury, using the APT-II programme. Brain Inj 14: 535–548. [DOI] [PubMed] [Google Scholar]
- Plenger PM, Dixon CE, Castillo RM, Frankowski RE, Yablon SA, Levin HS (1996). Subacute methylphenidate treatment for moderate to moderately severe traumatic brain injury: a preliminary double-blind placebo-controlled study. Arch Phys Med Rehabil 77: 536–540. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D (1993) The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation2nd edn. Neuropsychology Press: Tucson, AZ, USA. [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Shtasel DL, Flannery KA, Mozley LH et al (1995). Normative neuropsychological test performance: effects of age, education, gender and ethnicity. Appl Neuropsychol 2: 79–88. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A (1994). Development and validation of a multiple ability self-report questionnaire. J Clin Exp Neuropsychol 16: 93–104. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59: 22–33. [PubMed] [Google Scholar]
- Sohlberg MM, Avery J, Kennedy M, Ylvisaker M, Coelho C, Turkstra L et al (2003). Practice guidelines for direct attention training. J Med Speech Lang Pathol 11: xix–xxxix. [Google Scholar]
- Sohlberg MM, McLaughlin KA, Pavese A, Heidrich A, Posner MI (2000). Evaluation of attention process training and brain injury education in persons with acquired brain injury. J Clin Exp Neuropsychol 22: 656–676. [DOI] [PubMed] [Google Scholar]
- Spielberger CD (1983) State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc.: Palo Alto, CA. [Google Scholar]
- Tate RL (1997). Beyond one-bun, two-shoe: recent advances in the psychological rehabilitation of memory disorders after acquired brain injury. Brain Inj 11: 907–918. [DOI] [PubMed] [Google Scholar]
- The Psychological Corporation (1997) WAIS-III Wechsler Adult Intelligence Scale-Third Edition WMS-III Wechsler Memory Scale-Third Edition Updated Technical Manual. The Psychological Corporation: San Antonio, TX, USA.
- The Psychological Corporation (1999) Wechsler Abbreviated Scale of Intelligence (WASI) Manual. Harcourt-Brace & Company: San Antonio, TX, USA. [Google Scholar]
- Warden DL, Gordon B, McAllister TW, Silver JM, Barth JT, Bruns J et al (2006). Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma 23: 1468–1501. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM (1993). The PTSD checklist (PCL): reliability, validity, and diagnostic utility. Paper presented at the 9th Annual Convention of the International Society for Traumatic Stress Studies, San Antonio, TX..
- Westbrook A, Braver TS (2016). Dopamine does double duty in motivating cognitive effort. Neuron 89: 695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton P, Mathias JL, Vink R (2011). Impact of pharmacological treatments on cognitive and behavioral outcome in the postacute stages of adult traumatic brain injury: a meta-analysis. J Clin Psychopharmacol 31: 745–757. [DOI] [PubMed] [Google Scholar]
- Whyte J, Hart T, Schuster K, Fleming M, Polansky M, Coslett HB (1997). Effects of methylphenidate on attentional function after traumatic brain injury: a randomized, placebo-controlled trial. Am J Phys Med Rehabil 76: 440–450. [DOI] [PubMed] [Google Scholar]
- Whyte J, Hart T, Vaccaro M, Grieb-Neff P, Risser A, Polansky M et al (2004). Effects of methylphenidate on attention deficits after traumatic brain injury: a multidimensional, randomized, controlled trial. Am J Phys Med Rehabil 83: 401–420. [DOI] [PubMed] [Google Scholar]
- Whyte J, Vaccaro M, Grieg-Neff P, Hart T (2002). Psychostimulant use in the rehabilitation of individuals with traumatic brain injury. J Head Trauma Rehabil 17: 284–299. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ (2006) WRAT4 The Wide Range Achievement Test. Psychological Assessment Resources, Inc.: Lutz, FL, USA. [Google Scholar]
- Willmott C, Ponsford J (2009). Efficacy of methylphenidate in the rehabilitation of attention following traumatic brain injury: a randomised, crossover, double blind, placebo controlled inpatient trial. J Neurol Neurosurg Psychiatry 80: 552–557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.