Abstract
MxA is an antiviral protein induced by interferon (IFN)-α/β that is known to inhibit the replication of many RNA viruses. In these experiments, the 76-kDa MxA protein expressed in IFN-α-treated cells was shown to have antiviral activity against herpes simplex virus-1 (HSV-1), a human DNA virus. However, MxA was expressed as a 56-kDa protein in HSV-1-infected cells in the absence of IFN-α. This previously unrecognized MxA isoform was produced from an alternatively spliced MxA transcript that had a deletion of Exons 14–16 and a frame shift altering the C-terminus. The variant MxA (varMxA) isoform was associated with HSV-1 regulatory proteins and virions in nuclear replication compartments. varMxA expression enhanced HSV-1 infection as shown by a reduction in infectious virus titers from cells in which MxA had been inhibited by RNA interference and by an increase in HSV-1 titers when the 56-kDa varMxA was expressed constitutively. Thus, the human MxA gene encodes two MxA isoforms, which are expressed differentially depending on whether the stimulus is IFN-α or HSV-1. These findings show that alternative splicing of cellular mRNA can result in expression of a novel isoform of a host defense gene that supports instead of restricting viral infection.
Keywords: herpes simplex virus-1, IFN-α/β, immune evasion, mRNA alternative splicing
Mammalian Mx proteins are dynamin-like GTPases that are induced by interferon (IFN)-α/β and have antiviral activity against RNA viruses.1 The human MxA gene consists of 17 exons representing a 33-kb sequence on chromosome 21.2 When human cells are treated with IFN-α, MxA is expressed from an ~2700 bp mRNA as an ~76 kDa protein that oligomerizes and persists in the cytoplasm.3 MxA has a GTPase domain, a central interactive domain (CID) and a leucine zipper motif (Figure 1a).1 The induction of the Mx gene as an antiviral defense of mammalian cells depends on JAK/Stat signaling and is not triggered directly by RNA viruses or double-stranded RNA.4,5
Figure 1.
Inhibition of HSV-1 infection by MxA. (a) Schematic of MxA induced by IFN-α treatment. The 76-kDa MxA protein is expressed from Exons 5–17 and is organized in an N-terminal GTPase domain (residues 1–371) and a C-terminal region that can be divided into a central interactive domain (CID) (residues 372–540) and a leucine zipper domain (LZ) (residues 564–662). Folding back of the LZ region on the CID assembles the monomeric MxA into large oligomeric complexes and increases GTPase activity.1 (b) Effect of MxA on HSV-1 infection. Human fibroblasts that expressed full-length MxA were infected with HSV-1 and virus yields were evaluated by plaque assay at 24 h after infection. Constitutive expression of MxA reduced HSV-1 yields as shown; each bar represents the mean±s.e. of duplicate or triplicate titrations from two independent wells (two-tailed Student’s t-test, P<0.01).
Inhibition of RNA viruses by IFN-induced human MxA has been associated with copolymerization and sequestration of viral nucleoproteins in perinuclear complexes, which prevents genome amplification, as is observed in cells infected with LaCrosse virus and also with blocking nuclear import of capsids, as shown in studies of Thogoto virus.6,7 Mx1 is the murine protein that corresponds to human MxA, although Mx1 has a nuclear localization signal not present in MxA.3,8 Both human MxA and Mx1 interact with subunits of the influenza virus polymerase and nucleocapsid proteins to block viral genome transcription.9 Although human MxA is expressed exclusively in the cytoplasm of IFN-treated cells, Mx1 accumulates in nuclear bodies that express promyelocytic leukemia protein (PML) and associates with other ND10 components.10 How this nuclear expression of murine Mx1 contributes to restricting the infectious cycle of RNA viruses in this host is not known.11,12 In a recent report, African swine fever virus, which is a large double-stranded DNA virus, generated fewer progeny virions in MxA-expressing cells and MxA protein was recruited to the perinuclear virion assembly site in infected cells.13 Whether MxA is a component of innate immunity against herpes-viruses has not been established.
Herpes simplex virus-1 (HSV-1) is an ubiquitous human alpha-herpesvirus that infects mucosal epithelial cells and causes mucocutaneous lesions.14 On cell entry, viral genomic DNA and the virion tegument proteins that act as immediate early transactivators of herpesviral genes are transferred into the nucleus to initiate viral gene transcription, genome replication and capsid formation; assembly is completed by capsid envelopment in the cytoplasm. Innate cellular responses create a potent barrier against the subsequent spread of herpesviruses in the infected host. HSV-1 has been shown to have multifaceted strategies to modulate these innate antiviral defenses, many of which target IFN-regulated responses.15–18 The importance of IFN signaling in restricting the pathogenesis of HSV-1 infection in the infected host in vivo is demonstrated by the enhanced virulence that is observed when the IFN-α/β receptor is blocked and in IFN receptor knockout mice.16,19–21
Here, we report that MxA induced by IFN-α inhibits HSV-1. Further, we show that the antiviral activity of MxA is avoided in HSV-1-infected cells through a novel immune evasion mechanism involving the expression of an alternatively spliced transcript of the host cell gene. Finally, synthesis of variant MxA (varMxA) isoform, which undergoes nuclear translocation in infected cells, favors instead of interfering with HSV-1 replication.
RESULTS
HSV-1 replication is reduced by MxA
MxA protein is expressed from a spliced transcript of the human MxA gene in cells that are treated with IFN-α.1 The largest MxA mRNA found in IFN-α-treated cells corresponds to an open reading frame that extends from a translation start site in Exon 5 to a stop codon in Exon 172 (Figure 1a). In these experiments, the effect of MxA on the replication of HSV-1 was examined in MxA-expressing fibroblasts, which were infected with a recombinant retrovirus and selected for stable expression of full-length MxA. To assess whether HSV-1 titer was reduced in the presence of MxA, MxA-expressing cells or the control fibroblasts were inoculated with HSV-1 at a multiplicity of infection of 0.1 and 1; supernatants were collected from the monolayers 24 h after inoculation and HSV-1 titers were measured by plaque assay. An 11–12-fold reduction in HSV-1 titer was observed at multiplicities of infection of 0.1 and 1.0, with P-values of 0.005 and 0.001 (Student’s t-test), respectively, compared with viral titers in supernatants from infected control cells (Figure 1b). HSV-1 titers were also decreased in AD293 cells, which were transiently transfected with a plasmid containing full-length MxA cDNA (Supplementary Figure S1).
MxA is expressed in HSV-1-infected cells and exhibits altered intracellular localization compared with cells treated with IFN-α
As expected, MxA was expressed abundantly when primary human fibroblasts were treated with IFN-α and localized to the cytoplasm only, when detected with a murine monoclonal antibody against an epitope in the N-terminal GTPase domain of the 76-kDa MxA protein22 (Figure 2a).
Figure 2.
MxA expression and cellular localization in human fibroblasts after treatment with IFN-α, infection with HSV-1 or inoculation with inactivated HSV-1. (a) IFN-α treatment or HSV-1 infection. Human fibroblasts were treated with IFN-α (10 000 unit ml−1), mock or HSV-1 infected with HSV-1 (0.1 PFU per cell). After 12 and 24 h, cells were stained with M143 anti-MxA mAb (green) and antibody to the HSV-1 proteins, ICP8 (red) and analyzed by confocal microscopy (magnification ×400). MxA was detected with M143 mAb in nuclei that expressed HSV-1 ICP8 protein. (b) MxA protein was not detected in fibroblasts inoculated with UV-inactivated HSV-1 (1.5×108 PFU per ml) but was expressed in cells infected with the live HSV-1 at 1.0 PFU per cell. (c) Protein lysates from mock, HSV-1-infected and IFN-α-treated fibroblasts were separated into cytoplasmic (C) and nuclear (N) fractions; immunoblots were performed with polyclonal rabbit antibody to MxA protein and anti-α-tubulin antibody. MxA was not detected in mock-treated cells; MxA detected after IFN-α treatment was a 76-kDa band in the cytoplasmic fraction and MxA in HSV-1-infected cells was a smaller, ~56 kDa band in cytoplasmic and nuclear fractions. Shown is one representative experiment of five performed.
However, MxA was also detected using the same antibody in fibroblasts that were infected with HSV-1 in the absence of IFN-α treatment at 12 h and at 24 h after infection (Figure 2a). HSV-1-infected cells were identified by the expression of the viral protein, ICP8; HSV-1 ICP8 is a single-stranded DNA-binding protein that is also required for HSV DNA synthesis and enhances late gene transcription.23 ICP8 accumulates with ICP4, the immediate early protein, ICP27 and virion capsid proteins in replication compartments and has been shown to interact with cellular proteins found in these compartments, based on coimmunoprecipitation.24 MxA exhibited nuclear localization in fibroblasts that were infected with HSV-1, in contrast to its strictly cytoplasmic distribution when induced with IFN-α (Figure 2a). No MxA was detected in mock-treated human embryonic lung fibroblast (HELF) cells. MxA was not detected when HSV-1 was inactivated by ultraviolet irradiation before inoculation (Figure 2b). In addition, MxA was not found in cells inoculated with HSV-1 that were treated with the antiviral agent, phosphonoacetic acid, which is an inhibitor of viral DNA polymerase and thus blocks late viral gene synthesis (data not shown). These experiments indicated that the induction of MxA expression in HSV-1-infected cells required viral replication or its induction could be dependent on the expression of particular late viral genes. As HSV-1 is known to propagate efficiently in human fibroblasts, the MxA that was detected in virus-infected cells seemed to have no substantial inhibitory effect on HSV-1 replication.
The assessment of MxA protein expression in fibroblasts revealed that MxA was present as the expected 76 kDa band in the cytoplasmic fraction when fibroblasts were treated with IFN-α. In contrast, a smaller ~56 kDa band was present in both the nuclear and cytoplasmic fractions of HSV-1-infected fibroblasts and the 76-kDa form was not detected (Figure 2c). The smaller protein was designated varMxA as distinguished from the larger MxA protein induced by IFN-α.
Identification of an alternatively spliced MxA mRNA in herpesvirus-infected cells
As MxA protein was detected in HSV-1-infected fibroblasts in the absence of IFN-α treatment and appeared to be smaller than IFN-α-induced MxA, MxA transcripts were compared in fibroblasts that were uninfected, infected with HSV-1 or treated with IFN-α. Primer sets were designed to amplify regions of the MxA gene starting from Exon 9 and extending to sequences near the TAA stop codon in Exon 17. IFN-α-treated cells yielded a single product of ~1.6 kb, whereas a smaller ~1.0 kb product was detected in HSV-1-infected cells by reverse transcriptase polymerase chain reaction (RT-PCR) analysis (Figure 3a). Results were reproducible in four experiments and sequencing demonstrated that all products consisted of nucleotides corresponding to the MxA gene.
Figure 3.
Identification of an alternatively spliced MxA mRNA in HSV-1-infected cells. (a) Total RNA was isolated from mock-treated, IFN-α-treated (10 000 U ml−1) or HSV-1-infected (0.1 PFU per cell) human fibroblast cells at 24 h for RT-PCR; PCR products were cloned and sequenced and the MxA transcript was amplified using primer sets extending from Exon 9 to sequences near the TAA stop codon in Exon 17. A major 1.6 kb band was present in IFN-treated cells; a second ~1.0 kb band was observed in mock and HSV-1-infected cells. (b) Sequencing showed deletion of nucleotides 1564–2049 (Exons 14–16) from the smaller transcript and a frame shift in Exon 17. Sequence alignment of the larger and smaller products is shown beginning within Exon 13 and extending through Exon 17. Nucleotides present in MxA transcripts from IFN-α-treated cells that correspond to Exons 14–16, not found in those from HSV-1-infected cells, are shown in the box. Polypeptides predicted to be present in MxA made in IFN-α-treated cells but not in HSV-1-infected cells are indicated by the dotted line. The first amino acid encoded by the Exons 13–17 junctional sequence is a glycine (G). Differences in the coding sequences generated by the splice junction and giving rise to the frame shift are shown, with predicted amino acid changes. A diagram of the MxA exons in comparison with variant MxA is shown in (a). Data were reproducible with more than five independent bacterial colonies.
When the cDNAs corresponding to transcripts from IFN-α-treated and HSV-1-infected cells were sequenced, an alignment was observed between nucleotide sequences from the 5′ untranslated region to the end of Exon 13 (Figure 3a). However, sequencing of the smaller product from HSV-1-infected cells revealed a deletion of Exons 14–16; this deletion introduced a frame shift at Exon 17 that would be predicted to alter the C-terminus of the MxA protein (Figures 3a and b). Although HSV-1 is known to selectively target host mRNA for degradation, results from real-time PCR analysis showed that expression of MxA and the alternatively spliced MxA transcripts remained stable and increased slightly over an interval of at least 10 h before declining at 24 h after infection (Supplementary Figure S2).
The alignment of the nucleotide sequences corresponding to MxA transcripts induced by IFN-α and HSV-1, with the exception of the Exons 14–16 deletion, indicated that the smaller transcript in HSV-1-infected cells was generated in accordance with the ag/gt donor/acceptor rule of gene splicing.2 3′ Rapid amplification of cDNA ends and sequence analysis demonstrated that the smaller MxA transcript was polyadenylated and was 3′ coterminal with the larger MxA transcript induced by IFN-α (data not shown). These experiments showed that the truncated MxA transcript was not an abortive product but was the result of alternative splicing. Both the larger and alternatively spliced MxA transcripts were usually detectable at very low levels in fibroblasts that had not been infected or treated with IFN-α.
Expression of an MxA isoform with a novel C-terminal domain in herpesvirus-infected cells
On the basis of the transcript sequence, the 56-kDa MxA isoform found in HSV-1-infected cells is predicted to have a conserved N-terminal MxA region but lacks most of the CID (residues 424–586) of the MxA isoform produced in IFN-α-treated cells (Figure 4a). The frame shift also likely disrupts the leucine zipper motif (residues 564–662) and alters the C-terminal domain of varMxA compared with MxA expressed after IFN-α treatment.
Figure 4.
Translation of the alternatively spliced MxA mRNA and nuclear and cytoplasmic expression of the variant MxA isoform in HSV-1-infected cells. (a) The 56-kDa MxA isoform is predicted to lack most of the CID (residues 424–586) of the MxA isoform produced in IFN-α-treated cells. The frame shift disrupts the leucine zipper (residues 564–662) found in the IFN-α-induced isoform. (b) MxA expression in HSV-1-infected cells using antibodies to the N- and C-terminus. HSV-1-infected fibroblasts were stained with M143 mAb against an epitope within residues 1–363 or 2C12 mAb against an epitope in the CID (residues 432–471)22 and anti-HSV-1 ICP8 polyclonal rabbit antiserum, examined 24 h after inoculation by confocal microscopy (magnification ×400). MxA was detected with M143 but not with 2C12 mAb. (c) When lysates from IFN-α-treated or HSV-1-infected cells were analyzed with anti-varMxA-C antiserum, variant MxA was detected as a 56-kDa protein in HSV-1 infected but not in IFN-α-treated cells.
As shown in Figure 4b, MxA was detected when HSV-1-infected cells were stained with a murine monoclonal antibody, M143, against an N-terminal epitope of the MxA isoform known to be induced by IFN-α.22 The reactivity of M143 mAb with the protein expressed in HSV-1-infected cells was also shown by immunoblot (Supplementary Figure S3). However, no MxA expression was observed when HSV-1-infected cells, identified by expression of the viral protein, ICP8, were stained with a second murine monoclonal antibody, 2C12 (Figure 4b). This monoclonal antibody recognizes a C-terminal epitope in the CID of MxA protein made in IFN-α-treated cells, a region that is predicted to be disrupted in the varMxA detected in HSV-1-infected cells.
To further document that the MxA protein produced in HSV-1-infected cells was the varMxA isoform, a rabbit antiserum was generated against the altered C-terminus created by the frame shift associated with the deletion of MxA Exons 14–16. The specificity of this antibody for varMxA was shown by immunoprecipitation of the expected 56 kDa protein from human fibroblasts that had been transduced with a recombinant retrovirus expressing the alternatively spliced MxA (Supplementary Figure S3); the protein that was immunoprecipitated was verified as MxA by peptide sequencing. The M143 mAb also bound to the 56-kDa isoform in this cell line (Supplementary Figure S3). The varMxA expression was also detected when Vero cells were infected with HSV-1 (KOS strain) by immunoblot analysis (Supplementary Figure S4).
Both the nuclear and cytoplasmic fractions of lysates from HSV-1-infected cells contained proteins reactive with the rabbit antibody against the varMxA C-terminus (Figure 4c). Results were reproducible in five independent experiments. The estimated 56 kDa size of varMxA in HSV-1-infected cells was consistent with the deletion of Exons 14–16 from the alternatively spliced mRNA (Figure 3). The weakly reacting doublet seen in nuclear extracts of mock- and IFN-treated cells was likely to be a non-specific band rather than MxA, as it did not overlap with the bands detected in the cytoplasmic and nuclear fractions of HSV-1-infected cells. Thus, as was indicated in experiments using the monoclonal antibodies against the N- and C-terminal domains of IFN-α-induced MxA, HSV-1 infection of fibroblasts elicited production of an MxA isoform with a conserved N-terminus and a novel C-terminal domain.
varMxA protein is associated with replication compartments and virions in nuclei of herpesvirus-infected cells
MxA expression was prominent in the nuclei of HSV-1-infected cells in close proximity to ICP8 protein by confocal microscopy (Figure 2a). Analysis by transmission electron microscopy showed that nucleocapsids were present in HSV-1-infected fibroblasts at 12 h after inoculation and the HSV-1 protein, ICP4, was detected with nucleocapsids by immunoelectron microscopy (Figure 5a). Like ICP4, MxA was also observed in association with virion particles at 12 and 24 h (Figure 5a). As the MxA isoform induced by IFN-α was not detected in HSV-1-infected cells, these results suggested that the protein detected by both confocal and immunoelectron microscopy was varMxA.
Figure 5.
Variant MxA protein is associated with viral nucleocapsids and replication compartments in HSV-1-infected cells. (a) Human fibroblasts were infected with HSV-1 (0.1 PFU per cell) for 12 h and processed for standard electron microscopy. Numerous nucleocapsids were detected in the nuclei of HSV-1-infected cells (scale bars: 1 μm). Virally infected cells (0.1 PFU per cell) were also processed at 12–24 h, and cryosections were specifically labeled with protein-A gold (10 nm, PAG) conjugated anti-ICP4 (r74) to identify HSV-1 viral nucleocapsids (arrows). Separate sections were also labeled with rabbit anti-MxA antiserum; MxA was present in association with viral nucleocapsid structures within the nuclei of HSV-1-infected cells (10 nm PAG, arrows). Inserts show higher magnification (scale bars: 100 nm). (b) Human fibroblasts infected with HSV-1 (0.1 PFU per cell) were also stained with antiserum to the variant MxA C-terminus (green), anti-HSV-1 ICP4 (red) and DNA (Hoechst 33342) (blue) and analyzed by confocal microscopy at 12 h after infection. No variant MxA was detected either in mock-treated or IFN-α-treated HELF. Variant MxA was associated with ICP4, which localizes to viral replication compartments (indicated as RC) in HSV-1-infected cell nuclei but was not detected in IFN-α-treated cells by immunofluorescence. Cryosections of virally infected cells (0.1 PFU per cell) at 12 h and were specifically labeled with 10 nm PAG-conjugated rabbit anti-varMxA-C polyclonal antiserum. The photographs from immunoelectron microscopy analysis show the presence of variant MxA in replication compartments (scale bars: 0.2 μm).
When the rabbit antibody specific for the C-terminus of varMxA was used in confocal microscopy experiments, MxA was detected in nuclear bodies in HSV-1-infected cells, identified by ICP4 expression, but was not found in cells treated with IFN-α or in mock-treated cells (Figure 5b). The varMxA was also detected in nuclear replication compartments in HSV-1-infected cells by immunoelectron microscopy using the rabbit antibody to the varMxA C-terminus (Figure 5b).
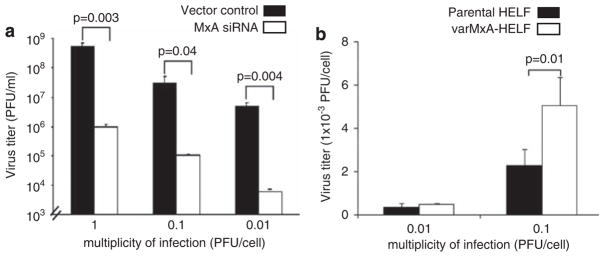
HSV-1 replication is reduced by inhibiting MxA transcription and enhanced by expression of varMxA protein
The expression and localization of varMxA to replication compartments in infected cells suggested that it might contribute to HSV-1 replication. To investigate this possibility, a recombinant retrovirus that produced short hairpin RNA from the U6 promoter was designed to knockdown MxA gene expression in human fibroblasts;25 its effects were confirmed by immunoblot and immunofluorescence (Supplementary Figure S5). RNA interference with MxA expression reduced the yields of infectious HSV-1 in all of three clones of cells that were inoculated with 1.0, 0.1 and 0.01 plaque-forming units per cell, whereas HSV-1 replication was not altered in fibroblasts infected with the cloned retrovirus vector control (Figure 6a). Conversely, to investigate whether the expression of varMxA protein could be shown to enhance HSV-1 infection, human fibroblasts were derived that constitutively expressed the varMxA isoform. When these cells were infected with HSV-1, infectious virus yields were increased two fold as compared with the control cell line (Figure 6b).
Figure 6.
HSV-1 replication is modulated by variant MxA protein. (a) Human fibroblasts were infected with a recombinant retrovirus that produces short hairpin RNA to inhibit MxA expression. MxA knockdown or control cells were infected with HSV-1 and virus yields were evaluated by plaque assay at 24 h after infection using three clones of MxA knockdown cells at low passages. Inhibition of MxA expression by siRNA reduced the yields of infections HSV-1 significantly after infection at 1, 0.1 and 0.01 PFU per cell. Each bar represents the mean±s.e. of triplicate titrations (two-tailed Student’s t-test, P=0.003, 0.04 and 0.004, respectively). Data were reproducible with independent clones of MxA knockdown cells and vector controls. (b) Human fibroblasts that expressed variant MxA were infected with HSV-1 and virus yields were evaluated by plaque assay at 24 h after infection. Constitutive expression of variant MxA enhanced HSV-1 yields are as shown; each bar represents the mean±s.e. of triplicate titrations from two independent experiments (two-tailed Student’s t-test, P=0.01).
DISCUSSION
The herpesviruses are ubiquitous mammalian pathogens that have evolved many strategies to escape the innate defense system during acute infection and reactivation and to achieve a balance between the virus and the host that allows their persistence in the population.16,18,26 IFN-α/β binding to the IFN receptor initiates a signaling cascade leading to the induction of the more than 300 IFN-stimulated genes that constitute a major component of cellular protection against viral pathogens.27 Some IFN-stimulated genes, including MxA and the related murine protein, Mx1, are known to function directly as antiviral effectors against RNA viruses. These experiments show that MxA induced in response to IFN signaling can also inhibit the replication of HSV-1, which is a common human alphaherpesvirus.
However, MxA was also detected in HSV-1-infected cells in the absence of IFN-α induction. This MxA protein was expressed as a smaller 56 kDa variant and transcriptional analyses showed that an alternatively spliced MxA mRNA was present in HSV-1-infected cells. In contrast to IFN-induced human MxA, which remains cytoplasmic, the varMxA protein was translocated into the nuclei of infected cells where it associated with viral replication compartments and virions. This nuclear translocation is presumed to require interaction with a viral or cellular protein, as human MxA differs from murine Mx1 in lacking a nuclear localization signal. Furthermore, instead of inhibiting infection, the expression of varMxA protein enhanced the production of infectious virus progeny in HSV-1-infected cells.
Many herpesviral immune evasion mechanisms target IFN signaling. For example, HSV-1 ICP0 interferes with IFN-α induction by the JAK/STAT signaling pathway and HSV-1 γ34.5 protein blocks PKR.26,28 IFN-α-stimulated IFN-stimulated gene-dependent and IFN-stimulated gene-independent genes including 2′,5′-OAS, IRF-1 and MxA are inhibited in fibroblasts and endothelial cells infected with human cytomegalovirus.29 Although the effect was less significant than MxA inhibition of RNA viruses, our experiments showed that HSV-1 replication was restricted in both MxA-expressing fibroblasts and transfected AD293 cells expressed MxA (Figure 1; Supplementary Figure S1). Although Pavlovic et al.30 reported HSV-1 infection of mouse fibroblasts transfected with MxA conferred no protection, the differences between these experiments and our findings may be due to the differing cell type and expression methods that were used; in addition, two of the three stable clones of MxA-transfected 3T3 cells yielded less HSV-1 than the control clones in their report. Thus, further investigation of MxA antiviral activity against herpes-viruses seems warranted.
In contrast to other immune evasion mechanisms, these investigations of MxA in HSV-1-infected cells suggest a different strategy by which innate antiviral defenses are counteracted through the induction and preferential expression of a varMxA isoform that lacks the antiviral effector function of the isoform induced by IFN. As transcripts for varMxA were detected at low levels in uninfected fibroblasts (Figure 3a; Supplementary Figure S2), HSV-1 infection could enhance translation of alternatively spliced MxA mRNA that is constitutively expressed.
The varMxA isoform discovered in HSV-1-infected cells is expressed from an alternatively spliced mRNA in which the nucleotide sequence from the 5′ non-coding sequence to the end of Exon 13 was present but Exons 14–16 were deleted and a frame shift was introduced. The protein product of the IFN-induced MxA transcript has an N-terminal region that constitutes the GTP-binding domain and a C-terminal effector domain, which is functionally divided into a CID and a leucin zipper (LZ) motif (Figure 1a). Activation of MxA GTPase activity requires backfolding of the LZ region on the CID, whereas GTP binding without GTP hydrolysis is sufficient for MxA antiviral effects.1 The mechanism by which this prototypic MxA inhibits RNA viruses has not been determined precisely. However, both the association of MxA with viral capsids and its antiviral activity are prevented by point mutations in the LZ region or by blocking an epitope in the CID region with the monoclonal antibody, 2C12, suggesting that functions of the C-terminal domain are required.6,22,31 The effect of the alternative splicing of the MxA transcript in HSV-1-infected cells was to remove most of the CID and disrupt the LZ region of the prototypic MxA C-terminus (Figure 4). As this varMxA lacks antiviral activity against HSV-1, these observations suggest that these MxA domains, which are necessary for its interference with RNA viruses, are also required to inhibit alphaherpesviruses.22,32,33
Alternative pre-mRNA splicing is a process by which multiple mRNA species are generated through combinatorial inclusion or exclusion of exons of a given gene.34 This process not only contributes significantly to protein diversity in mammalian cells but isoforms may be involved in regulating the prototype protein at the transcriptional or translational level. As the molecular basis for how alternative splice sites are selected remains largely unknown, it is difficult to predict the mechanism by which alternatively spliced MxA mRNA may be generated in HSV-1-infected cells. However, the differential expression of alternatively spliced variants in certain tissues, developmental stages or states of cell activation suggests that splice site choice is tightly controlled. For example, the CD44 gene, which encodes a T-cell homing protein, has 10 ‘variable’ cassette exons, all of which are omitted from the final mRNA transcript in resting naive T cells but are present in various combinations in activated T cells.35 Interleukin-15 is one of the several cytokines that are expressed as isoforms because of alternative mRNA splicing. Investigation of the expression of interleukin-15 suggests that the alternative isoform together with interleukin-15Rα has autocrine regulatory activity.36 As the prototypic full-length MxA protein was not detected in herpesvirus-infected cells, one hypothesis is that varMxA might regulate its expression.
The induction of varMxA expression occurred despite the fact that alphaherpesviruses encode virion host shut off proteins that block host cell protein synthesis.14 On the basis of analyses of the HSV-1 UL41 gene, which encodes an mRNA-specific RNAase, the virion host shut off function is now recognized as being selective and host cell mRNAs may be stabilized or degraded.37 Thus, in some cases, targeting mRNAs for rapid degradation is a mechanism to prevent the consequences of the activation of host cell gene promoters during viral replication and the continued transcription of host defense genes.38 This investigation of MxA transcription in HSV-1-infected cells suggests that modulating the splicing of cellular pre-mRNAs is another strategy for avoiding the production of antiviral proteins that may be associated with the continued expression of some host genes. Of interest, expression of a PML isoform was recently reported to be increased in human fibroblasts infected with HSV-1.39 This isoform, PMLb, was also generated from an alternatively spliced transcript and had altered intracellular localization.39 This PML isoform retained antiviral activity; in contrast, the synthesis of the varMxA isoform eliminated the restriction of HSV-1 replication caused by IFN-induced MxA. In both cases, alternative splicing was observed even though the HSV-1 ICP27 protein has inhibitory effects on host cell pre-mRNA processing.40
The fact that production of an isoform from an alternatively spliced transcript of MxA favored herpesviral replication, whereas this process reinforced the antiviral activity of PML suggests that examining the expression of various isoforms of host defense proteins in infected cells will reveal new insights about the interaction between herpesviruses and host cells. As it seems unlikely that varMxA is produced in only virus-infected cells, it will be of interest to explore whether varMxA has cellular functions and whether other stimuli can induce expression of this MxA isoform in human cells.
METHODS
Viruses and cells
The virus used was HSV-1 (F strain). AD293 cells were purchased from Stratagene Inc. (La Jolla, CA, USA) at passage 0; these cells are modified 293T cells with enhanced adherence characteristics. Primary HELFs, Vero and melanoma cells were maintained in MEM and AD293 were maintained in DMEM, with 10% fetal calf serum and antibiotics. HSV-1 (1.5×108 PFU per ml) was inactivated by UV irradiation (UV lamp wavelength, 254 nm; Kendro Laboratory Products, Waltham, MA, USA) for 30 min. Infectious virus was not recovered from cells inoculated with UV-irradiated HSV-1.
Antibodies and confocal microscopy
Antibodies to MxA included rabbit polyclonal antisera against IFN-induced MxA (#166)22 or the predicted varMxA C-terminus, generated for these experiments, and two murine monoclonal antibodies, M143, which recognizes an epitope within amino acids 1–363, and 2C12, against an epitope within amino acids 432–471 (kindly provided by Otto Haller, University of Freiburg, Germany). HSV-1 ICP4 polyclonal antibody (r74) and anti-ICP8 rabbit serum were kind gifts from Roger Everett (MRC Virology Unit, Glasgow, Scotland, UK) and David Knipe (Harvard University, Cambridge, MA, USA). ICP4 monoclonal antibody (10F1) was from Abcam Inc. (Cambridge, MA, USA). For confocal microscopy, cells were fixed in 4% paraformaldehyde at 24 h, permeabilized with 2% paraformaldehyde+0.2% Triton X-100 at room temperature for 30 min, and washed and stained with antibodies against MxA and HSV-1 proteins at room temperature for 1 h. Primary antibodies were diluted in PBS with 2% FBS as follows: anti-MxA mAb M143, 1:400; anti-MxA mAb 2C12, 1:400; anti-HSV-1 ICP4 antibodies: r74, 1:500 and 10F1, 1:5000; affinity purified polyclonal anti-varMxA-C antiserum, 1:100. After washing, cells were stained with FITC- or TexasRed-conjugated secondary antibody and visualized with a Zeiss LSM 510 confocal microscope. To further examine the localization of varMxA and HSV-1 ICP4, cells were stained using the HSV-1 primary antibodies followed by fluorophore (Alexa488, Alexa647)-conjugated donkey secondary antibody (Invitrogen Inc., Carlsbad, CA, USA) and analyzed with a Leica (Heidelberg, Germany) TCSSP2 confocal laser scanning microscope.
RNA extraction and RT-PCR
Uninfected, HSV-1-infected or IFN-α-treated HELFs (4×106) were homogenized by incubation with 1 ml of Trizol reagent (Invitrogen Inc.). RNA extraction was performed according to the manufacturer’s instructions. The first strand of cDNA was synthesized from 5 μg of total RNA by random hexamer and M-MLV RT (Invitrogen). Primer sets for RT-PCR are the upper primer starting from Exon 9 (Genbank BC032602, nt 937–955) and the lower primer located near the stop codon in Exon 17 (Genbank BC032602, nt 2217–2234): 5′-GCAAGCCGCTCCTTCAGG-3′. PCR was done with 1 cycle of 94 °C for 2 min for the pre-denaturation step; 35 cycles of 94 °C for 30 s, 58 °C for 30 s, 72 °C for 1–2 min and 72 °C for 5 min.
3′ Rapid Amplification of cDNA ends of varMxA
Total RNAs extracted from I HSV-infected cells with Tri-Reagent (Invitrogen) were subjected to 3′ rapid amplification of cDNA ends. An oligo-dT-containing adaptor primer (5′-ATGACCAATCAGATGGCAC(T)14-3′) was used to make the first-strand cDNA; PCR was then followed with varMxA-specific primer located at the Exon 13/17 junction region (5′-CAATTTTCAAGAAGGAGGCC-3′) and an adaptor primer (5′-ATGACCAATCAGATGGCAC-3′). Resulting PCR product from 3′ rapid amplification of cDNA ends was cloned and sequenced with a primer starting right after stop codon TAG (5′-GACGTGCA CGCACACTGTC-3′).
Lysate fractions and immunoblotting analysis
IFN-α-treated, HSV-infected and mock-treated HELFs were washed in cold PBS, removed by cell scraper and pelleted by centrifugation. To separate cytoplasmic (C) and nuclear (N) fractions, cells were incubated with hypotonic buffer containing 20 mM HEPES, pH 7.0, 10 mM KCl, 1.5 mM MgCl, 1 mM EDTA and 10% glycerol in the presence of protease inhibitors (Roche Inc., Nutley, NJ, USA) for 15 min on ice. Cytoplasmic protein was solubilized with NP-40 (final 0.2%), vortexed and collected from the supernatant after centrifugation. The cell pellet was washed in hypotonic buffer twice and resuspended in hypertonic buffer containing 20 mM HEPES, 450 mM NaCl, 10 mM KCl, 1.5 mM MgCl, 0.2 mM EDTA, 0.2% NP-40 and 20% glycerol with protease inhibitors. After 30 min on ice, the nuclear protein fraction was collected from the supernatant by centrifugation. To prepare whole-cell lysates, cells were incubated on ice for 15 min in RIPA buffer containing, 50 mM Tris-HCl, pH 7.0, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% sodium dodecyl sulfate and supernatants were collected by centrifugation at 13 000 r.p.m. for 15 min. Samples (20 μg) were subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with rabbit polyclonal anti-MxA antiserum (1:1000) or rabbit anti-varMxA antiserum (1:500) and detected by ECL Plus Western Blotting System (Amersham Biosciences Inc., Piscataway, NJ, USA). The membrane was stripped in buffer containing 62.5 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate and 100 mM β-mercaptoethanol at 68 °C and reprobed with anti-α-tubulin antibody (1:10 000) as a cytoplasmic protein control.
Electron microscopy
For standard electron microscopy (EM), cells were fixed in phosphate buffered 1% glutaraldehyde, pH 7.2 and post-fixed in 1% osmium tetroxid. The samples were dehydrated in a graded ethanol series and propylene oxide and embedded in epoxy-resin EMbed-812 (Electron Microscopy Sciences, Hatfied, PA, USA). Ultrathin sections (60 nm) were cut on an Ultracut S ultramicrotome (Leica), mounted on Formvar- and carbon-coated grids and counterstained with 1% aqueous uranyl acetate and 0.2% lead citrate. Processing for cryo-iEM and immunogold labeling was performed as described earlier.7 Samples were fixed in phosphate buffered 0.1% glutaraldehyde and 4% paraformaldehyde, pH 7.2, embedded in 10% gelatin and infiltrated with 2.3 M sucrose overnight. Sample blocks were mounted on cryoultramicrotomy pins and frozen in liquid nitrogen. Ultrathin sections (70 nm) were cut on an Ultracut Cryoultramicrotom (Leica) at −125 °C, picked up with a mixture (1:1) of 2% methylcellulose and 2.3 M sucrose and transferred to Formvar- and carbon-coated grids. Thawed cryosections were single labeled with rabbit polyclonal anti-varMxA-C antiserum (dilution 1:10) followed by incubation with Protein-A conjugated with 10 nm colloidal gold (University of Utrecht, The Netherlands). Sections were counterstained with 0.5% uranyl acetate in 2% methylcellulose for 10 min on ice and grids were looped out to dry. Samples were analyzed with a JEOL 1230 TEM (JEOL Ltd., Tokyo, Japan) at 80 kV and digital photographs were taken using a Gatan Multiscan 791 digital camera (Gatan Inc, Pleasanton, CA, USA).
Generation of polyclonal rabbit antiserum to the varMxA C-terminus
The sequence for the C-terminal half of varMxA protein at Exon 17 was cloned from pSPORT-MxA by PCR using an upper primer (5′-AAGGGATCCGGCCAGCAAGCGCATCTCCAG-3′), which introduced a BamHI site before the sequence for the first amino acid of the varMxA and a lower primer (5′-CGTGAATTCCTACGGGGCTGGACAGAGTGT-3′), which introduced an EcoRI site directly after the stop codon (TAG). The BamHI/EcoRI fragment was isolated and cloned into pGEX-2T at BamHI and EcoRI sites. The clone, pGEX-varMxA containing the sequence for 81 residues of varMxA peptide in frame with the glutathione-S-transferase coding region was verified by DNA sequencing. The pGEX-varMxA was transformed into BL21 DE3 bacterial cells (Invitrogen Inc.) and induced by IPTG. GST fusion protein was isolated using the MicroSpin GST Purification Module (Amersham Biosciences Inc.) as per the manufacturer’s protocol, dialyzed against PBS using Slide-A-Lyzer dialysis cassettes (Pierce Inc., Rockford, IL, USA), and concentrated using the Ultra Centrifugal filter device (Millipore Inc., Billerica, MA, USA). Rabbit polyclonal antisera to the GST-varMxA-C terminal protein was generated at Josman LLC (Palo Alto, CA, USA); antisera was Ig purified against GST-varMxA-C peptide by affinity chromatography using AminoLink Plus Immobilization kit (Pierce) according to the manufacturer’s instruction.
MxA knockdown by RNA interference
The vectors pS and pS-RT were used for retrovirus-based gene transfer (Ambion Inc., Austin, TX, USA). The nucleotide sequences, GTTCTTCCTGATAGATAAA corresponding to MxA 1407–1425 (Genbank BC032602) were chemically synthesized, annealed and inserted between the BamHI and HindIII sites of pS or pS-RT to produce a stem-loop RNA structure.25 The inserted nucleotide sequences were confirmed by DNA sequencing. Human fibroblasts were infected with the recombinant retrovirus that produces short hairpin RNA to inhibit MxA expression or no insert as control. Cells infected with control or MxA short hairpin RNA recombinant retrovirus were selected using puromycin (3 μg ml−1) and cloned into 96-well plates by limiting dilution. MxA knockdown was assessed by protein expression using immunoblot and immunofluorescence (Supplementary Figure S3). No resistance against HSV-1 infection was observed in retrovirus-infected cells.
Generation of the plasmid expressing varMxA
The pSPORT vector containing MxA cDNA (pSPORT-MxA, Genbank accession number BC032602) was purchased from ATCC Manassas, VA, USA. On the basis of the information given in Supplementary Figure S3, two sets of primers were designed for PCR using pSPORT-MxA as template to delete Exons 14–16 and generate the plasmid-expressing varMxA gene (pSPORT-varMxA). The oligonucleotide sequences for Set 1 are: upper primer 5′-GTGGTCCCCAGTAATGTGG-3′ (nt 937–955), lower primer: 5′-TTCTTGAAAATTGTTTTCAATTAT-3′ (nt 1540–1563); Set 2: upper primer 5′-GGAGGCCAGCAAGCGCATCT-3′ (nt 2049–2068), lower primer: 5′-CAGTGGCTACCCGGGAAC-3′ (nt 2329–2346). The 363 bp-BglII fragment and the 100 bp-SbFI fragment were isolated from the 626 and 298 bp PCR products amplified with Set 1 and Set 2 primer pairs, respectively. These products were used in a triple ligation with BglII/SbFI cut pSPORT-MxA to generate pSPORT-varMxA. Positive clones were verified by restriction enzyme digestion and deletion of Exons 14–16 was verified by DNA sequencing (Elim Biopharmaceuticals, Hayward, CA, USA). Expression of varMxA was confirmed by transient transfection of pSPORT-varMxA in melanoma cells.
Generation of fibroblasts expressing full-length MxA or varMxA
The full-length MxA was amplified from pSPORT-MxA by PCR using upper primer 5′-AGAGGATCCATGGTTGTTTCCGAAGTGGAC-3′ adding a BamHI site (underline) before the start codon and lower primer 5′-CAGAGAATTCTTAACCGGGGAACTGGGCAA-3′ adding an EcoRI site (underline) after the stop codon. The varMxA gene was amplified by PCR using pSPORT-varMxA described above as a template with an upper primer (5′-AGAGGATCCATGGTTGTTTCCGAAGTGGAC-3′) adding a BamHI site before the start codon and a lower primer (5′-CGTGAATTCCTACGGGGCTG GACAGAGTGT-3′) adding an EcoRI site following the varMxA stop codon. The resultant BamHI/EcoRI fragment containing MxA or varMxA was cloned into the retrovirus vector pBABEpuro (Strategen Inc.) at BamHI and EcoRI sites. Positive clones were screened by restriction enzyme digestion and sequenced to verify the presence of the MxA or the varMxA gene (Elim Biopharmaceuticals). Replication-defective virus particles were generated in 293T cells by cotransfection with the DNA mixture of pBABE vector containing the MxA gene or the varMxA gene, pVPack-VSV-G expressing VSV-G and pvPack-GP expressing gag-pol (Stratagene Inc.) prepared in Lipofectamine 2000 (Invitrogen Inc.) as described by the manufacturer. At 2 and 3 days after transfection, cell supernatants were collected and used to infect fibroblasts. Retrovirus-infected fibroblasts were selected and expanded in growth media containing puromycin (3 μg ml−1) to generate the MxA or the varMxA-expressing cell line. Expression of MxA or varMxA protein was verified by immunoblot and immunofluorescence.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH, AI20459 and AI053846 and from the National Science Council, Taiwan, NSC97-2320-B-002-005-MY3. We thank Drs Otto Haller and Georg Kochs, University of Freiburg, Freiburg, Germany, for discussions and reagents; Ilkka Julkunen, National Public Health Institute, Helsinki, Finland, David Knipe, Harvard University, Cambridge, MA, USA, and Roger Everett, MRC Virology Unit, Glasgow, UK, kindly provided anti-MxA and anti-HSV-1 antibodies. We thank Dr Chao-Jung Tu, Dr Jason Li, Abhinav Ponnaluru and Wen-Yuan Liu for technical assistance and Drs Barbara Berarducci and Stefan Oliver for discussions.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on Immunology and Cell Biology website (http://www.nature.com/icb)
References
- 1.Haller O, Kochs G. Interferon-induced Mx proteins: dynamin-like GTPases with antiviral activity. Traffic. 2002;3:710–717. doi: 10.1034/j.1600-0854.2002.31003.x. [DOI] [PubMed] [Google Scholar]
- 2.Tazi-Ahnini R, di Giovine FS, McDonagh AJ, Messenger AG, Amadou C, Cox A, et al. Structure and polymorphism of the human gene for the interferon-induced p78 protein (MX1): evidence of association with alopecia areata in the Down syndrome region. Hum Genet. 2000;106:639–645. doi: 10.1007/s004390000318. [DOI] [PubMed] [Google Scholar]
- 3.Aebi M, Fah J, Hurt N, Samuel CE, Thomis D, Bazzigher L, et al. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol Cell Biol. 1989;9:5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazzigher L, Pavlovic J, Haller O, Staeheli P. Mx genes show weaker primary response to virus than other interferon-regulated genes. Virology. 1992;186:154–160. doi: 10.1016/0042-6822(92)90069-2. [DOI] [PubMed] [Google Scholar]
- 5.Simon A, Fah J, Haller O, Staeheli P. Interferon-regulated Mx genes are not responsive to interleukin-1, tumor necrosis factor, and other cytokines. J Virol. 1991;65:968–971. doi: 10.1128/jvi.65.2.968-971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochs G, Janzen C, Hohenberg H, Haller O. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc Natl Acad Sci USA. 2002;99:3153–3158. doi: 10.1073/pnas.052430399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reichelt M, Stertz S, Krijnse-Locker J, Haller O, Kochs G. Missorting of LaCrosse virus nucleocapsid protein by the interferon-induced MxA GTPase involves smooth ER membranes. Traffic. 2004;5:772–784. doi: 10.1111/j.1600-0854.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- 8.Noteborn M, Arnheiter H, Richter L, Browning H, Weissmann C. Transport of the murine Mx protein into the nucleus is dependent on a basic carboxy-terminal sequence. J Interferon Res. 1987;7:657–669. doi: 10.1089/jir.1987.7.657. [DOI] [PubMed] [Google Scholar]
- 9.Turan K, Mibayashi M, Sugiyama K, Saito S, Numajiri A, Nagata K. Nuclear MxA proteins form a complex with influenza virus NP and inhibit the transcription of the engineered influenza virus genome. Nucleic Acids Res. 2004;32:643–652. doi: 10.1093/nar/gkh192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelhardt OG, Ullrich E, Kochs G, Haller O. Interferon-induced antiviral Mx1 GTPase is associated with components of the SUMO-1 system and promyelocytic leukemia protein nuclear bodies. Exp Cell Res. 2001;271:286–295. doi: 10.1006/excr.2001.5380. [DOI] [PubMed] [Google Scholar]
- 11.Haller O, Frese M, Rost D, Nuttall PA, Kochs G. Tick-borne thogoto virus infection in mice is inhibited by the orthomyxovirus resistance gene product Mx1. J Virol. 1995;69:2596–2601. doi: 10.1128/jvi.69.4.2596-2601.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlovic J, Haller O, Staeheli P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J Virol. 1992;66:2564–2569. doi: 10.1128/jvi.66.4.2564-2569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netherton CL, Simpson J, Haller O, Wileman TE, Takamatsu HH, Monaghan P, et al. Inhibition of a large double-stranded DNA virus by MxA protein. J Virol. 2009;83:2310–2320. doi: 10.1128/JVI.00781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roizman B, Knipe D, Whitley R. Herpes simplex viruses. In: Howley DKaP., editor. Fields Virol. Chapter 67. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 2503–2601. [Google Scholar]
- 15.Chee AV, Roizman B. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. J Virol. 2004;78:4185–4196. doi: 10.1128/JVI.78.8.4185-4196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ku CC, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med. 2004;200:917–925. doi: 10.1084/jem.20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin R, Noyce RS, Collins SE, Everett RD, Mossman KL. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J Virol. 2004;78:1675–1684. doi: 10.1128/JVI.78.4.1675-1684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mossman KL, Ashkar AA. Herpesviruses and the innate immune response. Viral Immunol. 2005;18:267–281. doi: 10.1089/vim.2005.18.267. [DOI] [PubMed] [Google Scholar]
- 19.Halford WP, Balliet JW, Gebhardt BM. Re-evaluating natural resistance to herpes simplex virus type 1. J Virol. 2004;78:10086–10095. doi: 10.1128/JVI.78.18.10086-10095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su YH, Oakes JE, Lausch RN. Ocular avirulence of a herpes simplex virus type 1 strain is associated with heightened sensitivity to alpha/beta interferon. J Virol. 1990;64:2187–2192. doi: 10.1128/jvi.64.5.2187-2192.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flohr F, Schneider-Schaulies S, Haller O, Kochs G. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett. 1999;463:24–28. doi: 10.1016/s0014-5793(99)01598-7. [DOI] [PubMed] [Google Scholar]
- 23.Gao M, Knipe DM. Potential role for herpes simplex virus ICP8 DNA replication protein in stimulation of late gene expression. J Virol. 1991;65:2666–2675. doi: 10.1128/jvi.65.5.2666-2675.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor TJ, Knipe DM. Proteomics of herpes simplex virus replication compartments: association of cellular DNA replication, repair, recombination, and chromatin remodeling proteins with ICP8. J Virol. 2004;78:5856–5866. doi: 10.1128/JVI.78.11.5856-5866.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang K-J, Ku C-C, Lehman IR. Endonuclease G: a role for the enzyme in recombination and cellular proliferation. Proc Natl Acad Sci USA. 2006;103:8995–9000. doi: 10.1073/pnas.0603445103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mossman KL, Smiley JR. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J Virol. 2002;76:1995–1998. doi: 10.1128/JVI.76.4.1995-1998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller DM, Zhang Y, Rahill BM, Waldman WJ, Sedmak DD. Human cytomegalovirus inhibits IFN-alpha-stimulated antiviral and immunoregulatory responses by blocking multiple levels of IFN-alpha signal transduction. J Immunol. 1999;162:6107–6113. [PubMed] [Google Scholar]
- 30.Pavlovic J, Zurcher T, Haller O, Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990;64:3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zurcher T, Pavlovic J, Staeheli P. Mechanism of human MxA protein action: variants with changed antiviral properties. EMBO J. 1992;11:1657–1661. doi: 10.1002/j.1460-2075.1992.tb05212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Numajiri A, Mibayashi M, Nagata K. Stimulus-dependent and domain-dependent cell death acceleration by an IFN-inducible protein, human MxA. J Interferon Cytokine Res. 2006;26:214–219. doi: 10.1089/jir.2006.26.214. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher B, Staeheli P. Domains mediating intramolecular folding and oligomerization of MxA GTPase. J Biol Chem. 1998;273:28365–28370. doi: 10.1074/jbc.273.43.28365. [DOI] [PubMed] [Google Scholar]
- 34.Smith CW, Valcarcel J. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem Sci. 2000;25:381–388. doi: 10.1016/s0968-0004(00)01604-2. [DOI] [PubMed] [Google Scholar]
- 35.Lynch KW. Consequences of regulated pre-mRNA splicing in the immune system. Nat Rev. 2004;4:931–940. doi: 10.1038/nri1497. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura H, Fujimoto A, Tamura N, Yajima T, Wajjwalku W, Yoshikai Y. A novel autoregulatory mechanism for transcriptional activation of the IL-15 gene by a nonsecretable isoform of IL-15 generated by alternative splicing. FASEB J. 2005;19:19–28. doi: 10.1096/fj.04-2633com. [DOI] [PubMed] [Google Scholar]
- 37.Esclatine A, Taddeo B, Roizman B. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc Natl Acad Sci USA. 2004;101:18165–18170. doi: 10.1073/pnas.0408272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esclatine A, Taddeo B, Evans L, Roizman B. The herpes simplex virus 1 UL41 gene-dependent destabilization of cellular RNAs is selective and may be sequence-specific. Proc Natl Acad Sci USA. 2004;101:3603–3608. doi: 10.1073/pnas.0400354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNally BA, Trgovcich J, Maul GG, Liu Y, Zheng P. A role for cytoplasmic PML in cellular resistance to viral infection. PLoS ONE. 2008;3:e2277. doi: 10.1371/journal.pone.0002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sciabica KS, Dai QJ, Sandri-Goldin RM. ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. EMBO J. 2003;22:1608–1619. doi: 10.1093/emboj/cdg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.