Abstract
Synaptic dysfunction is thought to play important roles in the pathophysiology of many neurological diseases, including Alzheimer’s disease, Parkinson’s disease, and schizophrenia. Over the past few decades, there have been systematic efforts to collect postmortem brain tissues via autopsies, leading to the establishment of dozens of human brain banks around the world. From cryopreserved human brain tissues, it is possible to isolate detached-and-resealed synaptic terminals termed synaptosomes, which remain metabolically and enzymatically active. Synaptosomes have become important model systems for studying human synaptic functions, being much more accessible than ex vivo brain slices or primary neuronal cultures. Here we review recent advances in the establishment of human brain banks, the isolation of synaptosomes, their biological activities, and various analytical techniques for investigating their biochemical and ultrastructural properties. There are unique insights to be gained by directly examining human synaptosomes, which cannot be substituted by animal models. We will also discuss how human synaptosome research has contributed to better understanding of neurological disorders, especially Alzheimer’s disease.
Keywords: Brain bank, Neurochemistry, Neurodegeneration, Neuron, Subcellular fractionation, Synapse
Introduction
Within the context of neural networks, the synapse is the region around the point of contact between two neurons, and there are two basic types depending on signal transmission mechanisms: chemical synapses and electrical synapses [1]. There are hundreds of neuron types and possibly thousands of synapse types in the mammalian brain. The number of chemical synapses in the neocortex alone exceeds 100 trillion [2]. It is, therefore, a monumental task to understand the sheer complexity and diversity of synaptic networks in the brain.
A very important method for studying synaptic terminals in the brain is to isolate them from tissues by subcellular fractionation techniques, and these preparations enriched in nerve endings are called “synaptosomes”. Synaptosomes were first isolated in the late 1950s [3], and extensively studied by electron microscopy to understand their structural properties and internal contents [4–6]. When brain tissues are homogenized, the shear force causes the nerve terminals to be torn apart from the axons and dendrites, and they reseal into spherical structures which can be isolated by centrifugation methods. The isolation procedure is schematically presented in Fig. 1, using excitatory synapses of pyramidal neurons as an example. The presynaptic terminal of the glutamatergic synapse is usually a varicosity (bouton) along the axon, while the postsynaptic terminal is usually a spine protruding from the dendrite. During the homogenization process, the synaptic terminals break off and reseal into vesicular structures around 0.5–1 μm in diameter [7]. By electron microscopy, one may find vesicular objects of synaptic origin, containing synaptic vesicles or postsynaptic density (PSD), as well as membrane-enclosed fragments of myelin, mitochondria, microscomes, lysosomes, and plasma membranes [8–10].
Fig. 1.
Isolation of human synaptosomes. When postmortem brain tissues are homogenized, the synaptic terminals often detach from neuronal processes and reseal into vesicular objects. The crude synaptosome pellet can be collected by sedimentation. Further enrichment is usually carried out by discontinuous sucrose gradient centrifugation
Synaptosomes freshly prepared from brain tissues are metabolically and enzymatically active. They contain the molecular machinery necessary for the uptake, storage, and release of neurotransmitters, as well as the channels and receptors required for signal transduction. They are active in protein synthesis, maintenance of plasma membrane potential, and ion homeostasis [11]. Synaptosomes can be further fractionated to collect various subcomponents such as synaptic vesicles, postsynaptic densities (PSD), and synaptic plasma membranes [4, 12]. The study of synaptosomes has been one of the cornerstones of neurochemistry research, being instrumental to our understanding of the molecular machinery of neurotransmission and synaptic protein–protein interaction networks [11]. The range of biochemical responses that remain active in synaptosomes continue to expand as more assays are being developed. For instance, recent studies have demonstrated the ability to measure synaptosome insulin response from frozen mouse brains [13], as well as the upregulation and downregulation of SUMO-lylation in mouse synaptosomes by delivering enzymes linked to TAT peptides [14]. While earlier works in synaptosome research used animal brains, a growing number of studies have been directly applied to human brain tissues. In this review we will discuss how to access human brain tissues, how to prepare human synaptosomes, how to study synaptosomes, and what may be learned from such studies. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Human Brain Banks
The collection of human brains to study psychiatric and neurological disorders can be traced back to the late nineteenth century, but more systematic approaches toward establishing human brain banks started in the 1960s in the United States [15]. Today, there are dozens of human brain banks around the world dedicated to the collection of postmortem brain tissues after autopsies. Regional consortiums of brain banks, such as BrainNet Europe [16] and the NeuroBioBank [17] under the National Institutes of Health, USA, have been established in order to standardize the diagnosis and tissue collection procedures to provide researchers better access to large cohorts of human subjects.
There are two fundamental questions about the study of synaptosomes using human brain tissues. The first is what can be gained by studying postmortem human tissues compared animal models. The second is the validity of such studies considering the issue of post-mortem interval (PMI) between the time of death and the collection of tissues by autopsy, which is inevitably much longer for human subjects than experimental animals.
The most compelling reason for studying human brain tissues in great detail is to understand the underlying neuropathology of various developmental, traumatic, and degenerative brain disorders. Neuropathology is the basis of accurate diagnosis, etiological understanding, mechanistic insight, and therapeutic development. For example, the identification of β-amyloid fragment in plaques was first made in postmortem frozen tissues [18], as well as the identification of α-synuclein in the Lewy bodies of Parkinson’s disease (PD) [19], tau in frontotemporal dementia (FTD) [20], and TDP-43 in amyotrophic lateral sclerosis (ALS) [21, 22]. A thorough understanding of human neuropathology provides the guiding principles for establishing animal models for various disorders.
It has been shown that, even under healthy conditions, the subcellular localization of huntingtin [23] and α-synuclein [24] are somewhat different in rodent and human brains. This really brings out the question whether current animal models of neurological disorders are truly satisfactory representations of the actual human disease. In the case of Alzheimer’s disease (AD), dozens of animal models have been developed [25, 26], and based on these models tens of billions of US dollars have been invested into finding disease-modifying therapies, but all such efforts have failed over the past decades. We need to address critically to what extent these animal models actually mimic the pathogenesis and pathology of AD in humans, beyond the superficial resemblance of accumulating plaques and tangles, which were artificially driven by overexpressing mutant proteins. For instance, when we first discovered the presence of tau oligomers inside human synaptic terminals [9, 27], we also checked a common animal model of tauopathy, rTg4510 mice overexpressing P301L human mutant tau [28], but its synaptosomes were free of tau oligomers (unpublished data). It is plausible that the mutant tau misfolded so aggressively that they became aggregated before reaching the synapse, causing a marked distinction between mouse and human neuropathology.
There are two basic methods to preserve postmortem brain tissues—formalin fixation and cryopreservation. The former is chemically crosslinked and hence more stable, providing easier handling for histochemical studies. The latter is more amenable to the isolation of various biomolecules and organelles, without the interference of extrinsic chemical modification. To isolate human synaptosomes, cryopreserved tissues are always used. The critical factor affecting the quality of human brain tissues is the PMI [29, 30]. Current trends in brain banking aim to reduce PMI to just a few hours, but for some cases it may still be as long as 24–30 h. Studies have shown that overall rRNA and mRNA stability is maintained for up to 30 h postmortem, without apparent correlation with pH changes due to tissue acidification [31], although specific mRNAs may be selectively degraded under the influence of long PMI or low pH [32]. On the other hand, dephosphorylation may occur on some proteins in less than 1 min, which is a significant problem even in animal experiments [33]. Ideally, the effect of PMI should be individually addressed for each assay condition, but this may not be practical in many experiments.
Isolation of Human Synaptosomes
The isolation of synaptosomes from mammalian brains has evolved relatively little over the last 30 years. This is based on subcellular fractionation techniques similar to those used for isolating other organelles such as mitochondria. In general, tissues are homogenized in a glass-Teflon (Potter–Elvehjem) grinder and an isotonic sucrose buffer (0.32 M). This is followed by low-speed centrifugation (500–1000×g) to remove nuclei and debris, and high-speed centrifugation (10–20K×g) to collect the crude synaptosome pellet (P2 fraction). The P2 fraction may be directly analyzed or further subjected to discontinuous density gradient centrifugation (0.3/0.8/1.2 M sucrose) to remove major contaminants such as myelin fragments and mitochondria, by collecting synaptosomes at the 0.8/1.2 M interface (Fig. 1) [6]. It is possible to collect synaptosomes by either sedimentation or flotation schemes in gradient centrifugation [34, 35]. Even after sucrose gradient centrifugation, the synaptosome faction still contains various organelle contaminants. Many of the vesicular objects from synaptosome preparations do not show clear organelle origin under electron microscopy and it is, therefore, rather difficult to assess quantitatively how presynaptic and postsynaptic terminals are resealed and collected by various isolation schemes.
Based on our experience of examining synaptically enriched preparations with electron microscopy, immunofluorescence microscopy [9, 27], flow cytometry, and super-resolution microscopy (unpublished data), with both human and mouse samples, it appears that at least five classes of nerve ending particles can be found (Fig. 2): (I) intact bipartite synapses, which show snowman-like structures; (II) presynaptic terminals with membrane-free PSD attached; (III) presynaptic terminals with membrane-enclosed PSD, but missing much of the postsynaptic cytoplasm; (IV) isolated presynaptic terminals; (V) isolated postsynaptic terminals. The underlying reason for the formation of class II and III objects is the numerous adhesion molecules linking the active zone to the PSD across the synaptic cleft. One may also occasionally find a single postsynaptic terminals with multiple presynaptic terminals attached, or vice versa.
Fig. 2.
Morphological classification of synaptically derived particles. From synapses composed of axonal boutons and dendritic spines, five different classes of nerve ending particles may be generated after detachment and resealing
The relative abundance of different classes of nerve ending particles are presumably affected by homogenization conditions, but detailed quantitative assessments have been very challenging using electron microscopy, immunofluorescence microscopy, and super-resolution techniques. It is generally assumed that isotonic homogenization buffers containing 0.32 M sucrose favor the formation of class II and IV objects. These are conventionally called “synaptosome” preparations, which are used by most laboratories working with isolated synaptic terminals [11]. A less common approach is to prepare isotonic homogenization buffers without sucrose, but with NaCl and other salts. Such preparations are generally called “synaptoneurosomes,” initially described as containing more class I, III, and V objects [36, 37]. In the early years of neurochemistry research, synaptosomes were supposedly presynaptic objects (class II and IV) and neurosomes were supposedly postsynaptic objects (class V), and synaptoneurosomes were supposedly bipartite (class I and III). However, later research revealed that the enrichment of various presynaptic and PSD markers examined by Western blot were hardly distinguishable between synaptosomes and synaptoneurosomes [38]. Our own experiments also confirmed that presynaptic markers (synaptophysin) and postsynaptic markers (PSD-95, MAP2) were similarly enriched in crude synaptosomes vs. synaptoneurosomes (unpublished data). Although sucrose and salt-based buffers may result in somewhat different resealing behavior of the postsynaptic terminal, the difference may not be as striking as initially assumed. Whittaker has suggested that all nerve ending particles obtained by subcellular fractionation may be generally described as “synaptosomes” [11]. On the other hand, “synaptoneurosomes” may be used under a more restricted context to describe synaptic fractions prepared in sucrose-free/high-salt homogenization buffers, which may be more suitable for studying postsynaptic components.
The major drawback of the sucrose gradient method is the change in osmotic pressure. As synaptosomes enter the hypertonic sucrose gradient, they will shrink in size and lose some cytoplasmic components. This can be overcome by using iso-osmotic medium such as Percoll (colloidal silica) [39, 40]. The advantages of Percoll include low viscosity, shorter centrifugation time, and maintaining constant osmolarity to prevent shrinkage/expansion [41]. However, synaptosomes collected from Percoll gradients (at 10/20% interface) are less enriched in synaptic terminals than those collected with sucrose gradients [42]. There is a tradeoff between synaptosome integrity and purity. Another method to carry out synaptosome isolation under isotonic condition is to employ Ficoll dissolved in 0.32 M sucrose [43, 44]. Ficoll is a high-mass, hydrophilic carbohydrate polymer crosslinked into spheres of 2–7 nm in diameter. However, the purity of synaptosomes prepared with Ficoll is also lower than those from sucrose gradients [45]. As such, the optimal choice among sucrose, Percoll, and Ficoll methods may be application-specific.
In our experience, synaptosome preparation protocols developed for rodent brains are equally applicable to postmortem human tissues [9, 27]. The only noticeable difference is that, after grinding brain tissues, human samples contain more insoluble debris than rodent samples, which can be removed by additional centrifugation or 20 μm nylon filters.
Studies of Human Synaptosomes
Studies of postmortem human synaptosomes started in the late 1970s [46]. Around this period, there were numerous studies on synaptosomes freshly prepared from experimental animals to study their morphology, metabolism, and functional activity [11]. Therefore, scientists began to examine the possibility of preparing synaptosomes from postmortem human tissues, comparing them to fresh brain tissues removed during neurosurgeries [47–49]. Compared to fresh tissues, synaptosomes prepared from postmortem tissues gradually lost their respiratory activity as PMI increases. Nonetheless, even with a PMI of 24 h, synaptosomes still retained respiratory activity and the ability to release neurotransmitters, and appeared to be morphologically indistinguishable from those from fresh tissues. It was also found that freezing human brain tissues slowly (without additional liquid) and thawing them rapidly were optimal for preserving metabolic activities in synaptosomes [49, 50]. Conversely, rapid freezing, adding sucrose solution as cryo-preservative, and slow thawing were all detrimental, which appeared to be somewhat counterintuitive and the underlying reasons remain unclear.
In the 1970s, researchers began to use postmortem tissues to explore the underlying pathological deficits in neurotransmitter systems in neurodegenerative disorders, especially AD [51]. Because AD is the most common of all neurodegenerative disorders, it was also easier to collect brain tissues from AD patients. In the 1980s, researchers started using synaptosomes prepared from postmortem brains to study AD-associated deficits in neurotransmission, including acetylcholine [52, 53], glutamate [54], and γ-aminobutyric acid (GABA) systems [55]. More recent synaptosome studies have examined the decrease of neprilysin in AD patients [56]. Likewise, the localization of huntingtin has been examined in the synaptosomes of Huntington’s disease patients [23]. For psychiatric disorders, postmortem synaptosome have been applied to study dopamine uptake in schizophrenia patients [57], SNAP-25 levels in bipolar disorder [58], and Na–K-ATPase levels in depressive disorders [59]. Overall, however, there are still very few studies that examine human synaptosomes for neurological disorders other than AD.
Expanding Analytical Techniques
Traditionally, human synaptosome studies have focused on individual aspects of neurotransmission or metabolism. With recent advances in high-throughput techniques such as transcriptomics (by deep RNA sequencing), proteomics (by shotgun mass spectrometry), and metabolomics (by liquid chromatography-tandem mass spectrometry), it should be possible to accelerate the pace of new discoveries with human synaptosomes. Unfortunately, this area of research remains underexplored, with only one preliminary study on the phosphoproteomics of normal human synaptosomes [60]. Moreover, neither transcriptomics nor metabolomics have been applied to study human and animal synaptosomes. The “Omics” approaches may be promising new avenues in human synaptosome research. They may also offer insights for biomarker discovery, because synaptic molecules and proteins are likely to be released into the cerebrospinal fluid or blood, and hence biochemical changes at synapses may be detectable in biological fluids by in vitro diagnostic methods of ever increasing sensitivity. While the presence of many synaptic proteins [61] in the cerebrospinal fluid [62] is well documented, less is known about the release of synaptic proteins into the blood. According to the Plasma Proteome Database [63], many classical synaptic proteins, such as synapotagmin, synaptophysin, SNAP-25, and syntaxin, have been identified in the serum. The serum levels of Aβ-42 and tau are increased in AD patients [64], and both proteins are known to be synaptically released [65, 66], although there may also be non-synaptic routes of their release into the serum.
Another opportunity for high-throughput characterization of synaptosomes is flow cytometry. Gylys and coworkers have shown that immunofluorescence staining in postmortem synaptosomes could be individually isolated or quantified at high speeds [67, 68]. In our experience, tens of thousands of synapses could be quantitatively analyzed in a few minutes on a flow cytometer, and up to three fluorescence channels is feasible for simultaneous detection (unpublished data). Gylys and coworkers have demonstrated that AD patients accumulated Aβ and hyperphosphorylated tau (p-tau) at synaptic sites in multiple brain regions, accompanied by increased synaptosome size, reduced numbers of PSD, and increased free cholesterol [69–71]. Co-localization of Aβ and p-tau was demonstrated by dual labeling experiments, and the synaptic accumulation of Aβ occured at the earliest plaque stages, before the accumulation of synaptic p-tau, which appeared at later stages [71, 72]. However, even in early disease stages, p-tau appeared to be elevated in individual Aβ-positive terminals. These results implied that soluble oligomers of Aβ may trigger tau pathology at synaptic terminals, which may be important for the onset of dementia [72]. They have also shown that c-terminal truncated tau was released by presynaptic terminals when postmortem synaptosomes were stimulated by depolarization, supporting the notion that tauopathy may spread across different regions through synaptic networks [66]. Flow cytometry of postmortem synaptosomes have also shown that dopamine transporter was decreased in the putamen region of dementia with Lewy body patients [73].
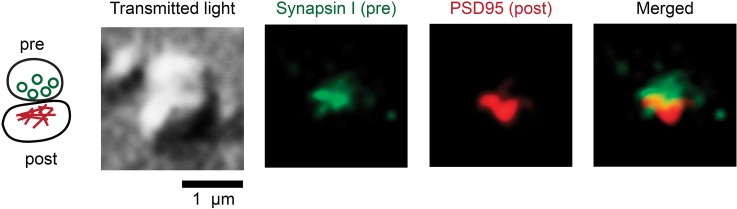
With the spatial resolution of current flow cytometers, it is not yet possible to differentiate proteins of presynaptic and postsynaptic origins. This differentiation is also very difficult in immunohistochemistry experiments because human tissues have much longer PMI than animals, resulting in less optimal preservation and hence higher background staining. Tai et al. have demonstrated that isolated human synaptic terminals fixed over glass slides could be immunostained to distinguish presynaptic from postsynaptic protein localization [27]. They discovered that normal human tau protein under normal conditions were equally abundant at presynaptic and postsynaptic terminals, against the common misconception that tau was predominantly presynaptic in healthy neurons. Under AD conditions, misfolded and hyperphosphorylated tau proteins were also equally abundant at presynaptic and postsynaptic terminals [9], which lent support to the hypothesis that misfolded tau may transmit trans-synaptically via “prion-like” mechanisms [74, 75]. Figure 3 shows the confocal image of an isolated human synaptosome immunostained with presynaptic and postsynaptic markers. A third immunofluorescence channel can be added to localize the target protein to presynaptic or postsynaptic sites. In fact, immunofluorescence imaging of fixed synaptosomes is technically similar than visualizing synaptic terminals in cultured neurons [9, 27]. Therefore, it is also possible to study synaptosomes by high-content imaging methods incorporating automated microscopes as well as automated image processing [76].
Fig. 3.
Immunofluorescence imaging of an isolated human synaptoneurosome fixed over the coverglass. Under the confocal laser scanning microscope, the bipartite synapse has a snowman-like appearance in the transmitted light channel (488 nm laser). With two-channel immunofluorescence detection, the upper structure is characterized by presynaptic marker synapsin I and the lower structure by postsynaptic marker PSD95. These images have been up-converted in pixel dimensions, smoothed, and contrast-enhanced (H. C. Tai, unpublished data)
The practical x–y resolution of confocal and epifluorescence microscopy is limited by diffraction to 150–200 nm. Compared to the size of synaptic terminals (500–1000 nm), this resolution is insufficient to reveal protein colocalization within the synapse. The application of direct stochastic optical reconstruction microscopy (dSTORM), a super-resolution microscopy technique based on immunofluorescence, can push the resolution limit down to 30 nm and reveal ultrastructural information within the synapse [77]. The dSTORM technique has yet to be applied to human synaptosomes, but it holds great promise for studying the ultrastructural distribution and colocalization of synaptic proteins.
There are multiple reasons as to why recent studies on human synaptosomes have focused on AD more than any other neurological disorder. First, AD is the most common of all neurodegenerative disorders and occurs frequently in the elderly. Therefore it is easier to build up large cohorts of AD patients in brain banks. Second, AD has often been attributed as a synaptic disorder [78]. Cognitive decline in AD patients shows stronger correlation with synapse loss than with neuronal loss, plaque deposition, tangles accumulation, or various measures of Aβ and tau misfolding [79, 80]. Third, key proteins in AD pathogenesis, including APP, Aβ, ApoE, and tau, have all been observed within human synaptosomes [9, 27, 71, 81–83]. Both Aβ and tau are secreted at human synaptic terminals upon activation or stimulation [65, 66]. The transmission of abnormal tau along synaptic networks has been demonstrated in several animal models [84–87], and its propagation into cortical regions is accelerated by misfolded Aβ [88]. We have also shown that hyperphosphorylated tau oligomers accumulated at synapses in AD subjects [9, 27], and subsequently observed that, among people with high plaque and tangle pathologies, those who did not accumulate tau oligomers at synaptic sites tended to remian congnitively normal, while those who did generally exhibited the congnitive symptoms of AD [89]. Therefore, the study of synaptic abnormalities is crucial for understanding the pathogenesis and progression of AD.
Moreover, current therapeutic agents for AD are also focused on preserving synaptic function. There are currently four FDA-approved drugs on the market for AD [90]. Three of them are cholinesterase inhibitors (donepezil, rivastigmine, galantamine) and the other is an N-methyl-d-aspartate receptor antagonist (memantine). The efficacy of cholinesterase inhibitors is explained by the massive degeneration of cholinergic neurons located at the nucleus basalis of Meynert in AD patients [91, 92]. However, currently available drugs only offer symptomatic relief for AD patients, without slowing down the progressive nature of neurodegeneration. Apparently, we need to further investigate the pathophysiology of synapses in AD patients, using the expanding analytical approaches mentioned above, to help us develop disease-modifying therapies which could slow down synaptic and neuronal loss.
Conclusion
The ultimate goal of neuroscience is to understand the human brain. However, due to practical limitations, most of the neuroscience research at the cellular and molecular levels have been conducted with animal models or cultured cells. The growing collection of tissues deposited in human brain banks provides tremendous opportunities for future research to directly investigate the human brain. This line of research will be most valuable for understanding human neurological disorders, especially for studying the “fine pathology” which has been largely overlooked. The “fine pathology” refers to a series of molecular and subcellular changes undetectable by conventional histology techniques, which may very well be quite different in human brains compared to animal models. The only way to tell if they are different is to apply the latest technologies and carefully examine both human and animal brain tissues.
The synapse plays crucial roles in the pathogenesis and pathology of many neurological disorders. The methods of isolating synaptosomes from animal tissues are equally applicable to human tissues. Human synaptosomes can be used to study neurotransmission, metabolism, protein synthesis, protein localization, and posttranslational modification. An increasing number of studies have been examining the synaptosomes of AD patients, and these analyses should be extended to other neurodegenerative and psychiatric disorders. To accelerate the pace of future discoveries, high-throughput analyses including transcriptomics, proteomics, metabolomics, high-content imaging, and flow cytometry should be increasingly applied to human synaptosomes. Ultrastructural studies based on super-resolution fluorescence imaging will offer important avenues for exploration, likely to reveal many exciting discoveries. We are just entering the dawn of human neuroscience and many new discoveries will undoubtedly originate from the direct examination of the human brain.
Acknowledgements
This work was supported by Grant 105-2113-M-002-016 from Ministry of Science and Technology, Taiwan. No funding or sponsorship was received for the publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Jia-Fong Jhou and Hwan-Ching Tai have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/CAD8F06039796B6E.
References
- 1.Eccles JC. The synapse: from electrical to chemical transmission. Annu Rev Neurosci. 1982;5:325–339. doi: 10.1146/annurev.ne.05.030182.001545. [DOI] [PubMed] [Google Scholar]
- 2.Tang Y, Nyengaard JR, De Groot DM, Gundersen HJ. Total regional and global number of synapses in the human brain neocortex. Synapse. 2001;41:258–273. doi: 10.1002/syn.1083. [DOI] [PubMed] [Google Scholar]
- 3.Hebb CO, Whittaker V. Intracellular distributions of acetylcholine and choline acetylase. J Physiol. 1958;142:187–196. doi: 10.1113/jphysiol.1958.sp006008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittaker V, Michaelson I, Kirkland RJA. The separation of synaptic vesicles from nerve-ending particles (‘synaptosomes’) Biochem J. 1964;90:293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Robertis E, De Iraldi AP, Garnaiz GRDL, Salganicoff L. Cholinergic and non-cholinergic nerve endings in rat brain. I. Isolation and subcellular distribution of acetylcholine and acetylcholinesterase. J Neurochem. 1962;9:23–35. doi: 10.1111/j.1471-4159.1962.tb07489.x. [DOI] [PubMed] [Google Scholar]
- 6.Gray E, Whittaker V. The isolation of nerve endings from brain: an electron microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- 7.Whittaker V. The morphology of fractions of rat forebrain synaptosomes separated on continuous sucrose density gradients. Biochem J. 1968;106:412. doi: 10.1042/bj1060412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones DG, Revell E. The postnatal development of the synapse: a morphological approach utilizing synaptosomes. I. General features. Z Zellforsch Mikrosk Anat. 1970;111:179–194. doi: 10.1007/BF00339784. [DOI] [PubMed] [Google Scholar]
- 9.Tai HC, Wang BY, Serrano Pozo A, Frosch MP, Spires-Jones TL, Hyman BT. Frequent and symmetric deposition of misfolded tau oligomers within presynaptic and postsynaptic terminals in Alzheimer inverted question marks disease. Acta Neuropathol Commun. 2014;2:146. doi: 10.1186/s40478-014-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotman C, Brown DH, Harrell BW, Anderson NG. Analytical differential centrifugation: an analysis of the sedimentation properties of synaptosomes, mitochondria and lysosomes from rat brain homogenates. Arch Biochem Biophys. 1970;136:436–447. doi: 10.1016/0003-9861(70)90215-8. [DOI] [PubMed] [Google Scholar]
- 11.Whittaker VP. Thirty years of synaptosome research. J Neurocytol. 1993;22:735–742. doi: 10.1007/BF01181319. [DOI] [PubMed] [Google Scholar]
- 12.Bermejo MK, Milenkovic M, Salahpour A, Ramsey AJ. Preparation of synaptic plasma membrane and postsynaptic density proteins using a discontinuous sucrose gradient. J Vis Exp. 2014 doi: 10.3791/51896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin W, Taglialatela G. A method to determine insulin responsiveness in synaptosomes isolated from frozen brain tissue. J Neurosci Methods. 2016;261:128–134. doi: 10.1016/j.jneumeth.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcelli S, Ficulle E, Iannuzzi F, Kövari E, Nisticò R, Feligioni M. Targeting SUMO-1ylation contrasts synaptic dysfunction in a mouse model of Alzheimer’s disease. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0176-9. [DOI] [PubMed] [Google Scholar]
- 15.Kretzschmar H. Brain banking: opportunities, challenges and meaning for the future. Nat Rev Neurosci. 2009;10:70–78. doi: 10.1038/nrn2535. [DOI] [PubMed] [Google Scholar]
- 16.Bell JE, Alafuzoff I, Al-Sarraj S, Arzberger T, Bogdanovic N, Budka H, et al. Management of a twenty-first century brain bank: experience in the BrainNet Europe consortium. Acta Neuropathol. 2008;115:497–507. doi: 10.1007/s00401-008-0360-8. [DOI] [PubMed] [Google Scholar]
- 17.Nichols L, Freund M, Ng C, Kau A, Parisi M, Taylor A, et al. The National Institutes of Health Neurobiobank: a federated national network of human brain and tissue repositories. Biol Psychiatry. 2014;75:e21–e22. doi: 10.1016/j.biopsych.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 19.Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 20.Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 21.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 22.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood JD, MacMillan JC, Harper PS, Lowenstein PR, Jones AL. Partial characterisation of murine huntingtin and apparent variations in the subcellular localisation of huntingtin in human, mouse and rat brain. Hum Mol Genet. 1996;5:481–487. doi: 10.1093/hmg/5.4.481. [DOI] [PubMed] [Google Scholar]
- 24.Kahle PJ, Neumann M, Ozmen L, Müller V, Jacobsen H, Schindzielorz A, et al. Subcellular localization of wild-type and Parkinson’s disease-associated mutant α-synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 26.Cuadrado-Tejedor M, García-Osta A. Current animal models of Alzheimer’s disease: challenges in translational research. Front Neurol. 2014;5:182. doi: 10.3389/fneur.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol. 2012;181:1426–1435. doi: 10.1016/j.ajpath.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blair JA, Wang C, Hernandez D, Siedlak SL, Rodgers MS, Achar RK, et al. Individual case analysis of postmortem interval time on brain tissue preservation. PLoS One. 2016;11:e0151615. doi: 10.1371/journal.pone.0151615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ervin JF, Heinzen EL, Cronin KD, Goldstein D, Szymanski MH, Burke JR, et al. Postmortem delay has minimal effect on brain RNA integrity. J Neuropathol Exp Neurol. 2007;66:1093–1099. doi: 10.1097/nen.0b013e31815c196a. [DOI] [PubMed] [Google Scholar]
- 32.Hynd MR, Lewohl JM, Scott HL, Dodd PR. Biochemical and molecular studies using human autopsy brain tissue. J Neurochem. 2003;85:543–562. doi: 10.1046/j.1471-4159.2003.01747.x. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed MM, Gardiner KJ. Preserving protein profiles in tissue samples: differing outcomes with and without heat stabilization. J Neurosci Methods. 2011;196:99–106. doi: 10.1016/j.jneumeth.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodd P, Hardy J, Oakley A, Edwardson J, Perry E, Delaunoy J-P. A rapid method for preparing synaptosomes: comparison, with alternative procedures. Brain Res. 1981;226:107–118. doi: 10.1016/0006-8993(81)91086-6. [DOI] [PubMed] [Google Scholar]
- 35.Lathia D, Wesemann W. Serotonin uptake and release by biochemically characterized nerve endings isolated from rat brain by concomitant flotation and sedimentation centrifugation. J Neural Transm. 1975;37:111–126. doi: 10.1007/BF01663628. [DOI] [PubMed] [Google Scholar]
- 36.Hollingsworth EB, McNeal ET, Burton JL, Williams RJ, Daly JW, Creveling CR. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3′:5′-monophosphate-generating systems, receptors, and enzymes. J Neurosci. 1985;5:2240–2253. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz RD, Skolnick P, Hollingsworth EB, Paul SM. Barbiturate and picrotoxin-sensitive chloride efflux in rat cerebral cortical synaptoneurosomes. FEBS Lett. 1984;175:193–196. doi: 10.1016/0014-5793(84)80597-9. [DOI] [PubMed] [Google Scholar]
- 38.Johnson MW, Chotiner JK, Watson JB. Isolation and characterization of synaptoneurosomes from single rat hippocampal slices. J Neurosci Methods. 1997;77:151–156. doi: 10.1016/S0165-0270(97)00120-9. [DOI] [PubMed] [Google Scholar]
- 39.Lagercrantz H, Pertoft H. Separation of catecholamine storing synaptosomes in colloidal silica density gradients. J Neurochem. 1972;19:811–823. doi: 10.1111/j.1471-4159.1972.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 40.Nagy A, Delgado-Escueta AV. Rapid preparation of synaptosomes from mammalian brain using nontoxic isoosmotic gradient material (Percoll) J Neurochem. 1984;43:1114–1123. doi: 10.1111/j.1471-4159.1984.tb12851.x. [DOI] [PubMed] [Google Scholar]
- 41.Dunkley PR, Jarvie PE, Heath JW, Kidd GJ, Rostas JA. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986;372:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- 42.Tenreiro P, Rebelo S, Martins F, Santos M, Coelho E, Almeida M, et al. Comparison of simple sucrose and percoll based methodologies for synaptosome enrichment. Anal Biochem. 2017;517:1–8. doi: 10.1016/j.ab.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Booth RF, Clark JB. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J. 1978;176:365–370. doi: 10.1042/bj1760365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurokawa M, Kato M, Sakamoto T. Distribution of sodium-plus-potassium-stimulated adenosine-triphosphatase activity in isolated nerve-ending particles. Biochem J. 1965;97:833–844. doi: 10.1042/bj0970833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joo F, Karnushina I. Morphometric assessment of the composition of the synaptosomal fractions obtained by the use of Ficoll gradients. J Neurochem. 1975;24:839–840. doi: 10.1111/j.1471-4159.1975.tb03880.x. [DOI] [PubMed] [Google Scholar]
- 46.Klemm N, Kuhar MJ. Post-mortem changes in high affinity choline uptake. J Neurochem. 1979;32:1487–1494. doi: 10.1111/j.1471-4159.1979.tb11089.x. [DOI] [PubMed] [Google Scholar]
- 47.Dodd P, Hardy JA, Oakley AE, Strong AJ. Synaptosomes prepared from fresh human cerebral cortex; morphology, respiration and release of transmitter amino acids. Brain Res. 1981;224:419–425. doi: 10.1016/0006-8993(81)90871-4. [DOI] [PubMed] [Google Scholar]
- 48.Hardy JA, Dodd PR, Oakley AE, Kidd AM, Perry RH, Edwardson JA. Use of post-mortem human synaptosomes for studies of metabolism and transmitter amino acid release. Neurosci Lett. 1982;33:317–322. doi: 10.1016/0304-3940(82)90392-5. [DOI] [PubMed] [Google Scholar]
- 49.Hardy JA, Dodd PR, Oakley AE, Perry RH, Edwardson JA, Kidd AM. Metabolically active synaptosomes can be prepared from frozen rat and human brain. J Neurochem. 1983;40:608–614. doi: 10.1111/j.1471-4159.1983.tb08024.x. [DOI] [PubMed] [Google Scholar]
- 50.Dodd P, Hardy J, Baig E, Kidd A, Bird E, Watson W, et al. Optimization of freezing, storage, and thawing conditions for the preparation of metabolically active synaptosomes from frozen rat and human brain. Neurochem Pathol. 1986;4:177–198. doi: 10.1007/BF02834357. [DOI] [PubMed] [Google Scholar]
- 51.Hardy J, Adolfsson R, Alafuzoff I, Bucht G, Marcusson J, Nyberg P, et al. Transmitter deficits in Alzheimer’s disease. Neurochem Int. 1985;7:545–563. doi: 10.1016/0197-0186(85)90050-6. [DOI] [PubMed] [Google Scholar]
- 52.Nordberg A, Winblad B. Reduced number of [3H] nicotine and [3H] acetylcholine binding sites in the frontal cortex of Alzheimer brains. Neurosci Lett. 1986;72:115–120. doi: 10.1016/0304-3940(86)90629-4. [DOI] [PubMed] [Google Scholar]
- 53.Rylett R, Ball M, Colhoun E. Evidence for high affinity choline transport in synaptosomes prepared from hippocampus and neocortex of patients with Alzheimer’s disease. Brain Res. 1983;289:169–175. doi: 10.1016/0006-8993(83)90017-3. [DOI] [PubMed] [Google Scholar]
- 54.Hardy J, Cowburn R, Barton A, Reynolds G, Lofdahl E, O’Carroll A-M, et al. Region-specific loss of glutamate innervation in Alzheimer’s disease. Neurosci Lett. 1987;73:77–80. doi: 10.1016/0304-3940(87)90034-6. [DOI] [PubMed] [Google Scholar]
- 55.Hardy J, Cowburn R, Barton A, Reynolds G, Dodd P, Wester P, et al. A disorder of cortical GABAergic innervation in Alzheimer’s disease. Neurosci Lett. 1987;73:192–196. doi: 10.1016/0304-3940(87)90016-4. [DOI] [PubMed] [Google Scholar]
- 56.Wang D-S, Lipton RB, Katz MJ, Davies P, Buschke H, Kuslansky G, et al. Decreased neprilysin immunoreactivity in Alzheimer disease, but not in pathological aging. J Neuropathol Exp Neurol. 2005;64:378–385. doi: 10.1093/jnen/64.5.378. [DOI] [PubMed] [Google Scholar]
- 57.Haberland N, Hetey L. Studies in postmortem dopamine uptake. II. Alterations of the synaptosomal catecholamine uptake in postmortem brain regions in schizophrenia. J Neural Transm. 1987;68:303–313. doi: 10.1007/BF02098505. [DOI] [PubMed] [Google Scholar]
- 58.Scarr E, Gray L, Keriakous D, Robinson P, Dean B. Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disord. 2006;8:133–143. doi: 10.1111/j.1399-5618.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- 59.Goldstein I, Levy T, Galili D, Ovadia H, Yirmiya R, Rosen H, et al. Involvement of Na(+), K(+)-ATPase and endogenous digitalis-like compounds in depressive disorders. Biol Psychiatry. 2006;60:491–499. doi: 10.1016/j.biopsych.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 60.DeGiorgis JA, Jaffe H, Moreira JE, Carlotti CG, Leite JP, Pant HC, et al. Phosphoproteomic analysis of synaptosomes from human cerebral cortex. J Proteome Res. 2005;4:306–315. doi: 10.1021/pr0498436. [DOI] [PubMed] [Google Scholar]
- 61.Dieterich DC, Kreutz MR. Proteomics of the synapse—a quantitative approach to neuronal plasticity. Mol Cell Proteom. 2016;15:368–381. doi: 10.1074/mcp.R115.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Guo Z, Zou L, Yang Y, Zhang L, Ji N, et al. Data for a comprehensive map and functional annotation of the human cerebrospinal fluid proteome. Data Brief. 2015;3:103–107. doi: 10.1016/j.dib.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nanjappa V, Thomas JK, Marimuthu A, Muthusamy B, Radhakrishnan A, Sharma R, et al. Plasma proteome database as a resource for proteomics research: 2014 update. Nucleic Acids Res. 2014;42:D959–D965. doi: 10.1093/nar/gkt1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiu MJ, Yang SY, Horng HE, Yang CC, Chen TF, Chieh JJ, et al. Combined plasma biomarkers for diagnosing mild cognition impairment and Alzheimer’s disease. ACS Chem Neurosci. 2013;4:1530–1536. doi: 10.1021/cn400129p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 66.Sokolow S, Henkins KM, Bilousova T, Gonzalez B, Vinters HV, Miller CA, et al. Pre-synaptic C-terminal truncated tau is released from cortical synapses in Alzheimer’s disease. J Neurochem. 2015;133:368–379. doi: 10.1111/jnc.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gylys KH, Fein JA, Yang F, Cole GM. Enrichment of presynaptic and postsynaptic markers by size-based gating analysis of synaptosome preparations from rat and human cortex. Cytom A. 2004;60:90–96. doi: 10.1002/cyto.a.20031. [DOI] [PubMed] [Google Scholar]
- 68.Sokolow S, Henkins KM, Williams IA, Vinters HV, Schmid I, Cole GM, et al. Isolation of synaptic terminals from Alzheimer’s disease cortex. Cytom A. 2012;81:248–254. doi: 10.1002/cyto.a.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gylys KH, Fein JA, Yang F, Wiley DJ, Miller CA, Cole GM. Synaptic changes in Alzheimer’s disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol. 2004;165:1809–1817. doi: 10.1016/S0002-9440(10)63436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gylys KH, Fein JA, Yang F, Miller CA, Cole GM. Increased cholesterol in Abeta-positive nerve terminals from Alzheimer’s disease cortex. Neurobiol Aging. 2007;28:8–17. doi: 10.1016/j.neurobiolaging.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 71.Fein JA, Sokolow S, Miller CA, Vinters HV, Yang F, Cole GM, et al. Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am J Pathol. 2008;172:1683–1692. doi: 10.2353/ajpath.2008.070829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bilousova T, Miller CA, Poon WW, Vinters HV, Corrada M, Kawas C, et al. Synaptic amyloid-beta oligomers precede p-tau and differentiate high pathology control cases. Am J Pathol. 2016;186:185–198. doi: 10.1016/j.ajpath.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Postupna NO, Keene CD, Latimer C, Sherfield EE, Van Gelder RD, Ojemann JG, et al. Flow cytometry analysis of synaptosomes from post-mortem human brain reveals changes specific to Lewy body and Alzheimer’s disease. Lab Invest. 2014;94:1161–1172. doi: 10.1038/labinvest.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70:304–310. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daniel JA, Malladi CS, Kettle E, McCluskey A, Robinson PJ. Analysis of synaptic vesicle endocytosis in synaptosomes by high-content screening. Nat Protoc. 2012;7:1439–1455. doi: 10.1038/nprot.2012.070. [DOI] [PubMed] [Google Scholar]
- 77.Heilemann M, Van De Linde S, Schüttpelz M, Kasper R, Seefeldt B, Mukherjee A, et al. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew Chem Int Ed. 2008;47:6172–6176. doi: 10.1002/anie.200802376. [DOI] [PubMed] [Google Scholar]
- 78.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 79.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 80.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 81.Arold S, Sullivan P, Bilousova T, Teng E, Miller CA, Poon WW, et al. Apolipoprotein E level and cholesterol are associated with reduced synaptic amyloid beta in Alzheimer’s disease and apoE TR mouse cortex. Acta Neuropathol. 2012;123:39–52. doi: 10.1007/s00401-011-0892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henkins KM, Sokolow S, Miller CA, Vinters HV, Poon WW, Cornwell LB, et al. Extensive p-tau pathology and SDS-stable p-tau oligomers in Alzheimer’s cortical synapses. Brain Pathol. 2012;22:826–833. doi: 10.1111/j.1750-3639.2012.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koffie RM, Hashimoto T, Tai HC, Kay KR, Serrano-Pozo A, Joyner D, et al. Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-β. Brain. 2012;135:2155–2168. doi: 10.1093/brain/aws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clarke H, et al. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 2014;127:667–683. doi: 10.1007/s00401-014-1254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dujardin S, Lecolle K, Caillierez R, Begard S, Zommer N, Lachaud C, et al. Neuron-to-neuron wild-type Tau protein transfer through a trans-synaptic mechanism: relevance to sporadic tauopathies. Acta Neuropathol Commun. 2014;2:14. doi: 10.1186/2051-5960-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, et al. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pooler AM, Polydoro M, Maury EA, Nicholls SB, Reddy SM, Wegmann S, et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol Commun. 2015;3:14. doi: 10.1186/s40478-015-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain. 2013;136:2510–2526. doi: 10.1093/brain/awt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41:615–631. doi: 10.3233/JAD-132690. [DOI] [PubMed] [Google Scholar]
- 91.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 92.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]