Abstract
Establishing the in vivo diagnosis of Alzheimer’s disease (AD) or other dementias relies on clinical criteria; however, the accuracy of these criteria can be limited. The diagnostic accuracy is 77% for a clinical diagnosis of AD, even among experts. We performed a review through PubMed of articles related to specific diagnostic modalities, including APOE genotyping, cerebrospinal fluid (CSF) testing, fludeoxyglucose F 18 positron emission tomography (PET), amyloid PET, tau PET, computed tomography (CT), single-photon emission CT, magnetic resonance imaging (MRI), and B12 and thyroid-stimulating hormone screening, to determine the specificity and sensitivity of each test used in the clinical diagnosis of AD. We added a novel immunomagnetic reduction assay that provides ultrasensitivity for analyzing the levels of plasma tau and beta amyloid 42 (Aβ42). The sensitivity and specificity of the current diagnostic approach (structural CT or MRI with screening labs) remain low for clinical detection of AD and are primarily used to exclude other conditions. Because of limited diagnostic capabilities, physicians do not feel comfortable or skilled in rendering a clinical diagnosis of AD. Compounding this problem is the fact that inexpensive, minimally invasive diagnostic tests do not yet exist. Biomarkers (obtained through CSF testing or PET imaging), which are not routinely incorporated in clinical practice, correlate well with pathologic changes. While PET is particularly costly and difficult to assess, CSF measures of tau and beta amyloid are not costly, and these tests may be worthwhile when the tiered approach proposed here warrants further testing. There is a need for developing bloodborne biomarkers that can aid in the clinical diagnosis of AD. Here we present a streamlined questionnaire-enriched, biomarker-enriched approach that is more cost-effective than the current diagnosis of exclusion and is designed to increase clinical confidence for a diagnosis of dementia due to AD.
Keywords: Biomarkers, Clinical assessment, Dementia, Diagnostic algorithm
Introduction
The original criteria described by the working group formed by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA) (referred to as the NINCDS-ADRDA criteria) [1] set forth tiers of probability for confidence in a diagnosis of Alzheimer’s disease (AD). The highest tier was “definite AD”, which was autopsy confirmed. The next tier was “probable AD”, which presented as a progressive amnestic disorder with dementia that affected two areas of cognition and functional impairment without other causes identified. “Possible AD” was described as a progressive amnestic syndrome that could have other contributors to the cognitive impairment (e.g., stroke, epilepsy).
These definitions stayed constant for almost 3 decades. In 2011, the National Institute on Aging–Alzheimer's Association (NIA-AA) working group met to review and update the diagnostic criteria (Table 1). The first principle is that, to have AD, one must have dementia [2]. The updated definition of dementia is cognitive impairment that interferes with the ability to function at work or at usual activities that is associated with a decline from a previous level of functioning and that is not caused by delirium or a psychiatric disorder [2]. Features discovered on history and examination should include the involvement of at least two cognitive domains, such as memory, reasoning and judgment, visuospatial, language, personality, behavior, and comportment. According to the 2011 NIA-AA guidelines, the diagnosis of AD requires that certain core criteria be met [2, 3]: report of cognitive concern by patient, caregiver, or clinician; gradual onset over months to years; evidence of longitudinal cognitive decline; differential diagnosis that rules out vascular, traumatic, and medical causes of cognitive decline; and objective evidence of impairment in two or more cognitive domains and inability to function at work or usual activities. Like the older NINCDS-ADRDA criteria, the tiers of probability included “proven AD”, “probable AD”, and “possible AD”. In this schema, proven AD is that which is confirmed by widely accepted neuropathology criteria at autopsy or after biopsy. Probable AD refers to evidence of amnestic predominant dementia that has an insidious onset, history of progressive worsening, and no evidence of cardiovascular disease, dementia with Lewy bodies, frontotemporal dementia, or aphasia. Possible AD refers to amnestic predominant dementia that has an atypical course or an etiologically mixed presentation, such as possible comorbidities that could contribute to the dementia presentation.
Table 1.
Diagnostic guidelines for Alzheimer’s disease from the National Institute on Aging–Alzheimer’s Association
Adapted from McKhann et al. [2]
| Disease state | Definition |
|---|---|
| Dementia core criteria |
Cognitive or behavioral symptoms that interfere with the ability to function at work or at usual activities, represent a decline from previous levels of functioning, and are not explained by delirium or major psychiatric disorder The cognitive or behavioral impairment involves a minimum of two of the following domains: impaired ability to acquire and remember new information, impaired reasoning and handling of complex tasks, impaired visuospatial abilities, impaired language functions, and changes in personality or behavior or comportment |
| Probable Alzheimer’s dementia | Meets criteria for dementia, in addition to insidious gradual onset, history of worsening cognition by report or observation, or initial and most prominent cognitive deficits in either amnestic (impaired learning or recent recall) or non-amnestic (language, visuospatial, or executive dysfunction) |
| Possible Alzheimer’s dementia |
Above criteria with: Atypical course (sudden onset, insufficient historical detail, or objective progressive decline) or etiologically mixed presentation (meets all core clinical criteria, but has evidence of cerebrovascular disease, features of dementia with Lewy bodies, or evidence of another neurologic or non-neurologic disease or medication that could affect cognition) |
| Proven Alzheimer’s dementia | Patient meets the clinical and cognitive criteria for AD dementia, and the neuropathologic examination demonstrates the presence of the AD pathology |
The NIA-AA working group reviewed biomarker evidence to support a clinical diagnosis of AD. They defined AD pathophysiology as either (1) beta amyloid (Aβ) seen on cerebrospinal fluid (CSF) testing or amyloid positron emission tomography (PET) or (2) neuronal injury as documented by demonstration of tau on CSF testing, fludeoxyglucose F 18 (18F-FDG) PET, or evidence of atrophy on structural magnetic resonance imaging (MRI). They concluded that probable AD with evidence of AD pathophysiology strengthened the probability of AD as the pathology but left this criterion as informative but not essential. In contrast, the International Working Group (IWG) [4] described the specific clinical phenotype (typical AD) as episodic memory impairment that occurred gradually and progressively and was reported by the patient or informant as having persisted for more than 6 months; in addition, the patient must display objective evidence of an amnestic syndrome of the hippocampal type based on significantly impaired performance on an episodic memory test. In contrast to the report of the NIA-AA working group, the IWG did consider biomarker evidence as not only supportive but as also essential. They defined in vivo evidence of Alzheimer’s pathology as one of the following: decreased Aβ1–42, together with increased total tau (T-tau) or phospho-tau (P-tau) in the CSF; increased tracer retention on amyloid PET; or AD autosomal dominant mutation present in PSEN1, PSEN2, or APP.
The consequence of guidelines that fail to incorporate biomarker evidence of support is that biomarker evidence is not routinely used in clinical practice. Historically, the diagnosis of AD has been approached by excluding other health conditions. In other words, the diagnosis of AD has been and continues to be a diagnosis of exclusion.
Inaccuracy of Diagnosis of Alzheimer’s Disease in Clinical Practice
The timely diagnosis of AD is an unmet need in clinical practice. Physicians are reluctant to make a diagnosis of dementia and, more specifically, a diagnosis of dementia caused by AD. The consequence of this reluctance is that diagnosis is delayed by an average of 2–3 years after symptom onset [5, 6]. In addition, one study evaluating the clinical diagnosis found that up to 50% of patients with any form of dementia are not formally diagnosed during life [7].
Not only is there a delay in diagnosis, but there is also evidence that a diagnosis of AD can often be quite inaccurate. Twenty-five percent of patients clinically diagnosed with probable AD during their lifetime did not have evidence of AD at autopsy [8]. In a clinical imaging/pathology series of 57 individuals clinically diagnosed with AD, 13 (23%) had no (n = 7) or sparse (n = 6) Aβ plaques at autopsy. Twelve of these individuals were diagnosed neuropathologically with a dementia disease other than AD, most frequently caused by aggregation of tau. Thus, diagnostic accuracy is 77% for a clinical diagnosis of AD, even among the experts. In another study, florbetaben PET was consistent with histopathologic results in all 12 patients for whom standard uptake value ratios (SUVRs) were available [9].
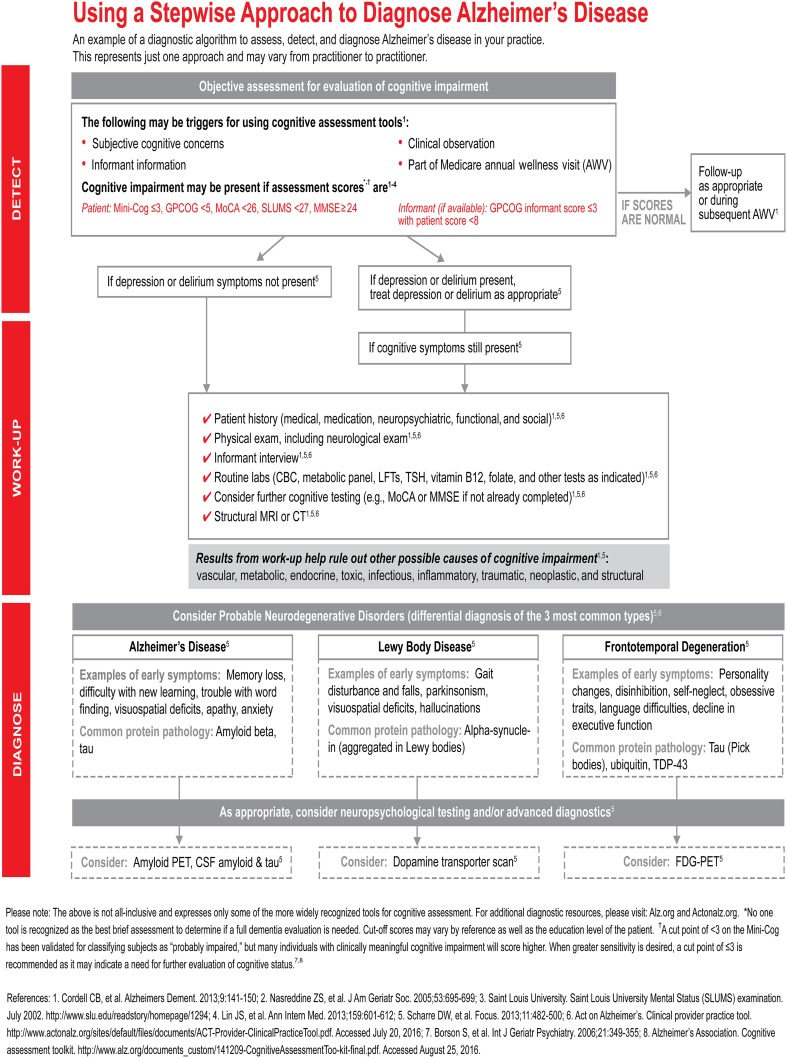
These data show that it is imperative that the clinical diagnosis be improved without a commensurate increase in cost. Thus, the diagnosis of AD dementia must transition from a diagnosis of exclusion to a diagnosis of inclusion. A solution might include a tiered approach with the incorporation of questionnaires and biomarkers. A representative outline of current practice is shown in Fig. 1.
Fig. 1.
A proposed stepwise approach to assessing a patient for dementia. It incorporates details that include the traditional diagnosis of exclusion while preparing the reader for the possibility of incorporating advanced biomarkers. CBC complete blood count, CSF cerebrospinal fluid, CT computed tomography, FDG-PET fludeoxyglucose F 18 positron emission tomography, GPCOG General Practitioner Assessment of Cognition, LFTs liver function tests, Mini-Cog Mini-Cognitive Assessment Instrument, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, MRI magnetic resonance imaging, PET positron emission tomography, SLUMS Saint Louis University Mental Status, TDP-43 TAR DNA-binding protein 43, TSH thyroid-stimulating hormone.
Copyright Eli Lilly and Co., all rights reserved. Used with permission
A New Tiered Approach to Diagnosing Alzheimer’s Disease
A new tiered approach to diagnosing AD could allow a more accurate diagnosis with lower cost and higher sensitivity. Herein, a revised approach to the current diagnostic algorithm is proposed. The rationale for each step is explained. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Step 1: Structured Questionnaires
Informant-based questionnaires could be incorporated into the diagnostic process. These questionnaires could be used routinely in both clinical and research settings to differentiate between individuals with amnestic mild cognitive impairment (MCI) and AD from individuals who are cognitively normal [10, 11]. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) and the Ascertain Dementia 8-Item Informant Questionnaire (AD8) have demonstrated good diagnostic accuracy for AD and have been found to correlate well with other conventional cognitive screening tests, such as the Mini-Mental State Examination (MMSE) [12, 13].
The Alzheimer’s Questionnaire (AQ) is a 21-item, informant-based assessment designed for ease of use in the clinical setting that has demonstrated high sensitivity and specificity for both amnestic MCI and AD [14, 15]. The concurrent validity of the AQ with other established measures of cognition was demonstrated by Malek-Ahmadi et al. [16], who found that the AQ correlates strongly with the Clinical Dementia Rating Sum of Boxes and correlates moderately with the MMSE and Montreal Cognitive Assessment (MoCA).
The AD8 is also a screening interview. It is a brief, sensitive screening measure that reliably differentiates between individuals with and without dementia. The AD8 comprises eight yes/no questions asked of an informant to rate change by querying memory, orientation, judgment, and function [12], and it takes approximately 2–3 min for the informant to complete. The AD8 has a sensitivity of 84% and specificity of 80% as well as excellent ability to discriminate between non-demented older adults and those with mild dementia (92%) regardless of the cause of impairment. The sensitivity and specificity of AD8 were determined in a 2006 study involving 255 patient-informant dyads, and these data were subsequently compared with the independently derived Clinical Dementia Rating and with patients’ performance on neuropsychologic tests [12]. Like the AQ, the AD8 is highly correlated with the Clinical Dementia Rating and neuropsychologic testing.
The IQCODE was developed as a way of measuring cognitive decline from a premorbid level using informant reports. Each item is rated on a 5-point scale from 1, meaning “much better”, to 5, meaning “much worse”, and the ratings are averaged over the 16 items to give a score ranging from 1 to 5, with 3 representing no change on any item. In clinical situations, a screening cutoff of 3.44+ on the Short IQCODE is a reasonable compromise for balancing sensitivity and specificity.
The informant-based questionnaires as a group have high sensitivity, specificity, and area under the curve (AUC) in differentiating normal controls from AD patients. The sensitivity and specificity of the AD8 are 85% and 86%, respectively, with an AUC of 0.83 [12]. The sensitivity and specificity of the IQCODE are 79% and 82%, respectively, with an AUC of 0.85 [13]. The sensitivity and specificity of the AQ are 99% and 96%, respectively, with an AUC of 0.99. All of these questionnaires are simple to administer, informant-based, and not time-consuming. More importantly, they allow structure for the primary care physician or specialist to capture incident cognitive decline.
For the purposes of specificity, additional questions to detect dementia with Lewy bodies (e.g., the Lewy Body Composite Risk Score [LBCRS]) [17] and frontotemporal dementia [18] could be added without significant increases in the burden of time.
Step 2: Aggregate Risk Analysis
Epidemiologic studies have shown that a variety of health conditions increase the risk for AD dementia. The patient’s medical history is gathered at the time of the consultation to ascertain whether medical conditions such as diabetes, hypertension, hypercholesterolemia, head injury, or cardiovascular disease are present. During the past decade, models have been proposed to quantify individual risk for developing AD on the basis of a patient’s individual demographics. These models incorporate age, family history, health conditions, and other factors to estimate risk (Table 2). On the basis of aggregate risk scoring, a score of less than 5 is low risk, 5-12 is moderate risk, and 12 and above is high risk [19, 20]. Doing an aggregate risk analysis during the consultation enriches the probability of AD if the score is high.
Table 2.
Aggregate risk scoring for risk factors for Alzheimer’s disease
| Risk factor | Odds ratio |
|---|---|
| A first-degree relative with AD | 3.0 |
| History of head injury with LOC | 2.0 |
| Age >65 years | 1.0 |
| Age >75 years | 4.0 |
| Age >85 years | 16.0 |
| Education <7 years | 3.6 |
| Female sex | 1.5 |
| Systolic BP >140 mmHg | 2.2 |
| BMI >30 kg/m2 | 2.3 |
| Cholesterol >6.5 mmol/l | 1.9 |
| APOE ε4 positivity | 4.0 |
| History of stroke | 2.4 |
| History of myocardial infarction | 2.5 |
| Untreated type 2 diabetes mellitus | 2.0 |
| Low physical activity (sedentary) | 1.7 |
| Continuation of smoking | 2.3 |
BMI body mass index, BP blood pressure, LOC loss of consciousness
Step 3: Bedside Cognitive Screening
A variety of brief cognitive tests were developed for assessing general cognitive function. The use of the AD8 in conjunction with a brief assessment, such as assessing the patient’s ability to remember a word list, could improve clinicians' ability to detect dementia in the primary care setting to 97% for dementia and 91% for MCI [12]. Below are a few of the more commonly used and easier to administer measures. Several diagnostic tests are now available for use in primary care as alternatives to the MMSE.
Mini-Mental State Examination (MMSE)
The MMSE is a copyrighted test that has frequently been used for the initial assessment of cognitive impairment. The MMSE has increasing sensitivity as the decline of the score over time is taken into account [21]. It is quick and easy to administer and can track the overall progression of cognitive decline, but it is not considered to be a good test for the definitive diagnosis of AD [22].
Mini-Cognitive Assessment Instrument (Mini-Cog)
The Mini-Cog combines an uncued three-item recall test with a clock-drawing test that serves as a recall distractor [23]. The Mini-Cog and the MMSE have similar sensitivity (76% vs. 79%, respectively) and specificity (89% vs. 88%, respectively) for dementia. The shortness of the Mini-Cog is a distinct advantage when the goal is to improve recognition of cognitive impairment in primary care [23].
Short Blessed Test (SBT)
The SBT is easily administered by a nonphysician and has been shown to discriminate among mild, moderate, and severe cognitive deficits [24, 25]. It consists of the items in the Blessed Orientation-Memory-Concentration Test, includes three orientation questions (month, year, and time of day), counting from 20 to 1, saying the months backward, and recalling a five-item name and address memory phrase [24]. The SBT is quite sensitive to early cognitive changes due to AD.
Saint Louis University Mental Status (SLUMS)
The SLUMS is a 30-point, 11-item, clinician-administered screening questionnaire that tests for attention, numeric calculation, immediate and delayed recall, animal naming, digit span, clock drawing, figure recognition/size differentiation, and immediate recall of facts from a paragraph [26]. In particular, the clock drawing test is designed to assess impairment in executive function. Due to copyright issues, the Veterans Administration has stopping using the MMSE, and they and others now use the SLUMS instead.
Montreal Cognitive Assessment (MoCA)
The MoCA is a brief cognitive screening tool with a high sensitivity and specificity for detecting MCI in patients who perform within the normal range of the MMSE [27]. The limitation of the MoCA may be its more complex interpretation, and it requires training to be administered properly.
Step 4: Physical and Neurologic Examination
The potential for reversible dementias is commonly pursued in the diagnostic consideration but the yield is quite low. In one meta-analysis, conditions requiring neuroimaging made up only 2.2% of cases with reversible cause seen in less than 10% of total cases. In fact, the meta-analysis found that only 0.6% of dementia cases actually reversed partially or fully [28].
A comprehensive physical and neurologic examination can detect incident focality (e.g., hemiparesis, asymmetry of tone or reflexes, hemi-sensory changes) and gait abnormalities. This examination can be used to detect cerebrovascular disease, mass lesions, parkinsonism, or communicating hydrocephalus [29]. Patients who show no salient neurologic abnormalities on examination have a lower probability that imaging studies will find an abnormality that needs intervention.
Though controversial and counter to conventional practice, we recommend that in the absence of abnormal neurologic findings on examination, structural imaging should not be obtained. The current practice guideline is to incorporate structural imaging as part of the diagnostic evaluation for AD [2, 3] to exclude other conditions. This practice comes from the previous American Academy of Neurology guidelines. In a class II study, 5% of patients had a clinically significant structural lesion (i.e., a potentially treatable lesion) but no features in their history or examination that would have predicted the lesion. Thus, the recommendation is to include CT or MRI in the patient’s initial evaluation to avoid missing any treatable conditions [30]. However, this percentage means the number needed to treat (NNT) is 1 in 20. At an estimated cost of $1000 per scan, that is $20,000 per identified treatable condition.
The obvious question is, “Does obtaining structural imaging add value?” In one study, data from MRI did not significantly improve discrimination performance in predicting all causes of dementia beyond that of a model that incorporated demographic, cognitive, health, lifestyle, physical function, and genetic data. In other words, clinical information might be just as good as structural imaging [31]. Another study found that CT impacted the diagnosis only 12% of the time and treatment 11% of the time [32]. Other recommendations include that structural imaging is useful only with the following caveats, as recommended by Wollman and Prohovnik: “we suggest that neuroimaging should be considered: (1) when clinical expertise is insufficient; (2) as a complement to specific likelihood ratios; and (3) in specific types of patients, for whom clinical evaluation is inappropriate or inadequate” [33]. Efforts are underway to develop algorithms to enhance the utility of structural imaging in clinical practice and to increase the sensitivity for detection and differentiation of dementia [34, 35]. Until such time as these technologies mature, our recommendation is to obtain structural imaging only when abnormal neurologic findings are found on examination for the purposes of an initial dementia evaluation. This position statement does not exclude the utility of structural imaging in the tiered approach. If there is any evidence of abnormality on neurologic examination that is referable to the central nervous system, then structural imaging is warranted.
Step 5: Laboratory Screening Tests Combined with Advanced Bloodborne Biomarkers
Like structural imaging, screening for deficiencies in B12 and thyroid stimulating hormone are low-cost, high-yield tests for identifying reversible causes of dementia [2, 36]. However, they are insufficient for detecting AD, because they are only used to exclude other conditions.
To move from a diagnosis of exclusion to a diagnosis of inclusion, one must consider including apolipoprotein E (APOE) genotyping. Also controversial, the rationale for doing so is as follows. The lifetime risk for developing AD for a patient who is homozygotic for the APOE ε4 is 91%, and the lifetime risk for a patient who is heterozygotic for APOE ε4 is 47% [37]. APOE ε4 carrier status is highly predictive of AD; an APOE ε4 carrier who is symptomatic has a 94–97% chance of having AD [38]. In one study, the clinical diagnosis of AD improved from 55% to 84% when APOE ε4 carrier status was added to the model [39]. In addition, 50% of MCI subjects who are APOE ε4 carriers progress to AD dementia in 3 years compared to 20% of non-APOE ε4 carriers [40]. MCI subjects (amnestic subtype) who are APOE ε4 carriers convert to AD >99% of the time [40]. APOE ε4 carriers are 26 times more likely to progress in cognitive decline. In addition, the presence of the APOE ε4 allele predicts AD pathology on PET imaging [41].
APOE genotyping could be added to disease-associated biomarkers to improve diagnostic yield. Disease-associated biochemical markers are present in the blood; however, the measurable amounts are only 10% of those present in the CSF. As a result, the sensitivity and accuracy of the technology used for measuring the levels of these disease-associated proteins in the blood are critical, in addition to other issues that also affect CSF biomarker measurements [42–45]. Recent advances in technology have improved the sensitivity and accuracy of the measurement of these disease biochemicals in the blood. Among these technologies, the immunomagnetic reduction (IMR) technology stands out in its ability to measure three important AD pathology-associated proteins (Aβ40, Aβ42, and tau) [46]. Due to the unique principles on which this technology is based, IMR assays show ultrasensitivity in the detection of low amounts of the proteins in the non-blood-cell fraction of blood samples, plasma, collected from subjects diagnosed with preclinical AD and clinical AD dementia [47–50]. In a pilot study, the main objective was to assess the Aβ40, Aβ42, and tau levels measured by the ultrasensitive IMR assays in plasma samples. When two cohorts were combined with a cutoff value of 382.68 (pg/ml)2, the product of Aβ2 and tau achieved 92% accuracy with a sensitivity of 96% and a specificity of 90% [46]. Although these new technologies have not yet been directly correlated with neuropathologic findings, there have been studies that attempt to correlate CSF levels and pathology. A 2008 study by Chiu et al. evaluated the combination of an abnormally low Aβ2 level in the CSF and an abnormally high tau level in the CSF and found that this combination predicted the presence of AD pathologic features with a sensitivity of 91.6%, a specificity of 85.7%, and an overall accuracy of 90.2% [48].
Step 6: Apply IWG Criteria in the Clinical Diagnosis of AD
As mentioned in the introduction, IWG [4] incorporates biomarker data into the clinical diagnosis. Biomarker evidence of AD increases the probability that a patient has AD. By the IWG-2 criteria, Kaplan-Meier survival probability estimates of progressing to AD dementia exceed 90% in 5 years, suggesting positive biomarker evidence enriches the probability of progression [4]. However, the question of which specific biomarker is most predictive remains unanswered.
Discussion
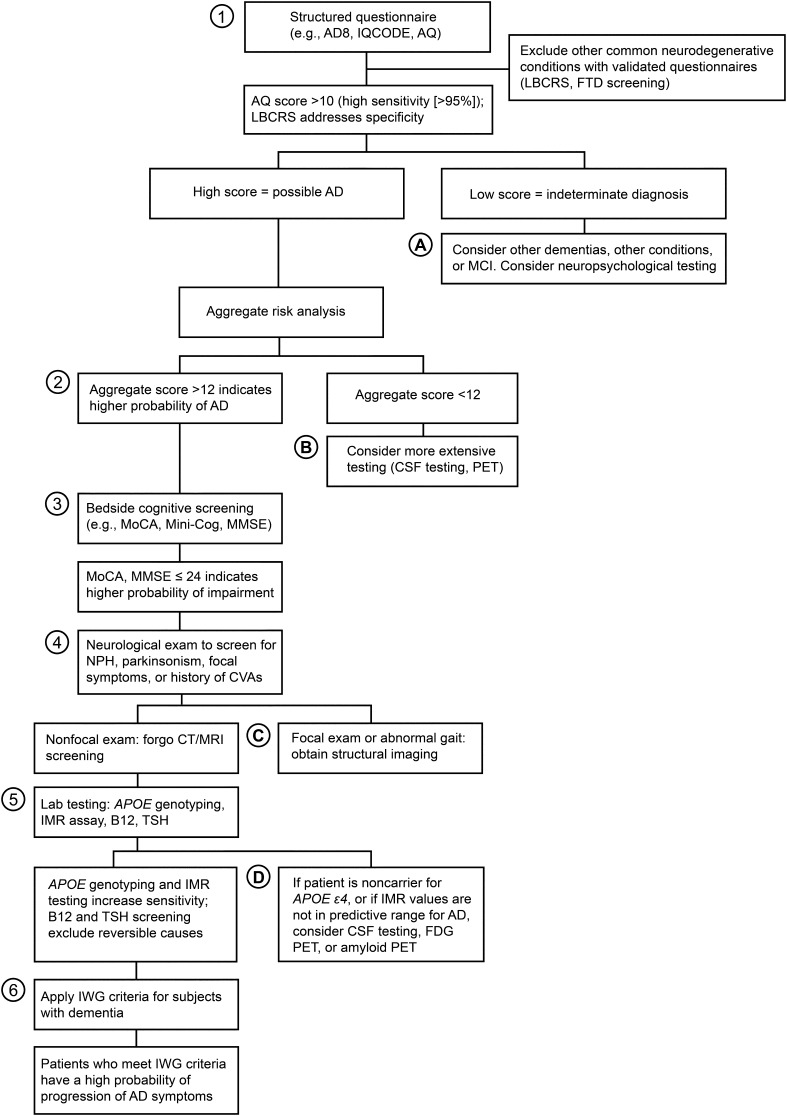
In this article, we propose a new algorithm for detecting dementia associated with AD. The rationale behind this algorithm is that the historical medical evaluation of dementia due to AD is inaccurate 25% of the time, that the diagnosis of AD has been, but should no longer be, a diagnosis of exclusion, that physicians do not feel confident in making a diagnosis of AD dementia, and that there is often a delay in diagnosis of 2–3 years [5, 6]. Technology is becoming available that greatly improves the diagnostic accuracy of AD. We present a novel algorithm that incorporates a structured history, an aggregate risk assessment, a cognitive screening measure, a through neurologic examination, and incorporation of biomarkers such as APOE and IMR assay. The use of this algorithm could improve the accuracy of a diagnosis of dementia due to AD to >90% without escalation of costs. This novel algorithm is summarized in Fig. 2. The algorithm must be assessed for efficacy but has the potential for accurately diagnosing a significant percentage of patients with AD, which could increase physician confidence. The resulting reduction in the time to diagnose patients might reduce the total morbidity. This outcome will certainly be the case with the advent and introduction of the disease-modifying therapies that are currently being developed.
Fig. 2.
New conceptual framework for assessment of dementia due to Alzheimer’s disease (AD). The primary diagnostic steps (tier 1) are indicated by the circled numbers. The supplemental diagnostic steps (tier 2) are indicated by the circled letters. The net cost per patient associated with tier 1 is less than $1200 USD. The sensitivity of tier 1 diagnosis is >90%; the specificity is yet to be determined. Because CT and MRI have a very low probability of supplying meaningful information, we advocate forgoing these studies in most patients. However, if normal pressure hydrocephalus (NPH), parkinsonism, focal symptoms, or a history of cerebrovascular accidents (CVAs) are present, these imaging studies are warranted. AD8 Ascertain Dementia 8-Item Informant Questionnaire, AQ Alzheimer’s Questionnaire, CSF cerebrospinal fluid, CT computed tomography, FDG fludeoxyglucose F 18, FTD frontotemporal dementia, IMR immunomagnetic reduction, IQCODE Informant Questionnaire on Cognitive Decline in the Elderly, IWG International Working Group, LBCRS Lewy Body Composite Risk Score, MCI mild cognitive impairment, Mini-Cog Mini-Cognitive Assessment Instrument, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, MRI magnetic resonance imaging, PET positron emission tomography, TSH thyroid-stimulating hormone.
Used with permission from Barrow Neurological Institute
A tiered approach allows additional tests to be added as needed. If there is focality on the examination (parkinsonism, gait abnormality, spasticity, or hemiparesis), then structural imaging could be added. If the subject is a non-APOE ε4 carrier, then amyloid PET or CSF testing for AD biomarkers could be attained. If the aggregate risk is low, then a more biomarker-intense approach could be taken. In short, this tiered system allows for flexibility. Since most AD dementia patients are likely to be APOE ε4 positive or are likely to have high screening scores for risk and impairment, in most cases, additional testing might not be necessary. Future research should include validation of the updated algorithm and determination of outcomes.
Acknowledgements
Funding for this study was provided by the National Institute on Aging Grant NIA P30 AG019610, the Arizona Alzheimer’s Research Consortium, and the Barrow Neurological Foundation. No direct funding was provided by external sources for the drafting of this manuscript. No funding was received for the publication of this article. Editorial assistance in the preparation of this manuscript was provided by the staff of Neuroscience Publications at Barrow Neurological Institute. Support for this assistance was funded by Barrow Neurological Institute. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Marwan N. Sabbagh, Lih-Fen Lue, Daniel Fayard, and Jiong Shi declare that they have no conflicts of interest pertaining to this project. Dr. Marwan N. Sabbagh holds stock or has ownership of Muses Labs, Inc.; Versanum, Inc.; and Brain Health, Inc. He serves in an advisory capacity for Biogen; Eli Lilly and Co.; and vTv Therapeutics, Inc. He is a Research Investigator for studies supported by AC Immune; Eli Lilly and Co.; Biogen; Merck & Co., Inc; vTv Therapeutics, Inc.; F. Hoffmann-La Roche, Ltd.; Lundbeck; Avid Radiopharmaceuticals; and Axovant Sciences, Inc. Dr. Lih-Fen Lue has research funding for a contracted research project from MagQu Co., Ltd. Dr. Jiong Shi is the Principal Investigator for studies supported by Merck & Co., Inc; Lundbeck; Genentech, Inc.; Biogen; Eli Lilly and Co.; and INC Research, LLC.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/CAD8F06039796B6E.
References
- 1.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 2.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 5.Balasa M, Gelpi E, Antonell A, Rey MJ, Sanchez-Valle R, Molinuevo JL, et al. Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology. 2011;76(20):1720–1725. doi: 10.1212/WNL.0b013e31821a44dd. [DOI] [PubMed] [Google Scholar]
- 6.Boise L, Camicioli R, Morgan DL, Rose JH, Congleton L. Diagnosing dementia: perspectives of primary care physicians. Gerontologist. 1999;39(4):457–464. doi: 10.1093/geront/39.4.457. [DOI] [PubMed] [Google Scholar]
- 7.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN, Force USPST Screening for dementia in primary care: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2003;138(11):927–937. doi: 10.7326/0003-4819-138-11-200306030-00015. [DOI] [PubMed] [Google Scholar]
- 8.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71(4):266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabbagh MN, Schauble B, Anand K, Richards D, Murayama S, Akatsu H, et al. Histopathology and florbetaben PET in patients incorrectly diagnosed with Alzheimer’s disease. J Alzheimers Dis. 2017;56(2):441–446. doi: 10.3233/JAD-160821. [DOI] [PubMed] [Google Scholar]
- 10.Sabbagh MN, Malek-Ahmadi M, Belden CM. The use of informant-based questionnaires in differentiating mild cognitive impairment from normal aging. Expert Rev Neurother. 2012;12(6):637–639. doi: 10.1586/ern.12.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry. 2005;20(9):827–834. doi: 10.1002/gps.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942–1948. doi: 10.1212/01.wnl.0000247042.15547.eb. [DOI] [PubMed] [Google Scholar]
- 13.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16(3):275–293. doi: 10.1017/S1041610204000390. [DOI] [PubMed] [Google Scholar]
- 14.Sabbagh MN, Malek-Ahmadi M, Kataria R, Belden CM, Connor DJ, Pearson C, et al. The Alzheimer’s questionnaire: a proof of concept study for a new informant-based dementia assessment. J Alzheimers Dis. 2010;22(3):1015–1021. doi: 10.3233/JAD-2010-101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malek-Ahmadi M, Davis K, Belden C, Laizure B, Jacobson S, Yaari R, et al. Validation and diagnostic accuracy of the Alzheimer’s questionnaire. Age Ageing. 2012;41(3):396–399. doi: 10.1093/ageing/afs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malek-Ahmadi M, Davis K, Belden CM, Sabbagh MN. Comparative analysis of the Alzheimer questionnaire (AQ) with the CDR sum of boxes, MoCA, and MMSE. Alzheimer Dis Assoc Disord. 2014;28(3):296–298. doi: 10.1097/WAD.0b013e3182769731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galvin JE. Improving the clinical detection of Lewy body dementia with the Lewy body composite risk score. Alzheimers Dement (Amst). 2015;1(3):316–324. doi: 10.1016/j.dadm.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scharre DW, Trzepacz PT. Evaluation of cognitive impairment in older adults. Focus J Lifelong Learn Psychiatry. 2013;11(4):482–500. doi: 10.1176/appi.focus.11.4.482. [DOI] [Google Scholar]
- 19.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5(9):735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 20.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive status of patients for the clinicians. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.de Souza LC, Sarazin M, Goetz C, Dubois B. Clinical investigations in primary care. Front Neurol Neurosci. 2009;24:1–11. doi: 10.1159/000197897. [DOI] [PubMed] [Google Scholar]
- 23.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: comparison of the mini-cog and mini-mental state examination in a multiethnic sample. J Am Geriatr Soc. 2005;53(5):871–874. doi: 10.1111/j.1532-5415.2005.53269.x. [DOI] [PubMed] [Google Scholar]
- 24.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 25.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/WNL.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 26.Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900–910. doi: 10.1097/01.JGP.0000221510.33817.86. [DOI] [PubMed] [Google Scholar]
- 27.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 28.Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163(18):2219–2229. doi: 10.1001/archinte.163.18.2219. [DOI] [PubMed] [Google Scholar]
- 29.Sabbagh MN, Nair AK. Approach to the geriatric neurology patient: the neurologic examination. In: Nair A, Sabbagh MN, editors. Geriatric neurology. Wiley: Oxford; 2014. pp. 71–84. [Google Scholar]
- 30.Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. doi: 10.1212/WNL.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 31.Stephan BC, Tzourio C, Auriacombe S, Amieva H, Dufouil C, Alperovitch A, et al. Usefulness of data from magnetic resonance imaging to improve prediction of dementia: population based cohort study. BMJ. 2015;350:h2863. doi: 10.1136/bmj.h2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Condefer KA, Haworth J, Wilcock GK. Clinical utility of computed tomography in the assessment of dementia: a memory clinic study. Int J Geriatr Psychiatry. 2004;19(5):414–421. doi: 10.1002/gps.1028. [DOI] [PubMed] [Google Scholar]
- 33.Wollman DE, Prohovnik I. Sensitivity and specificity of neuroimaging for the diagnosis of Alzheimer’s disease. Dialogues Clin Neurosci. 2003;5(1):89–99. [PMC free article] [PubMed] [Google Scholar]
- 34.Harper L, Barkhof F, Scheltens P, Schott JM, Fox NC. An algorithmic approach to structural imaging in dementia. J Neurol Neurosurg Psychiatry. 2014;85(6):692–698. doi: 10.1136/jnnp-2013-306285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6(2):67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobson SA. Laboratory medicine in psychiatry and behavioral science. Arlington, VA: American Psychiatric Association Publishing; 2012.
- 37.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 38.Relkin NR, Kwon YJ, Tsai J, Gandy S. The National Institute on Aging/Alzheimer’s Association recommendations on the application of apolipoprotein E genotyping to Alzheimer’s disease. Ann N Y Acad Sci. 1996;802:149–176. doi: 10.1111/j.1749-6632.1996.tb32608.x. [DOI] [PubMed] [Google Scholar]
- 39.Mayeux R, Saunders AM, Shea S, Mirra S, Evans D, Roses AD, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s Disease Centers Consortium on Apolipoprotein E and Alzheimer’s Disease. N Engl J Med. 1998;338(8):506–511. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- 40.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 41.Jack CR, Jr, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, et al. Age, sex, and APOE epsilon4 effects on memory, brain structure, and beta-amyloid across the adult life span. JAMA Neurol. 2015;72(5):511–519. doi: 10.1001/jamaneurol.2014.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31(3):357–367. doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 43.Henriksen K, O’Bryant SE, Hampel H, Trojanowski JQ, Montine TJ, Jeromin A, et al. The future of blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement. 2014;10(1):115–131. doi: 10.1016/j.jalz.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang JH, Vanderstichele H, Trojanowski JQ, Shaw LM. Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer’s disease. Methods. 2012;56(4):484–493. doi: 10.1016/j.ymeth.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 45.Chouraki V, Beiser A, Younkin L, Preis SR, Weinstein G, Hansson O, et al. Plasma amyloid-beta and risk of Alzheimer’s disease in the Framingham Heart Study. Alzheimers Dement. 2015;11(3):249–57 e1. doi:10.1016/j.jalz.2014.07.001. [DOI] [PMC free article] [PubMed]
- 46.Lue LF, Sabbagh MN, Chiu MJ, Leung N, Snyder N, Schmitz C et al. Plasma levels of Ab40, Ab42, and tau measured by ultra-sensitive immunomagnetic reduction assays identified probable Alzheimer’s dementia: Findings from two cohorts. Front Neurosci (in Press).
- 47.Chiu MJ, Yang SY, Chen TF, Chieh JJ, Huang TZ, Yip PK, et al. New assay for old markers-plasma beta amyloid of mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res. 2012;9(10):1142–1148. doi: 10.2174/156720512804142967. [DOI] [PubMed] [Google Scholar]
- 48.Chiu MJ, Chen YF, Chen TF, Yang SY, Yang FP, Tseng TW, et al. Plasma tau as a window to the brain-negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer’s disease. Hum Brain Mapp. 2014;35(7):3132–3142. doi: 10.1002/hbm.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tzen KY, Yang SY, Chen TF, Cheng TW, Horng HE, Wen HP, et al. Plasma Abeta but not tau is related to brain PiB retention in early Alzheimer’s disease. ACS Chem Neurosci. 2014;5(9):830–836. doi: 10.1021/cn500101j. [DOI] [PubMed] [Google Scholar]
- 50.Yang CC, Yang SY, Chieh JJ, Horng HE, Hong CY, Yang HC, et al. Biofunctionalized magnetic nanoparticles for specifically detecting biomarkers of Alzheimer’s disease in vitro. ACS Chem Neurosci. 2011;2(9):500–505. doi: 10.1021/cn200028j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.