Abstract
OBJECTIVES
The cognitive characteristics of individuals with Hoarding Disorder (HD) are not well understood. Existing studies are relatively few and somewhat inconsistent but suggest that individuals with HD may have specific dysfunction in the cognitive domains of categorization, speed of information processing, and decision-making. However, there have been no studies evaluating the degree to which cognitive dysfunction in these domains reflects clinically significant cognitive impairment (CI).
METHODS
Participants included 78 individuals who met DSM-V criteria for HD and 70 age- and education-matched controls. Cognitive performance on measures of memory, attention, information processing speed, abstract reasoning, visuospatial processing, decision-making, and categorization ability was evaluated for each participant. Rates of clinical impairment for each measure were compared, as were age and education corrected raw scores for each cognitive test.
RESULTS
HD participants showed greater incidence of CI on measures of visual memory, visual detection, and visual categorization relative to controls. Raw score comparisons between groups showed similar results with HD participants showing lower raw score performance on each of these measures. In addition, in raw score comparisons HD participants also demonstrated relative strengths compared to control participants on measures of verbal and visual abstract reasoning.
CONCLUSIONS
These results suggest that HD is associated with a pattern of clinically significant CI in some visually mediated neurocognitive processes including visual memory, visual detection, and visual categorization. Additionally these results suggest HD individuals may also exhibit relative strengths, perhaps compensatory, in abstract reasoning in both verbal and visual domains.
Keywords: Hoarding Disorder, cognitive impairment, executive dysfunction, categorization, information processing speed, memory, attention
INTRODUCTION
Hoarding disorder (HD) is a disabling behavioral syndrome that is defined as the excessive acquisition of and inability or unwillingness to discard personal possessions, even if they are apparently useless or have limited value (e.g., hoarding), causing significant distress or functional impairment, and resulting in living and/or work spaces that are either unusable for their intended purposes, or would be unusable if not for the intervention of outside agencies or individuals [1–4]. In addition to the core feature of problematic hoarding behavior, many individuals with HD also exhibit traits of indecisiveness, difficulty with categorization, disorganization, slowness in completing tasks, and often report difficulties with memory, concentration and attention [3, 5–12]. Although clinical and anecdotal evidence provides support for cognitive dysfunction in HD, there has been little research on the neuropsychological correlates of HD. The neurocognitive studies that do exist support the concept that executive dysfunction is a prominent feature of HD, although the data in specific cognitive domains (e.g., decision making, attention, working memory, etc) is somewhat inconsistent [6–8, 10–21].
Most studies of executive function in HD have examined only one or a few cognitive domains (typically categorization, decision-making, and speed of information processing) [6, 14, 16, 18–21]. In addition to the use of different cognitive measures across studies, even within the same cognitive domain, the subject populations (individuals with OCD and hoarding symptoms, and individuals with HD with or without OCD) also vary between studies, which could contribute to the inconsistency of findings. Perhaps most importantly, none of the existing studies of cognitive functioning in HD have evaluated the presence of clinically significant cognitive impairment (CI) and have instead relied on raw score comparisons. Of note, the performance inefficiencies attributed to HD in these existing studies are often subtle and usually fall within the normal range of cognitive functioning. As such, it is difficult to determine the extent to which the observed cognitive inefficiencies associated with HD are clinically significant, often defined as performance falling 1.5 standard deviations below age matched peers. In other patient populations, a diagnosis of CI has been shown to be strongly associated with functional outcomes and has led to the development of specific treatment accommodations [22–25].
The aim of this study was to evaluate cognitive functioning in individuals with HD across the range of cognitive domains to specifically evaluate the incidence of CI in each domain. Based on the current literature on HD and neurocognitive function along with our previous work [15], we hypothesized that individuals with HD would exhibit greater incidence of CI on measures of visual categorization, visual learning and memory, visual detection, and speed of information processing when compared to age and education matched controls. Further, we hypothesized that HD would not differ from controls in other cognitive domains, including attention, verbal learning and memory, abstract reasoning, and planning ability.
METHODS
Participants
Participants were ages 18 and older and were recruited through mental health clinics throughout the Bay Area, the Mental Health Association of San Francisco (MHA-SF), and through media advertisements. Individuals with schizophrenia, intellectual disability, known dementia, or any acute medical condition that is known or suspected to affect cognitive function (including, but not limited to, head trauma or active substance abuse) were excluded. Participants with a history of substance use disorders were required to be sober from all substances (except tobacco) for a minimum of three months prior to participation in the study. All participants provided verbal and written informed consent to participate in the study and were financially compensated for their participation. The study was approved by the UCSF Institutional Review Board.
HD participants were included if they met DSM-V criteria for HD [26], plus two out of three of the following: 1) a score of ≥40 on the Saving Inventory, Revised (SI-R), 2) a score of ≥20 on the UCLA Hoarding Symptom Scale (UHSS), and 3) a score of ≥12 on the Clutter Image Rating Scale-Revised (CI-R) [27–29]. We excluded participants with co-occuring OCD. Control participants were included if they did not have hoarding symptoms, as defined by two of three of the following: scores of ≤20 on the SI-R, ≤10 on the UHSS, and ≤ 8 on the CI-R plus clinical interview. Control participants with a lifetime history of OCD were excluded; control participants with a lifetime history of DSM-V Axis I psychiatric disorders other than HD and OCD were not excluded, as long as those disorders were in remission at the time of the assessment. Control participants who had first-degree biological relatives with a diagnosis of HD or who had a first-degree relative with clinically significant hoarding symptoms by report were also excluded.
Diagnostic assessments
Psychiatric diagnoses were made by a licensed psychologist or psychiatrist utilizing DSM-V criteria [26]. Diagnostic assessments were made blinded to group status (HD vs. control), using all available information, including the self-administered symptom rating scales (described below), a structured clinical interview, and medical records when available. Hoarding symptoms were assessed using the SI-R, the UHSS, and the CI-R, along with the Structured Interview Hoarding Disorder (SIHD) [30]. The Saving Inventory-Revised (SI-R) [27] is a 26-item self-report questionnaire that measures hoarding symptoms and their impact. The UCLA Hoarding Severity Scale (UHSS) [28] is a 10-item clinician-administered instrument that was designed to be used in conjunction with a clinician interview. The UHSS assesses clutter, acquisition, and difficulty discarding, as well as the individual’s level of shame and impairment in social relationships due to hoarding. The Clutter Image Rating Scale (CI-R) [29] is a series of 9 photographs depicting varying levels of clutter, and is used to visually assess hoarding severity[29]. The Structured Interview for Hoarding Disorder (SIHD) [30] is a semi-structured clinical interview instrument designed to assess for Hoarding Disorder (HD) according to DSM-V criteria. The SIHD assesses hoarding behaviors, and distress and interference associated with hoarding behaviors.
Other psychiatric symptoms were assessed using self-report questionnaires for acute anxiety, depressive, and obsessive-compulsive symptoms (the Beck Anxiety Inventory (BAI)[31], the Beck Depression Inventory (BDI) [32], and the Yale Brown Obsessive Compulsive Scale (YBOCS) [33]), along with the Structured Clinical Interview for Diagnosis of DSM disorders (SCID) for lifetime diagnoses [26].
Neuropsychological assessment
Measures of cognitive functioning were administered by trained research staff under the supervision of a licensed neuropsychologist. Research staff were blinded to participant group status.. CI was defined as performance falling 1.5 standard deviations below age matched normative data (Scaled Score < 6). Demographic information (age, gender, years of education) was obtained for each participant at the time of assessment. Estimated IQ was obtained for all participants using the National Adult Reading test [34].
Visual memory and learning was measured using the Brief Visuospatial Memory Test - Revised (BVMT-R) [35]. For the BVMT, the outcome variables utilized were number of total correct responses on the learning trials and on the delayed free recall trial (memory). Verbal memory and learning was measured using the Hopkins Verbal Learning Test (HVLT) [36]. The two outcome variables utilized were number of total correct responses on the learning trials (learning) and on the delayed free recall trial (memory). Visual spatial processing was measured using the WAIS III Block Design subtest total correct [37]. Abstract reasoning was measured using the WAIS Similarities subtest (verbal) and the WAIS Matrix subtest (visual) [38]. The total number of correct responses was used as the outcome measure for both subtests. Attention and working memory was assessed using two WAIS subtests: Digit Span and the Letter Number Sequencing subtests. For each test, total number of correct responses was utilized as the outcome variable. Speed of information processing was measured using the Conners Continuous Performance Test II Reaction time [39], along with the Symbol Digit Modalities Test- Oral version total correct (SDMT) (a motor-free measure of information processing speed and visual working memory) [40], and the Stroop Color Word Test total correct score (SCWT) [41]. The CPT detection score was used as a measure of visual detection or visual discrimination ability, the CPT Perseveration score was used as a measure of perseverative responses, and the CPT Hit Rate score was used as an overall measure of visual response to stimuli. Visual categorization and problem solving was measured using the Delis Kaplan Executive Function System (DKEFS) Sorting Test, Set 1 [42, 43]. The outcome measures for the DKEFS Sorting Test were the time for the first sort, the total number of correct sorts divided by the total number of attempted sorts, and the total number of incorrect sorts. Decision-making and Planning was measured using the Iowa Gambling Test (IGT) [44] with total net score was used as the outcome variable. The DKEFS Tower Test total achievement score was also used as a measure of planning ability.
Procedures
Participants completed a variety of self-report questionnaires to assess hoarding behaviors and other psychiatric symptoms and subsequently participated in a clinical interview. Upon determination of eligibility for the study, participants completed the battery of neuropsychological tests. All participants were compensated for their participation.
284 total individuals were screened for study participation (188 HD, 96 Control). 16 potential HD participants were deemed ineligible to participate because of psychotic disorder diagnosis, recent illicit substance abuse, or not meeting screening criteria and 56 met criteria for OCD. 4 potential control participants were ineligible because of recent substance use. Of the 116 HD participants that were eligible to participate, 102 were enrolled in the study and 24 participants dropped out or declined participation: 78 fully completed the study and are included in analyses. There were no significant differences in demographic characteristics of individuals dropping out of study in comparison to those who participated. Of the 92 control participants that were eligible to participate, 70 enrolled in the study.
Statistical Analyses
We first conducted analysis of variance (ANOVA) for independent samples (for continuous variables) and Pearson’s chi-square tests (for categorical variables) to evaluate group differences (HD vs. control) on the demographic and clinical variables (gender, age, education, depression and anxiety severity, and estimated premorbid intelligence). We next examined the incidence of CI in HD compared to controls for each specific domain as a categorical variable. Incidence of CI was calculated for both HD and control participants and was compared using Pearson’s chi square tests. A significance level of p < 0.05 was utilized for each statistical comparison. For tests which showed significant differences between controls and HD participants on chi square tests, we subsequently completed logistic regressions adjusting for age, education, gender, and FSIQ. We next conducted one-way analyses of variance for independent samples to compare HD and control participants on raw score measures of cognitive functioning for each of the outcome variables, controlling for age, gender, education and estimated full scale IQ. Of note, scaled scores were used as outcome variables for the CPT test for increased interpretability. Because increased anxiety and depressive symptoms are key features of HD, we did not control for these symptoms in the analyses. We calculated effect sizes (Cohen’s d) for each cognitive measure.
RESULTS
Demographic and clinical characteristics
There were more women in the HD groups than in the control group (χ2 = 4.73; p = 0.03) and mean estimated full scale IQ was higher for the HD group than controls (χ2 = 2.01; p = 0.05). HD participants did not differ from controls with respect to age or education (Table 1). As expected, HD participants reported significantly more anxiety (χ2 = 7.41; p <0.001) and depressive symptoms (χ2 = 6.37; p <0.001) than did control participants. [INSERT TABLE 1 HERE]
Table 1.
Participant characteristics (n=148)
| Controls (N = 70) | HD (N = 78) | t/χ2; p value | |
|---|---|---|---|
| Gender (% female) | 53 | 80 | 4.73; .03 |
| Mean age (SD) | 58 | 58.17 | .08; .94 |
| Mean education (SD) | 15.97 | 15.48 | 1.25; .21 |
| Mean IQ (SD) | 116.15 | 119.02 | 2.01; .05 |
| Mean BAI score (SD) | 2.79 | 14.11 | 7.41; < .001 |
| Mean BDI score (SD) | 2.21 | 17.94 | 6.37; <.001 |
Note: HD = Hoarding Disorder, BAI = Beck Anxiety Inventory, BDI = Beck Depression Inventory
Incidence of Cognitive Impairment
A significantly greater proportion of HD participants demonstrated CI relative to control participants on the measures of visual memory (BVMT delayed recall; χ2 = 4.14, p = 0.04), visual detection (CPT detectability score; χ2 = 3.81, p=0.05), and categorization ability (D-KEFS Card Sorting Task ratio of correct to attempted trials; χ2 = 4.13, p = 0.04, and number of incorrect attempts; χ2 = 5.07, p = 0.02 (Figure 1). Secondary logistic regressions controlling for age, education, gender, and IQ showed similar results for categorization ability (D-KEFS Card Sort ratio of correct to attempted, β=0.52, std error = 0.22, χ2 = 5.51, p = 0.02; D-KEFS Incorrect; β = 0.65, std error = 0.27, χ2 = 5.84, p = 0.02 visual memory (BVMT; β = 0.45, Std error = 0.24, p = 0.06). Due to the low incidence of CI in the detectability task a logistic regression with covariates did not result in a stable model. The incidence of cognitive impairments for the remaining neuropsychological measures did not differ between the two groups (Table 2)
Figure 1.
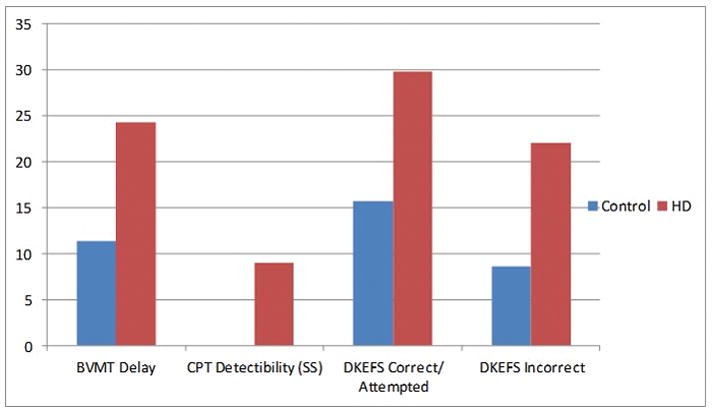
Percent of participants with clinically significant impairment on selected neuropsychological measures.
Note: HD = Hoarding Disorder, BVMT = Brief Visuospatial Memory Test, DKEFS = Delis
Kaplan Executive Function System
Blue = HD participants: Red = Control participants
Table 2.
Rates of Cognitive Impairment in HD relative to control groups
| NP test | Control: % impaired <6 | HD: % impaired <6 | Chi-Square; p |
|---|---|---|---|
| Visual Learning and Memory | |||
| BVMT-D | 11.43 | 24.36 | 4.14; 0.04 |
| BVMT-L | 17.14 | 25.64 | 1.57; 0.21 |
| Verbal Learning and Memory | |||
| HVLT- D | 6.25 | 6.41 | 0.00; 0.97 |
| HVLT-L | 7.14 | 6.41 | 0.03; 0.86 |
| Visuospatial Processing | |||
| Block Design | 0.00 | 1.30 | 0.52; 0.47 |
| Abstract Reasoning | |||
| Similarities | 1.45 | 4.35 | 0.68; 0.41 |
| Matrix Reasoning | 1.85 | 3.51 | 0.29; 0.59 |
| Attention/Working Memory | |||
| Digit-Span | 0.00 | 0.00 | na |
| Letter Number Sequencing | 2.90 | 0.00 | .68; 0.41 |
| Information Processing Speed | |||
| SDMT | 7.14 | 15.38 | 2.47; 0.12 |
| Stroop CW | 2.94 | 5.13 | 0.44; 0.51 |
| Visual Detection/Perseveration | |||
| CPT Hit Rate (SS) | 12.50 | 26.92 | 3.20; 0.07 |
| CPT Perseveration (SS) | 17.50 | 17.95 | 0.00; 0.96 |
| CPT Detectability (SS) | 0.00 | 8.97 | 3.81; 0.05 |
| Visual Categorization and Problem Solving | |||
| DKEFS-Correct/Attempted | 15.71 | 29.87 | 4.13; 0.04 |
| DKEFS Incorrect | 8.57 | 22.08 | 5.07; 0.02 |
| Tower Test | 2.50 | 8.33 | 1.142; 0.29 |
Note: HD = Hoarding Disorder, HVLT= Hopkins Verbal Learning Test, BVMT = Brief Visuospatial Memory Test, SDMT= Symbol Digit Modalities Test, CPT= Conner’s Continuos Performance Test, DKEFS = Delis Kaplan Executive Function System
Neuropsychological function
Table 3 shows age- and education-corrected raw scores for the HD and control groups on measures of neuropsychological functioning for eight domains: visual learning and memory, verbal learning and memory, visual spatial processing, abstract reasoning, attention and working memory, speed of information processing, detection and perseveration, visual categorization, and decision making/problem solving. In group comparisons of raw scores, individuals with HD scored significantly worse on the BVMT delayed recall task, F(2,147) = 4.18, p=.04, the CPT detection test F(2,116) = 3.90, p=.05 and measures of categorization ability, as evidenced by the ratio of correct/attempted F(2,145) = 6.42, p=.01) and the number of incorrect sorts F(145) = 6.74, p < 0.01. HD participants exhibited stronger performance on measures of verbal abstract reasoning (Similarities) F(2,91) F=5.06, p= .03 and visual abstract reasoning (WAIS Matrix Reasoning) F(2,110) F=7.19, p<.01.
Table 3.
Neuropsychological functioning in individuals with Hoarding Disorder without co-occurring OCD (HD) and matched controls
| Neuropsychological measure | Control | HD | Control | HD | Group F | Group p | Effect size |
|---|---|---|---|---|---|---|---|
| N | N | Mean (SD) | Mean (SD) | ||||
| Visual learning and memory | |||||||
|
| |||||||
| BVMT Delayed recall | 70 | 78 | 8.89 (0.31) | 8.01 (0.50) | 4.18 | 0.04 | 0.54 |
| BVMT Learning | 70 | 78 | 22.1 (1.03) | 21.06(0.98) | 1.02 | 0.31 | 0.12 |
|
| |||||||
| Verbal learning and memory | |||||||
|
| |||||||
| HVLT-Delayed recall | 64 | 78 | 9.69 (0.23) | 9.71 (0.21) | 0.21 | 0.27 | 0.61 |
| HVLT Learning | 64 | 78 | 26.13 (0.51) | 27.27(0.48) | 1.63 | 0.20 | 0.30 |
|
| |||||||
| Visuospatial processing | |||||||
|
| |||||||
| WAIS Block Design | 40 | 77 | 41.40 (1.70) | 38.84(1.31) | 0.58 | 0.45 | 0.34 |
|
| |||||||
| Abstract reasoning | |||||||
|
| |||||||
| WAIS Similarities | 69 | 23 | 24.05 (0.62) | 27.02(1.16) | 5.06 | 0.03 | 0.65 |
| WAIS Matrix Reasoning | 54 | 57 | 16.56 (0.59) | 18.87(0.62) | 7.19 | <0.01 | 0.42 |
|
| |||||||
| Attention/working memory | |||||||
|
| |||||||
| WAIS Digit Span Total | 40 | 58 | 19.56 (0.61) | 20.03(0.54) | 0.31 | 0.58 | 0.16 |
|
| |||||||
| Information processing speed | |||||||
|
| |||||||
| SDMT | 70 | 78 | 53.01 (1.21) | 51.26(.97) | 0.66 | 0.42 | 0.22 |
| Stroop Color-Word Score | 68 | 78 | 40.75 (1.04) | 38.81(0.98) | 0.80 | 0.37 | 0.23 |
| CPT Reaction Time Scale Score | 40 | 78 | 8.55 (.52) | 8.09 (0.37) | 0.02 | 0.47 | 0.68 |
|
| |||||||
| Detection/Perseveration | |||||||
|
| |||||||
| CPT HRT | 40 | 78 | 8.24 (.55) | 8.16 (.40) | 0.01 | 0.90 | 0.15 |
|
| |||||||
| CPT Detection Scale Score | 40 | 78 | 11.19 (.51) | 9.91 (.37) | 3.90 | 0.05 | 0.43 |
| CPT Perseverations Scale Score | 40 | 78 | 9.43 (0.61) | 8.79 (0.43) | 0.57 | 0.45 | 0.17 |
|
| |||||||
| Visual categorization | |||||||
|
| |||||||
| DKEFS Time of First Sort | 70 | 77 | 8.80 (.69) | 7.07 (0.68) | 3.23 | 0.07 | 0.31 |
| DKEFS Correct/Attempted | 70 | 77 | 0.82 (0.02) | 0.73(0.20) | 6.42 | 0.01 | 0.32 |
| DKEFS Incorrect | 70 | 77 | 1.28 (.20) | 2.16(.20) | 6.74 | <0.01 | 0.42 |
|
| |||||||
| Decision making and Problem Solving | |||||||
|
| |||||||
| Iowa Gambling Task | 40 | 54 | 18.77 (4.59) | 30.43(4.25) | 3.23 | 0.08 | 0.18 |
|
| |||||||
| DKEFS Tower Test Achievement Score |
40 | 24 | 16.95 (0.78) | 17.08 (1.05) | 0.17 | 0.68 | 0.03 |
Note: HD = Hoarding Disorder, BVMT = The Brief Visuospatial Memory Test, HVLT = Hopkins Verbal Learning Test, WAIS = Wechsler Adult Intelligence Scale, SDMT = Symbol Digit Modalities Test, CPT = Conners Continuous Performance Test, TMT = Trail Making Test, DKEFS = Delis Kaplan Executive Function System.
Means shown are controlled for age, education, and IQ. Unless otherwise indicated, means represent age- and education-adjusted raw scores. Effect sizes are reported as Cohen’s d.
DISCUSSION
Although a number of previous studies have examined cognitive function in individuals with Hoarding Disorder (HD), this study is the first to examine the incidence of clinically significant cognitive impairment (CI) in HD across a wide range of cognitive domains. As hypothesized, we found that HD was associated with increased incidence of clinically significant cognitive impairment on measures of visual memory, visual detection, and visual categorization, although contrary to expectation, we saw no differences on measures of visual learning or speed of information processing. We found similar patterns using raw score comparisons, where HD was associated with poorer performance on several measures of visual memory, detection, and categorization compared to controls. Unexpectedly, in our raw score comparisons, HD was also associated with relative cognitive strengths in abstract reasoning skills in both verbal and visual tasks. Each of these findings will be discussed below.
Perhaps the most significant result arising from this study is the finding that HD participants demonstrated a higher incidence of clinically significant CI in visual memory, detection and categorization tasks than our control participants. Twenty-four percent of individuals with HD demonstrated impairment in visual memory, with up to 30% of HD individuals showing impairment on a visual categorization task. Of note, while statistically significant, the incidence of CI on measures of visual detection was much lower with 8% of the sample showing impairment on this measure. In secondary analyses we controlled for the effects of age, education, gender, and IQ and saw very similar results, although the group differences in visual memory was slightly attenuated. Further, due to the sample characteristics, a logistic regression model was not possible for the detection task.
These findings are important for several reasons. First, as clinically significant cognitive deficits are by definition severe enough to impact functional status, these findings provide further support for the conceptualization that functional impairment in HD may be associated with specific cognitive impairments. Second, although previous studies have suggested that executive dysfunction in general is a prominent feature of HD, only a few have examined categorization ability in HD. However, the current literature does not allow for the determination of whether any observed deficits in categorization represent a primary characteristic of HD or whether they are part of a more global dysexecutive syndrome. Our use of a more comprehensive neuropsychological assessment allowed us to examine this question, and our results suggest that categorization deficits, along with deficits in visual memory may be a core feature of an HD-related dysexecutive syndrome. Given the relatively low incidence of impairment on visual detection tasks, it is likely that this type of impairment may not be a central aspect of HD, however a lack of impairment on this task for controls suggests that further investigation is warranted.
In addition to the finding that HD participants have a high incidence of cognitive impairment in specific domains, our findings that HD participants demonstrated poorer performance on raw score comparisons with control participants on measures of visual memory, visual detection, and categorization ability are also important. First, these findings highlight the degree to which visual categorization and visual memory dysfunction represents a significant aspect of HD. However, we did not see group differences on other measures of executive function, such as planning ability, information processing speed, or attention between groups that have been previously reported [45, 46]. Despite anecdotal reports of problems with decision-making among individuals with HD, and an initial study showing impairment on the IGT associated with hoarding symptoms among individuals with OCD [8], we found in our sample that the HD participants performed better than controls on the IGT, although not significantly so. These findings, in contrast to the initial study of hoarding symptoms in OCD sample, is consistent with the majority of studies showing no statistically significant difference in IGT performance in HD samples [7, 16, 18, 19]. Similarly, there were no differences between groups on measures of visuospatial processing, as measured by the WAIS Block Design subtest. This is in line with previous studies that have reported either better performance, or no difference between individuals with hoarding symptoms and controls on the ROCFT immediate copy task [6, 20] and other tasks of visuospatial processing [17], although performance on the Block Design task has not been previously reported.
An unexpected finding, and one that has not been previously reported, is that HD participants in our sample demonstrated relative strengths when compared to control participants on measures of abstract reasoning in both verbal and visual tasks. While these strengths are seen on raw score comparisons, the clinical significance of these findings remains to be determined. Nevertheless, when all measures were examined more globally, including both comparison of means and comparison of impairment rates, a pattern that differentiates HD from control participants begins to emerge. This pattern consists of relative deficits in tasks involving some aspects of visual processing (visual memory and visual categorization), and relative strengths in abstract reasoning). These results suggest that a key feature of HD may be deficits in some aspects of visual processing, perhaps partially compensated for by strong abstract reasoning abilities. Although these data are preliminary, and the sample sizes are small, the identified deficits in visual processing are consistent with what has been previously reported [6, 7, 15, 17].
The identification of specific deficits in visually rather than verbally mediated processes is in line with anecdotal reports that in addition to difficulty discarding, individuals with HD have difficulty organizing possessions within their home. This leads to excessively cluttered and unusable spaces, and this disorganization and clutter is what differentiates hoarding from collecting [47]. Hoarding is an inherently visual process, and impairments in visual categorization and memory may lead to difficulty processing, cataloging, and remembering where objects are placed, if not immediately visible, ultimately leading to clutter. Thus, although requiring replication in larger samples, the results of study begin to provide a framework for understanding the neurocognitive basis for hoarding behaviors.
LIMITATIONS
The primary limitation of this work relates to sample size and our results clearly require replication in larger samples. Somewhat paradoxically, the comparatively small sample sizes make the results that we did obtain even more remarkable; the effect sizes for all measures that showed a trend for a difference between HD and control participants were moderate to large, and the patterns were consistent across multiple analytic approaches (e.g., assessment of raw scores vs. comparison of impairment rates). Similarly, as we had several specific hypotheses we did not correct our results to account for multiple comparisons so our findings should be interpreted cautiously.
SUMMARY
The results of this study provide ongoing evidence for neurocognitive dysfunction among individuals with HD, and extend the current literature by 1) evaluating the incidence of clinically significant CI in HD, and 2) more comprehensively assessing neurocognitive function across multiple domains, and 3) integrating the findings across domains (and across studies) under a model that considers the potential of cognitive strengths in specific domains in HD as well as the incidence of cognitive impairment. To the best of our knowledge, this is the first study to suggest that there may be a specific pattern of cognitive functioning in HD characterized by CI in visually mediated processes, and perhaps also by compensatory strengths in abstract reasoning abilities. If borne out in additional samples, these results provide the beginnings of a comprehensive model of executive dysfunction in HD that has implications both for understanding the underlying pathophysiology of HD and eventually for treatment planning.
Acknowledgments
The authors would like to acknowledge Kera Mallard, David Bickford, Ross Crothers, Allison Siu, and Samuel Stark for their role in the manuscript preparation. This manuscript and information contained in this manuscript have not been published previously. This research was supported by NIMH Grants R21 MH087748, K08 MH081065, R01 0977669, the Althea Foundation, and a Patient-Centered Outcomes Research Institute (PCORI) Award (contract # 6000). The authors have no conflict of interest.
Footnotes
DISCLAIMER
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee
FINANCIAL DISCLOSURE: This research was supported by NIMH Grants R21 MH087748, K08 MH081065, R01 0977669, the Althea Foundation, and a Patient-Centered Outcomes Research Institute (PCORI) Award (contract # 6000).
References
- 1.APA. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Mueller A, et al. The prevalence of compulsive hoarding and its association with compulsive buying in a German population-based sample. Behav Res Ther. 2009;47(8):705–9. doi: 10.1016/j.brat.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Frost RO, Gross RC. The hoarding of possessions. Behav Res Ther. 1993;31(4):367–81. doi: 10.1016/0005-7967(93)90094-b. [DOI] [PubMed] [Google Scholar]
- 4.Steketee G, Frost R. Compulsive hoarding: current status of the research. Clin Psychol Rev. 2003;23(7):905–27. doi: 10.1016/j.cpr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Hartl TL, et al. Relationships among compulsive hoarding, trauma, and attention-deficit/hyperactivity disorder. Behav Res Ther. 2005;43(2):269–76. doi: 10.1016/j.brat.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Hartl TL, et al. Actual and perceived memory deficits in individuals with compulsive hoarding. Depress Anxiety. 2004;20(2):59–69. doi: 10.1002/da.20010. [DOI] [PubMed] [Google Scholar]
- 7.Grisham JR, et al. Neuropsychological impairment associated with compulsive hoarding. Behav Res Ther. 2007;45(7):1471–83. doi: 10.1016/j.brat.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence NS, et al. Decision making and set shifting impairments are associated with distinct symptom dimensions in obsessive-compulsive disorder. Neuropsychology. 2006;20(4):409–19. doi: 10.1037/0894-4105.20.4.409. [DOI] [PubMed] [Google Scholar]
- 9.Mataix-Cols D, et al. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61(6):564–76. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 10.Luchian SA, McNally RJ, Hooley JM. Cognitive aspects of nonclinical obsessive-compulsive hoarding. Behav Res Ther. 2007;45(7):1657–62. doi: 10.1016/j.brat.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Wincze JP, Steketee G, Frost RO. Categorization in compulsive hoarding. Behav Res Ther. 2007;45(1):63–72. doi: 10.1016/j.brat.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Tolin DF, et al. An exploratory study of the neural mechanisms of decision making in compulsive hoarding. Psychol Med. 2009;39(2):325–36. doi: 10.1017/S0033291708003371. [DOI] [PubMed] [Google Scholar]
- 13.Tolin DF, Villavicencio A. Inattention, but not OCD, predicts the core features of hoarding disorder. Behav Res Ther. 2011;49(2):120–5. doi: 10.1016/j.brat.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayers CR, et al. Executive functioning in older adults with hoarding disorder. Int J Geriatr Psychiatry. 2013;28(11):1175–81. doi: 10.1002/gps.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackin RS, et al. Cognitive functioning in individuals with severe compulsive hoarding behaviors and late life depression. Int J Geriatr Psychiatry. 2011;26(3):314–21. doi: 10.1002/gps.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolin DF, Villavicencio A. An exploration of economic reasoning in hoarding disorder patients. Behav Res Ther. 2011;49(12):914–9. doi: 10.1016/j.brat.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolin DF, et al. Neuropsychological functioning in hoarding disorder. Psychiatry Res. 2011;189(3):413–8. doi: 10.1016/j.psychres.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blom RM, et al. Cognitive functioning in compulsive hoarding. J Anxiety Disord. 2011;25(8):1139–44. doi: 10.1016/j.janxdis.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Grisham JR, et al. Categorization and cognitive deficits in compulsive hoarding. Behav Res Ther. 2010;48(9):866–72. doi: 10.1016/j.brat.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Pinto PS, et al. Influence of specific obsessive-compulsive symptom dimensions on strategic planning in patients with obsessive-compulsive disorder. Rev Bras Psiquiatr. 2011;33(1):40–6. doi: 10.1590/s1516-44462011000100009. [DOI] [PubMed] [Google Scholar]
- 21.McMillan SG, Rees CS, Pestell C. An investigation of executive functioning, attention and working memory in compulsive hoarding. Behav Cogn Psychother. 2013;41(5):610–25. doi: 10.1017/S1352465812000835. [DOI] [PubMed] [Google Scholar]
- 22.Alexopoulos GS, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Arch Gen Psychiatry. 68(1):33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackin RS, Arean P, Elite-Marcandonatou A. Problem solving therapy for the treatment of depression for a patient with Parkinson’s disease and mild cognitive impairment: a case study. Neuropsychiatr Dis Treat. 2006;2(3):375–9. doi: 10.2147/nedt.2006.2.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackin RS, Arean PA. Cognitive and psychiatric predictors of medical treatment adherence among older adults in primary care clinics. Int J Geriatr Psychiatry. 2007;22(1):55–60. doi: 10.1002/gps.1653. [DOI] [PubMed] [Google Scholar]
- 25.Mackin RS, et al. The effect of cognitive impairment on mental healthcare costs for individuals with severe psychiatric illness. Am J Geriatr Psychiatry. 19(2):176–84. doi: 10.1097/JGP.0b013e3181e56cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.First M, et al. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I), Clinician Version. Arlington, VA: American Psychiatric Publishing; 1997. [Google Scholar]
- 27.Frost RO, Steketee G, Grisham J. Measurement of compulsive hoarding: saving inventory-revised. Behav Res Ther. 2004;42(10):1163–82. doi: 10.1016/j.brat.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Saxena S, et al. Paroxetine treatment of compulsive hoarding. J Psychiatr Res. 2007;41(6):481–7. doi: 10.1016/j.jpsychires.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolin DF, Frost RO, Steketee G. An open trial of cognitive-behavioral therapy for compulsive hoarding. Behav Res Ther. 2007;45(7):1461–70. doi: 10.1016/j.brat.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mataix-Cols D, et al. The London field trial for hoarding disorder. Psychol Med. 2013;43(4):837–47. doi: 10.1017/S0033291712001560. [DOI] [PubMed] [Google Scholar]
- 31.Beck AT, Steer RA. Beck Anxiety Inventory. San Antonio, TX: The Psychological Corporation; 1988. [Google Scholar]
- 32.Beck AT, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 33.Goodman WK, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 34.Nelson HE. The National Adult Reading Test (NART): Test Manual. 1982. [Google Scholar]
- 35.Benedict RH. Brief Visuospatial Memory Test-Revised. Lutz: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- 36.Shapiro AM, et al. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13(3):348–58. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 37.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 38.Wechsler D. Wechsler Memory Scale: Administration and Scoring Manual. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 39.Conners CK, editor. Conners’ Continuous Performance Test II: Computer program for Windows technical guide and software manual. Multi-Health Systems; North Tonawanda, NY: 2000. [Google Scholar]
- 40.Smith A. Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- 41.Golden CJ, Freshwater SM. Stroop Color and Word Test: Revised examiner’s manual. Wood Dale, IL: Stoelting Co; 2002. [Google Scholar]
- 42.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: Harcourt Assessment; 2001. [Google Scholar]
- 43.Parmenter BA, et al. Validity of the Wisconsin Card Sorting and Delis-Kaplan Executive Function System (DKEFS) Sorting Tests in multiple sclerosis. J Clin Exp Neuropsychol. 2007;29(2):215–23. doi: 10.1080/13803390600672163. [DOI] [PubMed] [Google Scholar]
- 44.Bechara A, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 45.Grisham JR, et al. Categorization and cognitive deficits in compulsive hoarding. Behav Res Ther. 48(9):866–72. doi: 10.1016/j.brat.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Morein-Zamir S, et al. The profile of executive function in OCD hoarders and hoarding disorder. Psychiatry Res. 215(3):659–67. doi: 10.1016/j.psychres.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nordsletten AE, et al. Finders keepers: the features differentiating hoarding disorder from normative collecting. Compr Psychiatry. 2013;54(3):229–37. doi: 10.1016/j.comppsych.2012.07.063. [DOI] [PubMed] [Google Scholar]


