Abstract
Dendritic cells are equipped for sensing danger signals, capturing, processing and presenting antigens to naïve or effector cells and are critical in inducing humoral and adaptive immunity. Successful vaccinations are those that activate DC to elicit both cellular and humoral responses as well as long lasting memory response against the target of interest. Recently it has become apparent that tumor cells can provide new sources of antigens through non-synonymous mutations or frame-shift mutations, leading to potentially hundreds of mutation-derived tumor antigens (MTA) or neoantigens. T cells recognizing MTA have been detected in cancer patients and can even lead to tumor regression. Designing MTA-specific vaccination strategies will have to take into account the adjuvant activity of DC subsets and the best formulation to elicit an effective immune response. Here we discuss the potential of human DC to prime MTA-specific responses.
Keywords: Dendritic cell(s), vaccination, mutation derived tumor Antigen, tumor associated antigen
DC immunotherapy
A successful vaccination strategy should elicit a strong cellular as well as humoral response against the target antigen, which is essential for a long-term protection 1. Antigen capture and presentation to naïve T and B cells are critical for the control of pathogens as well as malignancies. Dendritic cells (DC) are a heterogeneous population of leukocytes, a link between the innate and adaptive immune system, that play a critical role in the initiation and regulation of immune responses. As professional antigen presenting cells they efficiently uptake antigens, process them and present antigenic peptides on MHC class I to activate CD8 T cells. Likewise, they present antigens to CD4 T cells through MHC II, which are critical for providing helper functions to CD8 T cells for cytolytic activity, and to B cells for antibody production. This unique ability of DCs has made them an attractive candidate for cell-based therapy.
The past two decades have witnessed the execution of hundreds of DC based vaccine trials, primarily in cancer but also in infectious diseases such as HIV-1 infection2,3. Typically DCs have been enriched from blood or generated from precursors (monocytes or CD34+ stem cells), loaded with antigens and administrated through different routes to patients to induce immunity 4. A variety of antigens have been deployed, and DCs are now even being given in combination with checkpoint blockade 5 (Table 1) with evidence of clinical efficacy. Altogether these studies have demonstrated that DCs can be safely administered and evidence of immunological and clinical responses in patients has been described 2,3. Sipuleucel-T, a partially enriched preparation of blood DC was the first approved cell based vaccine therapy and is used to treat castrate resistant prostate cancer 6. An improvement in overall survival of four months was noted, with the vaccine eliciting immunity towards the priming antigen as well as evidence of epitope spreading 7. Evidence is accumulating that the vaccine may have more efficacy in earlier stages of prostate cancer 8.
Table 1.
List of Clinical Trials With Combination of DC Vaccines and Check Point Inhibitors
| Check point inhibitors | Cancer type | Type of DC vaccine | ClinicalTrials.gov Identifier: |
|---|---|---|---|
| PD-1 | Acute myelogenous leukemia | DC/AML fusion | NCT01096602 |
| PD1 (CT-011) | Myeloma | DC/myeloma fusion | NCT01067287 |
| PD-1 (CT011) | Renal cell carcinoma | DC/Renal cell carcinoma fusion | NCT01441765 |
| PD-1 (Nivolumab) | Brain tumor | CMV pp65-LAMP mRNA pulsed autologous DCs | NCT02529072 |
| CTLA-4 (ipilimumab) | Melanoma | TriMix-DC (MAGE-A3, MAGE-C2, tyrosinase, and gp100) | NCT01302496 |
| CTLA-4 (CP-675,206) | Melanoma | MART-1 peptide pulsed DC | NCT00090896 |
Despite these advances, confirmation that DCs can effectively control advanced tumors remains limited. One of the major reasons may be that trials are usually conducted on patients in the late stage of disease. Clinical trials appear to show better responses in patients at early stage of diseases compared to late stage 9. Furthermore, most of the clinical trials are conducted with DCs generated from precursor cells such as CD14+ monocytes or CD34+ cells with a combination of cytokines yielding monocyte-derived DCs (MoDCs) which do not strictly resemble circulating blood DCs 10, 11 or Langerhans like cells which are considered by some to be more efficient at inducing cytolytic T cells in vivo 12, respectively. In vitro generation also requires prolonged cultures and some studies suggest an advantage of short term DC generation protocols to elicit better responses 13. Another major drawback of the in vitro generated DCs that are injected into the skin are their poor ability to migrate toward lymph node, with efficiencies of only 10–20%, which compromises the initiation of an effective immune response14, 15. Investigators are thus employing approaches to enhance DC migration through skin priming and inflammation of injected sites 16, Comparative genomics approaches have now in fact confirmed predictions that there are inherent differences between MoDCs and bonafide DC subsets present in the steady state in the body10,11. These differences account for altered functional properties and may yield different clinical results.
The heterogeneous population of DCs can be classified into different subsets, based on the expression of unique phenotypic markers and gene expression profile, as conventional DC type 1 (cDC1), cDC2, plasmacytoid DC (pDC), Langerhans cells (LCs) and inflammatory dendritic cells (inf-DCs) as summarized in the Table 2. There are limited numbers of clinical trials conducted using circulating DC subsets for vaccination, but there are several in progress, that will compare blood derived cDC and pDC as vaccine adjuvants, delivered IV, in cancers such as melanoma (NCT02692976, NCT02692976). The clinical outcomes although not tested in large studies yet suggest evidence of immunogenicity and possibly clinical response 17,18,17,19. These exciting new approaches may ultimately indicate the use of the right DC candidates for vaccination, 17,18, 19 how best to activate and load them with antigens and answer the question of how best to deliver DCs in vivo. DCs derived from induced pluripotent stem cells (iPS) 20, 21 or from iPS modified for the expression of antigens of interest is another potential approach for DC based cancer immunotherapy 22.
Table 2.
DC Subsets in Human
| Dendritic cell subset | Alternative names | Key surface markers | Associated gene signature | Functions |
|---|---|---|---|---|
| Conventional DC type 1-cDC1 | BDCA3+ DC/CD141+ DC | HLADR+CD11c+CD141+Clec9a+XCR1+CADM1+ | IRF8, batf3, Bc16, Flt3, CLNK | Cross presentation of dead cell associated antigens to CD8 T cells, Type III IFN production under TLR-3 triggering |
| Conventional DC type 2-CDc2 | BDCA1 DC/CD1c+ DC | HLADR+CD11c+CD1c+CD172+ | IRF4, Notch2, Rbpj, Klf4 | Activation of CD4 T cells, IL-12 production |
| plasmacytoid DC - pDC | BDCA2+ DC | HLADR+CD11c-CD123+CD303+CD304+CD45RA+ | IRF8, Bcl11a, Spi-B, E2-2, Runx1 | Major producer of the type I IFN, Anti viral immunity |
| Langerhans cells-LC | HLADR+CD11c+CD1a+CD207+ | EPCAM, ABCC4 and BMPR1A | Major component of skin immunity, | |
| inflammatory dendritic cells-inf-DC | MoDCs | HLADR+CD11c+CD11b+CD206+CD209 +/− | MAFB, FCGR2B, TPI1 | Major source of Nitric oxide and tumor necrosis factor during infection and inflammation |
Studies on the maturation status of DCs confirmed that immature DCs could generate tolerance instead of immunity against the antigen of interest 23, 24. The critical role of DCs to produce inflammatory cytokines which are necessary for generating CD8 T cell response are not always considered in most vaccination trials 25. The role of IL-12 and the amount produced by antigen loaded DCs on initiating strong antitumor response in clinical settings has been validated 26. Other key cytokines include type I IFN members, produced by pDC and DC1, and IL-15 27. In addition efficient antigen loading is essential to prime T cells. DC1 are the most efficient at cross presenting antigens from cellular sources and thus targeting antigen to these cells has to be considered when one is thinking about using tumor antigens, in particular MTAs, the focus of discussion in this review.
Improving the efficiency of DC immunotherapy in cancer
As alluded to above, many variables exist that must be considered to improve the efficiency and clinical outcome of DC based immunotherapy in cancer. These include the choice and production approach of DC subsets(s), selection and delivery of antigens to DCs, choice of ideal adjuvants, delivery approach (eg skin vs IV vs intranodal vs intratumoral) and other combinatorial approaches to synergize the vaccination efficiency.
Neo-Antigens a promising targets for personalized DC vaccines
Tumor-associated antigens (TAA) were first identified in melanoma patients and demonstrated by the identification of MAGEA1 specific T cell clones in the tumor infiltrating lymphocytes 28. The classical TAAs are non-mutated antigens and have a restricted expression or over expression in tumor cells. They might be expressed at low levels by normal cells, restricted to only germ line cells and through epigenetic modulation, or be over expressed or heterogeneously expressed in cancer cells. Many such TAA have now been identified, one of the most prominent being members of the cancer-testis (CT) gene family and include antigen such as MAGEA3 and NY-ESO-1. What is significant is that host T cells are capable of recognizing these epitopes29, indicating that host immunity can be induced spontaneously, and that it is feasible to break tolerance. Other TAA include differentiation antigens (eg Melan-A/MART-1), altered antigens (e.g. MUC-1) over expressed antigens (e.g. Her2-Neu) or oncoviral antigens (e.g. HPV, EBV). Most DC vaccine trials have targeted these types of TAAs and there are different approaches used for loading these antigens to DCs 30, 31. The known TAA, however, account for only a small fraction of the endogenous anti-tumor response32,33. Further studies revealed the presence of antigens arising from somatic mutations that result in proteins with altered sequence34. These mutation-derived tumor antigens (MTA) are generally believed to be patient-specific, although in specific circumstances common mutations such as KRAS p.G12D 35,36 and Calreticulin p.K385fs 37 can also be antigenic.
Landmark studies in advanced-stage cancer patients have demonstrated the clinical utility of immune checkpoint inhibitors targeting CTLA-438 and PD-1/PD-L1 39. Retrospective analyses suggest that the number of somatic mutations found in a tumor prior to treatment may be a predictive biomarker in patients treated with inhibitors of CTLA-440,41, PD-139 and PD-L142. Encouraged by these results, attempts have been made to target MTA directly with DC-based therapeutic vaccines and adoptive cell transfer, generating encouraging preliminary results43,44,45. Recent pre-clinical studies of alternative MTA-specific therapeutic vaccination approaches, including peptide and nucleic acid based vaccines also exhibit potential 46, 47,48,49,50. These animal models highlight the importance of lymph node resident dendritic cells which accumulate antigen in response to vaccination and then initiate the MTA-specific immune response51. Combining the potential of neoantigens and DC vaccines may be a promising strategy for future immunotherapy approaches.
Loading DCs with TAA
One of the most common methods to load DCs is with short peptides that are predicted to efficiently bind the major histocompatibility complex I (MHC I) and capable of priming CD8 T cell responses. Alternatively loading long peptides (lengths of 15 to 30 amino acids) with epitopes targeting both CD4 and CD8 T cells are theoretically more useful for anti-tumor therapy, because CD4 helper cells can have anti-tumor activity51 and provide help for CTLs52. Proof of principle that DCs can prime and boost neoantigen responses in subjects with a history of melanoma was published last year from the University of Washington 43
Pilot studies in patients also show the safety and potential for targeting MTA to DCs for eliciting immune responses51. The Sahin group in particular have shown that stabilized RNA encoding MTA can safely elicit T cell responses in melanoma patients with about 30% of their predicted epitopes eliciting T cell responses. Interestingly most of these are CD4 in nature, indicating a need to improve vaccine approaches to gain a greater breadth of CD8 responses. One could argue given the less than complete ability to immunize against all MTAs, one should consider approaches that deliver the entire TAA repertoire to DCs. In order to maximize the target epitope and personalize the vaccines, different approaches like loading DCs with whole tumor lysate 53 or loading DCs with total RNA from tumor cells 54 or fusing the DCs with tumor cells etc are being investigated 2, 3,55. These approaches may be helpful to broaden the target epitopes but possible problems could be frequency of antigens, expression, cross-presentation capacity, etc. and laborious procedures for vaccine generation.
In vivo targeting of dendritic cells-selection of delivery vehicles
DCs can be targeted with various approaches and a widely used method is the use of different antibodies tagged with antigen of interest. The antibody-based approach can be used for targeting all dendritic cell subsets or any specific DC subsets. Intracutaneous administration of antibody targeting the C-type lectin receptor DEC205 with NY-ESO-1 fusion protein and TLR adjuvants R848 or poly IC-LC or a combination against different malignancies including melanoma, ovarian cancer, sarcoma, small lung cancer could induce cellular and humoral response and confirms the safety and feasibility of the approach56. DEC-205 expression is not only restricted to DCs but also expressed by monocytes, NK cells and lymphocytes 57, hence the approach is less selective and an alternative is targeting unique molecules expressed on specific DC subsets. One of the well-studied systems with different murine 58 and primate models 59 is the targeting of the mouse CD8a+ DC or the human cDC1 60 subset with the C-type lectin receptor Clec9a. The studies show encouraging results on eliciting strong cellular and humoral responses and appear to be critical for initiating long-term protection characteristic of a successful vaccination 61, 62. Another approach might be to target the chemokine receptor XCR1 on the same DC subset using an antibody 63 or XCL1 vaccibody tagged with antigen of interest 63, 64,65. Studies in animal models show encouraging results on initiating strong immune response by this approach.
Delivering RNA from TAAs with lipid based vehicles (RNA-Lipoplex) can target the lymph node resident DCs and initiate immune responses. Studies in mouse models and pilot studies in patients show the safety and feasibility of this approach 49. Another potential approach is the use of albumin hitchhiking to deliver the antigens to the lymph node resident DCs 66. In all these cases targeting DCs would require an individualized approach and the expense and the associated cost would need to be considered.
In vivo amplification of DC subsets with Flt3L administration
At steady state human blood contains three DC subsets and they are pDCs (0.2%), cDC1 (0.02%) and cDC2 (0.2%)67. A limited number of clinical trials are being conducted by isolating pDCs or cDC2 and injecting them back into patients after in vitro antigen loading17–18. One of the major bottlenecks of the current strategy is that the method is mainly restricted to pDCs or cDC2 which are present at relatively higher frequencies compared to the cDC1 (<0.02%). cDC1 are the human homologue of the mouse cross presenting DCs and a potential exciting candidate for DC immunotherapy. Methods to expand or produce these cells for cell-based therapies are being aggressively pursued. One approach utilizes Flt3L which is a key cytokine for DC generation and Flt3L administration in healthy donors increases the frequency of DC subsets pDCs (6–16 fold), cDC1 (48 fold) and cDC2 (130 fold) 68, even in patients who have had cancer 69. This approach therefore may facilitate the isolation of different subsets in sufficient numbers for multiple rounds of DC vaccination as well as provide an option for testing the potential of cDC1 for immunotherapy. On the other hand it can facilitate the in vivo targeting of DCs by increasing the available targets as illustrated in Fig 1. The increased frequency of DC subsets may improve uptake of targeted antigen and increase migration towards lymph nodes for eliciting successful immune responses. Flt3-L administration and subsequent intratumoral Poly I: C injection induced the expansion and activation of CD103+ DCs in mouse melanoma models and elicited antitumor responses. 70 Clinical trials in melanoma patients using a combination of Flt3-L, DEC205/NY-ESO-1 fusion protein and poly IC-LC confirms the safety and immunogenicity of the approach (NCT02129075). Flt3L in combination with Intratumoral administration of Poly I:C-LC and irradiation is also safe and potentially clinically active (NCT01976585-J Brody, personal communication) in B cell lymphoma.
FIGURE 1.
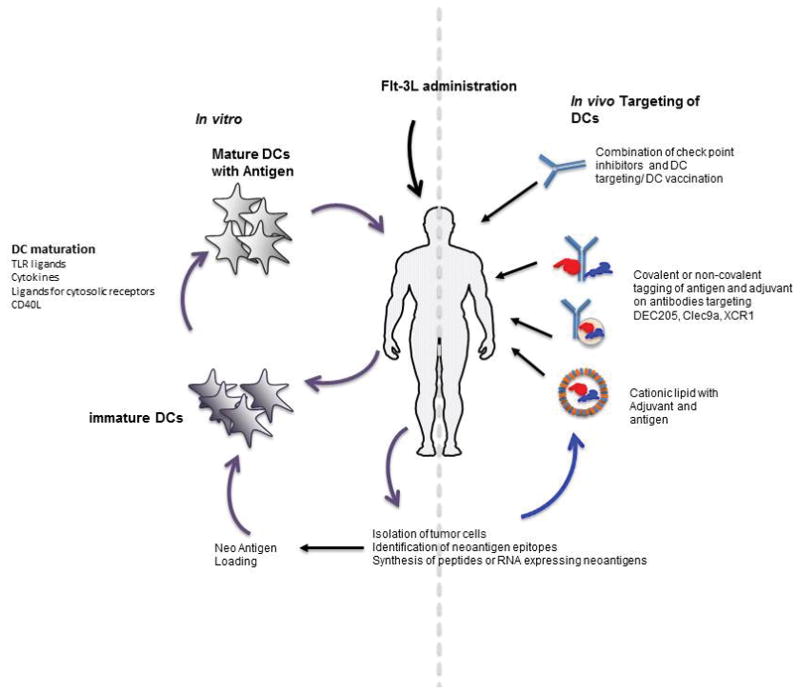
Illustration of the potential of Flt-3L administration for improving the DC based cancer immunotherapy.
Selection and delivery of adjuvants
Selection of suitable adjuvants for activating DCs is important for eliciting a desired immune response. There are different adjuvants used for their activation including TLR agonists (eg Poly I:C/Poly I:C:LC, R848, Imiquimod, CpG, LPS), cytokine cocktails (TNF, IFN alpha, IFN gamma, IL1beta) or ligands targeting the co-stimulatory molecules (CD40L) 25. DC subsets exhibit a differential expression profile of TLRs and it is difficult to select a single TLR agonist for activating all DC subsets 71. There are no comprehensive comparative studies conducted to select the ideal adjuvants or a combination for activating all DC subsets or specific DC subsets and testing their immune outcome. Dhodapkar et, al., did not observe any synergistic effect upon administration of a combination of TLR3 and TLR7/8 in comparison with the individual ligands in DEC-205/NY-ESO-1 study 56. The observation may be due to the lack of response in limited numbers of patients (n=8) or the combination of ligand may not be taken up by the same DCs. Focusing on the combination of different TLR ligands or TLR ligands with cytosolic receptors like STING 72 or combining them with CD40 ligands may be a better option for inducing strong immune response to the target of interest. These approaches are applicable for both in vitro antigen loading as well as for in vivo targeting of DCs for immunotherapy.
One of the critical aspects to be considered for designing DC vaccination strategies for future studies is the mode of delivery of the adjuvants. Current approaches are non-targeted as adjuvants are injected at the site antigen delivery. This may result in a systemic immune response and toxicity by activation of non-targeted cells expressing the TLRs. Also it can activate non-antigen loaded DCs which may lead to autoimmunity or T cell anergy. The ideal situation will be activating the DCs that are taken up the antigen of interest. Antigen of interest can be co-delivered through covalent or non-covalent linkage to the delivery vehicles 73. This may ensure the delivery of antigen and adjuvant to the cells of interest as well as ensure the activation of DCs to avoid or reduce the unintended outcomes.
Identifying the best subset for vaccination
DCs are heterogeneous populations of professional antigen presenting cells. It is still a question whether all the subsets are equally efficient in initiating immune response or if they have any functional specialization and relative advantage on initiating immune response. Studies in mouse models shows that the intra-tumoral and lymph node presence of CD8a+ cross presenting DCs is important for initiating antitumor immune response and specific deletion of these subsets abrogate these response 74,75. In vitro studies with human DC subsets are more controversial. A few reports have shown the specific advantage of cDC1 for cross presenting dead cell associated antigen 76,77,78,79 while some other reports claim all subsets are equally efficient for antigen presentation 80. A comprehensive approach is still required to unravel the functional specialization of the subsets for antigen presentation. Even though each subset may have a very specific role under different conditions of infection or at physiological state; vaccination strategies are based on eliciting a strong cellular and humoral response. Hence identifying the most appropriate subsets may ease the development of tools for targeted delivery and identification of suitable adjuvants to achieve a strong and long lasting immune response.
Combination with checkpoint inhibitors
Presence of tumor antigen specific CD8 T cells in patients and the failure of the immune system to control the tumor, led to unraveling the role of check point molecules as a tool for the immune evasion strategies by the tumor. Various studies in different malignancies treated with checkpoint inhibitors have unquestionably confirmed their role in tumor progression 81. Checkpoint inhibitors are a new class of attractive agents in immunotherapy and the antibodies targeting and inhibiting the immune checkpoints induce antitumor response in various malignancies most probably by amplifying the antigen experienced T cells, but also blocking regulatory T cells. Most commonly used targets are CTLA-4, PD1 and PDL1 and there are other antagonist and agonist checkpoint molecules with therapeutic potential currently being tested. The major drawbacks are unintended autoimmune responses and toxicity. The checkpoint molecules may also be playing a critical role in blocking the priming and amplifying the effector cells by the DCs present in the tumor niche, in addition to blocking checkpoint molecule activity on DCs themselves. The tumor may use these molecules to modulate DC vaccines and block the initiation of antitumor immunity; especially when we target the DCs present in the tumor niche. A combination of check point inhibitor and DC vaccination may be helpful in broadening the anti-tumor response by amplifying the antigen experienced T cells and DCs may specifically prime naïve T cells with less immunogenic neoantigens. There are number of clinical trials in progress with combinations of DC vaccines and checkpoint inhibitors and are summarized in Table 1. A phase II study shows the combination of autologous DCs and anti-CTLA-4 antibody could improve the over all survival in advanced melanoma patients 5. DC vaccination loaded with neoantigens on patients who have undergone ipilimumab treatments could elicit antigen specific CD8 T cells and indicate the role of amplifying the response to multiple antigens 43.
Other approaches
There is potential for an integrated approach for using DC immunotherapy with other conventional treatments such as chemotherapy or radiation therapy, which could release or even create more mutated tumor antigens. Other than the conventional approach, DC immunotherapy can be combined with other small molecules to inhibit the tolerogenic molecules like IDO which can induce regulatory T cells (Treg), or depletion of Tregs, targeting or neutralizing the immune suppressive cytokines in tumor niches with anti-IL-10 or with anti-TGF beta which may enhance the anti tumor response and generate a long lasting immune response 82,83.
Summary
DCs are natural adjuvants for vaccination and combining them with neoantigens can pave a new scenario in cancer immunotherapy. Current neoantigen vaccinations mainly depend on the dominant antigen epitopes and available TCR pool. DCs may be able to prime naïve T cells against subdominant epitopes and broaden the antigen pool as well as expand both CD8 and CD4 T cell clones to elicit a strong immune response against a wide number of tumor antigens. Some of the studies with neo-antigen based immunotherapy show a CD4 T cell mediated bias rather than CD8 T cells 51. A strong CD8 mediated immune response may be critical for eliminating the tumor. The future DC based neoantigens vaccines should focus on eliciting both CD8 and CD4 T cells response by optimizing different steps like the selection of antigen, adjuvant and loading or targeting the ideal DC subset. DC based neoantigen vaccination are highly personalized and developing a large scale vaccination strategy will require the development of faster and cheaper methods for identifying neoantigens and loading/delivering to DCs for treating the patients. Nevertheless this approach has significant potential and can be a milestone in cancer immunotherapy.
Acknowledgments
Some of the studies cited in this review were supported by the Cancer Research Institute, the Melanoma Research Alliance, and the NIH (AI044628, CA180913 and AI081848). Nina Bhardwaj is an extramural member of the Parker Institute for Cancer Immmunotherapy.
References
- 1.Garbi N, Kurts C. The Hierarchy of Antigen Delivery. EBioMedicine. 2016;5:7–8. doi: 10.1016/j.ebiom.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257–67. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 3.Tagliamonte M, Petrizzo A, Tornesello ML, Buonaguro FM, Buonaguro L. Antigen-specific vaccines for cancer treatment. Hum Vaccin Immunother. 2014;10:3332–46. doi: 10.4161/21645515.2014.973317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong L, Brockstedt D, Benike C, Wu L, Engleman EG. Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol. 2001;166:4254–9. doi: 10.4049/jimmunol.166.6.4254. [DOI] [PubMed] [Google Scholar]
- 5.Wilgenhof S, Corthals J, Heirman C, et al. Phase II Study of Autologous Monocyte-Derived mRNA Electroporated Dendritic Cells (TriMixDC-MEL) Plus Ipilimumab in Patients With Pretreated Advanced Melanoma. Journal of Clinical Oncology. 2016;34:1330–8. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 6.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 17:3520–6. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 7.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 8.Hu R, George DJ, Zhang T. What is the role of sipuleucel-T in the treatment of patients with advanced prostate cancer? An update on the evidence. Ther Adv Urol. 2016;8:272–8. doi: 10.1177/1756287216645314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bol KF, Aarntzen EH, Hout FE, et al. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. Oncoimmunology. 2016;5:e1057673. doi: 10.1080/2162402X.2015.1057673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins SH, Walzer T, Dembélé D, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biology. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vu Manh T-P, Elhmouzi-Younes J, Urien C, et al. Defining Mononuclear Phagocyte Subset Homology Across Several Distant Warm-Blooded Vertebrates Through Comparative Transcriptomics. Frontiers in Immunology. 2015;6 doi: 10.3389/fimmu.2015.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratzinger G, Baggers J, de Cos MA, et al. Mature Human Langerhans Cells Derived from CD34+ Hematopoietic Progenitors Stimulate Greater Cytolytic T Lymphocyte Activity in the Absence of Bioactive IL-12p70, by Either Single Peptide Presentation or Cross-Priming, Than Do Dermal-Interstitial or Monocyte-Derived Dendritic Cells. The Journal of Immunology. 2004;173:2780–91. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 13.Burdek M, Spranger S, Wilde S, Frankenberger B, Schendel DJ, Geiger C. Three-day dendritic cells for vaccine development: antigen uptake, processing and presentation. J Transl Med. 2010;8:90. doi: 10.1186/1479-5876-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara S, Wada H, Miyata H, et al. Clinical trial of the intratumoral administration of labeled DC combined with systemic chemotherapy for esophageal cancer. J Immunother. 2012;35:513–21. doi: 10.1097/CJI.0b013e3182619cb4. [DOI] [PubMed] [Google Scholar]
- 15.Verdijk P, Aarntzen EH, Lesterhuis WJ, et al. Limited amounts of dendritic cells migrate into the T-cell area of lymph nodes but have high immune activating potential in melanoma patients. Clin Cancer Res. 2009;15:2531–40. doi: 10.1158/1078-0432.CCR-08-2729. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–9. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prue RL, Vari F, Radford KJ, et al. A phase I clinical trial of CD1c (BDCA-1)+ dendritic cells pulsed with HLA-A*0201 peptides for immunotherapy of metastatic hormone refractory prostate cancer. J Immunother. 2015;38:71–6. doi: 10.1097/CJI.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 18.Schreibelt G, Bol KF, Westdorp H, et al. Effective Clinical Responses in Metastatic Melanoma Patients after Vaccination with Primary Myeloid Dendritic Cells. Clin Cancer Res. 2016;22:2155–66. doi: 10.1158/1078-0432.CCR-15-2205. [DOI] [PubMed] [Google Scholar]
- 19.Tel J, Aarntzen EH, Baba T, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–75. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 20.Takacs E, Boto P, Simo E, et al. Immunogenic Dendritic Cell Generation from Pluripotent Stem Cells by Ectopic Expression of Runx3. J Immunol. 2016 doi: 10.4049/jimmunol.1600034. [DOI] [PubMed] [Google Scholar]
- 21.Silk KM, Silk JD, Ichiryu N, et al. Cross-presentation of tumour antigens by human induced pluripotent stem cell-derived CD141(+)XCR1+ dendritic cells. Gene Ther. 2012;19:1035–40. doi: 10.1038/gt.2011.177. [DOI] [PubMed] [Google Scholar]
- 22.Zeng J, Wu C, Wang S. Antigenically Modified Human Pluripotent Stem Cells Generate Antigen-Presenting Dendritic Cells. Sci Rep. 2015;5:15262. doi: 10.1038/srep15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Vries IJ, Lesterhuis WJ, Scharenborg NM, et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–100. [PubMed] [Google Scholar]
- 24.McIlroy D, Gregoire M. Optimizing dendritic cell-based anticancer immunotherapy: maturation state does have clinical impact. Cancer Immunol Immunother. 2003;52:583–91. doi: 10.1007/s00262-003-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carreno BM, Becker-Hapak M, Huang A, et al. IL-12p70-producing patient DC vaccine elicits Tc1-polarized immunity. J Clin Invest. 2013;123:3383–94. doi: 10.1172/JCI68395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linette GP, Carreno BM. Dendritic cell-based vaccines: Shining the spotlight on signal 3. Oncoimmunology. 2013;2:e26512. doi: 10.4161/onci.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munz C, Dao T, Ferlazzo G, de Cos MA, Goodman K, Young JW. Mature myeloid dendritic cell subsets have distinct roles for activation and viability of circulating human natural killer cells. Blood. 2005;105:266–73. doi: 10.1182/blood-2004-06-2492. [DOI] [PubMed] [Google Scholar]
- 28.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 29.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015;125:3413–21. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin Cancer Res. 2016;22:1897–906. doi: 10.1158/1078-0432.CCR-15-1399. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Bosch ML, Salgaller ML. Current methods for loading dendritic cells with tumor antigen for the induction of antitumor immunity. J Immunother. 2002;25:289–303. doi: 10.1097/00002371-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Andersen RS, Thrue CA, Junker N, et al. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 2012;72:1642–50. doi: 10.1158/0008-5472.CAN-11-2614. [DOI] [PubMed] [Google Scholar]
- 33.Linnemann C, van Buuren MM, Bies L, et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nature Medicine. 2014;21:81–5. doi: 10.1038/nm.3773. [DOI] [PubMed] [Google Scholar]
- 34.Verdegaal EM, de Miranda NF, Visser M, et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;536:91–5. doi: 10.1038/nature18945. [DOI] [PubMed] [Google Scholar]
- 35.Tran E, Ahmadzadeh M, Lu YC, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–90. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran E, Robbins PF, Lu Y-C, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. New England Journal of Medicine. 2016;375:2255–62. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmström MO, Riley CH, Svane IM, Hasselbalch HC, Andersen MH. The CALR exon 9 mutations are shared neoantigens in patients with CALR mutant chronic myeloproliferative neoplasms. Leukemia. 2016;30:2413–6. doi: 10.1038/leu.2016.233. [DOI] [PubMed] [Google Scholar]
- 38.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyder A, Makarov V, Merghoub T, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. New England Journal of Medicine. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2016 doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carreno BM, Magrini V, Becker-Hapak M, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–8. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran E, Robbins PF, Lu YC, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med. 2016;375:2255–62. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castle JC, Kreiter S, Diekmann J, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72:1081–91. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 47.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–81. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yadav M, Jhunjhunwala S, Phung QT, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–6. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 49.Kranz LM, Diken M, Haas H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 50.Kreiter S, Diken M, Selmi A, et al. FLT3 ligand enhances the cancer therapeutic potency of naked RNA vaccines. Cancer Res. 2011;71:6132–42. doi: 10.1158/0008-5472.CAN-11-0291. [DOI] [PubMed] [Google Scholar]
- 51.Kreiter S, Vormehr M, van de Roemer N, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–6. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 53.Chiang CL, Kandalaft LE, Tanyi J, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res. 2013;19:4801–15. doi: 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251–63. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 55.Rosenblatt J, Stone RM, Uhl L, et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Science Translational Medicine. 2016;8:368ra171–368ra171. doi: 10.1126/scitranslmed.aag1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhodapkar MV, Sznol M, Zhao B, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med. 2014;6:232ra51. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato M. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. International Immunology. 2006;18:857–69. doi: 10.1093/intimm/dxl022. [DOI] [PubMed] [Google Scholar]
- 58.Lahoud MH, Ahmet F, Kitsoulis S, et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J Immunol. 2011;187:842–50. doi: 10.4049/jimmunol.1101176. [DOI] [PubMed] [Google Scholar]
- 59.Li J, Ahmet F, Sullivan LC, et al. Antibodies targeting Clec9A promote strong humoral immunity without adjuvant in mice and non-human primates. Eur J Immunol. 2015;45:854–64. doi: 10.1002/eji.201445127. [DOI] [PubMed] [Google Scholar]
- 60.Tullett KM, Leal Rojas IM, Minoda Y, et al. Targeting CLEC9A delivers antigen to human CD141+ DC for CD4+ and CD8+T cell recognition. JCI Insight. 2016;1:e87102. doi: 10.1172/jci.insight.87102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato Y, Zaid A, Davey GM, et al. Targeting Antigen to Clec9A Primes Follicular Th Cell Memory Responses Capable of Robust Recall. J Immunol. 2015;195:1006–14. doi: 10.4049/jimmunol.1500767. [DOI] [PubMed] [Google Scholar]
- 62.Park HY, Light A, Lahoud MH, Caminschi I, Tarlinton DM, Shortman K. Evolution of B cell responses to Clec9A-targeted antigen. J Immunol. 2013;191:4919–25. doi: 10.4049/jimmunol.1301947. [DOI] [PubMed] [Google Scholar]
- 63.Hartung E, Becker M, Bachem A, et al. Induction of potent CD8 T cell cytotoxicity by specific targeting of antigen to cross-presenting dendritic cells in vivo via murine or human XCR1. J Immunol. 2015;194:1069–79. doi: 10.4049/jimmunol.1401903. [DOI] [PubMed] [Google Scholar]
- 64.Terhorst D, Fossum E, Baranska A, et al. Laser-assisted intradermal delivery of adjuvant-free vaccines targeting XCR1+ dendritic cells induces potent antitumoral responses. J Immunol. 2015;194:5895–902. doi: 10.4049/jimmunol.1500564. [DOI] [PubMed] [Google Scholar]
- 65.Fossum E, Grodeland G, Terhorst D, et al. Vaccine molecules targeting Xcr1 on cross-presenting DCs induce protective CD8+ T-cell responses against influenza virus. Eur J Immunol. 2015;45:624–35. doi: 10.1002/eji.201445080. [DOI] [PubMed] [Google Scholar]
- 66.Liu H, Moynihan KD, Zheng Y, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–22. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rovati B, Mariucci S, Manzoni M, Bencardino K, Danova M. Flow cytometric detection of circulating dendritic cells in healthy subjects. Eur J Histochem. 2008;52:45–52. doi: 10.4081/1185. [DOI] [PubMed] [Google Scholar]
- 68.Anandasabapathy N, Breton G, Hurley A, et al. Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers. Bone Marrow Transplant. 2015;50:924–30. doi: 10.1038/bmt.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nina Bhardwaj PA, Marc Ernstoff, Brent Hanks, Mark Albertini, Jason Luke, Michael Yellin, Tibor Keler, Thomas Davis, Andrea Crocker, Laura Vitale, Chihiro Morishima, Philip Friedlander, Martin Cheever, Steven Fling. A Phase II Randomized Study of CDX-1401, a Dendritic Cell Targeting NY-ESO-1 Vaccine, in Patients with Malignant Melanoma Pre-Treated with Recombinant CDX-301, a Recombinant Human Flt3 Ligand. Journal of clinical oncology. 2016;34(suppl) abstr 9589. [Google Scholar]
- 70.Salmon H, Idoyaga J, Rahman A, et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44:924–38. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hemont C, Neel A, Heslan M, Braudeau C, Josien R. Human blood mDC subsets exhibit distinct TLR repertoire and responsiveness. J Leukoc Biol. 2013;93:599–609. doi: 10.1189/jlb.0912452. [DOI] [PubMed] [Google Scholar]
- 72.Corrales L, Glickman LH, McWhirter SM, et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015;11:1018–30. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sehgal K, Ragheb R, Fahmy TM, Dhodapkar MV, Dhodapkar KM. Nanoparticle-mediated combinatorial targeting of multiple human dendritic cell (DC) subsets leads to enhanced T cell activation via IL-15-dependent DC crosstalk. J Immunol. 2014;193:2297–305. doi: 10.4049/jimmunol.1400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hildner K, Edelson BT, Purtha WE, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fuertes MB, Kacha AK, Kline J, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bachem A, Guttler S, Hartung E, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–81. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crozat K, Guiton R, Contreras V, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–92. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiang MC, Tullett KM, Lee YS, et al. Differential uptake and cross-presentation of soluble and necrotic cell antigen by human DC subsets. Eur J Immunol. 2016;46:329–39. doi: 10.1002/eji.201546023. [DOI] [PubMed] [Google Scholar]
- 79.Flinsenberg TW, Compeer EB, Koning D, et al. Fcgamma receptor antigen targeting potentiates cross-presentation by human blood and lymphoid tissue BDCA-3+ dendritic cells. Blood. 2012;120:5163–72. doi: 10.1182/blood-2012-06-434498. [DOI] [PubMed] [Google Scholar]
- 80.Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med. 2013;210:1035–47. doi: 10.1084/jem.20121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 82.Sathyanarayanan V, Neelapu SS. Cancer immunotherapy: Strategies for personalization and combinatorial approaches. Mol Oncol. 2015;9:2043–53. doi: 10.1016/j.molonc.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gardner A, Ruffell B. Dendritic Cells and Cancer Immunity. Trends in Immunology. 2016;37:855–65. doi: 10.1016/j.it.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


