Abstract
Background
Preclinical data suggest that early life stress has detrimental effects on the brain’s dopaminergic system, particularly the mesocorticolimbic pathway. Altered dopamine function is thought to contribute to the development of stress-related pathologies; yet, little is known about the impact of early stress on dopamine systems during childhood and adolescence, when stress-related disorders frequently emerge. Here, we evaluate the impact of early threat exposure (violence, abuse) on functional connectivity of putative dopaminergic midbrain regions, the ventral tegmental area (VTA) and substantia nigra (SN), giving rise to mesocorticolimbic and nigrostriatal pathways, respectively.
Methods
Resting-state functional magnetic resonance imaging scans were completed in 43 trauma-exposed and 43 matched comparison youth (ages 7–17). Functional connectivity of the VTA and SN were compared between groups.
Results
The trauma group demonstrated lower functional connectivity between the VTA and hippocampus. No group differences in SN connectivity were observed. Across all participants, there were age-related decreases in connectivity of both VTA and SN with the hippocampus, suggesting that age-related attenuations in VTA-hippocampal circuitry may be exacerbated in trauma-exposed youth. Higher levels of anxiety symptomology were associated with reduced SN–nucleus accumbens connectivity.
Conclusions
Prior research suggests that VTA-hippocampal circuitry is critical for the gating of new information into long-term memory. Lower connectivity in this circuitry suggests a novel mechanism that may serve to adaptively prevent the overwriting of a previously stored trauma memory, but at the same time contribute to the broad range of cognitive and emotional difficulties linked to early stress exposure.
Keywords: reward, fMRI, resting-state, early stress, dopamine, early adversity
INTRODUCTION
Exposure to stress early during development has detrimental effects on the brain’s dopaminergic system (1–3). For instance, animals exposed to early stress show reductions in dopamine release, metabolism, and receptor densities, as well as increased anhedonic-like behaviors (4–7). As such, blunted dopamine neuromodulation is implicated in the pathogenesis of anxiety and stress-related pathologies, including major depressive disorder and posttraumatic stress disorder (PTSD; 8).
Preclinical studies suggest that stress selectively impacts the mesocorticolimbic pathway (see 9), one of two major dopaminergic pathways in the brain. The mesocorticolimbic pathway projects from the midbrain ventral tegmental area (VTA) to hippocampus, amygdala, ventral striatum (including nucleus accumbens; NAcc), and prefrontal regions (10). The second major dopaminergic pathway, the nigrostriatal pathway, projects from an adjacent midbrain region – the substantia nigra (SN) – primarily to sensorimotor regions. Although VTA and SN share some overlapping projections, the nigrostriatal pathway appears to be relatively spared in the presence of stress (9).
While mesocorticolimbic dopamine is classically implicated in reward processing, current theory suggests that dopamine also modulates motivated behavior and learning-related processes more broadly (11–14). Human neuroimaging studies indicate that the VTA responds to both anticipated gains as well as losses (15), consistent with electrophysiological data in nonhuman primates (16, 17). Thus, dopamine in the mesocorticolimbic system is involved in the processing and learning of novel or motivationally salient (both aversive and appetitive) events. This broad role in motivational control has led to core theory that the mesocorticolimbic dopamine system is critical for adapting to ever-changing environmental conditions (18).
Importantly, mesocorticolimbic dopamine signaling also enhances memory consolidation in the hippocampus, allowing long-term storage of learned environmental contingencies (19). In this framework, dopaminergic projections from VTA to hippocampus regulate the entry of novel information into long-term memory. This allows past experiences to adaptively guide present behavior, and allows new information to gain access into long-term memory, thus updating stored memory. Entry of new information into memory is, however, regulated, to prevent overwriting of pre-existing information and to prevent encoding of all novel environmental information that could overwhelm memory capacity (20). Atypical elevations or attenuations within this circuitry could lead to either pathological overwriting or persistence of stored memory, respectively. Alterations in this key circuitry may therefore contribute to the broad range of cognitive and emotional disturbances associated with early stress exposure (by, for e.g., persistence of trauma-related memory).
Most human research on early stress to date has focused on mesolimbic target regions (amygdala, ventral striatum, and hippocampus) during threat or reward processing. Studies have found increased amygdala response to negative emotional stimuli (e.g., fearful facial expressions), and decreased ventral striatal response to positive and rewarding cues in children, adolescents, or adults with histories of early stress exposure (e.g., 21, 22–24). There is also evidence for reduced hippocampal activity and poorer (verbal) declarative memory retrieval in adolescents exposed to early stress (25), suggesting poorer integration of novel information into long-term memory. Importantly, regions assessed in these prior studies (amygdala, ventral striatum, hippocampus) are critical but downstream targets of dopamine neurons. The effects of early stress on connectivity of putative midbrain dopaminergic regions has yet to be tested in humans.
We report on a pediatric neuroimaging study that tested the impact of childhood trauma exposure on resting-state functional connectivity of the VTA, which gives rise to the mesocorticolimbic pathway. Childhood trauma is defined here as experiences of early threat (i.e., violence, abuse), a unique but central dimension of early adversity (26). We focused on threat here given the preponderance of threat experiences in this urban sample, and that no participants endorsed experiencing only neglect - which more closely relates to the deprivation dimension. Given structural and functional alterations are frequently documented in mesolimbic regions in trauma-exposed youth and core theory that stress-related pathologies (depression, PTSD) are characterized by downregulation of mesolimbic dopaminergic pathways (6), we predicted lower connectivity of the VTA with mesolimbic target regions (i.e., amygdala, ventral striatum, hippocampus) in trauma-exposed relative to matched comparison youth. Given preclinical data suggesting that stress impacts the mesocorticolimbic while sparing the nigrostriatal system (9), we will examine specificity of group related effects by examining SN functional connectivity and predicting non-significant differences. However, functional boundaries between SN and VTA may be less clear in humans and other primates (27) and trauma effects may not be specific to connectivity of the VTA.
MATERIALS AND METHODS
Participants
This study examined 86 children and adolescents, ages 7–17 (M = 11.89, SD = 2.50). Trauma-exposed (n = 43) and comparison (n = 43) groups were drawn from a larger youth cohort, and matched on age and sex. Both groups were recruited from an urban, low resource, minority community (Detroit-area) at increased risk for trauma (28) and associated psychopathology (29). Thus, a majority of study participants (55%) reported annual incomes less than $40,000, and 45% were African American (Table 1). Importantly, trauma and comparison groups were additionally matched on sociodemographic factors (see Table 1), allowing us to better disentangle the effects of trauma from chronic socioeconomic adversity (see 30). Recruitment of participants was accomplished via advertisements posted on the Wayne State University website, Craigslist (Detroit), printed flyers, or through Metro Detroit mental health clinics. Individuals exhibiting any of the following criteria were excluded: contraindication for magnetic resonance imaging (MRI), history of brain injury, neurological or movement disorders, or English as a second language. Written parental informed consent as well as child/adolescent assent were obtained prior to participation. The experimental design and all study procedures were approved by the Institutional Review Board of Wayne State University.
Table 1.
Participant information, by group.
| Variable | Trauma (n=43) | Comparison (n=43) | p-value |
|---|---|---|---|
| Age, mean (SD) | 12.28 (2.65) | 11.51 (2.29) | 0.153 |
| Sex (female), n (%) | 26 (60.47%) | 30 (69.77%) | 0.25 |
| IQ, mean (SD) | 97.57 (16.62) | 102.97 (17.17) | 0.86 |
| Puberty (Tanner Stage), mean (SD) | 3.33 (1.34) | 2.8 (1.46) | 0.09a |
| Annual income, n (%) | 0.052 | ||
| <$40,000 | 27 (62.8%) | 20 (46.5%) | |
| $40–60,000 | 10 (23.3%) | 10 (23.3%) | |
| $60–80,000 | 1 (2.3%) | 6 (14.0%) | |
| $80–100,000 | 1 (2.3%) | 1 (2.3%) | |
| >$100,000 | 2 (4.7%) | 5 (11.6%) | |
| Not Reported | 2(4.7%) | 1 (2.3%) | |
| Race/ethnicity, n (%) | 0.085 | ||
| African American | 20 (46.51%) | 19 (44.19%) | |
| Caucasian | 13 (30.2%) | 16 (37.2%) | |
| Hispanic | 2 (4.7%) | 1 (2.3%) | |
| Other | 0 | 1 (2.3%) | |
| Biracial | 4 (9.3%) | 3 (7.0%) | |
| Not Reported | 4 (9.3%) | 3 (7.0%) | |
| Parental Marital Status, n (%) | 0.102 | ||
| Unpartnered | 26 (60.5%) | 19 (44.2%) | |
| Partnered | 16 (37.2%) | 24 (55.8%) | |
| Not Reported | 1 (2.3%) | 0 | |
| Trauma Type Endorsed, n (%) | |||
| Exposure to domestic violence | 35 (81%) | ||
| Exposure to other violence | 15 (35%) | ||
| Sexual abuse | 10 (23%) | ||
| Neglect | 11 (26%) | ||
| Physical abuse | 4 (9%) | ||
| Emotional abuse | 1 (2%) | ||
| Movement | |||
| Max FD | 5.09 (7.91) | 3.23 (4.51) | 0.18 |
| Mean FD | 0.54 (0.69) | 0.39 (0.60) | 0.28 |
| Anxiety (SCR) | 23.23 (17.78) | 18.66 (15.10) | 0.23 |
| Depressive Symptoms (CDI) | 3.26 (3.47) | 2.95 (3.51) | 0.69 |
| Reward Sensitivity (BIS/BAS) | 26.08 (4.00) | 25.92 (4.69) | 0.87 |
Abbreviations: FD, framewise displacement; IQ, intelligence quotient; SCR, Screen for Anxiety Related Emotional Disorders; CDI, Children’s Depression; BIS/BAS, Behavioral Inhibition and Activation Scales.
Demographics and clinical measures
IQ was estimated using the Kaufman Brief Intelligence Test (KBIT 2; 31). Two participants (one trauma-exposed, one comparison) scored <70 on the KBIT; results excluding these two participants were consistent with findings reported here. Puberty was evaluated via self-reported Tanner staging (32). Three self-reported symptom dimensions were estimated: anxiety (Screen for Child Anxiety-Related Emotional Disorders, SCR; 33), depression (Children’s Depression Inventory - short form, CDI; 34) and reward sensitivity (Behavioral Inhibition and Activation Scales, BIS/BAS; 35). Demographic and clinical measures are presented in Table 1, by group. Statistical analyses were two-tailed and effects were considered significant at p < 0.05. Analysis was implemented in IBM Statistical Package for the Social Sciences Statistics v.21 (SPSS).
Trauma exposure
Based on parent and/or child endorsements, participants who experienced at least one trauma indicated on the Children’s Trauma Assessment Center Screen Checklist (36) were classified as “trauma-exposed” (see Table 1). Three checklist items were excluded to narrow trauma type to deprivation (e.g., neglect) and threats to safety (e.g., abuse, violence exposure), distinct, but central trauma types (26). Removed items were: (i) exposure to drug activity, (ii) parental/caregiver drug use/substance abuse, and (iii) frequent and multiple moves or homelessness. Notably, 26% of participants in the trauma group reported experiencing neglect, but none reported experiencing neglect only.
Seed-based functional connectivity analysis
See Supplemental Material for information on magnetic resonance imaging (MRI) data acquisition, processing, and mitigation of potential motion-related artifact. Bilateral VTA and SN seed regions were defined via probabilistic anatomical masks, drawn from an average of 50 healthy adults (37). Prior studies using similar methodology and conventional imaging parameters demonstrate distinct but overlapping patterns of VTA and SN resting-state connectivity in adults (37) as well as children as young as 7 (38). Functional connectivity of VTA and SN was computed using the CONN toolbox (v. 15.h; https://www.nitrc.org/projects/conn/). Semi-partial correlation was used to compute unique functional connectivity of each seed region, controlling for contributions of the other.
Statistical analysis
To establish the basic network topology and compare this to prior research, we first performed one-sample and paired-sample t-tests to evaluate connectivity of VTA, SN, and VTA vs. SN across the sample. We expected to see distinct but overlapping patterns of connectivity that resemble prior neuroimaging studies (e.g., 37, 38) as well as known neuroanatomical projections (10). Our main analysis evaluated connectivity of VTA and of SN between groups, using two-sample t-tests. We also explored age-related change and age x trauma interactions in connectivity of VTA and SN across the sample, using regression analyses. All group-level analyses were performed in SPM8. Given our a priori interest in effects of trauma on mesolimbic connectivity, we evaluated bilateral amygdala, hippocampus, and NAcc regions of interest (ROIs). ROIs were defined using the Harvard-Oxford atlas distributed with FSL software (http://www.fmrib.ox.ac.uk/fsl/), and a small-volume family-wise error corrected threshold of pFWE < 0.05 was applied. A complementary whole brain threshold was also applied, using a combined voxelwise and cluster-level threshold of p < 0.001 and cluster extent > 79 voxels, as determined by AFNI’s 3dFWHMx and 3dClustSim (https://afni.nimh.nih.gov). The spatial autocorrelation of data and non-Gaussian nature of the fMRI signal were accounted for when computing this threshold.
Relation to clinical measures
For regions showing significant group differences (trauma vs. comparison), we evaluated possible associations between functional connectivity in these regions and clinical measures (anxiety, depressive symptoms, reward sensitivity) using Pearson bivariate correlation. Neural responses were extracted for each participant at peak regions of significant group difference using 6 mm radii spheres, and submitted to IBM SPSS software for correlation analyses. In addition, we evaluated possible effects of clinical measures on connectivity of VTA and SN, using whole brain regression analyses in SPM8. Results were considered significant at the corrected whole brain threshold.
RESULTS
Demographics and clinical measures
Trauma and comparison groups were matched on age, sex, IQ, race, income, parental marital status, and motion during the scan (see Table 1). They also did not differ on self-reported anxiety, depressive symptoms, or reward sensitivity. Notably, 56% participants in the trauma group endorsed experiencing more than one type of trauma. This is consistent with prior reports (e.g., 39). Given the high co-occurrence of trauma types and that no participants in the sample endorsed exposure to deprivation only, we focused on effects of threat exposure rather than dissociating effects of threat and deprivation. Follow-up analyses included neglect as a covariate of no interest.
VTA vs. SN connectivity across the sample
One sample t-tests across the sample showed substantial overlap in connectivity maps for VTA and SN, across widespread subcortical, cortical, and brainstem regions (e.g., thalamus, temporal lobe, cerebellum, basal ganglia, insula; see Figure S1 and Table S1). Paired sample t-test (VTA vs. SN) revealed preferential connectivity of the VTA with hippocampus, caudate, and orbitofrontal cortex, and preferential connectivity of the SN with supplemental motor area, thalamus, and basal ganglia (Figure 1 and Table S1). Overall, this pattern is consistent with previous resting-state functional connectivity studies in adults and children (37, 38).
Figure 1. Connectivity of ventral tegmental area (VTA) vs. substantia nigra (SN) across the sample.
Left panel displays location of VTA and SN seed regions. Right panel shows regions more connected to VTA than SN (red) and vice versa (blue). Results of the paired sample t-test are shown here at p < 0.005, 10 voxel threshold for display purposes. See Table S1 for summary of results surviving whole brain correction.
Group differences in VTA connectivity
ROI analyses demonstrated lower VTA connectivity with the right hippocampus in trauma-exposed relative to comparison youth (247 voxels; x = 34, y = −22, z = −16; Z = 4.23, pFWE = 0.013; see Figure 2). Probabilistic cytoarchitectonic maps (40) estimated that this hippocampal peak was centered in the DG (dentate gyrus; 40% probability), CA1 (cornus ammonis; 30%), or CA3 (17%). We observed a similar effect for left hippocampus, but it did not reach significance (84 voxels; x = −30, y = −22, z = −18; Z = 3.86, pFWE = 0.05). No group differences were observed for VTA connectivity with amygdala or NAcc. The effect on VTA-hippocampal connectivity was also significant at the corrected whole brain threshold, remained significant when additionally controlling for puberty, income, neglect, and was not related to movement across the scan (mean or maximum FD). Given groups did not differ on self-reported symptomology, not surprisingly, hippocampal-VTA connectivity was not associated with anxiety, depression symptoms, or reward sensitivity either across the sample or within groups (p’s > 0.1). Whole brain analyses indicated no other areas showing between-group differences in VTA connectivity.
Figure 2. Reduced functional connectivity between ventral tegmental area (VTA) and hippocampus in trauma-exposed relative to comparison children and adolescents.
Left panel displays location of VTA seed region. Results of the two sample t-test (trauma vs. comparison) are shown here at p < 0.005, 10 voxel threshold for display purposes, and significant using small-volume family-wise error correction as well as at the corrected whole brain threshold.
Group differences in SN connectivity
In contrast to findings for VTA connectivity, ROI analyses demonstrated increased SN connectivity with the right hippocampus in trauma-exposed relative to comparison youth (212 voxels; x = 34, y = −26, z = −16; Z = 3.93, pFWE = 0.039). However, this effect no longer reached significance when additionally controlling for income (pFWE = 0.069). There was also an effect of increased SN connectivity with right amygdala in the trauma relative to comparison group, but this effect did not reach significance (46 voxels; x = 18, y = 2, z = −14; Z = 3.61, pFWE = 0.056). There were no group differences in SN connectivity at the corrected whole brain threshold.
Effects of age on VTA and SN connectivity
Effects of age on connectivity of VTA and SN across all participants are shown in Figure 3. Notably, age was negatively associated with VTA-hippocampal (2 hippocampal clusters: 1: 543 voxels; x = 38, y = −32, z = −12; Z = 4.66, pFWE = 0.002; 2: 580 voxels; x = −34, y = −24, z = −18; Z = 4.2, pFWE = 0.015) as well as SN-hippocampal connectivity (2 hippocampal clusters: 1: 663 voxels; x = −30, y = −30, z = −12; Z = 4.63, pFWE = 0.003; 2: 479 voxels; x = −28, y = −28, z = −14; Z = 4.48, pFWE = 0.005) in ROI analyses and at the corrected whole brain threshold (see Table S2). These effects remained significant when additionally controlling for trauma exposure. Age was negatively associated with VTA-amygdala (2 amygdalar clusters: 1: 144 voxels; x = −34, y = −4, z = −22; Z = 3.17, pFWE = 0.008; 2: 115 voxels; x = 28, y = −10, z = −22; Z = 3.79, pFWE = 0.032) and SN-amygdala (139 voxels; x = −34, y = −4, z = −18; Z = 3.61, pFWE = 0.059) connectivity, though the latter did not reach significance. Whole brain analyses showed that midbrain and inferior parietal lobe were other areas of significant age-related change (see Table S2 for summary).
Figure 3. Age-related change in connectivity of ventral tegmental area (VTA; top) and substantia nigra (SN; bottom) midbrain regions across the sample.
Positive correlations are shown in red; negative correlations are shown in blue. Results of age regressions are shown here at a p < 0.005, 10 voxel threshold for display purposes. Circles designate areas of significant age-related change. Region of interest (ROI) analyses indicated that age was negatively associated with VTA-hippocampal, SN-hippocampal, and VTA-amygdalar connectivity. Whole brain analyses showed that age was negatively associated with VTA and SN connectivity with inferior parietal lobe, and with SN connectivity with midbrain and cerebellum.
Age x trauma interactions in VTA and SN connectivity
Given that trauma was associated with decreased VTA-hippocampal and increased SN-hippocampal connectivity, we tested for age x trauma interactions. No ROIs showed significant age x trauma effects for SN or VTA connectivity. Whole brain analyses indicated significant age x trauma effect for VTA connectivity with precuneus (Brodmann Area 7; 103 voxels; x = 8, y = −62, z = 66; Z = 4.03). No other regions were significant at the corrected whole brain threshold.
Effects of clinical measures on VTA and SN connectivity
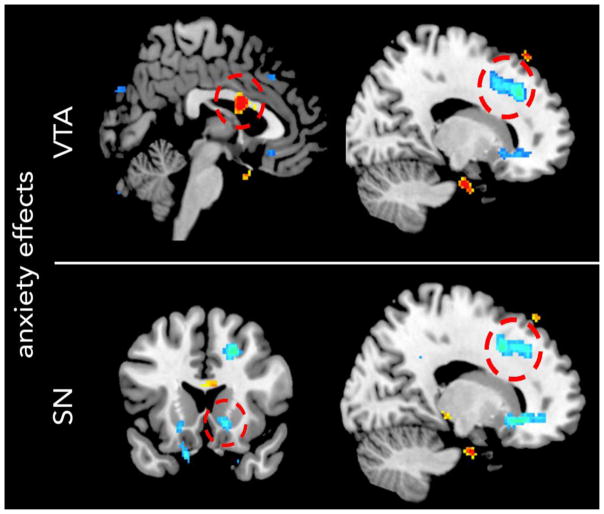
ROI analyses showed lower SN-NAcc connectivity in more anxious youth, (82 voxels; x = 16, y = 24, z = −8; Z = 3.59, pFWE = 0.026; Figure 4). SN-NAcc connectivity was not related to trauma exposure, p = 0.5. No other ROIs showed effects of clinical measures (RS, anxiety or depressive symptoms). At the corrected whole brain threshold, we observed increased VTA connectivity with middle cingulate cortex, and reduced VTA and SN connectivity with dorsomedial prefrontal cortex in more anxious youth (see Figure 4 and Table S3). In addition, we observed increased SN connectivity with occipital lobe in youth with higher reward sensitivity (Table S3).
Figure 4. Effects of anxiety symptoms on connectivity of ventral tegmental area (VTA; top) and substantia nigra (SN; bottom) midbrain regions across the sample.
Positive correlations are shown in red; negative correlations are shown in blue. Results of symptom regressions are shown here at a p < 0.005, 10 voxel threshold for display purposes. Circles designate areas of significant anxiety-related change. Region of interest (ROI) analyses indicated that anxiety was negatively associated with SN connectivity with nucleus accumbens. Whole brain analyses showed that anxiety was positively associated with VTA connectivity with middle cingulate cortex, and negatively associated with VTA and SN connectivity with dorsomedial prefrontal cortex.
DISCUSSION
Here, we evaluated effects of early threat exposure (i.e., violence, abuse) on functional connectivity of two putative dopaminergic midbrain regions, VTA and SN, which give rise to mesocorticolimbic and nigrostriatal pathways, respectively. In line with prior resting-state functional connectivity studies (37, 38), we observed distinct but overlapping patterns of connectivity in VTA and SN, with VTA more strongly connected with hippocampus and orbitofrontal cortex, and SN more strongly connected with sensorimotor regions. Consistent with our hypothesis, we found lower connectivity within the mesolimbic pathway in trauma-exposed relative to matched comparison youth. In particular, we observed reduced resting-state connectivity in the trauma group between the VTA and hippocampus, a circuitry critical for novelty detection and the modulation of memory by behavioral significance (20). A blunting within VTA-hippocampal circuitry may underlie the broad range of cognitive, emotional, learning, and memory impairments linked to childhood trauma exposure.
The VTA-hippocampal loop is thought to control the entry of new information into long-term memory, thereby protecting previously stored information (see 20). The ‘downward’ arc of the loop carries novelty signals from the hippocampus to the VTA, which, in turn, activates novelty-dependent firing of VTA dopaminergic neurons (41). The ‘upward’ arm of the loop is characterized by direct dopaminergic projections from the VTA to the hippocampus (42, 43) that enhance long-term potentiation (LTP) and long-term memory (19). There are also indirect projections from VTA to hippocampus through the ventral striatum that are thought to provide information on behavioral and motivational relevance of novel information (44). VTA-hippocampal circuitry may also be critical for contextual modulation of aversive/appetitive learning, and in fear extinction learning (45). Together, these connections are thought to be essential for the modulation of memory by novel information, allowing for experience-dependent modification of old memories.
Although our data cannot resolve whether it is the upward and/or downward arc of the loop that is altered in trauma-exposed youth, they do indicate blunted coordination within this key circuitry. Attenuation of the VTA-hippocampal loop in youth who have experienced trauma might reflect the restricted entry of new information into long-term memory. This may relate to a previously hypothesized disrupted mesolimbic circuit involving a hypo-dopaminergic state in trauma-exposed individuals that prevents the extinction of trauma memories in those who do not return to baseline (see 46). Attenuation of the VTA-hippocampal loop may explain the persistence of fear- and trauma-related memory (as new information regarding safety is less likely to be appended into memory), as well as enduring difficulties in learning new information in trauma-exposed individuals (see 47). As this circuitry regulates motivational learning and memory for aversive as well as rewarding stimuli, a dampening in this circuitry may underlie the broad range of reward- and threat-related alterations previously reported in individuals exposed to early stress (for a review, see 30). Here, we found that increasing age was also associated with lower VTA-hippocampal connectivity, suggesting that trauma exposure may exacerbate age-related attenuations within this circuitry. While we did not observe a significant age x trauma interaction on VTA-hippocampal connectivity in this developmental sample, the negative effect of trauma exposure on VTA-hippocampal connectivity may be more apparent in older youth who may already have relatively attenuated VTA-hippocampal connectivity, or for trauma-exposed youth who continue to show low connectivity over time. Nonetheless, these findings are broadly consistent with contemporary neurobiological theories suggesting that early stress alters development of limbic circuitry, which may have detrimental consequences later in life (see 48 for a review). If attenuation of VTA-hippocampal connectivity does indeed reflect increased gating of previously stored memory, then memory may be more resistant to modification and trauma interventions with increasing age.
Given groups did not differ on self-reported symptomology, not surprisingly, hippocampal-VTA connectivity was not associated with anxiety, depression symptoms, or reward sensitivity either across the sample or within groups. Future research should test whether this neural pattern or a lack of recovery over time predicts later emergence of psychopathology, or if it relates to more proximal processes or behavioral phenotypes that may serve to increase risk in some individuals. For example, attenuations in learning and memory circuitry may relate to more vivid, frequent and uncontrollable memories of a traumatic event and lead to behavioral disruption (e.g., avoidance, hyperarousal). It may also relate to poor recall of extinction learning, which is a core component of cognitive-behavioral therapy (CBT) - the primary behavioral intervention for trauma-exposed youth. ROI analyses did demonstrate, however, reduced SN-NAcc connectivity in more anxious youth. This is surprising given that the mesolimbic pathway is more typically implicated in affective and motivational processes. However, the nigrostriatal pathway may contribute to affective behavior by performing motor activities to approach or avoid stimuli with known affective value (11).
In line with a prior pediatric study (38), we observed regionally-specific age-related changes in connectivity of dopamine target regions. Results showed age-related reductions in VTA and SN connectivity with hippocampus, and age-related decreases in VTA connectivity with amygdala. Age-related changes were not observed for NAcc. An age-related decrease in VTA-amygdala connectivity is in contrast to a prior pediatric study, which found increased VTA-amygdala connectivity in adults relative to typically developing children and adolescents (ages 12 ± 3; 38). Thus, midbrain pathways may show continued reorganization into at least young adulthood.
While this study has a number of strengths including relatively large sample size, sociodemographic matching of trauma and comparison groups, and evaluation of a high-risk urban cohort, there are limitations. First, functional connectivity analyses cannot determine directionality (i.e., hippocampus→VTA or vice versa) or rule out the possibility of indirect pathways contributing to observed changes in connectivity (i.e., through a third region, e.g., ventral striatum). Second, as noted, group differences in connectivity were examined during rest. Thus, it remains to be tested how this circuitry operates during the processing and encoding of new information and importantly how this relates to behavior. Our results are, however, fitting with prior reports of reduced hippocampal activity, which is associated with poorer declarative memory retrieval in trauma-exposed adolescents (25). Third, we did not dissociate threat and deprivation here, despite recent conceptual framework asserting that threat and deprivation have distinct effects of neural development (26). We focused on threat and did not dissociate these here, given (1) their high co-occurrence, and (2) that no participants endorsed experiencing only neglect. Although group differences in VTA-hippocampal connectivity remained significant when neglect was added as a nuisance covariate, future studies including more discrete experiences of neglect (e.g., institutionalization; 21) should be better able to dissociate these. Future studies may also evaluate how the developmental timing (49), duration (17), and temporal proximity of the exposure to scanning influences connectivity of this circuit. For example, traumatic stress may transiently increase dopamine activity, which may enhance consolidation of the trauma memory (46). This transient increase may be followed by a more sustained hypo-dopaminergic state that fails to recover in some individuals, which may prevent successful extinction of trauma-related fear – a process that requires new learning (50).
Conclusions
Our results suggest a novel neurobiological mechanism of childhood trauma wherein new information is less able to gain access into long-term memory. While this framework could explain the broad range of cognitive and emotional alterations observed in trauma-exposed youth, VTA-hippocampal circuitry has yet to be tested in the context of a learning and memory paradigm. It will be important to evaluate whether learning signal in VTA is dulled and/or the transfer of new information into storage via the hippocampus. Moreover, it will be important to identify whether effects of trauma are specific to signaling related to certain types of information (e.g., cues vs. contexts), motivational relevance (e.g., value, salience, changes in the environment), or of novelty (e.g., stimulus novelty, associative novelty) – all of which have been linked to dopamine signaling (11, 19, 51). Results could have exciting new implications for interventions. For example, cognitive remediation or trauma therapies could be coupled with interventions that boost dopamine signaling, thus increasing the brain’s novelty signal associated with new information and/or increase information retention by enhancing access into long term memory. There is already evidence to support this possibility; for example, pharmacological enhancement of dopamine signaling in healthy adults and adults with PTSD (via, for e.g., L-DOPA administration) promotes fear extinction and may reduce the return of fear (see review by 52). Perhaps most relevant for pediatric populations, behavioral interventions that boost dopamine transmission might be beneficial. For example, exploration of a novel environment increases dopamine signaling in VTA-hippocampal circuitry and enhances learning to subsequent events in rodents (53).
Supplementary Material
Acknowledgments
The authors thank Yashwanth Katkuri, Pavan Jella, and Zahid Latif of Wayne State University (WSU) for their assistance in neuroimaging data acquisition, Laura Crespo, Kelsey Sala-Hamrick, Farrah Elrahal, Clara Zundel, Shelley Paulisin, Sajah Fakhoury, Allesandra Iadipaolo, Craig Peters, Farah Sheikh, Brian Silverstein, Suzanne Brown, and Caitlin Waters of WSU for assistance in participant recruitment and data collection, and thank the children and their families who generously shared their time.
Research reported in this publication was supported, in part, by the Merrill Palmer Skillman Institute, Department of Pediatrics and Department of Pharmacy Practice of Wayne State University (WSU), NIH National Institute of Environmental Health Sciences awards P30 ES020957 and R21 ES026022 (MET), NIH National Institute of Mental Health award R01 MH110793 (MET), and a NARSAD Young Investigator Award (MET). Dr. Marusak is supported by American Cancer Society award 129368-PF-16-057-01-PCSM. Dr. Rabinak is supported by National Institute of Mental Health grant K01 MH101123.
Footnotes
FINANCIAL DISLOCUSRES
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- 2.Puglisi-Allegra S, Kempf E, Cabib S. Role of genotype in the adaptation of the brain dopamine system to stress. Neuroscience and biobehavioral reviews. 1990;14:523–528. doi: 10.1016/s0149-7634(05)80078-8. [DOI] [PubMed] [Google Scholar]
- 3.Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Molecular psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- 4.Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. The European journal of neuroscience. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- 5.Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, et al. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacology, biochemistry, and behavior. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 6.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual review of clinical psychology. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neuroscience and biobehavioral reviews. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 8.Bogdan R, Nikolova YS, Pizzagalli DA. Neurogenetics of depression: a focus on reward processing and stress sensitivity. Neurobiology of disease. 2013;52:12–23. doi: 10.1016/j.nbd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabib S, Puglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology. 1996;128:331–342. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- 10.Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Critical reviews in neurobiology. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- 11.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neuroscience and biobehavioral reviews. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- 13.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain research Brain research reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 14.Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends in neurosciences. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- 15.Carter RM, Macinnes JJ, Huettel SA, Adcock RA. Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Frontiers in behavioral neuroscience. 2009;3:21. doi: 10.3389/neuro.08.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holly EN, Miczek KA. Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology. 2016;233:163–186. doi: 10.1007/s00213-015-4151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wise RA. Dopamine, learning and motivation. Nature reviews Neuroscience. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 19.Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends in neurosciences. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental science. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015;40:1250–1258. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, et al. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanson JL, Hariri AR, Williamson DE. Blunted Ventral Striatum Development in Adolescence Reflects Emotional Neglect and Predicts Depressive Symptoms. Biological psychiatry. 2015;78:598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrión VG, Haas BW, Garrett A, Song S, Reiss AL. Reduced Hippocampal Activity in Youth with Posttraumatic Stress Symptoms: An fMRI Study. Journal of Pediatric Psychology. 2010;35:559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience and biobehavioral reviews. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Düzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott BH, Tobler PN. Functional imaging of the human dopaminergic midbrain. Trends in neurosciences. 2009;32:321–328. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. General hospital psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowe SR, Galea S, Uddin M, Koenen KC. Trajectories of posttraumatic stress among urban residents. American journal of community psychology. 2014;53:159–172. doi: 10.1007/s10464-014-9634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomason ME, Marusak HA. Toward understanding the impact of trauma on the early developing human brain. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test: KBIT 2; Manual. Pearson; 2004. [Google Scholar]
- 32.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annual review of medicine. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 33.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs M. Children’s Depression Inventory. Multi-Health Systems, Incorporated; 1992. [Google Scholar]
- 35.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. (behavioral inhibition system; behavioral activation system) Journal of personality and social psychology. 1994:319. [Google Scholar]
- 36.Marusak HA, Etkin A, Thomason ME. Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. NeuroImage Clinical. 2015;8:516–525. doi: 10.1016/j.nicl.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA. Resting state networks distinguish human ventral tegmental area from substantia nigra. NeuroImage. 2014;100:580–589. doi: 10.1016/j.neuroimage.2014.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cerebral cortex. 2014;24:935–944. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British journal of psychiatry: the journal of mental science. 2010;197:378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 41.Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. Journal of neurophysiology. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- 42.Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain research bulletin. 1994;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 43.Samson Y, Wu JJ, Friedman AH, Davis JN. Catecholaminergic innervation of the hippocampus in the cynomolgus monkey. The Journal of comparative neurology. 1990;298:250–263. doi: 10.1002/cne.902980209. [DOI] [PubMed] [Google Scholar]
- 44.Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 45.Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: a convergence of theory with fear and reward circuitry. Neurobiol Learn Mem. 2014;108:65–77. doi: 10.1016/j.nlm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corral-Frias NS, Lahood RP, Edelman-Vogelsang KE, French ED, Fellous JM. Involvement of the ventral tegmental area in a rodent model of post-traumatic stress disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:350–363. doi: 10.1038/npp.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, et al. Mindfulness: A Proposed Operational Definition. Clinical Psychology: Science and Practice. 2004;11:230–241. [Google Scholar]
- 48.Callaghan BL, Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in human neuroscience. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 51.Kafkas A, Montaldi D. Striatal and midbrain connectivity with the hippocampus selectively boosts memory for contextual novelty. Hippocampus. 2015;25:1262–1273. doi: 10.1002/hipo.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menezes J, Alves N, Borges S, Roehrs R, de Carvalho Myskiw J, Furini CR, et al. Facilitation of fear extinction by novelty depends on dopamine acting on D1-subtype dopamine receptors in hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E1652–1658. doi: 10.1073/pnas.1502295112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.