Abstract
Short elements in mammalian mRNA can control gene expression by activating the RNA-dependent protein kinase PKR that attenuates translation by phosphorylating cytoplasmic eukaryotic initiation factor 2α (eIF2α). We demonstrate a novel, positive role for PKR activation and eIF2α phosphorylation in human globin mRNA splicing. PKR localizes in splicing complexes and associates with splicing factor SC35. Splicing and early-stage spliceosome assembly on β-globin pre-mRNA depend strictly on activation of PKR by a codon-containing RNA fragment within exon 1 and on phosphorylation of nuclear eIF2α on Serine 51. Nonphosphorylatable mutant eIF2αS51A blocked β-globin mRNA splicing in cells and nuclear extract. Mutations of the β-globin RNA activator abrogated PKR activation and profoundly affected mRNA splicing efficiency. PKR depletion abrogated splicing and spliceosome assembly; recombinant PKR effectively restored splicing. Excision of the first intron of β-globin induces strand displacement within the RNA activator of PKR by a sequence from exon 2, a structural rearrangement that silences the ability of spliced β-globin mRNA to activate PKR. Thus, the ability to activate PKR is transient, serving solely to enable splicing. α-Globin pre-mRNA splicing is controlled likewise but positions of PKR activator and silencer are reversed, demonstrating evolutionary flexibility in how PKR activation regulates globin mRNA splicing through eIF2α phosphorylation.
Keywords: globin mRNA splicing, eIF2α phosphorylation, PKR, RNA activator of PKR, spliceosome assembly
Introduction
The RNA-dependent protein kinase R (PKR) is an intracellular sensor of stress that plays a central role in antiviral defense and innate immunity1. Double-stranded RNA generated during viral replication activates PKR, which inhibits protein synthesis by phosphorylating the α-subunit of eukaryotic translation initiation factor 2 (eIF2α) on Ser51; phosphorylation of eIF2α blocks GTP/GDP exchange needed for recycling of eIF2 between rounds of translation initiation2. eIF2α phosphorylation is critical for mounting the integrated cellular stress response3,4,5. Activation of PKR in the cytoplasm leads to eIF2α phosphorylation that results in a block of translation of mRNAs such as globin mRNA6,7.
Effective engagement of the tandem RNA-binding domains of PKR needed for trans-autophosphorylation of the PKR dimer and the subsequent kinase activation8,9 requires double-helical RNA of minimally 16-18 base pairs (bp)10 and optimally 40-80 bp11. However, short elements within mammalian mRNA can regulate specific gene expression by activating PKR, as shown for the human genes encoding inflammatory cytokines, such as tumor necrosis factor-α (TNF-α)12 and interferon-γ (IFN-γ)13. IFN-γ mRNA activates PKR through a 5′-proximal pseudoknot, resulting in eIF2α phosphorylation and attenuation of its own translation through a negative feedback loop13,14. Efficient splicing of TNF-α pre-mRNA is dependent on a short element in the 3′-untranslated region (3′-UTR) that potently activates PKR12. This cis-acting RNA element, conserved from teleost fish to humans, folds into a pseudoknot that is critical for PKR activation and mRNA splicing. Splicing enabled by the TNF-α RNA activator of PKR depends strictly on phosphorylation of eIF2α on Ser51 that yet does not repress translation. Indeed, eIF2α phosphorylation is not only necessary but also sufficient to achieve efficient splicing of TNF-α mRNA (manuscript in submission). eIF2α phosphorylation positively controls TNF-α mRNA splicing in primary human immune cells, showing its physiological relevance, but the mechanism behind this need remains to be resolved.
Hemoglobin production depends on a massive expression of globin mRNA15 that demands highly efficient splicing of precursor transcripts. We report here that splicing of α- and β-globin pre-mRNAs depends strictly on the activation of PKR by intragenic RNA activators that overlap with translated codons. Splicing of β-globin mRNA is abrogated by PKR depletion, which can be restored by adding back recombinant PKR. The RNA activator of PKR in β-globin pre-mRNA resides in the first exon; mutations of a short helix within this activator profoundly affected both PKR activation and mRNA splicing. Efficient globin mRNA splicing requires phosphorylation of eIF2α. PKR activation and eIF2α phosphorylation mediate splicing at an early step in β-globin spliceosome assembly.
The activator of PKR in β-globin pre-mRNA is generated from 5′-UTR sequence and the start of the open reading frame (ORF), as in the case of IFN-γ mRNA13,14. However, we show that excision of the first intron of β-globin induces strand displacement within exon 1 by a sequence from exon 2 that affects base pairing in the short helix critical for PKR activation, resulting in silencing of the PKR activator within the spliced mRNA. In this manner, the ability to activate PKR is transient, solely to enable splicing.
Our results link a mechanism that controls mRNA translation in the cytoplasm, i.e., phosphorylation of the eIF2α chain upon RNA-mediated activation of PKR, to a critical step in globin mRNA splicing in the nucleus. The finding that splicing of the globin mRNAs is mediated by intragenic RNA activators of PKR shows that this stress response kinase is used more widely beyond the immune system to effect post-transcriptional upregulation of specific genes.
Results
PKR inhibitors block splicing of β-globin mRNA
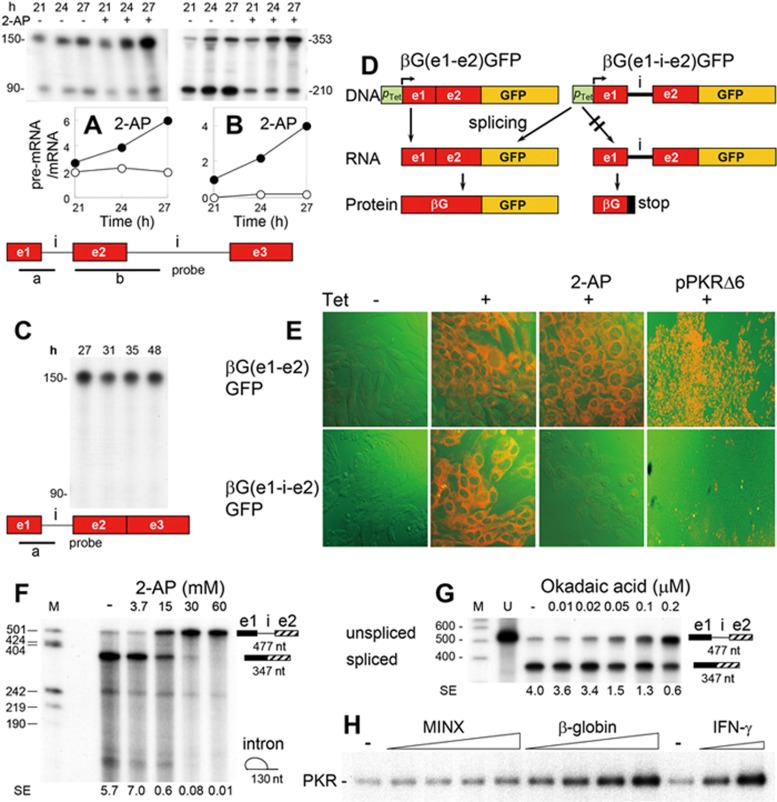
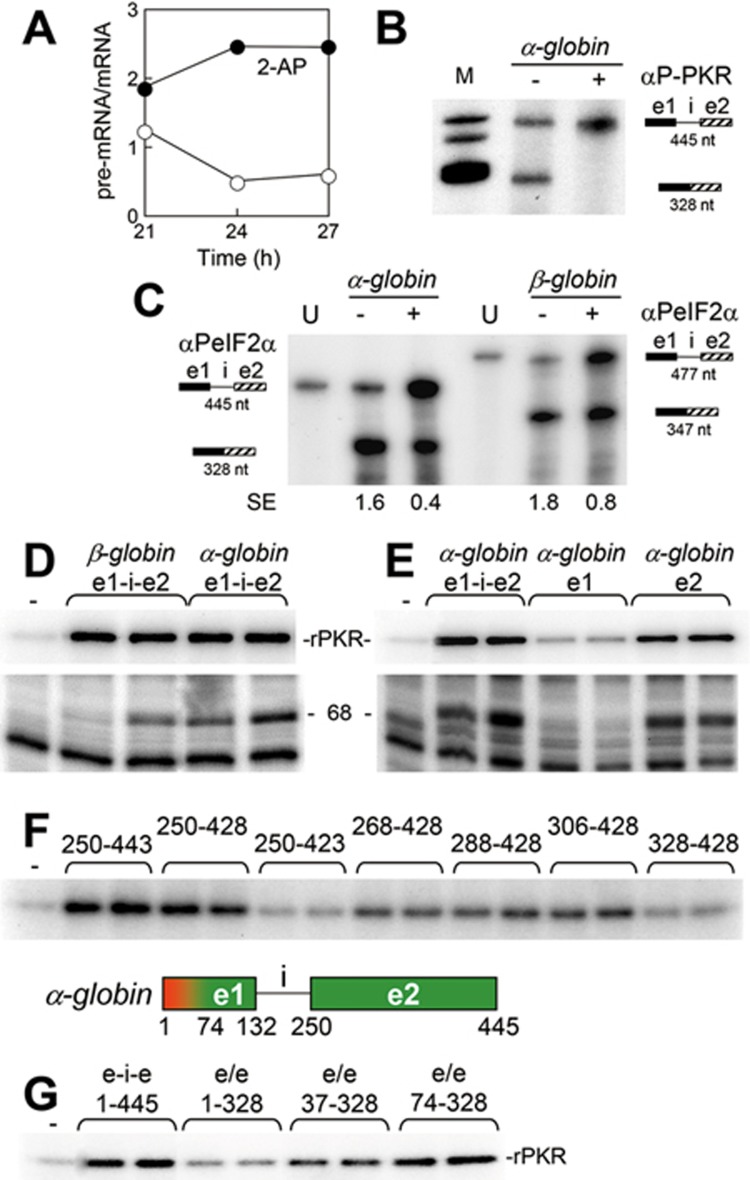
We followed excision of human β-globin introns 1 (Figure 1A) and 2 (Figure 1B) in transfected cells by means of antisense RNA probes. Addition of 2-aminopurine (2-AP), an inhibitor of eIF2α kinases16 that blocks splicing of TNF-α mRNA12, induced accumulation of unspliced β-globin pre-mRNA at the expense of spliced mRNA, leading to an increase in pre-mRNA/mRNA ratio (Figure 1A-1B). When we expressed a β-globin gene from which intron 2 had been deleted, intron 1 failed to be excised and only unspliced pre-mRNA was recovered (Figure 1C), in agreement with earlier observations17. This result implies that intron 1 must be excised before intron 2 deletion, rendering removal of intron 1 the critical first step in β-globin mRNA splicing. Once intron 1 has been excised, splicing is completed efficiently (Figure 1B, lanes without 2-AP).
Figure 1.
Splicing of β-globin mRNA is blocked by PKR inhibitors. (A, B) Expression of unspliced pre-mRNA (reflected by the 150-nt (A) or 353-nt (B) band) and spliced mRNA of β-globin (reflected by the 90-nt (A) or 210-nt (B) band) in HEK293 cells transfected with the human β-globin gene, assayed by RNase protection analysis using RNA probes a (A) or b (B) (e, exon; i, intron). Where indicated, 15 mM 2-AP was added 18 h after the transfection. Ratio of pre-mRNA/mRNA in the absence (○) or presence of 2-AP (•) is plotted. (C) Expression of β-globin RNA in HEK293 cells transfected with the β-globin gene lacking intron 2, assayed with probe a. (D, E) Inhibition of β-globin intron 1 excision by 2-AP and a dominant-negative mutant of PKR. BHK-21 cells were stably transfected with fusion constructs pβG(e1-e2)GFP and pβG(e1-i-e2)GFP lacking and containing intron 1, respectively, under the tetracycline-activatable promoter (pTet) (D). Expression of β-globin/GFP protein was induced by the treatment with 12.5 pg/ml Tet for 6 h. Where indicated, 20 mM 2-AP was added 14 h before induction, or cells were transfected with 2 g pPKRΔ6 DNA9 17 h before induction. Confocal microscopy of fluorescence is shown (GFP, orange); PKRΔ6 panels are at lower magnification (E). (F, G) In vitro splicing of β-globin pre-mRNA is blocked by 2-AP and okadaic acid. Splicing of capped, 32P-labeled 477-nt β-globin precursor transcript in HeLa cell nuclear extract was analyzed as described in Materials and Methods section, in the presence of 2-AP (F) or okadaic acid (G). Position of intron lariat is indicated in F. M, marker DNA; U, input unspliced RNA. Splicing efficiency (SE), defined as a ratio of spliced/unspliced RNA, is provided under each lane. (H) Activation of PKR by β-globin pre-mRNA. rPKR was incubated with the 477-nt β-globin pre-mRNA template or 226-nt MINX pre-mRNA (0.75, 2.25, 3.75 and 7.5 nM) or with 203-nt 5′-terminal fragment of human IFN-γ mRNA (0.75 and 2.25 nM) in the presence of [γ-32P]ATP as described in Materials and Methods section. Autoradiogram shows phosphorylated PKR.
We next created constructs containing β-globin exons 1 and 2 wherein the β-globin coding sequence is fused in-frame to green fluorescent protein (GFP) ORF (Figure 1D). When splicing is blocked, the construct containing intron 1 would generate an RNA product having a stop codon in the intron, precluding expression of GFP. Cells transfected with the intronless construct expressed GFP regardless of the addition of 2-AP or co-expression of PKRΔ6, a dominant-negative mutant of human PKR (Figure 1E). By contrast, these treatments abolished GFP expression when intron 1 was present (Figure 1E). By forming heterodimers with wild-type (wt) PKR, kinase-dead PKRΔ6 blocks its activation18. Therefore, 2-AP and PKRΔ6 blocked excision of the β-globin intron in globin-GFP pre-mRNA. Unlike the case of β-globin pre-mRNA (Figure 1C), globin-GFP pre-mRNA splicing was not impeded by lack of the second intron; apparently, due to the lack of exon 3, the downstream sequence does not interfere with upstream splicing. Moreover, in a HeLa cell nuclear extract, splicing of a β-globin pre-mRNA template consisting of exon 1, intron 1 and exon 2 but lacking downstream sequence19 was inhibited completely by 2-AP (Figure 1F). The effect of 2-AP resembled that of okadaic acid, a known splicing inhibitor20 (Figure 1G). These results suggest that splicing of β-globin pre-mRNA requires activation of PKR.
To examine the hypothesis that β-globin pre-mRNA functions as an activator of PKR, we studied its ability to activate recombinant human holo-PKR expressed in an RNA-activatable form (rPKR)14. We used adenovirus MINX pre-mRNA, an efficient template for in vitro splicing21, and the 5′-terminal fragment of human IFN-γ mRNA, which is a potent activator of PKR13,14 as negative and positive controls, respectively. In contrast to MINX pre-mRNA, the β-globin pre-mRNA template strongly activated PKR, manifested by autophosphorylation of the kinase (Figure 1H).
Splicing of β-globin mRNA is dependent on PKR activation
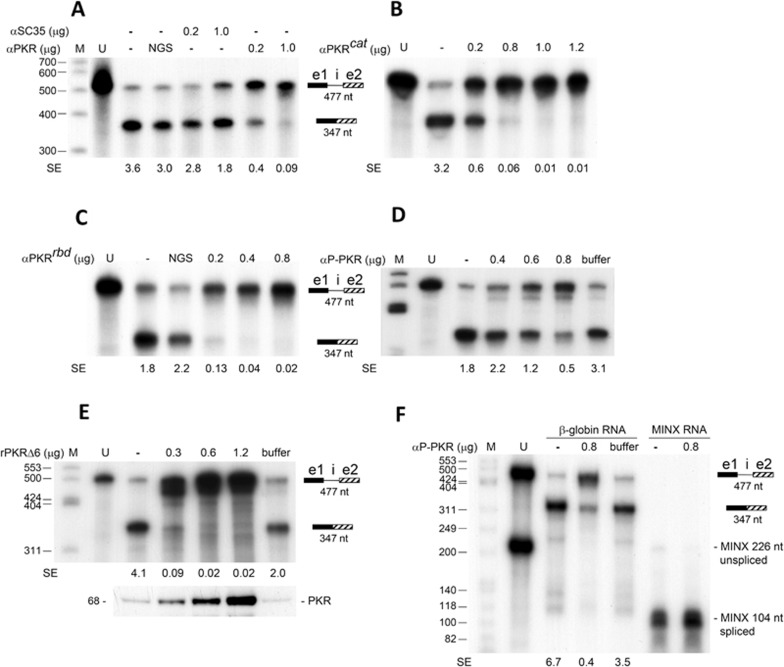
Similar to an antibody (Ab) against splicing factor SC35, an Ab against total PKR blocked splicing of β-globin pre-mRNA template in vitro (Figure 2A). Anti-PKR Abs against the catalytic domain (Figure 2B) or the RNA-binding domain of the kinase (Figure 2C) blocked splicing of β-globin pre-mRNA template, as did a monoclonal antibody (mAb) specific for phosphorylated PKR (Figure 2D). Thus, β-globin mRNA splicing not only requires PKR but the kinase must be phosphorylated and hence activated. Indeed, splicing was also abrogated by PKRΔ6 (Figure 2E). Whereas anti-phospho-PKR mAb inhibited splicing of β-globin pre-mRNA template, it did not affect splicing of MINX pre-mRNA, showing specificity (Figure 2F). In the presence of anti-phospho-PKR mAb, unspliced β-globin pre-mRNA accumulated yet there was no accumulation of unspliced MINX RNA (Figure 2F).
Figure 2.
Splicing of β-globin mRNA depends on PKR activation. (A-F) Splicing of β-globin pre-mRNA template is blocked by anti-PKR Abs and the dominant-negative mutant of PKR. In vitro splicing was performed as in Figure 1F. Where indicated, normal goat serum (NGS, 1 μg) (A, C), polyclonal Ab against SC35 (αSC35), holo-PKR (αPKR) (A), the catalytic domain of PKR (αPKRcat) (B) or the RNA-binding domain of PKR (αPKRrbd) (C), anti-phospho-PKR mAb (αP-PKR) specific for phospho-Thr446 (D, F), or rPKRΔ6 (E) was added in the amounts shown. Western blot of PKR in reaction mixtures is shown at bottom in E. Effect of αP-PKR on splicing of the β-globin pre-mRNA template and MINX pre-mRNA is compared in F. Buffer, corresponding protein storage buffer. SE, splicing efficiency.
PKR is found in splicing complexes and is needed for spliceosome formation
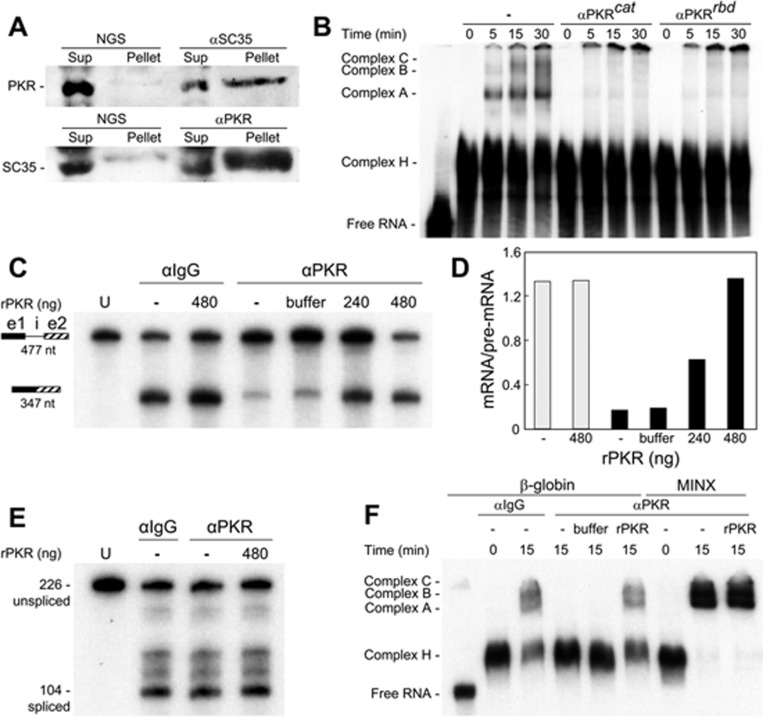
To investigate whether PKR associates with the β-globin pre-mRNA splicing complex, we performed the in vitro splicing reaction for 30 min and then immunoprecipitated holo-PKR or SC35, the latter of which is present in active splicing complexes22. Abs against SC35 precipitated PKR from HeLa cell nuclear extract and vice versa, Abs against PKR precipitated SC35 (Figure 3A). Confocal microscopy of intact cells confirmed that PKR is present in both cytoplasm and nucleus, and it colocalizes with SC35 to the nuclear speckles (Supplementary information, Figure S1A), sites of mRNA splicing23, supporting the inhibition of splicing by Abs against PKR and SC35 (Figure 2A) and the co-immunoprecipitation of these two proteins (Figure 3A). To investigate whether PKR is essential for splicing complex formation, we studied kinetics of ribonucleoprotein formation during in vitro splicing using labeled β-globin pre-mRNA template. Appearance of the spliceosomal complex A preceded that of complexes B and C (Figure 3B). Preincubation of HeLa cell nuclear extract with Abs against the catalytic or RNA-binding domain of PKR sufficed to abrogate formation of complex A yet did not affect formation of early complex H, which is not committed to splicing24 (Figure 3B). We conclude that PKR associates with splicing complexes and is needed early in spliceosome formation.
Figure 3.
PKR is in splicing complexes and required for splicing and spliceosome formation. (A) β-globin pre-mRNA template was incubated in HeLa cell nuclear extract for 30 min before immunoprecipitation with NGS, αSC35 or αPKR. Western blots of PKR (top) and SC35 (bottom) are shown for soluble (Sup) and precipitated fractions (Pellet). (B) Anti-PKR Abs inhibit spliceosome formation. Labeled β-globin pre-mRNA template was incubated in HeLa cell nuclear extract for the times shown. Where indicated, αPKRcat or αPKRrbd was added 20 min before incubation. Ribonucleoprotein splicing complexes A, B, C and H were resolved by native PAGE. Left lane shows input unspliced RNA. (C-D) Recombinant PKR (rPKR) reconstitutes β-globin pre-mRNA splicing upon PKR depletion. (C) After adding NaCl to 500 mM, nuclear extract was incubated at 4 °C with αIgG or αPKR Ab for 18 h; the immunoprecipitate was removed using protein A/G Sepharose beads. The depleted extract was centrifuged through Sephadex G-25 before in vitro splicing was performed for 2 h, with rPKR or storage buffer (buffer) added as shown. (D) Plot of mRNA/pre-mRNA ratio in C; αIgG, grey bars; αPKR, black bars. (E) In parallel, splicing of MINX RNA was assayed as in C. U, input unspliced RNA. (F) Reconstitution of spliceosomes on β-globin pre-mRNA template with rPKR. Formation of spliceosomes was assayed as in B, using extract depleted of PKR as in C. Where indicated, 480 ng of rPKR was added back. Three lanes on right show MINX RNA, assayed in parallel.
Recombinant PKR (rPKR) reconstitutes β-globin mRNA splicing upon PKR depletion
Splicing of the β-globin pre-mRNA template was virtually eliminated when PKR was depleted by immunoprecipitation with anti-PKR Ab, whereas anti-IgG had no effect (Figure 3C). Splicing in PKR-depleted nuclear extract was restored in full by adding back rPKR (Figure 3C-3D). By contrast, splicing of MINX RNA was completely resistant to PKR depletion, showing specificity (Figure 3E). Likewise, formation of splicing complexes A-C on β-globin pre-mRNA template, but not on MINX RNA, was abolished by PKR depletion, and was restored by adding back rPKR (Figure 3F). Of note, to avoid co-immunoprecipitation of splicing factors with PKR, we performed the immunoprecipitation in the presence of 500 mM NaCl25, and desalted the nuclear extract by gel filtration before the splicing reaction. Splicing and spliceosome formation likewise were restored in full by rPKR when the nuclear extract was desalted by dialysis (Supplementary information, Figure S1B-S1C). These reconstitution experiments validate the specificity of anti-PKR Ab that blocks splicing (Figures 2 and 3) and demonstrate directly that splicing of β-globin mRNA is PKR-dependent.
Addition of rPKR did not further stimulate splicing in αIgG-treated nuclear extract (Figure 3D), indicating that unlike the RNA-mediated activation of PKR, the level of this kinase, an abundant protein in the cell (Supplementary information, Figure S1A), does not limit β-globin mRNA splicing
eIF2α phosphorylation is required for splicing of β-globin mRNA
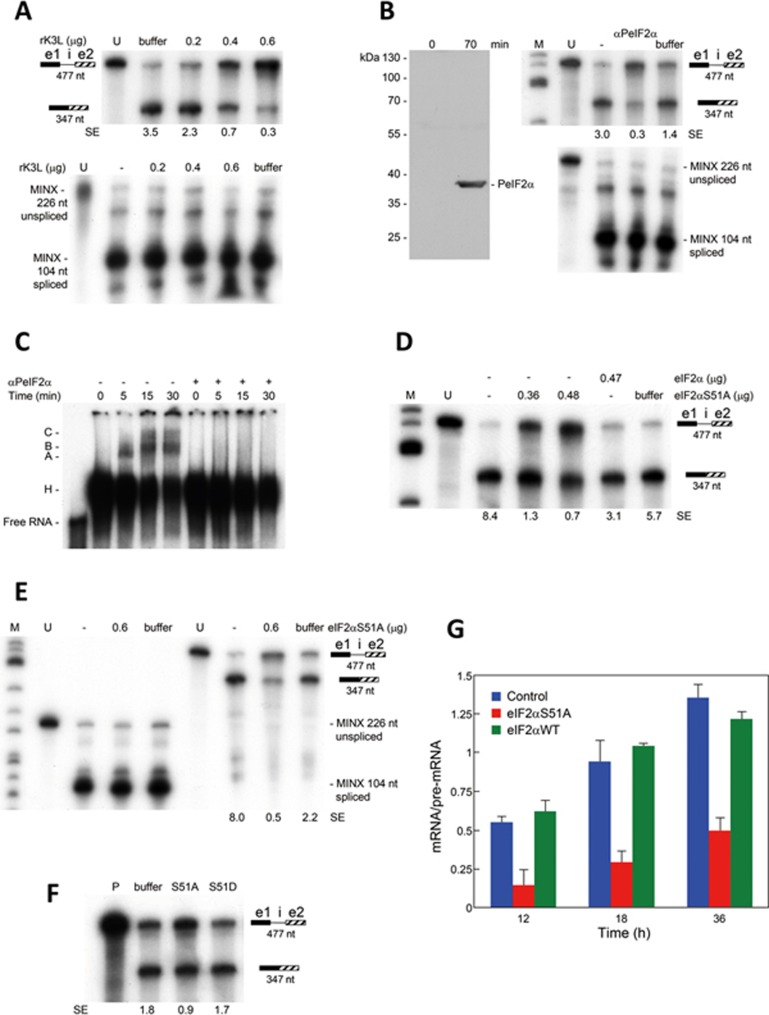
PKR inhibits translation initiation by phosphorylating its substrate eIF2α. We considered the possibility that eIF2α phosphorylation might play a role in β-globin mRNA splicing. First, we studied splicing of β-globin pre-mRNA template in nuclear extract in the presence of recombinant vaccinia virus K3L protein, which acts as a pseudosubstrate of PKR that binds the kinase and thus blocks its catalytic site26,27. K3L effectively inhibited splicing of β-globin pre-mRNA template but not that of MINX pre-mRNA (Figure 4A).
Figure 4.
β-Globin pre-mRNA splicing depends on eIF2α phosphorylation. (A) K3L inhibits splicing of β-globin mRNA. In vitro splicing was performed as in Figure 1F, using β-globin pre-mRNA template or MINX pre-mRNA. Before incubation, reaction mixtures were supplemented with recombinant K3L (rK3L) in the amounts shown. (B) Splicing of β-globin mRNA requires eIF2 phosphorylation. Right, splicing was assayed as in A, in the presence of anti-phospho-eIF2α mAb (αPeIF2α 3 μg). Left, western blot of splicing reaction mixture with αPeIF2α shows mAb specificity and eIF2α phosphorylation at 70 min. (C) αPeIF2α inhibits early spliceosome formation. Labeled β-globin pre-mRNA template was incubated in HeLa cell nuclear extract for the times shown. Where indicated, αPeIF2α was added 30 min before addition of the RNA. Ribonucleoprotein complexes A, B, C and H were resolved by native PAGE. Left lane shows input unspliced RNA (Free RNA). (D-F) eIF2αS51A inhibits splicing of β-globin mRNA. Splicing was assayed as in B, in the presence of yeast eIF2α or eIF2αS51A (D, E) in the amounts shown, or of 0.5 μg eIF2αS51A or eIF2αS51D (F). SE, splicing efficiency. (G) Expression of non-phosphorylatable mutant eIF2αS51A inhibits splicing of β-globin mRNA. HEK293T cells were co-transfected with equal amounts of β-globin gene vector and vector expressing human full-length eIF2αS51A or eIF2αWT, or empty vector (Control). At intervals after transfection, levels of spliced and unspliced β-globin transcripts were determined by qRT-PCR; splicing efficiency is expressed as the ratio of spliced RNA over unspliced RNA (means + SEM; n = 3).
Because K3L blocks autophosphorylation of PKR and phosphorylation of eIF2α26, we next examined the role of eIF2α phosphorylation in β-globin mRNA splicing. Indeed, eIF2α was phosphorylated during the splicing reaction (Figure 4B). The anti-phospho-eIF2α mAb, which did not recognize unphosphorylated eIF2α (Figure 4B, zero time point), blocked splicing of β-globin pre-mRNA template but not that of MINX pre-mRNA (Figure 4B) and completely inhibited formation of spliceosomal complex A on β-globin pre-mRNA template, showing a need for phosphorylated eIF2α in the early stages of spliceosome assembly (Figure 4C). By contrast, spliceosomal complex assembly on MINX RNA was not affected by this mAb (Supplementary information, Figure S2A), demonstrating the selective requirement for phospho-eIF2 in β-globin mRNA splicing. Consistently, addition of recombinant non-phosphorylatable mutant eIF2S51A strongly inhibited splicing of β-globin pre-mRNA template (Figure 4D-4E), whereas addition of wt eIF2α did not (Figure 4D). Splicing of MINX pre- mRNA, by contrast, was unaffected by eIF2αS51A (Figure 4E). Whereas eIF2αS51A inhibited β-globin mRNA splicing, phosphomimetic mutant eIF2αS51D did not (Figure 4F), reinforcing the need for phospho-eIF2α.
We next followed splicing of β-globin mRNA in cells. Expression of non-phosphorylatable mutant eIF2αS51A13 strongly reduced the splicing efficiency of β-globin mRNA (Figure 4G and Supplementary information, Figure S2B), whereas expression of wt human eIF2α did not (Figure 4G). These results extend the in vitro splicing data and show that phosphorylation of eIF2α is needed for efficient splicing of full-length β-globin mRNA in the cell. We conclude that eIF2α plays an essential role in splicing of β-globin mRNA and must be phosphorylated on Ser51 for splicing to occur.
The RNA activator of PKR resides in β-globin exon 1
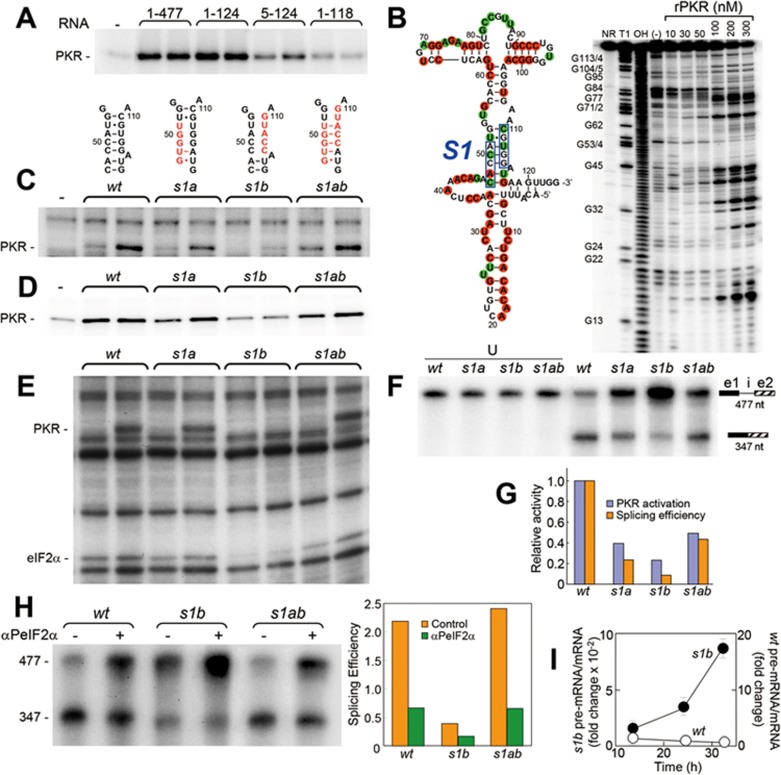
The β-globin pre-mRNA splicing template activates PKR (Figure 1H), raising the question of where this property maps within the 477-nucleotide (nt) RNA molecule. Progressive truncation from the 3′ end showed that the ability to activate PKR was fully maintained in the 5′-terminal 124-nt RNA fragment but lost almost completely in the 5′-terminal 118-nt fragment; removal of the first 4 nts from the 5′ end compromised PKR activation (Figure 5A). The RNA activator of PKR thus maps to the first 124 nts of β-globin pre-mRNA comprising the 50-nt 5′-UTR and the first 74 nts of the ORF, and is made up solely of exon 1 sequence.
Figure 5.
Splicing efficiency is coupled to activation of PKR by the activator in β-globin exon 1. (A) Activation of PKR by β-globin RNA. Phosphorylation of rPKR was assayed in the presence of [γ-32P]ATP, and 5 and 10 nM intact β-globin pre-mRNA template (nts 1-477) or truncated RNAs (nts 1-124, 5-124 and 1-118). (B) In-line structure probing of the 5′-terminal 124 nts of the β-globin pre-mRNA template. Gel shows representative pattern of spontaneous cleavages in 5′ end-labeled RNA incubated alone (−) or with rPKR (nM) as shown. G (T1) and alkali (OH) ladders serve as size markers; NR, input RNA. Structure on left is based on repeated in-line probing. Red and green colors denote nt showing enhanced and reduced cleavage, respectively, induced by rPKR. In helix S1, boxes denote nts mutated in s1a and s1b. (C-E) In s1a, positions 47-51 were mutated to GUGGU; in s1b, positions 110-114 were mutated to GUACC (red sequences in C). Activation of rabbit reticulocyte PKR (C, E) or rPKR (D) by wt or mutant forms of the 477-nt (C, D) or 124-nt (E) RNA was assayed; eIF2α phosphorylation is shown in E. For activation of rabbit reticulocyte PKR, 1.2 and 2 nM RNA was used, and for rPKR, 5 and 10 nM RNA was used. (F, G) Splicing efficiency is coupled to the ability to activate PKR. (F) Splicing of wt and mutant forms of β-globin pre-mRNA template in HeLa cell nuclear extract was analyzed as in Figure 1F; left four lanes, input unspliced RNA (U). (G) Comparison of the ability to activate PKR at the lower RNA concentration in D and splicing efficiency (ratio of spliced RNA over pre-mRNA) in F. (H) Splicing of s1b pre-mRNA template is restored by compensatory mutation. In vitro splicing was assayed in the absence or presence of αPeIF2α (1 μg); splicing efficiency is plotted in the bar graph. (I) β-globin precursor RNA processing is coupled to the ability to activate PKR. HEK293T cells were transfected with wt- or s1b-mutant β-globin gene and excision of intron 1 was assayed by quantitative RT-PCR; ratio of unspliced over spliced RNA is plotted (means + SEM; n = 3).
Probing of the 124-nt RNA element by spontaneous cleavage analysis28 supports the structure shown in Figure 5B. rPKR induced increased cleavage or protection from cleavage at various positions in the RNA element (Figure 5B), indicating that large sections of the activator RNA interact directly with PKR or undergo conformational changes upon binding of the kinase.
Splicing efficiency is coupled to the ability of the RNA to activate PKR
Our results so far show that β-globin pre-mRNA splicing template potently activates PKR (Figure 1H) through its 5′-terminal domain (Figure 5A) and that activation of PKR is needed for splicing of this RNA (Figures 2, 3B-3F and Supplementary information, Figure S1). We next asked whether during splicing, activation of PKR is accomplished by the splicing template itself. We mutated the short central helix S1 consisting of 7 bp within the 124-nt RNA element (Figure 5B) to yield s1a and s1b mutations. The s1a and s1b mutations disrupt 3 and 5 bp in helix S1, respectively; this can explain why the s1a mutation partially reduced the ability of the 477-nt β-globin pre-mRNA template to activate PKR, as revealed by assays using rabbit reticulocyte ribosomal fraction containing native PKR (Figure 5C) or rPKR (Figure 5D), whereas the s1b mutation severely impaired PKR activation. Ability to activate PKR was restored, at least in part, in the double mutant s1ab which retains 5 out of 7 bp in helix S1 (Figure 5C-5D).
The failure of the s1b pre-mRNA template to activate PKR (Figure 5C-5D) and to induce eIF2α phosphorylation (Supplementary information, Figure S3A) was not due to a lack of the ability to bind PKR, because it competed as well as wt RNA for PKR binding in electrophoretic mobility shift analysis (Supplementary information, Figure S3B). Thus, binding of the β-globin RNA to PKR is insufficient for kinase activation, resembling the properties of the RNA activators of PKR in IFN-γ13,14 and TNF-α (manuscript in submission) genes. The phenotype of these mutations was conserved in the 124-nt RNA element with respect to the ability to activate PKR and to induce eIF2α phosphorylation (Figure 5E). We conclude that helix S1 shown in Figure 5B is maintained in the β-globin pre-mRNA splicing template
We next analyzed the effect of these mutations on splicing of the β-globin pre-mRNA template. s1a and s1b mutations severely impaired splicing efficiency and this was more pronounced for s1b. In contrast, the double mutation s1ab restored splicing in part (Figure 5F-5G). Notably, the relative splicing efficiency for wt, s1a, s1b and s1ab β-globin pre-mRNA templates correlated closely with their ability to activate rPKR at the lower RNA concentration, which was more sensitive to mutations (Figure 5D and 5G). Thus, splicing efficiency in HeLa cell nuclear extract containing all components needed for spliceosome formation reflects the ability of the RNA to activate PKR in an assay where the only macromolecules present are RNA and rPKR.
Reflecting their similar ability to activate native PKR (Figure 5C and 5E), in an independent experiment, wt and s1ab mutant pre-mRNA templates were spliced with similar efficiency, which was inhibited by anti-phospho-eIF2α mAb to a similar extent (Figure 5H). Thus, compensatory mutation in s1ab RNA not only restores PKR activation and splicing to s1b mutant RNA but such splicing remains as dependent on eIF2α phosphorylation as splicing of wt RNA. Even though s1b RNA is severely impaired in splicing, its low residual activity likewise requires phospho-eIF2α (Figure 5H).
Mutation of the PKR activator also affected β-globin mRNA splicing in the cell, where the s1b mutation strongly impaired intron 1 excision (Figure 5I). The ratio of pre-mRNA/mRNA declined over time for the wt gene as expected yet increased for the s1b gene, attesting to a block in pre-mRNA processing. These results show that the splicing efficiency of the β-globin pre-mRNA template is regulated by its ability to activate PKR through the 124-nt exonic RNA element.
PKR activation and eIF2α phosphorylation enable splicing of Aγ-globin mRNA
Fetal γ-globin genes are related to the adult β-globin gene. Except for nt 25 in the 5′-UTR, Gγ- and Aγ-globin gene sequences are identical through exon 229. We examined whether activation of PKR might also regulate excision of Αγ-globin intron 1. Indeed, as for β-globin (Figure 2E), PKRΔ6 strongly inhibited splicing of Αγ-globin pre-mRNA template containing exon 1, intron 1 and the first 172 nts of exon 2 (Supplementary information, Figure S3C). Αγ-Globin and β-globin pre-mRNA templates activated PKR comparably (Supplementary information, Figure S3D). Αγ-Globin exon 1 RNA was sufficient to activate the kinase and induce eIF2α phosphorylation (Supplementary information, Figure S3E). Splicing of Αγ-globin and β-globin pre-mRNA templates was similarly inhibited by anti-phospho-eIF2α mAb (Supplementary information, Figure S3F). Thus, PKR activation and eIF2 phosphorylation also enable splicing of Aγ-globin mRNA.
The ability of β-globin pre-mRNA to activate PKR is silenced upon splicing
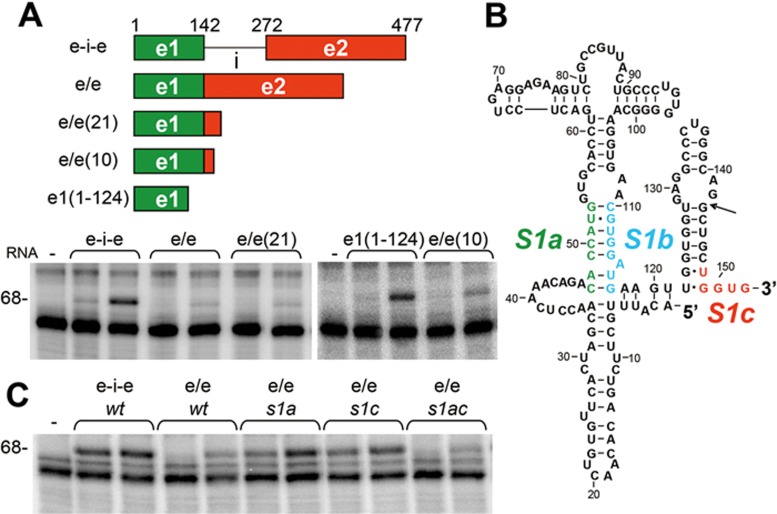
During erythroid development, the cellular globin content rises from < 0.1% of the total protein amount in proerythroblasts to 95% in reticulocytes, reflecting a massive translation of globin mRNA15. Erythropoiesis would be compromised severely if mature β-globin mRNA were to activate PKR and thereby inhibit its own translation, in a manner resembling that for IFN-γ mRNA13. In reticulocyte lysates, rabbit globin mRNA does not detectably activate PKR30. Because the activator of PKR in β-globin pre-mRNA splicing template is made up solely of exon sequence (Figure 5A), there is a need for a mechanism by which the ability to activate PKR is blocked in mature human β-globin mRNA. Indeed, whereas the 477-nt β-globin pre-mRNA template strongly activated PKR (Figure 6A, e-i-e), the spliced mRNA product consisting of exon 1 and the first 205 nts of exon 2 activated PKR weakly (at high RNA concentration) or not at all (at low RNA concentration) (Figure 6A, e/e). Apparently, upon excision of intron 1, the RNA activator of PKR in β-globin pre-mRNA template is silenced.
Figure 6.
The ability to activate PKR is silenced upon splicing. (A) Excision of β-globin intron 1 causes silencing of the ability to activate PKR. Rabbit reticulocyte ribosome fraction was incubated with 1.2 and 2 nM RNAs, including 477-nt β-globin pre-mRNA template (e-i-e), RNA comprised of 142-nt exon 1 joined to the first 205 (e/e), 21 (e/e(21)) or 10 nts (e/e(10)) of exon 2, or the 5′-terminal 124 nts of exon 1 (e1(1-124)) (see map). PKR activation was then determined by its autophosphorylation level (68 kDa band). (B) Schematic shows complementarity of S1b and S1c with S1a in e/e(10) RNA sequence; arrow indicates exon 1/2 splice junction. (C) A mutation in β-globin exon 2 relieves silencing. In s1c, positions 148-152 were mutated to ACCAC. Activation of PKR by 1.2 and 2 nM pre-mRNA template (e-i-e) and wt or mutant forms of spliced RNA (e/e) was assayed using rabbit reticulocyte ribosome fraction.
To explore the silencing mechanism, we first truncated the second exon from the 3′ end. β-globin mRNA fragment containing exon 1 and the first 21 nts of exon 2 failed to activate PKR significantly; even truncation that left only 10 nts of exon 2 fused to exon 1 sufficed to impair the ability to activate PKR (Figure 6A). This result suggests that when juxtaposed to exon 1, the first 10 nts of exon 2 are able to silence the PKR activator in exon 1. The first 10 nts of exon 2 sequence contains a 5′-UGGUG-3′ motif (S1c) that in principle could anneal with the 3′-ACCAC-5′ motif in S1a, forming 5 stable bp and displacing the corresponding S1b motif (Figure 6B, modeled in Supplementary information, Figure S4A). To test this concept, we mutated the 5′-CACCA-3′ motif in S1a to 5′-GUGGU-3′ and/or changed the S1c motif to 5′-ACCAC-3′ in the spliced e/e RNA. e/e RNA fragments with the resulting s1a or s1c mutation induced activation of PKR. The double mutation, e/e s1ac, which restores base pairing between S1a and S1c, also restored silencing of PKR activation (Figure 6C).
The 477-nt β-globin pre-mRNA template with the s1a mutation, which partially retains the base pairing within helix S1, still possessed partial ability to activate PKR (Figure 5C). As the s1a mutation abrogates base pairing with the S1c silencer sequence, this could prevent the disruption of base pairing between S1a and S1b sequences after splicing (Figure 6B), and thus allow for residual PKR activation, as is indeed observed in Figure 6C.
We conclude that upon excision of intron 1, base pairing between S1a and S1b sequences within helix S1, critical for activation of PKR by β-globin pre-mRNA template (Figure 5C-5E), is abrogated through displacement of S1b by S1c sequence located just downstream of the exon junction, within the first 10 nts of exon 2. This results in silencing of the ability of the mature mRNA product to activate PKR. Silencing thus requires strand replacement within helix S1 and as shown by e/e s1ac, this process tolerates sequence inversion of the two complementary strands. Phylogenetic analysis shows that except for mouse β-globin major RNA, the potential for S1a-S1b base pairing through the AUG codon is conserved, even in teleost fish (Supplementary information, Figure S4B).
An RNA activator of PKR resides in α-globin exon 2
A hallmark of normal erythropoiesis is efficient synthesis of α- and β-globin in an equimolar ratio. We therefore investigated whether splicing of α-globin mRNA is also regulated by activation of PKR and phosphorylation of eIF2α. In cells transfected with the human α2-globin gene, addition of 2-AP led to prompt accumulation of unspliced pre-mRNA (Figure 7A and Supplementary information, Figure S5). Splicing of the α-globin pre- mRNA template comprising exon 1, intron 1 and all but 9 nts of exon 2 in HeLa cell nuclear extract was blocked by anti-phospho-PKR mAb (Figure 7B) and was at least as sensitive as splicing of β-globin pre-mRNA template to anti-phospho-eIF2α mAb (Figure 7C). α-Globin pre-mRNA template was as potent an activator of PKR as β-globin pre-mRNA template (Figure 7D). In contrast to β-globin, however, the α-globin RNA activator of PKR mapped to exon 2 (Figure 7E). 3′-truncation of exon 2 RNA showed that a full PKR activation requires the first 179 nts of exon 2 (nts 250-428) and was largely lost upon removal of five additional nts from the 3′ end; 5′-truncations defined a 123-nt core (nts 306-428) as the minimal activator (Figure 7F).
Figure 7.
Excision of α2-globin intron 1 depends on PKR activation and eIF2α phosphorylation. (A) 2-AP inhibits splicing of α-globin mRNA. HEK293T cells were transfected with the human α2-globin gene. 2-AP (15 mM) was added at 18 h after transfection. At times shown, expression of unspliced and spliced RNA was assayed by RNase protection analysis (Supplementary information, Figure S5). Pre-mRNA/mRNA ratio is plotted. (B, C) mAbs against phospho-PKR and phospho-eIF2α block splicing of α-globin pre-mRNA template. Splicing of capped, 32P-labeled 445-nt α-globin pre-mRNA template in HeLa cell nuclear extract was analyzed as in Figure 2D, in the absence or presence of 1 μg αP-PKR mAb (B) and for splicing of α- and β-globin pre-mRNA templates, in the absence or presence of 3 μg αPeIF2α mAb (C). SE, splicing efficiency. (D, E) The α-globin RNA activator of PKR maps to exon 2. Activation of rPKR (top) or rabbit reticulocyte PKR (bottom) was assayed in the presence of 445-nt α-globin or 477-nt β-globin pre-mRNA template (e1-i-e2) (D) or in the presence of 445-nt pre-mRNA template (e1-i-e2), 132-nt exon 1 RNA (e1) or 196-nt exon 2 RNA (e2) of α-globin (E). For activation of rabbit reticulocyte PKR, 1.2 and 2 nM RNA was used and for rPKR, 5 and 10 nM RNA was used. (F) Definition of the minimal α-globin RNA activator of PKR. Activation of rPKR was analyzed in the presence of 5 and 10 nM α-globin exon 2 RNA truncated to the positions shown (see map at bottom). (G) Excision of α-globin intron 1 causes silencing of the ability to activate PKR. Activation of rPKR was analyzed in the presence of 5 and 10 nM α-globin RNA as shown (e/e, 328-nt spliced form). The silencer maps to nts 1-74 in exon 1, shown in red in the map.
A silencer of PKR activation resides in α-globin exon 1
Excision of intron 1 almost totally eliminated the ability of α-globin RNA to activate PKR (Figure 7G). PKR activation was restored in part by truncation of the first 36 nts of exon 1 and fully by deletion of the first 73 nts, placing the silencer of PKR activation within the 5′-proximal region of α-globin exon 1 (Figure 7G). Thus, for both α- and β-globin genes, splicing of intron 1 depends on PKR activation and eIF2α phosphorylation, but the respective positions of activator and silencer are reversed between the adjoining exons.
Discussion
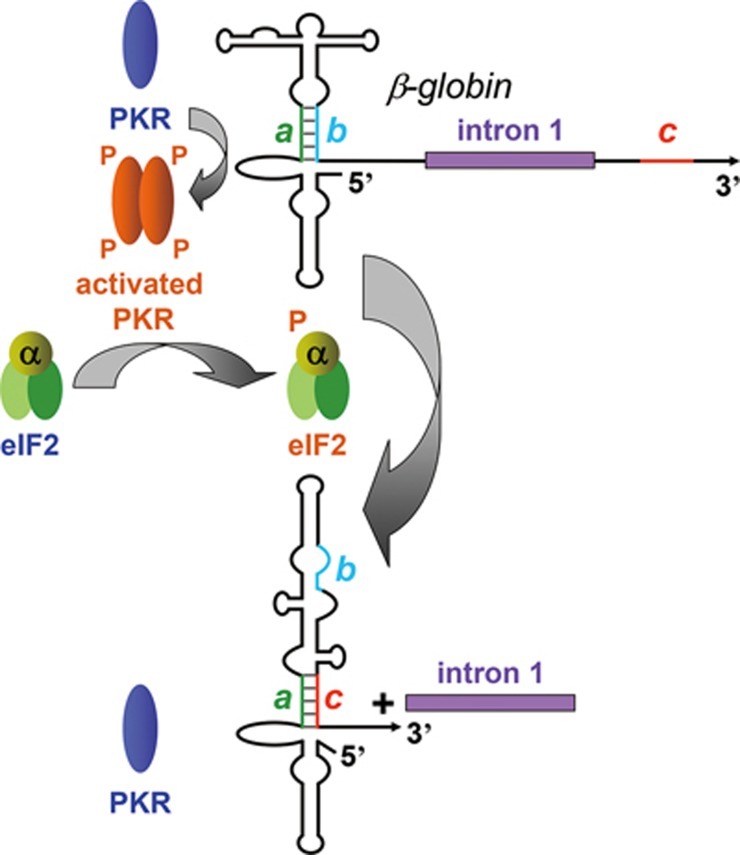
Our finding is that splicing of globin mRNA requires not only activation of PKR by an intragenic RNA activator but also phosphorylation of the PKR substrate, eIF2α. There are similar requirements for the TNF-α gene encoding an inflammatory cytokine (manuscript in submission) suggesting the generality of our findings. Hemoglobin synthesis depends on optimal translation of globin mRNA in reticulocytes and globin reaches 95% of the total protein amount under conditions where no further transcription takes place15. Hence, local activation of PKR by globin mRNA, resulting in phosphorylation of eIF2α and translational downregulation, would be detrimental. It is thus surprising that globin mRNA splicing depends strictly on activation of PKR as well as on phosphorylation of eIF2α, with the RNA activator of PKR not only being intrinsic to the globin pre-mRNA transcript but also located within an exon and therefore retained in the mature mRNA. The solution to this paradox is provided by a mechanism, demonstrated here, in which excision of the first intron induces an interaction between nucleotides in the adjacent exons, causing a structural rearrangement in the mRNA that silences the activator of PKR. Thus, whereas β-globin pre-mRNA potently activates PKR and induces phosphorylation of eIF2α, both required in the early stages of spliceosome assembly, the spliced mRNA no longer activates PKR. Use of RNA-mediated activation of PKR and eIF2α phosphorylation to enable mRNA splicing reveals a dual function for this mechanism associated with the integrated stress response, yielding opposite outcomes in cytoplasm and nucleus.
On the basis of analysis in cells and in vitro, our data support a splicing control mechanism depicted in Figure 8. β-globin pre-mRNA exon 1 functions as a potent activator of PKR. Mutation of the activator profoundly affected splicing efficiency of β-globin pre-mRNA, in tight correlation with its ability to activate PKR. PKR was co-immunoprecipitated with SC35, showing that it associates with splicing complexes. PKR activation is a prerequisite for the first step in β-globin mRNA splicing, excision of intron 1, which was blocked specifically by a dominant-negative mutant of PKR and by Abs against PKR and phospho-PKR, including an mAb that specifically targets phospho-Thr446, a pivotal residue in the kinase activity segment. Critically, both splicing and early spliceosome formation were abrogated by depletion of PKR and restored by adding back recombinant RNA-activatable holo-PKR. Should eIF2 kinases that are not activated by RNA2 be capable of supporting β-globin mRNA splicing, these approaches would not have yielded an inhibition. Once PKR is activated, eIF2α must be phosphorylated on Ser51 to allow formation of early-stage spliceosomal complex A. Splicing was inhibited by K3L, a viral eIF2α pseudosubstrate of PKR, by anti-phospho-eIF2α mAb and by non-phosphorylatable mutant eIF2αS51A, yet not by phosphomimetic eIF2αS51D. As for TNF-α (manuscript in submission), expression of eIF2αS51A blocked splicing of full-length β-globin mRNA in cells. The dependence of splicing on eIF2α phosphorylation early during β-globin spliceosome assembly provides a mechanistic explanation for the need for PKR activation in splicing.
Figure 8.
Model for regulation of β-globin mRNA splicing by an RNA activator of PKR in exon 1 and silencing of the PKR activator upon intron 1 excision. The activator of PKR in exon 1 relies on 5 bp a-b pairing in helix S1. Activation of PKR results in phosphorylation of eIF2α, a prerequisite for excision of intron 1 (top). Excision of intron 1 juxtaposes exon 2 sequence c and exon 1 sequence a, causing strand displacement in helix S1 that silences the ability to activate PKR (bottom).
Upon excision of β-globin intron 1, a 5-nt exon 2 sequence c, located just downstream of the exon junction, displaces strand b of short helix a-b in the RNA activator of PKR in exon 1, which is critical for the ability of β-globin pre-mRNA to activate PKR (Figures 6 and 8). This displacement results in silencing of the ability of the exonic RNA element to activate PKR. Mutation of either strand a or c in this helix was sufficient to allow the spliced RNA product to activate PKR, whereas double mutation that restored base pairing resulted in restoration of silencing of the ability to activate PKR. In this manner, activation of PKR is eliminated once intron 1 has been excised. This RNA-mediated silencing mechanism ensures that the local activation of PKR serves solely to enable splicing.
IFN-γ mRNA attenuates its own translation by locally activating PKR13 through a structure comprised of the 5′-UTR and the first 26 codons, causing it to be disrupted by ribosomes during translation and requiring dynamic refolding to restore the activator14. Although the β-globin activator of PKR likewise spans the 5′-UTR and the first 25 codons of the ORF, β-globin mRNA does not activate PKR after splicing owing to the silencer mechanism.
α- and β-globin proteins are produced in an equimolar ratio but their genes differ in terms of chromosomal location, gene dosage, transcriptional control and mRNA stability31,32 as well as in the ability of their mRNAs to compete in translation33,34. We have shown that α- and β-globin genes share a regulatory strategy, i.e., dependence of mRNA splicing on activation of PKR and phosphorylation of eIF2α, but differ in the location of the elements that activate PKR and silence this property. Whereas the β-globin RNA activator of PKR is located in exon 1, the α-globin activator is composed solely of protein-encoding sequence in exon 2. Conversely, in β-globin mRNA the silencer of PKR activation is located in exon 2, whereas in α-globin mRNA it resides in the 5′-half of exon 1. These distinct solutions demonstrate evolutionary flexibility in achieving the common aim of bringing mRNA splicing under the control of PKR activation. The first introns of α-and β-globin thus serve a novel function, to separate the PKR activator required for splicing from the silencer of PKR activation needed to enable translation.
Our experiments link eIF2α phosphorylation to an early step in spliceosome assembly on globin mRNA, a complex and dynamic process in which the roles of multiple individual proteins remain to be delineated35,36. Whereas eIF2α phosphorylation inhibits translation, we demonstrate a positive role for phosphorylation of eIF2α on Ser51: it is necessary for splicing of globin mRNA. This requirement is shared with splicing enabled by the RNA activator of PKR in the TNF-α 3′-UTR, which is likewise abrogated by eIF2αS51A expression (manuscript in submission). Splicing efficiency of TNF-β pre-mRNA, which lacks a PKR activator, is strongly enhanced not only by inserting the TNF-α activator but also by merely increasing the level of phospho-eIF2α globally in the cell by using an inhibitor of eIF2α dephosphorylation, demonstrating that eIF2α phosphorylation is both necessary and sufficient to achieve efficient splicing. Control of TNF mRNA splicing by eIF2α phosphorylation operates in primary human immune cells, showing its physiological relevance (manuscript in submission).
Our results demonstrate a novel nuclear role for PKR and eIF2α phosphorylation. A nuclear extract displays multiple important properties: the need for PKR in complex A formation on β-globin splicing template; inhibition of β-globin pre-mRNA splicing upon depletion of PKR and restoration of splicing by recombinant human PKR; restoration of complex A formation on a β-globin splicing template; phosphorylation of eIF2α and the need for phospho-eIF2α in complex A formation.
Differentiating human erythroleukemia cells do not express β-globin; very low levels of β-globin mRNA were detected, relative to α-globin mRNA, in conditions inhibiting cell proliferation37. Even before induction of differentiation, K562 cells expressed fully spliced α-globin mRNA, whereas pre-mRNA was not detectable (LSN, data not shown). We overcame the limitations of studying splicing of adult human globin mRNA in human cell lines by in vitro splicing assays and by genetic approaches, including gain-of-function mutations that provide mechanistic insight. Data from in vitro analysis of globin mRNA splicing were validated in transfected human and hamster cells.
Whereas human TNF-α and IFN-γ genes contain potent intragenic activators of PKR, these elements display structural defects in the mouse (manuscript in submission,14). The mouse also differs significantly in terms of β-globin gene organization, expressing major and minor species38, impeding facile comparison with the human gene.
Synthesis of heme and globin is coordinated through a feedback loop wherein heme is needed to block activation of the eIF2 kinase HRI, thereby preventing attenuation of globin mRNA translation39,40. Phosphorylation of eIF2α by two distinct kinases, PKR and HRI, thus regulates separate steps in globin gene expression in nucleus and cytoplasm, i.e., mRNA splicing and translation, respectively. In the cytoplasm, eIF2α is found in complex with eIF2β and γ chains, forming holo-eIF2 active as translation factor41. Whether eIF2β and γ subunits are involved in globin mRNA splicing is not yet known.
Materials and Methods
Gene transfection
Human embryonic kidney HEK293T cells were transiently transfected with 2 μg pBS vector carrying the 5 kb PstI-PstI fragment of genomic β-globin DNA including the upstream and downstream regulatory sequences. β-globin gene lacking intron 2 was constructed by replacing sequence downstream from the PmlI site in exon 2 with cDNA sequence. pβG(e1-i-e2)GFP and pβG(e1-e2)GFP contain β-globin exons 1 and 2, with and without intron 1, respectively, under the tetracycline-inducible promoter, exon 2 being fused in-frame to the EGFP ORF at the BamHI site 18 bp upstream from the 3′ end of exon 2. These two vectors were used to stably transfect BHK-21 cells together with pTet-tTAK (Gibco BRL) in a ratio of 1:10. After four passages in DMEM medium, transfected cells were induced with 12.5 pg/ml tetracycline; cells showing over 70% fluorescence were selected. Expression of PKRΔ6 was performed as described13.
Splicing assay by RNase protection analysis
To generate β-globin intron 1 splicing probe (probe a), genomic DNA was digested with NcoI and NspI and the filled-in fragment ligated into HincII-digested pBS vector. To generate β-globin intron 2 splicing probe (probe b), 5′-CGGAATTCTACCCTTGGACCCAGAG-3′ and 5′-CCCAAGCTTCGATCCTGAGACTTCCAC-3′ were used as PCR primers on genomic DNA; the EcoRI-HindIII fragment was excised from the product and inserted into pBS vector. Total RNA was isolated with TRIzol Reagent (Invitrogen) and subjected to RNase protection analysis with RNases A and T112,42. Band intensity in autoradiograms was quantitated with a Fujifilm BAS 1800II Phosphoimager.
Splicing assay by quantitative real-time PCR
Total cellular RNA was harvested using TRIzol Reagent (Invitrogen) and cDNA synthesized from 1 μg RNA using Verso RT-PCR Kit (ABgene) in a 20-μl reaction volume. Quantitative real-time PCR was performed using 0.25 μg cDNA, 300 nM primers/probe Double-Dye (Taqman style) mix and Absolute Blue QPCR Mix (ABgene). Primers and probes specific for spliced (primers: 5′-AAGGTGAACGTGGATGAAGTT-3′, 5′-AGCATCAGGAGTGGACAGAT-3′ probe: 5′-CCTGGGCAGGCTGCTGGTGGTC-3′) and unspliced (primers: 5′- TATCAAGGTTACAAGACAGGTTTAAG-3′, 5′-GCCTAAGGGTGGGAAAATAGAC-3′ probe: 5′-CTCTTGGGTTTCTGATAGGCACTGACTCT-3′) forms of β-globin RNA and normalizing human β-actin gene were from PrimerDesign. Reactions were performed for 40 cycles (95 °C for 15 s, 60 °C for 30 s and 72 °C for 15 s) using a Rotor-Gene 6000 instrument (Corbett Life Science). The ΔΔCt method was used to evaluate mRNA-to-pre-mRNA ratio. Amplification efficiency of spliced and unspliced forms, determined by CT slope, was similar and at least 99%.
In vitro splicing
HeLa cell nuclear extract (Promega) and capped, 32P-labeled 477-nt human β-globin precursor SP6 transcript generated from BamHI-cut DNA template (0.17 pmol, 1.25 × 105 cpm/pmol) or adenovirus MINX SP6 transcript generated from BamHI-cut DNA template21 were incubated for 3 h at 30 °C in splicing buffer (reaction volume, 25 μl) following instructions of the manufacturer (Promega). When the effect of αP-PKR (Calbiochem) or αP-eIF2α (Abcam) was studied, the reaction was preincubated with Ab for 15 min at 30 °C and 15 min at 4 °C before RNA and RNasin were added; for αPKR (Cell Signaling), αPKRcat, and αPKRrbd (Santa Cruz), preincubation was carried out for 15 min at 4 °C. RNA was extracted, precipitated and analyzed in 4% polyacrylamide/8 M urea gels. For analysis of spliceosome formation, 4-μl samples were collected at time intervals, incubated for 10 min with 50 μg heparin at 4 °C, made 10% glycerol and separated on 3.75% native polyacrylamide gels (polyacrylamide:bis, 80:1; 50 mM Tris-base, 50 mM glycine, pH 8.8). The α-globin pre-mRNA template for in vitro splicing, generated from HincII-digested human α2-globin DNA, was a 445-nt SP6 transcript. The Αγ-globin pre-mRNA template was a 439-nt T3 transcript. 5′-end labeled HinfI-digested φX174 single-stranded DNA (Fermentas) served as size marker, except in Figures 1G and 2A where boiled 100-bp DNA ladder (New England Biolabs) was used.
Co-immunoprecipitation of PKR and SC35
Antibodies were bound to protein A-Sepharose (GE Healthcare) by incubation for 2 h at 4 °C. Immunoprecipitation from HeLa nuclear extract was performed by addition of 12 μg αPKR, 26.4 μg αSC35 (Santa Cruz) or 60 μg NGS followed by incubation for 5 h at 4 °C. Complexes were washed five times with the buffer containing 50 mM Tris-HCl (pH 7.4), 5 mM EDTA, 150 mM NaCl, 1 M KCl, 0.2% bovine serum albumin, and 0.5% Triton X-100.
Mutagenesis
β-globin s1a mutant was generated by replacing the AleI-AleI fragment in wt 1-477 bp DNA with 5′-CTGTGTTCACTAGCAACCTCAAACAGAGTGGT-3′ annealed to 5′-ACCACTCTGTTTGAGGTTGCTAGTGAACACAG-3′. s1b was created by PCR using phosphorylated primers 5′-GAAGTACCATGAAGTTGGTGGTGAGG-3′ and 5′-ACCTTGCCCCACAGGG-3′ with the wt 1-477 bp DNA template and KOD polymerase (Novagen) to generate linear full-length plasmid DNA carrying blunt ends; the product was purified on a 1% agarose gel and self-ligated. Mutant e/e s1c was generated from β-globin cDNA under the SP6 promoter by PCR, using primers 5′-TGCACCACGTCTACCCTTGGACCCAGAGG-3′ and 5′-GCCTGCCCAGGGCCTCA-3′. Truncations were created using restriction enzyme digestion, ligation to annealed complementary oligonucleotides, or PCR; primer sequences will be supplied on request. Truncation of α2-globin and γ-globin exon 1/intron 1/exon 2 DNA was done by PCR using phosphorylated DNA primers with KOD polymerase as above.
In-line structure probing of RNA
For in-line probing of RNA, we adapted a previously described protocol28. About 1 nM 5′ 32P-labeled RNA was incubated for 40 h at 25 °C in the buffer containing 20 mM MgCl2/50 mM Tris-HCl pH 8.3, 100 mM KCl, 1.5 mM dithiothreitol and 6% (v/v) glycerol in the absence or presence of rPKR. RNA cleavage products were resolved by 10% PAGE.
Recombinant proteins
PKR, PKRΔ6 and K3L were cloned into pHTT7K43 and expressed in Escherichia coli Rosetta(DE3)pLysS (Invitrogen) as full-length N-terminally hexahistidine-tagged proteins. PKR was expressed in RNA-activatable form and was > 98% pure on SDS-12% PAGE14. K3L was recovered from inclusion bodies by solubilization in 6 M urea buffer and loaded onto a Ni-column that was eluted stepwise with imidazole buffer containing 6 M urea; next, urea concentration was reduced 10-fold in six dialysis steps. K3L was renatured by dialysis against 0.5 M arginine, 20% glycerol, phosphate-buffered saline before arginine concentration was reduced five-fold in four dialysis steps; it was stored in the final buffer. Saccharomyces cerevisiae eIF2α, eIF2αS51A and eIF2αS51D were cloned into pET15b and expressed as C-terminally truncated hexahistidine-tagged 25 kDa proteins (manuscript in submission).
Activation of PKR
Uncapped RNA transcripts were purified by Sephadex G-50 gel chromatography, washed with ethanol and eluted with water as described44. Rabbit reticulocyte ribosomal fraction was prepared and activation of PKR by RNA was assayed as described45. Activation of 100 ng rPKR by RNA was determined as described14. Unless otherwise noted, RNA concentrations used were 1.2 and 2 nM for rabbit reticulocyte ribosome fraction and 5 and 10 nM for rPKR.
Immunodepletion and reconstitution of PKR
For immunodepletion of PKR from HeLa nuclear extract, 200 μl of the extract was brought to 500 mM NaCl25, first incubated with 12 μg of αIgG or αPKR Ab for 18 h at 4 °C with head-over-tail rotation, and then incubated with washed protein A/G Sepharose beads for 2 h at 4 °C with head-over-tail rotation before the nuclear extract was separated from the beads. To remove NaCl, the depleted nuclear extract was either centrifuged through Sephadex G-25 or desalted by overnight dialysis in buffer (20 mM Hepes pH 7.9, 0.5 mM dithiothreitol, 20% (v/v) glycerol, and 0.5 mM phenylmethylsulfonyl fluoride) before in vitro splicing was performed for 2 h with or without rPKR or storage buffer.
Author Contributions
LI, FO, LSN and RK designed research; LI, FO, LSN, EE, SC-C, YB-A and YB performed research and analyzed data; RK, LI and LSN wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
Acknowledgments
We thank Blanka Shanitzki for assistance in quantitative real-time PCR. This work was supported by grants from the Israel Science Foundation.
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Material
Colocalization of PKR with SC35 and reconstitution of β-globin pre-mRNA template splicing and spliceosome formation by recombinant PKR.
Spliceosome formation on MINX RNA is re sistant to inhibition by αPeIF2α mAb and eIF2αS51A expression inhibits β-globin mRNA splicing.
Properties of s1b mutant β-globin RNA and PKR- and phospho-eIF2α-dependent splicing of Aγ-globin mRNA.
Structure model and phylogenetic conservation of base pairing in unspliced and spliced β-globin mRNA.
2-Aminopurine inhibits splicing of α-globin mRNA.
References
- Nallagatla SR, Toroney R, Bevilacqua PC. Regulation of innate immunity through RNA structure and the protein kinase PKR. Curr Opin Struct Biol 2011; 21:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 2009; 136:731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 2003; 11:619–633. [DOI] [PubMed] [Google Scholar]
- Muaddi H, Majumder M, Peidis P, et al. Phosphorylation of eIF2α at serine 51 is an important determinant of cell survival and adaptation to glucose deficiency. Mol Biol Cell 2010; 21:3220–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 2011; 332:91–94. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. Identification and RNA-binding properties of an initiation factor capable of relieving translational inhibition induced by heme deprivation or double-stranded RNA. Biochem Biophys Res Commun 1974; 61:591–597. [Google Scholar]
- Farrell PJ, Balkow K, Hunt T, Jackson RJ, Trachsel H. Phosphorylation of initiation factor eIF-2 and the control of reticulocyte protein synthesis. Cell 1977; 11:187–200. [DOI] [PubMed] [Google Scholar]
- Zhang F, Romano PR, Nagamura-Inoue T, et al. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J Biol Chem 2001; 276:24946–24958. [DOI] [PubMed] [Google Scholar]
- Dey M, Cao C, Dar AC, et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2α substrate recognition. Cell 2005; 122:901–913. [DOI] [PubMed] [Google Scholar]
- Bevilacqua PC, Cech TR. Minor-groove recognition of double-stranded RNA by the double-stranded RNA-binding domain from the RNA-activated protein kinase PKR. Biochemistry 1996; 35:9983–9994. [DOI] [PubMed] [Google Scholar]
- Manche L, Green SR, Schmedt C, Mathews MB. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol Cell Biol 1992; 12:5238–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman F, Jarrous N, Ben-Asouli Y, Kaempfer R. A cis-acting element in the 3′-untranslated region of human TNF-α mRNA renders splicing dependent on the activation of protein kinase PKR. Genes Dev 1999; 13: 3280–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Asouli Y, Banai Y, Pel-Or Y, Shir A, Kaempfer R. Human interferon-gamma mRNA autoregulates its translation through a pseudoknot that activates the interferon-inducible protein kinase PKR. Cell 2002; 108:221–232. [DOI] [PubMed] [Google Scholar]
- Cohen-Chalamish S, Hasson A, Weinberg D, et al. Dynamic refolding of IFN-γ mRNA enables it to function as PKR activator and translation template. Nat Chem Biol 2009; 5:896–903. [DOI] [PubMed] [Google Scholar]
- Nienhuis AW, Benz EJ. Regulation of hemoglobin synthesis during the development of the red cell. N Engl J Med 1977; 297:1318–1328. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Role of the RNA-dependent protein kinase in the regulated expression of genes in transfected cells. Pharmacol Ther 1992; 54:307–317. [DOI] [PubMed] [Google Scholar]
- Custódio N, Carvalho C, Condado I, et al. In vivo recruitment of exon junction complex proteins to transcription sites in mammalian cell nuclei. RNA 2004; 10:622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koromilas AE, Roy S, Barber GN, Katze MG, Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science 1992; 257:1685–1689. [DOI] [PubMed] [Google Scholar]
- Krainer AR, Maniatis T, Ruskin B, Green MR. Normal and mutant human β-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell 1984; 36:993–1005. [DOI] [PubMed] [Google Scholar]
- Mermoud JE, Cohen P, Lamond AI. Ser/Thr-specific protein phosphatases are required for both catalytic steps of pre-mRNA splicing. Nucleic Acids Res 1992; 20:5263–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmuth K, Urlaub H, Vornlocher HP, et al. Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc Natl Acad Sci USA 2002; 99:16719–16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XD, Maniatis T. The 35-kDa mammalian splicing factor SC35 mediates specific interactions between U1 and U2 small nuclear ribonucleoprotein particles at the 3′ splice site. Proc Natl Acad Sci USA 1992; 89:1725–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector DL, Fu XD, Maniatis T. Associations between distinct pre-RNA splicing components and the cell nucleus. EMBO J 1991; 10:3467–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud S, Reed R. An ATP-independent complex commits pre-mRNA to the mammalian spliceosome assembly pathway. Genes Dev 1991; 5:2534–2546. [DOI] [PubMed] [Google Scholar]
- Makarova OV, Makarov EM, Lührmann R. The 65 and 110 kDa SR-related proteins of the U4/U6.U5 tri-snRNP are essential for the assembly of mature spliceosomes. EMBO J 2001; 20:2553–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K, Elroy-Stein O, Moss B, Jagus R. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor 2 α-specific protein kinase. J Biol Chem 1993; 268:12837–12842. [PubMed] [Google Scholar]
- Dar AC, Sichieri F. X-ray crystal structure and functional analysis of vaccinia virus K3L reveals molecular determinants for PKR subversion and substrate recognition. Mol Cell 2002; 10:295–305. [DOI] [PubMed] [Google Scholar]
- Soukup GA, Breaker RR. Relationship between internucleotide linkage geometry and the stability of RNA. 1999; RNA 5:1308–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom JL, Blechl AE, Smithies O. Human fetal Gγ- and Aγ-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell 1980; 21:627–638. [DOI] [PubMed] [Google Scholar]
- Rosen H, Di Segni G, Kaempfer R. Translational control by messenger RNA competition for eukaryotic initiation factor 2. J Biol Chem 1982; 257:946–952. [PubMed] [Google Scholar]
- Higgs DR, Garrick D, Anguita E, et al. Understanding α-globin gene regulation: aiming to improve the management of thalassemia. Ann NY Acad Sci 2005; 1054:92–102. [DOI] [PubMed] [Google Scholar]
- Mahajan MC, Karmakar S, Weissman SM. Control of beta globin genes. J Cell Biochem 2007; 102:801–810. [DOI] [PubMed] [Google Scholar]
- Lodish HF. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem 1971; 246:7131–7138. [PubMed] [Google Scholar]
- Di Segni G, Rosen H, Kaempfer R. Competition between α- and β-globin messenger ribonucleic acids for eukaryotic initiation factor 2. Biochemistry 1979; 18:2847–2854. [DOI] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell 2009; 136:701–718. [DOI] [PubMed] [Google Scholar]
- Hoskins AA, Moore MJ. The spliceosome: a flexible, reversible macromolecular machine. Trends Biochem Sci 2012; 37:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi M, Yamamoto Y, Hirooka N, et al. A high concentration of triiodothyronine attenuates the stimulatory effect on hemin-induced erythroid differentiation of human erythroleukemia K562 cells. Endocr J 2015; 62:431–440. [DOI] [PubMed] [Google Scholar]
- Hoffmann FG, Opazo JC, Storz JF. New genes originated via multiple recombinational pathways in the beta-globin gene family of rodents. Mol Biol Evol 2008; 25:2589–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R, Kaufman J. Translational control of hemoglobin synthesis by an initiation factor required for recycling of ribosomes and for their binding to messenger RNA. Proc Natl Acad Sci USA 1972; 69:3317–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2α kinase: relevance to anemias. Blood 2007; 109:2693–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken CE, Lorsch JR. A mechanistic overview of translation initiation in eukaryotes. Nat Struct Mol Biol 2012; 19:568–576. [DOI] [PubMed] [Google Scholar]
- Jarrous N, Osman F, Kaempfer R. 2-Aminopurine selectively inhibits splicing of tumor necrosis factor alpha mRNA. Mol Cell Biol 1996; 16:2814–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada C, Eder PS, Gopalan V, Altman S. Purification and characterization of Rpp25, an RNA-binding protein subunit of human ribonuclease P. RNA 2002; 8:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circle DA, Neel OD, Robertson HD, Clarke PA, Mathews MB. Surprising specificity of PKR binding to delta agent genomic RNA. RNA 1997; 3:438–448. [PMC free article] [PubMed] [Google Scholar]
- Ben-Asouli Y, Banai Y, Hauser H, Kaempfer R. Recognition of 5′-terminal TAR structure in human immunodeficiency virus-1 mRNA by eukaryotic translation initiation factor 2. Nucleic Acids Res 2000; 28:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Colocalization of PKR with SC35 and reconstitution of β-globin pre-mRNA template splicing and spliceosome formation by recombinant PKR.
Spliceosome formation on MINX RNA is re sistant to inhibition by αPeIF2α mAb and eIF2αS51A expression inhibits β-globin mRNA splicing.
Properties of s1b mutant β-globin RNA and PKR- and phospho-eIF2α-dependent splicing of Aγ-globin mRNA.
Structure model and phylogenetic conservation of base pairing in unspliced and spliced β-globin mRNA.
2-Aminopurine inhibits splicing of α-globin mRNA.