Abstract
Neurodegeneration in Alzheimer’s disease (AD) is extensively studied, and the involvement of astrocytes and other cell types in this process has been described. However, the responses of astrocytes themselves to amyloid β peptides ((Aβ; the widely accepted major toxic factor in AD) is less well understood. Here, we show that Aβ(1-42) is toxic to primary cultures of astrocytes. Toxicity does not involve disruption of astrocyte Ca2+ homeostasis, but instead occurs via formation of the toxic reactive species, peroxynitrite. Thus, Aβ(1-42) raises peroxynitrite levels in astrocytes, and Aβ(1-42) toxicity can be inhibited by antioxidants, or by inhibition of nitric oxide (NO) formation (reactive oxygen species (ROS) and NO combine to form peroxynitrite), or by a scavenger of peroxynitrite. Increased ROS levels observed following Aβ(1-42) application were derived from NADPH oxidase. Induction of haem oxygenase-1 (HO-1) protected astrocytes from Aβ(1-42) toxicity, and this protective effect was mimicked by application of the carbon monoxide (CO) releasing molecule CORM-2, suggesting HO-1 protection was attributable to its formation of CO. CO suppressed the rise of NADPH oxidase-derived ROS caused by Aβ(1-42). Under hypoxic conditions (0.5% O2, 48 h) HO-1 was induced in astrocytes and Aβ(1-42) toxicity was significantly reduced, an effect which was reversed by the specific HO-1 inhibitor, QC-15. Our data suggest that Aβ(1-42) is toxic to astrocytes, but that induction of HO-1 affords protection against this toxicity due to formation of CO. HO-1 induction, or CO donors, would appear to present attractive possible approaches to provide protection of both neuronal and non-neuronal cell types from the degenerative effects of AD in the central nervous system.
The progression of Alzheimer’s disease (AD), from early loss of functional synapses1, 2, 3 to the loss of neurones through apoptosis and other pathways4, 5, 6, 7 has been described extensively, yet remains to be fully understood. The association of disease progression with increased levels of amyloid β peptide (Aβ; predominantly the 1–42 form, Aβ1–42) is also well established: this peptide is neurotoxic, and is also an important constituent of disease-characterising plaques.8, 9
Astrocytes are as numerous as neurons in the central nervous system10 and their role in neurodegenerative diseases has also been extensively explored.11, 12, 13 These cells are diverse in structure and function, regulating CNS homeostasis in general and shaping activity at individual synapses.11, 14, 15 More recently there is a growing appreciation of the heterogeneity of astrocytes.16 In AD, and transgenic murine models of AD, atrophy of astrocytes is widely reported, and reactive astrocytes (in part defined as staining positively for glial fibrillar acidic protein; GFAP) have long been anatomically associated with plaques (reviewed in ref. 11). Atrophic loss of morphology clearly undermines the ability of astrocytes to regulate synaptic activity, and disrupts the ‘neurovascular unit’ in which astrocytes play a central role in balancing local blood flow to neuronal activity.17, 18
Numerous in vitro studies have also established that astrocytes can, at least in part, mediate the neurotoxic effects of Aβ. Thus, for example, extrasynaptic glutamate release from astrocytes in response to Aβ exposure leads to synaptic damage and loss.19 Evidence exists that suggests Aβ disrupts astrocyte [Ca2+]i and by doing so activates reactive oxygen species (ROS) production by NADPH oxidase (reviewed in ref. 20). This increases lipid peroxidation in both astrocytes and neurones, which in turn depletes glutathione (GSH) levels in both cell types. Since neurones require delivery of GSH precursors from astrocytes, they are preferentially susceptible to continued oxidative stress and so die, whereas astrocytes have a greater antioxidant capacity to survive. It should be noted, however, that this model is not universally accepted, and others have indicated that astrocytic Ca2+ signalling is not disrupted by Aβ, at least over the same timecourse, and that different downstream signalling pathways are evoked.21, 22
Despite the extensive literature on the involvement of astrocytes in the progression of neurodegeneration in AD, information available concerning the molecular mechanisms underlying responses of astrocytes themselves to Aβ is relatively limited and appears seemingly contradictory in some respects. Thus, for example, astrocyte viability has been reported to be unaffected or even slightly potentiated following exposure to sub-micromolar Aβ1-42 for 24 h.23 By contrast, others have shown that astrocytes are susceptible to micromolar Aβ−induced death over similar time periods.24 In the present study, we demonstrate that cortical astrocytes can undergo apoptotic death when exposed to sub-micromolar levels of Aβ1-42, and that this occurs via formation of peroxynitrite (ONOO–). Furthermore, we demonstrate a complex pattern of modulation of this process of Aβ toxicity by the induction of the antioxidant enzyme haem oxygenase-1 (HO-1).
Results
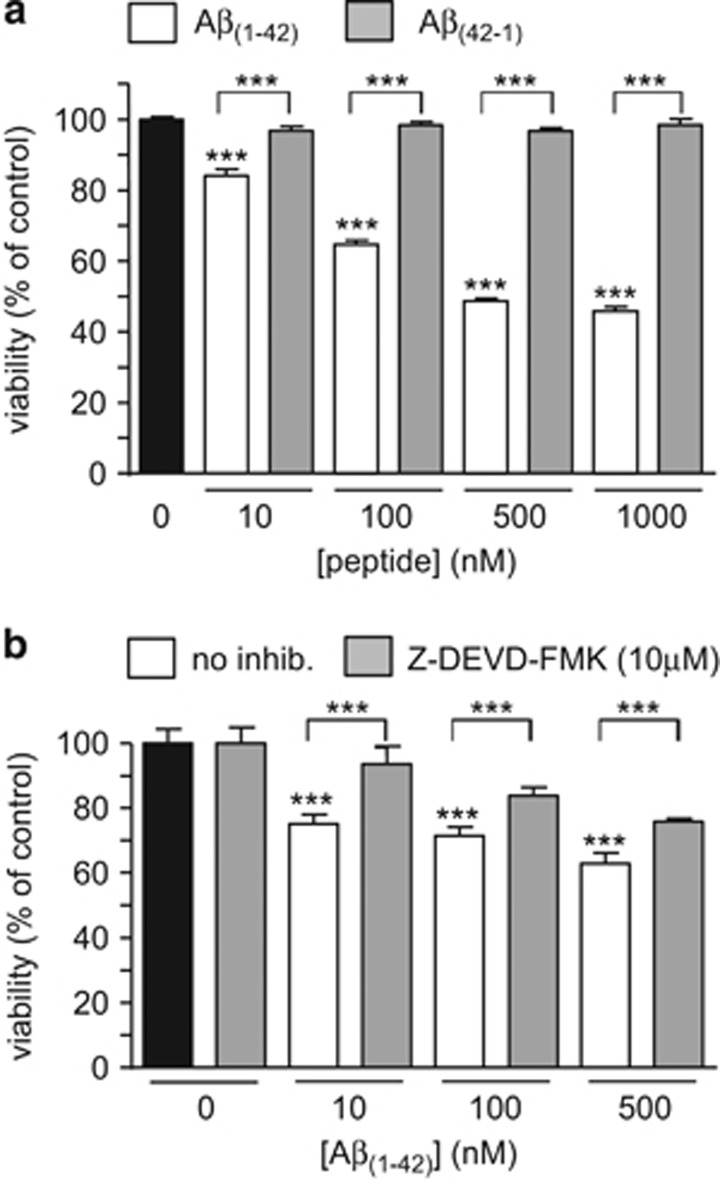
Exposure of astrocytes to Aβ1-42 (10 nM-1 μM) for 24 h caused a concentration-dependent loss of viability, as shown in Figure 1a. This toxic effect of Aβ appeared selective, since the reverse sequence peptide, Aβ42-1, was without effect over the same concentration range (Figure 1a). In the presence of the caspase-3 inhibitor Z-DEVD-FMK (10 μM) the toxic effects of Aβ1–42 were partially reversed, suggesting the toxicity of Aβ1–42 was at least partly due to the induction of apoptosis.
Figure 1.
Amyloid peptide Aβ1–42 is toxic to astrocytes in part via inducing apoptosis. (a) Effect on cell viability of a 24 h exposure of astrocytes to Aβ1–42 (10–1000 nM, white bars) and the reverse peptide Aβ42-1 (grey bars) using the mitochondrial activity-based MTT assay. Bars represent the mean±S.E.M. data of cells from 4 repeats (each performed in duplicate) with cells from different preparations. (b) as (a), except cells were exposed either to Aβ1–42 alone (10–500 nM, white bars) or Aβ1–42 in the additional presence of 10 μM Z-DEVD-FMK, a caspase-3 inhibitor (grey bars). Bars represent the mean±S.E.M. data of cells from 3 repeats (each performed in duplicate) with cells from different preparations. Significant difference: ***P<0.001 effects of peptide alone compared to control, or between amyloid peptide and reverse peptide (a), or effects of Z-DEVD-FMK at each amyloid concentration, as indicated
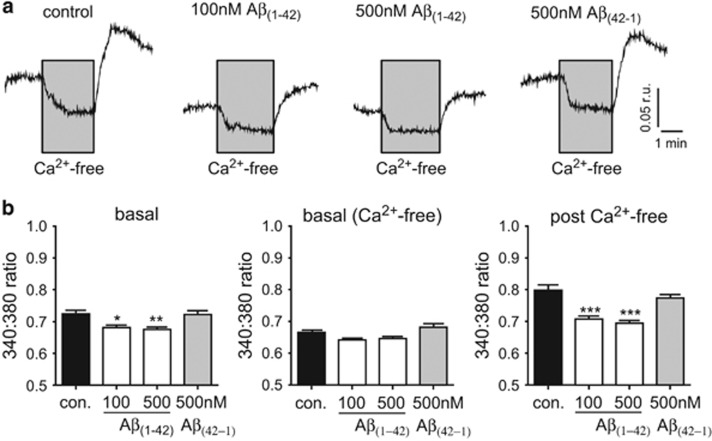
Since it has previously been suggested that amyloid peptides disrupt [Ca2+]i in astrocytes, we next examined whether [Ca2+]i was altered in astrocytes at the levels we found to be toxic. As shown by the examples in Figure 2a, and the mean data of Figure 2b, exposure of astrocytes to 100 nM or 500 nM Aβ1–42 for 24 h caused a modest but significant reduction in resting [Ca2+]i whereas 500 nM reverse peptide (Aβ42-1) was without significant effect. Removal of extracellular Ca2+ (replaced with 1 mM EGTA) caused a reversible fall of [Ca2+]i in all cell groups examined, and basal [Ca2+]i under these conditions were similar across the 4 groups (Figure 2b, middle). Restoration of extracellular Ca2+ caused a transient overshoot of [Ca2+]i in control and reverse-peptide treated astrocytes, and this was significantly suppressed in cells exposed to Aβ1-42. This difference aside, our results are not consistent with the idea that Aβ1–42 is toxic due to its ability to raise [Ca2+]i as has previously been suggested.20
Figure 2.
Aβ1–42 does not dramatically alter [Ca2+]i in astrocytes. (a) Example microfluorimetric measurements of [Ca2+]i in astrocytes under control conditions, or exposed to Aβ1–42 or Aβ42-1 as indicated, for 24 h. Scale bars apply to all traces. In each case, for the period indicated by the grey area, extracellular Ca2+ was replaced with 1mM EGTA. (b) Mean±S.E.M. levels of [Ca2+]i measured in 8–9 recordings under normal conditions (left), during exposure to Ca2+-free solution (containing 1 mM EGTA; middle) and following replacement of Ca2+ in the perfusate (right). Significance: *P<0.05; **P<0.01; ***P<0.001 as compared with controls
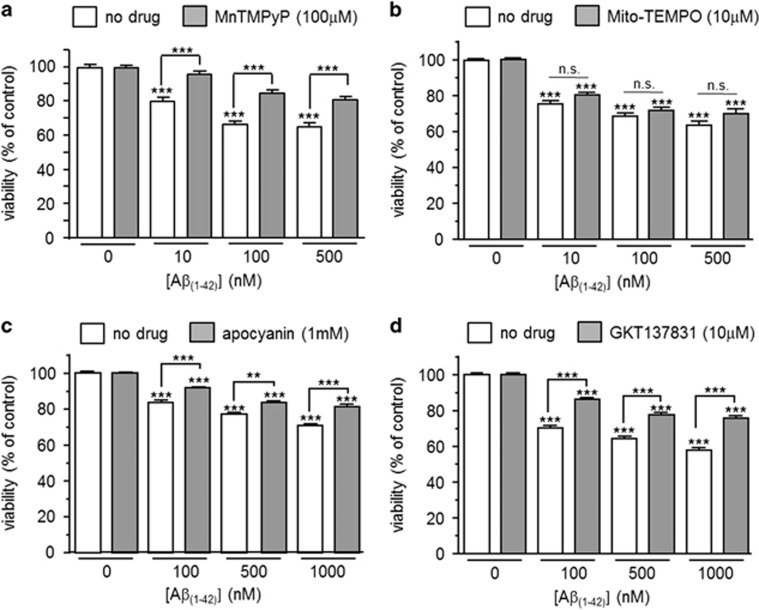
ROS generation has often been associated with amyloid toxicity.25, 26 To investigate any potential role for ROS in amyloid-mediated loss of astrocyte viability, we first examined the effects of MnTMPyP, a superoxide dismutase mimetic. As shown in Figure 3a, MnTMPyP significantly ameliorated the toxic effects of Aβ1–42. However, treatment of cells with mito-TEMPO, a mitochondria targeted antioxidant, was unable to modify significantly the toxic effects of Aβ1–42, suggesting that ROS were not derived from mitochondria (Figure 3b). Instead, a significant reduction in the toxicity of Aβ1–42 was observed in the presence of either apocyanin, a non-specific inhibitor of NADPH oxidase (Figure 3c), or the NOX1/NOX4 inhibitor, GKT13783127 (Figure 3d). These data are consistent with NADPH oxidase as being at least partly responsible for the production of toxic ROS following exposure to Aβ1–42.
Figure 3.
Evidence for the involvement of NADPH oxidase-derived ROS in Aβ1–42 toxicity. (a) Effect on cell viability of a 24 h exposure of astrocytes to Aβ1–42 alone (10–500 nM, white bars) or Aβ1–42 in the additional presence of 100 μM MnTMPyP (grey bars). Bars represent the mean±S.E.M. data of cells from 3 repeats (each performed in duplicate) with cells from different passages. (b) as (a), except cells were either treated with Aβ1–42 alone (10–500 nM, white bars) or Aβ1–42 in the additional presence of 10 μM Mito-TEMPO (grey bars). Bars represent the mean±S.E.M. data of cells from 3 repeats (each performed in duplicate). Significance: ***P<0.001 as compared with respective controls, or between cells without drug versus MnTMPyP (a). NS, not significant. (c) Effect on cell viability of a 24 h exposure of astrocytes to Aβ1–42 alone (100–1000 nM, white bars) or Aβ1–42 in the additional presence of 1 mM apocyanin (grey bars). Bars represent the mean±S.E.M. data of cells from 3 repeats (each performed in duplicate) with cells from different preparations. (d) as (c), except cells were either treated with Aβ1–42 alone (100–1000 nM, white bars) or Aβ1–42 in the additional presence of 10 μM GKT137831 (grey bars). Bars represent the mean±S.E.M. data of cells from 2 repeats (each performed in duplicate) with cells from different preparations. Significance: **P<0.01; ***P<0.001 as compared either with respective controls, or between drug treatment or no treatment, as indicated
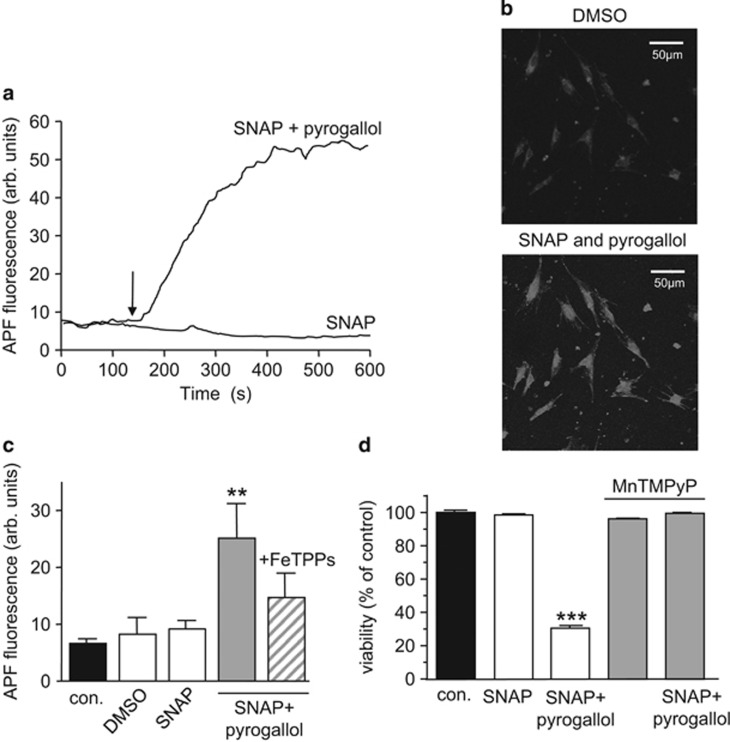
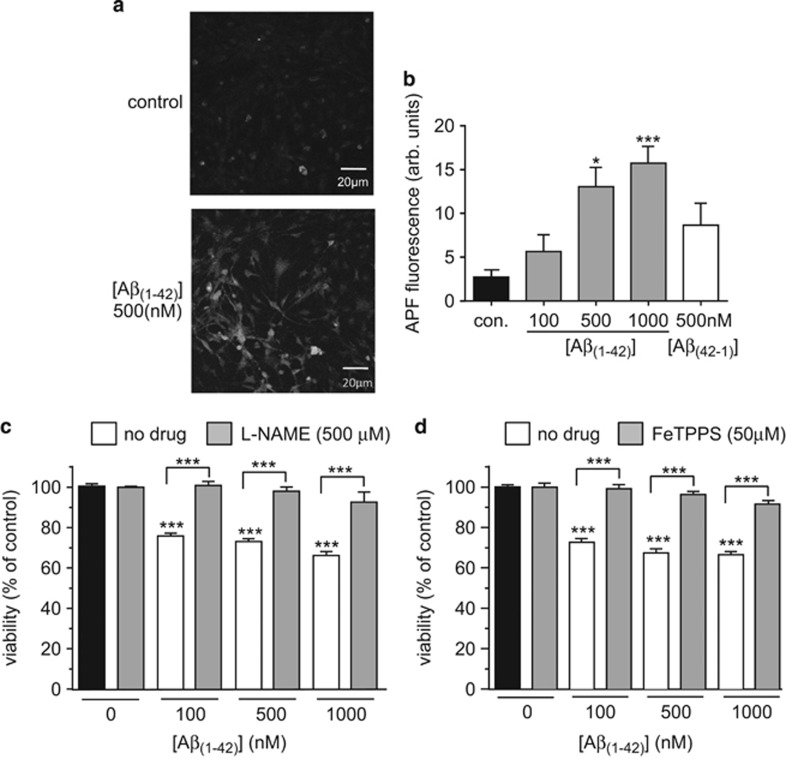
It was noteworthy in the studies reported in Figures 2 and 3 that ROS inhibition could not fully reverse the toxic effects of Aβ1–42, suggesting the involvement of other factors. Amyloid peptides have long been known to increase nitric oxide (NO) formation via induction of iNOS in astrocytes and neurones.24, 28, 29, 30 Increased formation of NO, in the presence of elevated ROS levels, can lead to formation of the highly toxic ROS, peroxynitrite (ONOO–). To explore a possible role for ONOO– we first examined its effects on astrocyte viability. ONOO– formation was monitored using 2-[6-(4′-amino) phenoxy-3H-xanthen-3-on-9-yl]benzoic acid (APF) fluorescence. Exposure of cells to the NO donor S-nitrosopenacillamine (SNAP; 200 μM) alone was without effect on APF fluorescence (Figure 4a), but when applied with pyrogallol (100 μM), which auto-oxidises to form superoxide, a clear rise of APF fluorescence was apparent (Figures 4a–c). Figure 4b illustrates two images of APF fluorescence, before and during exposure to SNAP together with pyrogallol. Mean data are plotted in Figure 4c, which also illustrates the ability of the ONOO– scavenger FeTPPs (5,10,15,20-tetrakis-[4-sulfonatophenyl]-porphyrinato-iron[III] 50 μM), which converts ONOO– rapidly to nitrate,31 to reduce the SNAP / pyrogallol rise of ONOO–. Figure 4d shows that ONOO– is highly toxic to astrocytes: exposure to SNAP alone was without significant effect on astrocyte viability, but together with pyrogallol it caused a striking loss of cells. This effect was prevented by the superoxide dismutase mimetic MnTMPyP (Figure 4d).
Figure 4.
Peroxynitrite is highly toxic to astrocytes. (a) Fluorescence images of astrocytes loaded with the ONOO– sensitive dye, 2-[6-(4′-amino) phenoxy-3H-xanthen-3-on-9-yl]benzoic acid (APF). Cells were first treated with vehicle (DMSO; 1:1000) and then SNAP (200 μM) alone or together with pyrogallol (100 μM). (b) Example timecourses of the changes in APF fluorescence seen in cells exposed to SNAP alone (10 μM) or SNAP together with pyrogallol (100 μM). (c) Mean±S.E.M. peak fluorescence detected in astrocytes exposed to vehicle, SNAP (200 μM), SNAP together with pyrogallol (100 μM) or both agents together with the ONOO– scavenger FeTPPS (50 μM). **P<0.01 as compared with control. (d) Effect on cell viability of a 24 h exposure of astrocytes to SNAP (200 μM), SNAP together with pyrogallol (100 μM) or both agents together with the antioxidant MnTMPyP (100 μM). Each bar represents the mean±S.E.M. taken from between 4 and 8 observations. Significance; ***P<0.001 as compared with control
The data in Figure 4 show clearly that formation of ONOO– in astrocytes is detectable, and is highly toxic. To investigate whether Aβ1–42 can exert its toxic actions through the formation of ONOO– we first examined its ability to increase APF fluorescence. As exemplified in the images of Figure 5a and the mean data of Figure 5b, APF fluorescence was indeed increased following a 24 h exposure to Aβ1–42. Consistent with the idea that ONOO– may contribute to its toxicity, we also found that Aβ1–42 induced loss of astrocyte viability was essentially completely prevented in the presence of L-NAME to prevent NO formation (Figure 5c). Similarly, in the presence of the ONOO– scavenger FeTPPS, Aβ1–42 was without significant effect on astrocyte viability (Figure 5d. Together, these findings suggest that Aβ1–42 is deleterious to astrocytes due to stimulation of elevated levels of both ROS and NO, which subsequently form ONOO−.
Figure 5.
Aβ1-42toxicity involves peroxynitrite formation. (a) Separate fluorescence images of astrocytes loaded with the ONOO– sensitive dye, 2-[6-(4′-amino) phenoxy-3H-xanthen-3-on-9-yl]benzoic acid (APF). Cells were either untreated or exposed to 500 nM Aβ1-42 for 24 h. (b) Mean±S.E.M. (taken from 5-10 recordings) APF fluorescence detected in astrocytes without treatment, or following a 24 h treatment with either 100–1000 nM Aβ1–42 or reverse peptide (500 nM), as indicated. *P<0.05; **P<0.01 as compared with control. (c) Effect on cell viability of 24 h exposure of astrocytes to Aβ1–42 alone (100–1000 nM, white bars) or Aβ1–42 in the additional presence of 500 μM L-NAME (grey bars). Bars represent the mean±S.E.M. data of cells from 6 repeats (each performed in duplicate) with cells from different preparations. Significance: ***P<0.001 as compared either with respective controls, or between drug treatment or no treatment, as indicated. (d) as (c), except that cells were exposed either to Aβ1–42 alone (100–1000 nM, white bars) or Aβ1–42 in the additional presence of 50 μM FeTPPs (grey bars). Bars represent the mean±S.E.M. data of cells from 3 repeats. Significant difference: ***P<0.001 effects of peptide alone compared to control, or between amyloid peptide with or without FeTPPS at each amyloid concentration, as indicated
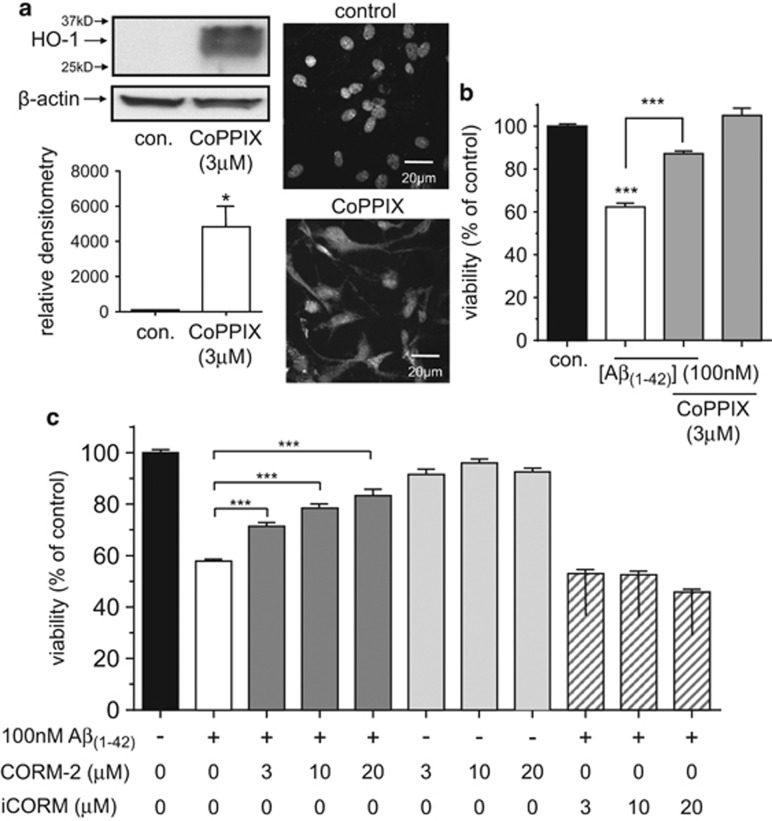
We have previously shown that induction of HO-1 affords protection in neurons against the toxicity of Aβ1–42.32 To investigate whether HO-1 was similarly protective in astrocytes, we first induced its expression by exposing astrocytes to an HO-1 inducer, cobalt protoporphyrin IX (CoPPIX; 3 μM) for 24 h. As shown in Figure 6a such treatment caused a strong induction of HO-1, and in CoPPIX-treated astrocytes, the toxic effects of Aβ1–42 (added for the same 24 h exposure to CoPPIX) were significantly attenuated (Figure 6b). Earlier studies have shown that carbon monoxide (CO), a product of HO-1-mediated haem degradation, can provide protection against apoptosis33, 34 and so we investigated such a role for CO in astrocytes. As illustrated in Figure 6c, exposure of cells to the CO donor CORM-2 (3–20 μM) caused a concentration-dependent reversal of Aβ1–42 toxicity without significantly affecting the viability of astrocytes not exposed to Aβ1–42. The control compound iCORM (which does not release CO) was unable to affect the toxicity of Aβ1–42. Neither CORM-2 nor iCORM induced significant levels of HO-1 themselves (Supplementary Figure 1). Similarly, L-NAME did not alter HO-1 expression significantly (Supplementary Figure 1). Biliverdin, another HO-1 product, was without effect on the toxicity of Aβ1–42 (Supplementary Figure 2).
Figure 6.
HO-1 induction protects astrocytes from Aβ1–42 toxicity via CO formation. (a) Left, western blot for HO-1 taken from control astrocytes and astrocytes exposed to CoPPIX (3 μM) for 24 h, as indicated. β-actin was also probed to confirm approximately equal protein loading of lanes. Below, mean±S.E.M. (n=3) relative densitometric readings for control and CoPPIX-treated cells, as indicated. *P<0.05. Right, images of control and CoPPIX-treated cells, immunostained for HO-1. Scale bar applied to both images. (b) Effect on cell viability of a 24 h exposure of astrocytes to Aβ1–42 alone (100 nM, white bar) or Aβ1–42 in the additional presence of 3 μM CoPPIX. Also shown is the lack of effect of CoPPIX alone. Bars represent the mean±S.E.M. data of cells from 5 repeats (each performed in duplicate) with cells from different preparations. ***P<0.001. (c) Effect on cell viability of a 24 h exposure of astrocytes to 100 nM Aβ1–42 in the absence (white bar) or presence (dark grey bars) of the CO donor CORM-2 (3–20 μM). The effects of CORM-2 alone are also presented (light grey bars), along with the effects of 100 nM Aβ1–42 in the additional presence of the inactive form of CORM-2, iCORM (hatched bars). Bars represent the mean±S.E.M. data of cells from 6 repeats (each performed in duplicate) with cells from different preparations. ***P<0.001
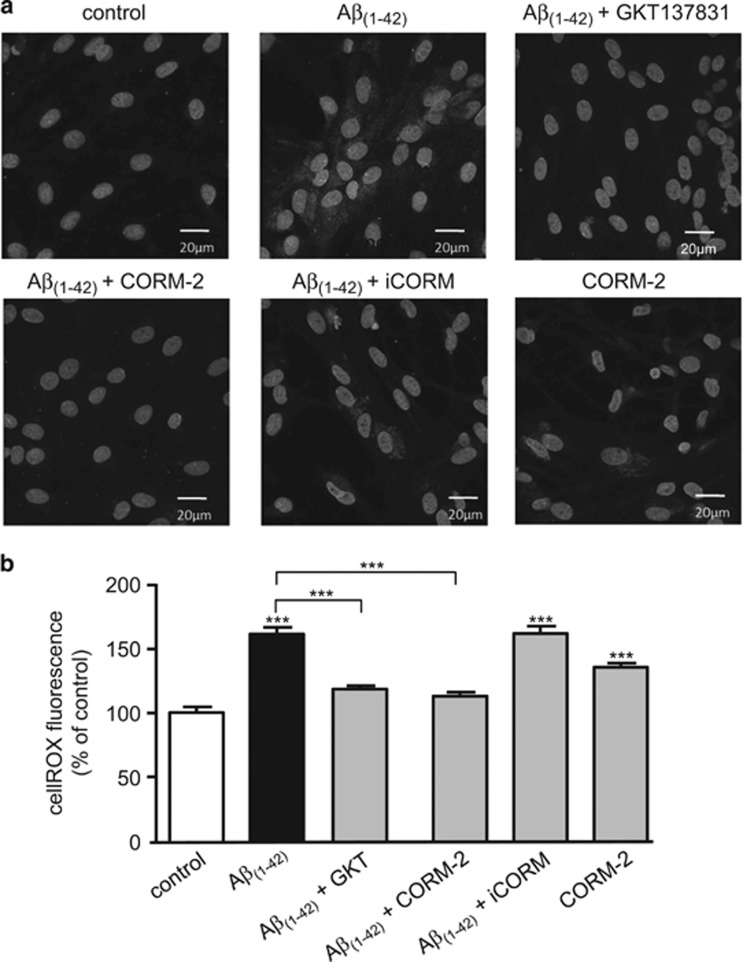
A previous study has reported that CO inhibits NADPH oxidase in proliferating smooth muscle.35 Since NADPH oxidase was a significant source of ROS mediating Aβ1–42 toxicity (Figure 3), we investigated whether CO inhibition of NADPH oxidase activity accounted for its protective effects against Aβ1–42 toxicity. To do this, we examined ROS formation using CellROX deep Red, a fluoroprobe which emits fluorescence upon oxidation. Representative images are shown in Figure 7a, and quantified in Figure 7b. As compared with untreated cells, those exposed to Aβ1–42 showed a significant increase in ROS production (increased cytoplasmic fluorescence) and this was significantly reduced by the NOX1/NOX4 inhibitor, GKT137831 (10 μM). Fluorescence was also significantly suppressed by 20 μM CORM-2 but not by iCORM. Interestingly, CORM-2 alone caused a modest rise in fluorescence, presumably because it is known to stimulate ROS formation from mitochondria.36
Figure 7.
Aβ1–42 increases ROS formation from NADPH oxidase. (a) Representative CellROX images of astrocytes under control conditions, or following a 24 h treatment with 100 nM Aβ1–42 alone, or together with GKT137831 (10 μM), or CORM-2 (20 μM) or iCORM (20 μM), as indicated. Bottom right image shows the effects of 20 μM CORM-2 alone. All images also show DAPI (nuclear) staining. Scale bar applies to all images. (B) Mean±S.E.M. fluorescence determined under the conditions exemplified in a. Data taken from 10 regions of interest, measured in 3–5 images from 3 experimental repeats. Significance: ***P<0.001 as compared with control or compared with 100 nM Aβ1–42 treatment, as indicated
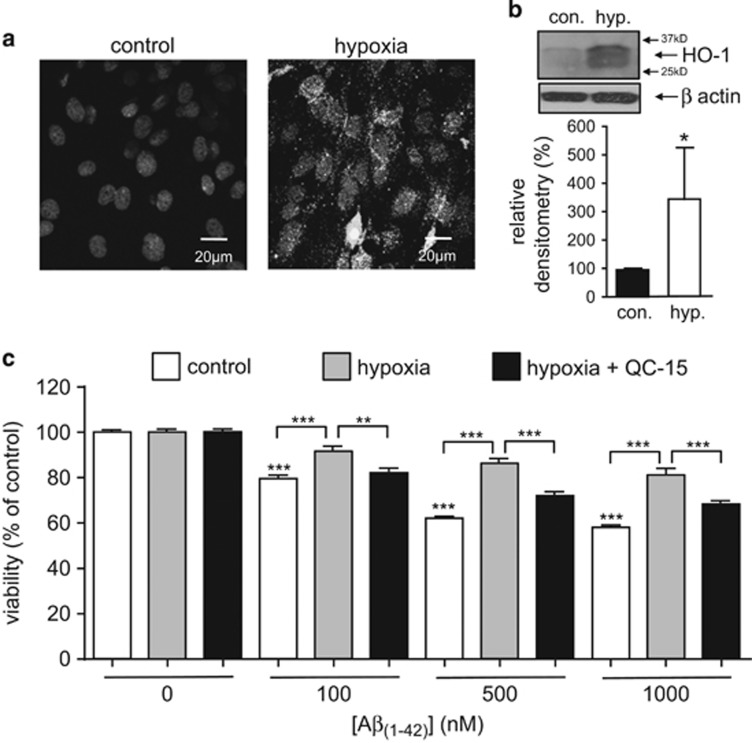
HO-1 is induced by several forms of cellular stress, prominent amongst which is hypoxia.37, 38 We maintained astrocytes in hypoxia (0.5% O2) for 48 h and found this induced HO-1 strongly, as observed using both immunohistochemistry (Figure 8a) and western blotting (Figure 8b). Astrocytes maintained in hypoxia for a subsequent 24 h exposure to Aβ1–42 were significantly more resistant to toxicity than those maintained under control (normoxic) conditions (Figure 8c). Hypoxic resistance to Aβ1–42 toxicity appeared to be due specifically to HO-1 induction, since it was largely prevented by application of the selective HO-1 inhibitor QC-1539 (Figure 8c).
Figure 8.
Hypoxia protects against Aβ1–42 toxicity via HO-1 induction. (a) Representative images of astrocytes immunostained for HO-1 under control conditions, or following a 48 h exposure to hypoxia (0.5% O2) as indicated. Scale bar applies to both images. (b) Example of western blot showing induction of HO-1 by hypoxia (hyp., 48 h, 0.5% O2). Bar graph plots mean±S.E.M. (n=3) densitometry (relative to control (normoxia)) taken from blots. *P<0.05. (c) Effect on cell viability of a 24 h exposure of astrocytes to Aβ1–42 alone (100–1000 nM, white bars) or Aβ1–42 under hypoxic conditions in the absence (grey bars) or additional presence of 10 μM QC-15 (black bars). Bars represent the mean±S.E.M. data of cells from 3 repeats (each performed in duplicate) with cells from different preparations. Significance: **P<0.01; ***P<0.001 as compared with control or as compared between treatments, as indicated
Discussion
The present study demonstrates that sub-micromolar levels of Aβ1–42 have a significant impact on astrocyte viability over a 24 h period in comparison to a control peptide. Whilst these findings at least superficially agree with some previous studies,24 others have indicated that Aβ1–42 does not alter astrocyte viability,23 but can disrupt [Ca2+]i20. These latter findings contrast with our observations both on viability (Figure 1) and alterations in [Ca2+]i (Figure 2). At present we cannot account for such different responses, but one likely possibility is the peptide preparation used. Our studies have employed peptides that were maintained for 24 h at 37 °C in medium before being applied to cells. We have previously shown that under these conditions cells are exposed to a mixture of monomers, small globular assemblies and protofibrils, as assessed by electron microscopy.32 By contrast, elevations in astrocytic [Ca2+]i were observed following acute application of 10–50 μM of Aβ1–42 which was prepared in ultrapure water, reducing the likelihood of aggregation.40, 41 Despite these reported differences, what is clear from this study and others is that Aβ1–42 can elevate astrocytic ROS levels. This is likely to be pathologically important, and precedes amyloid-induced increases of ROS in neurons.42 ROS derived from mitochondria have been implicated in ageing and associated with Ca2+ mobilisation in astrocytes.43 In the present study, NADPH oxidase(s) appear to be major contributors to the increased ROS levels (see ref. 44 and Figure 3). Although we have not explored the mechanism underlying amyloid-mediated stimulation of NADPH oxidase activity, it has previously been suggested that this occurs via Ca2+-dependent activation of protein kinase Cβ.44, 45 Whether or not the same process underlies NADPH oxidase activation as reported here is unclear, but it is noteworthy that no elevation of [Ca2+]i was observed in the present study (Figure 2).
Astrocytes respond to pro-inflammatory signals with the release of toxic species, including ONOO– (see refs 46, 47). It has previously been reported that astrocytes are relatively resistant to ONOO– toxicity when applied exogenously or derived from iNOS.48, 49 Indeed, although NO modulates mitochondrial function in astrocytes, no overt toxicity has been reported50 in agreement with this study (Figure 4d). However, all three isoforms of nitric oxide synthase (NOS) are elevated in AD,51, 52 and Lipton and colleagues have produced a number of studies which collectively provide compelling evidence that much of the toxicity of amyloid peptides arises due to stimulation of NO production.53, 54, 55, 56 Aberrant, or excessive nitrosylation of target proteins (that is, conversion of cysteine –SH groups to –SNO groups) accounts for many of these deleterious effects.30, 55, 56 However, amyloid peptides have also been reported to increase ONOO– levels in vivo46, 47 and this can also impact on protein function through nitration of tyrosine residues.57 Indeed, Aβ1–42 has been reported to be a target of nitration, which can result in increased peptide aggregation.58 Given these reported differential sensitivities to NO and NO-related ROS, we examined the involvement of both NO and ONOO–. Consistent with previous studies our data highlighted that NO alone does not mediate amyloid toxicity. However, when added with a source of superoxide, pyrogallol, a dramatic rise of ONOO– was observed, together with a large reduction in cell viability (Figure 4). These effects could be reversed by the ONOO– scavenger, FeTPPs, the superoxide dismutase mimetic MnTMPyP, and also by L-NAME-mediated inhibition of NO formation (Figures 3, 4 and 5). As we also demonstrated that Aβ1–42 could directly raise ONOO– levels (Figure 5b), our data strongly suggest that the toxic effects of Aβ1–42 on astrocytes are due to its ability to promote both ROS and NO formation and hence increase ONOO– levels.
The cytotoxic actions of ONOO– have been linked to intracellular glutathione levels and also to haem oxygenase activity.59 Induction of HO-1 in both neurons and astrocytes is well known to be associated with AD60, 61 although whether this is beneficial, or contributes to disease progression, is subject to debate: HO-1 induction specifically in glia has been shown to be detrimental because of the oxidative activity of iron liberated by haem degradation.62, 63 However, we32 and others64, 65 have provided evidence that HO-1 induction is protective. In the present study, HO-1 induction (either chemically or via exposure to hypoxia) is clearly protective against the toxic effects of Aβ1–42 and this protection is attributable to the formation of CO. Similarly in neurons we have shown that CO is protective against Aβ1–42 toxicity, but significantly the underlying mechanisms are quite distinct: in neurons, protection was attributable to inhibition of AMP-dependent protein kinase activation.32 In contrast, the present study demonstrates that CO provides protection via inhibition of ROS production in astrocytes specifically by NADPH oxidase. This correlates well with observations in smooth muscle cells and macrophages, where HO-1 induction, and resultant CO formation suppresses NADPH oxidase activity.35 This result is perhaps surprising, given that CO can itself increase ROS formation (Figure 7), not from NADPH oxidase but from mitochondria.36 Furthermore, CO can increase formation of NO in various cell types (e.g. refs 66, 67) and can in some instances itself be damaging through formation of ONOO–, as shown in neuroblastoma (SH-SY5Y) cells.66 The source of ROS and / or the specific cell type is therefore likely to be key to outcomes. Clearly, the present study shows that CO is protective against Aβ1–42 toxicity in astrocytes, and this is mediated through suppression of NADPH oxidase activity.
In summary, we have shown that sub-micromolar concentrations Aβ1–42 are toxic to astrocytes, due to the activation of NADPH oxidase and subsequent elevation of ROS. In the presence of tonic NOS activity, ONOO– formation is increased. Induction of HO-1 provides protection against Aβ(1–42) toxicity primarily via inhibition of NADPH oxidase. Our findings therefore further support the idea that HO-1 / CO is protective in the central nervous system and reveals potential mechanisms by which neuroprotection may be enhanced in the face of Aβ1–42 cellular toxicity of AD.
Materials and Methods
Astrocyte preparation
To obtain primary cultures of astrocytes, cerebral cortices were removed from 5–7-day-old Wistar rats and placed in ice-cold phosphate-buffered solution (PBS) containing no Ca2+ or Mg2+ (Gibco, Thermo-Fisher, Paisley, UK). Meninges were removed and cortices were minced with a razor blade and dispersed into the same buffer containing 0.25 mg/ml trypsin, at 37 °C for 15 min. Trypsin digestion was halted by the addition of an equal volume of buffer supplemented with 16 μg/ml soy bean trypsin inhibitor (type I-S; Sigma Aldrich (Gillingham, UK), UK), 0.5 μg/ml DNase I (EC 3.1.21.1 type II from bovine pancreas; 125 kU/ml; Sigma Aldrich) and 0.3 mM MgSO4. The digested tissue was then pelleted by centrifugation at 400 × g for 5 min and the supernatant decanted before resuspending the cell pellet in 6.8 ml of buffer solution containing 100 μg/ml soy bean trypsin inhibitor, 0.5 μg/ml DNase I and 1.5 mM MgSO4. Tissue was subsequently triturated gently with a 10 ml stripette (10 ×). The cloudy cell suspension was pipetted into 120 ml of media. The culture medium consisted of Eagle’s minimal essential medium supplemented with 10% foetal calf serum (v/v) and 1% (v/v) penicillin-streptomycin (Invitrogen, Paisley, UK). The cell suspension was then aliquoted into 75 cm2 flasks. Cells were then kept in a humidified incubator at 37 °C (95% air, 5% CO2). Six hours after plating out the cell suspension, cells were washed with fresh media to remove non-adhered cells and debris. This resulted in a culture of astrocytes (GFAP positive) as previously described.68, 69 Culture medium was exchanged every 7 days and cells were grown in culture for up to 14 days.
MTT assays
Cell viability was investigated using MTT assays, as previously described.32 This technique compares well with the ATP-based CellTiter-Glo Luminescent Cell Viability Assay (Promega; SI Figure 3). Cells were cultured in poly-lysine coated 96-well plates to ~50% confluence or greater. Experiments were only carried out when all of the cell groups showed a similar confluency when viewed under the microscope. The final volume of each well after any treatment was kept at 100 μl. Cells were treated for 24 h with different concentrations of either Aβ1–42 or the reverse peptide Aβ42-1 made up in serum-free media (SFM). The media in the control cells was also replaced with SFM for 24 h to ensure that all observations made were due to the application of Aβ rather than the result of serum withdrawal. This was done for all the experiments involving Aβ application.
When applying the tricarbonyldichlororuthenium(II) dimer (CORM-2) for 24 h, cells were treated twice a day (0930am and 1700 hours) to replenish the amount of CO in the media. Some cells were treated in parallel identically with iCORM, the inactive, control compound which cannot release CO. Following the 24 h treatments with Aβ and CORM-2, the media was discarded and the cells washed gently with PBS. This step was repeated to get rid of all the CORM-2 as it reacts with the MTT. Then the PBS was replaced with 100 μl of fresh cell culture media in each well. For the MnTMPyP experiments, the cells were pre-treated with MnTMPyP for 30 min prior to applying Aβ1–42. For the L-NAME experiments, the cells were pre-treated with L-NAME for 1 h prior to treating with Aβ1–42. Next, 11 μl of Thiazolyl Blue Tetrazolium Bromide (5 mg/ml, MTT, Sigma) made up in sterile PBS was added to each well (10% by volume) and the cells were incubated at 37 °C for 3 h. An equal volume (111 μl per well) of solubilizing solution consisting of isopropanol and HCl (24 ml propan-1-ol/isopropyl alcohol (Sigma) + 1 ml 1M HCl) was added to each well to lyse the cells and the contents of each well was thoroughly mixed by pipetting. Absorbance was measured at 570 nm and at 630 nm using a spectrophotometer. The experiments were done in duplicate and repeated using cells from at least 3 different rat preparations to ensure the reliability of results. All of the results were normalised to untreated control cells and shown as a percentage change in cell viability compared to the corresponding controls.
APF fluorescence
APF (2-[6-(4′-amino) phenoxy-3H-xanthen-3-on-9-yl]benzoic acid) fluorescence was used to detect peroxynitrite (ONOO–) formation as previously described.66 Cells were plated on to coverslips in 24-well plates and when needed for experiments coverslips containing cells were incubated with 100 nM, 500 nM, 1 μM Aβ1–42 or 500 nM Aβ42-1 for 24 h. Following the 24 h treatments the cells were incubated with 10 μM APF (Sigma Aldrich, UK) made up in HEPES buffered saline and incubated in the dark for 1 h at 37 °C. Following the 1 h incubation period, the coverslip was cut into fragments and one fragment was placed on a glass slide containing 200 μl of HEPES- buffered saline with 10 μM APF. APF was used due to its limited nonselective reactivity and resistance to light-induced auto-oxidation. Oxidation causes bright green fluorescence with an excitation/emission maxima of around 490/515 nm. Fluorescence increases when APF reacts with ONOO– and the changes in fluorescence intensity were measured using a ZEISS (Oberkochen, Germany) laser scanning confocal microscope (LSM 510). The change in fluorescence was measured continuously for a total of 10 min. The fluophore was excited at 488 nm (emission was at 510 nm) by sequential scanning with argon lasers and the Zeiss AIM software was used to obtain the images. The same brightness, contrast and gamma settings were used for each condition.
Western blotting
Cells used for immunoblotting were cultured in T75 flasks and when confluent, were treated with Aβ1–42, Aβ42-1 or cobalt protoporphyrin (CoPPIX) at the concentrations indicated in the Results for 24 h. For the hypoxic experiments, cells were exposed to hypoxia (0.5% O2) for 48 h. Following the treatments, cells were washed in PBS and then lysed in situ with 600 μl of mammalian protein extraction reagent (M-PER, Pierce) containing complete protease inhibitor tablets (Roche) for 30 min at room temperature. Protein levels in the lysates were assessed using a BCA assay (Pierce). Cell proteins (typically 30 μg protein per lane) were separated on 12.5%, 0.75 mm thick polyacrylamide SDS gels and electrophoretically transferred onto 0.2 μm PVDF membranes (BioRad). The blots were blocked for 1 h with 10% milk protein in Tris-buffered saline with 0.05% Tween (TBST) then probed with primary antibody raised against HO-1 (1:200, rabbit polyclonal, Santa Cruz technologies or 1:1000 rabbit polyclonal, GeneTEX) at 4 °C overnight. Next, membranes were washed with TBST for 3 × 10 min prior to incubating with anti-rabbit horse radish peroxidise-conjugated secondary antibody (1:2000; Amersham Pharmacia Biotech, Buckinghamshire UK) for 1 h at room temperature. Following this incubation, membranes were washed in TBST for 3 × 10 min and bands visualised using an enhanced chemiluminescence detection system and hyperfilm ECL (Merck, UK).
Immunofluorescence
Cells were cultured on poly-lysine coated glass coverslips in 6-well plates at >50% confluence prior to treatment with Aβ1–42, Aβ42-1 or cobalt protoporphyrin (CoPPIX) at the concentrations indicated in the Results for 24 h, or prior to exposure to hypoxia (0.5% O2, 48 h). Following said treatments, cells were immunostained for HO-1 expression. Briefly, media was discarded and the cells were washed (3 × 5 min) with Dulbecco’s PBS. Cells were then fixed with paraformaldehyde (4% in PBS) for 20 min, following which they were permeabilized with PBS containing 0.22% Triton X100 supplemented with 10% normal goat serum (NGS; Sigma). Following 3 × 5 min washes with Dulbecco’s PBS containing 1% NGS, cells were then incubated overnight at 4 °C with the primary antibody; rabbit polyclonal anti-HO-1 (1:100, Santa Cruz) in Dulbecco’s PBS containing 1% NGS. The following day, cells were washed with Dulbecco’s PBS containing 1% NGS (3 × 5 min). Antibody binding was visualised by incubating the cells with a secondary antibody; Alexa Fluor-488 conjugated anti-rabbit IgG (1:1000, Invitrogen), for 1 h in the dark. Post-incubation, and following 3 × 5 min washes with Dulbecco’s PBS, coverslips were mounted on slides using VectashieldR mounting media containing DAPI (Vector Laboratories, CA). The slides were then examined using a Zeiss laser scanning confocal microscope (LSM 700).
Amyloid beta preparation
Aβ1–42 and Aβ42-1 (r-Peptides, Bogart USA) were dissolved in DMEM (Gibco, Paisley, UK) to make up 100 μM stock solutions and kept at −20 °C. In order to form protofibrils prior to treating the cells, the Aβ peptide was maintained at 37 °C for 24 h, as previously described.32
CellROX assay
Cells were cultured on poly-lysine coated glass coverslips in 6-well plates at ⩾50% confluence prior to treatment as described in the Results section. Following treatment the media was removed, cells were washed with PBS and 5 μM CellROX deep red regent (Molecular Probes, Life Technologies, Paisley, UK) was applied for 30 min in the dark at 37 °C. Thereafter, cells were washed three times with PBS and fixed with 10% buffered formaldehyde (Sigma) for 15 min. Cells were then washed with PBS and the coverslips were mounted on slides using Vectorshield mounting media containing DAPI (Vector Laboratories, Burlingame, CA, USA). Slides were then examined using a Zeiss laser scanning confocal microscope (LSM 700). Images for all the treatments on a particular day were taken using identical settings. ImageJ software (NIH, Bethesda, USA) was used to analyse the images. To do this, 10 regions of interest were obtained for each image and 3–5 images each were taken for any given treatment on any given experimental day.
Statistical analysis
Data are shown as mean±S.E.M. Statistical analysis was carried out using one-way ANOVA followed by either the Dunnett’s or Bonferroni post test, as appropriate. P-values of <0.05 were considered significant. CellROX results were analysed using a two-way ANOVA followed by a Bonferroni post test. P<0.05 was considered to be significant.
Acknowledgments
This work was supported by grants from the Alzheimer’s Society and Alzheimer’s Research UK.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis).
Edited by A Verkhratsky
The authors declare no conflict of interest.
Supplementary Material
References
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science 2002; 298: 789–791. [DOI] [PubMed] [Google Scholar]
- Coleman PD, Yao PJ. Synaptic slaughter in Alzheimer's disease. Neurobiol Aging 2003; 24: 1023–1027. [DOI] [PubMed] [Google Scholar]
- Conforti L, Adalbert R, Coleman MP. Neuronal death: where does the end begin? Trends Neurosci 2007; 30: 159–166. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem Biophys Res Commun 2005; 331: 761–777. [DOI] [PubMed] [Google Scholar]
- LeBlanc AC. The role of apoptotic pathways in Alzheimer's disease neurodegeneration and cell death. Curr Alzheimer Res 2005; 2: 389–402. [DOI] [PubMed] [Google Scholar]
- Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature 2006; 443: 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Landshamer S. Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr Alzheimer Res 2006; 3: 269–283. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science 1992; 256: 184–185. [DOI] [PubMed] [Google Scholar]
- Hardy J, Bogdanovic N, Winblad B, Portelius E, Andreasen N, Cedazo-Minguez A et al. Pathways to Alzheimer's disease. J Intern Med 2014; 275: 296–303. [DOI] [PubMed] [Google Scholar]
- Lent R, Azevedo FA, Andrade-Moraes CH, Pinto AV. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur J Neurosci 2012; 35: 1–9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer's disease. Neuroscience 2016; 323: 170–182. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Olabarria M, Chvatal A, Verkhratsky A. Astroglia in dementia and Alzheimer's disease. Cell Death Differ 2009; 16: 378–385. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Olabarria M, Noristani HN, Yeh CY, Rodriguez JJ. Astrocytes in Alzheimer's disease. Neurotherapeutics 2010; 7: 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature 1994; 369: 744–747. [DOI] [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG et al. Glial cells in (patho)physiology. J Neurochem 2012; 121: 4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett JA. Determinants of regional and local diversity within the astroglial lineage of the normal central nervous system. J Neurochem 2009; 110: 1717–1736. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron 2011; 71: 782–797. [DOI] [PubMed] [Google Scholar]
- Otsu Y, Couchman K, Lyons DG, Collot M, Agarwal A, Mallet JM et al. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci 2015; 18: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S et al. Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci USA 2013; 110: E2518–E2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova PR, Abramov AY. Interaction of neurons and astrocytes underlies the mechanism of Abeta-induced neurotoxicity. Biochem Soc Trans 2014; 42: 1286–1290. [DOI] [PubMed] [Google Scholar]
- Lim D, Iyer A, Ronco V, Grolla AA, Canonico PL, Aronica E et al. Amyloid beta deregulates astroglial mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia 2013; 61: 1134–1145. [DOI] [PubMed] [Google Scholar]
- Lim D, Ronco V, Grolla AA, Verkhratsky A, Genazzani AA. Glial calcium signalling in Alzheimer's disease. Rev Physiol Biochem Pharmacol 2014; 167: 45–65. [DOI] [PubMed] [Google Scholar]
- Tomasini MC, Borelli AC, Beggiato S, Ferraro L, Cassano T, Tanganelli S et al. Differential effects of palmitoylethanolamide against amyloid-beta induced toxicity in cortical neuronal and astrocytic primary cultures from wild-type and 3xTg-AD mice. J Alzheimers Dis 2015; 46: 407–421. [DOI] [PubMed] [Google Scholar]
- Aguirre-Rueda D, Guerra-Ojeda S, Aldasoro M, Iradi A, Obrador E, Mauricio MD et al. WIN 55,212-2, agonist of cannabinoid receptors, prevents amyloid beta1-42 effects on astrocytes in primary culture. PLoS ONE 2015; 10: e0122843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J, Lloret A, Giraldo E, Badia MC, Alonso MD. Antioxidant pathways in Alzheimer's disease: possibilities of intervention. Curr Pharm Des 2011; 17: 3861–3864. [DOI] [PubMed] [Google Scholar]
- Sutherland GT, Chami B, Youssef P, Witting PK. Oxidative stress in Alzheimer's disease: Primary villain or physiological by-product? Redox Rep 2013; 18: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K et al. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med 2012; 53: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Scuderi C, Savani C, Steardo L Jr, De FD, Cottone P et al. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br J Pharmacol 2007; 151: 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 2009; 324: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Lipton SA. Protein S-nitrosylation as a therapeutic target for neurodegenerative diseases. Trends Pharmacol Sci 2016; 37: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko TP, Highkin MK, Veenhuizen AW, Manning PT, Stern MK, Currie MG et al. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J Biol Chem 1998; 273: 15646–15653. [DOI] [PubMed] [Google Scholar]
- Hettiarachchi N, Dallas M, Al-Owais M, Griffiths H, Hooper N, Scragg J et al. Heme oxygenase-1 protects against Alzheimer's amyloid-beta(1-42)-induced toxicity via carbon monoxide production. Cell Death Dis 2014; 5: e1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Owais MM, Scragg JL, Dallas ML, Boycott HE, Warburton P, Chakrabarty A et al. Carbon monoxide mediates the anti-apoptotic effects of heme oxygenase-1 in medulloblastoma DAOY cells via K+ channel inhibition. J Biol Chem 2012; 287: 24754–24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas ML, Boyle JP, Milligan CJ, Sayer R, Kerrigan TL, McKinstry C et al. Carbon monoxide protects against oxidant-induced apoptosis via inhibition of Kv2.1. FASEB J 2011; 25: 1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taille C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem 2005; 280: 25350–25360. [DOI] [PubMed] [Google Scholar]
- Scragg JL, Dallas ML, Wilkinson JA, Varadi G, Peers C. Carbon monoxide inhibits L-type Ca2+ channels via redox modulation of key cysteine residues by mitochondrial reactive oxygen species. J Biol Chem 2008; 283: 24412–24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BJ, Laderoute KR, Short SM, Sutherland RM. The identification of heme oxygenase as a major hypoxic stress protein in Chinese hamster ovary cells. Br J Cancer 1991; 64: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL et al. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 1997; 272: 5375–5381. [PubMed] [Google Scholar]
- Kinobe RT, Dercho RA, Nakatsu K. Inhibitors of the heme oxygenase - carbon monoxide system: on the doorstep of the clinic? Can J Physiol Pharmacol 2008; 86: 577–599. [DOI] [PubMed] [Google Scholar]
- Abramov AY, Canevari L, Duchen MR. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci 2003; 23: 5088–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canevari L, Abramov AY, Duchen MR. Toxicity of amyloid beta peptide: tales of calcium, mitochondria, and oxidative stress. Neurochem Res 2004; 29: 637–650. [DOI] [PubMed] [Google Scholar]
- Narayan P, Holmstrom KM, Kim DH, Whitcomb DJ, Wilson MR St, George-Hyslop P et al. Rare individual amyloid-beta oligomers act on astrocytes to initiate neuronal damage. Biochemistry 2014; 53: 2442–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson J, Duchen MR. Mitochondrial oxidative stress and cell death in astrocytes—requirement for stored Ca2+ and sustained opening of the permeability transition pore. J Cell Sci 2002; 115: 1175–1188. [DOI] [PubMed] [Google Scholar]
- Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci 2005; 25: 9176–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci 2004; 24: 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MH, Yamada K, Nakajima A, Mizuno M, He J, Kamei H et al. Tyrosine nitration of a synaptic protein synaptophysin contributes to amyloid beta-peptide-induced cholinergic dysfunction. Mol Psychiatry 2003; 8: 407–412. [DOI] [PubMed] [Google Scholar]
- Malinski T. Nitric oxide and nitroxidative stress in Alzheimer's disease. J Alzheimers Dis 2007; 11: 207–218. [DOI] [PubMed] [Google Scholar]
- Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 2004; 6: 45–51. [DOI] [PubMed] [Google Scholar]
- Dringen R. Glutathione metabolism and oxidative stress in neurodegeneration. Eur J Biochem 2000; 267: 4903. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Almeida A. Modulation of astroglial energy metabolism by nitric oxide. Antioxid Redox Signal 2006; 8: 955–965. [DOI] [PubMed] [Google Scholar]
- Thorns V, Hansen L, Masliah E. nNOS expressing neurons in the entorhinal cortex and hippocampus are affected in patients with Alzheimer's disease. Exp Neurol 1998; 150: 14–20. [DOI] [PubMed] [Google Scholar]
- Luth HJ, Holzer M, Gartner U, Staufenbiel M, Arendt T. Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer's disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res 2001; 913: 57–67. [DOI] [PubMed] [Google Scholar]
- Gu Z, Nakamura T, Lipton SA. Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Mol Neurobiol 2010; 41: 55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Lipton SA. S-nitrosylation of critical protein thiols mediates protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Antioxid Redox Signal 2011; 14: 1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Nakamura T, Cao G, Holland EA, McKercher SR, Lipton SA. S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc Natl Acad Sci USA 2011; 108: 14330–14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tu S, Akhtar MW, Sunico CR, Okamoto S, Lipton SA. Aberrant protein s-nitrosylation in neurodegenerative diseases. Neuron 2013; 78: 596–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD et al. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys 1992; 298: 431–437. [DOI] [PubMed] [Google Scholar]
- Kummer MP, Hermes M, Delekarte A, Hammerschmidt T, Kumar S, Terwel D et al. Nitration of tyrosine 10 critically enhances amyloid beta aggregation and plaque formation. Neuron 2011; 71: 833–844. [DOI] [PubMed] [Google Scholar]
- Srisook K, Kim C, Cha YN. Cytotoxic and cytoprotective actions of O2- and NO (ONOO-) are determined both by cellular GSH level and HO activity in macrophages. Methods Enzymol 2005; 396: 414–424. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Cisse S, Stopa EG. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann Neurol 1995; 37: 758–768. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Heme oxygenase expression in human central nervous system disorders. Free Radic Biol Med 2004; 37: 1995–2011. [DOI] [PubMed] [Google Scholar]
- Song W, Zukor H, Lin SH, Liberman A, Tavitian A, Mui J et al. Unregulated brain iron deposition in transgenic mice over-expressing HMOX1 in the astrocytic compartment. J Neurochem 2012; 123: 325–336. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem 2009; 110: 469–485. [DOI] [PubMed] [Google Scholar]
- Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem 2000; 75: 304–313. [DOI] [PubMed] [Google Scholar]
- Imuta N, Hori O, Kitao Y, Tabata Y, Yoshimoto T, Matsuyama T et al. Hypoxia-mediated induction of heme oxygenase type I and carbon monoxide release from astrocytes protects nearby cerebral neurons from hypoxia-mediated apoptosis. Antioxid Redox Signal 2007; 9: 543–552. [DOI] [PubMed] [Google Scholar]
- Hettiarachchi NT, Boyle JP, Bauer CC, Dallas ML, Pearson HA, Hara S et al. Peroxynitrite mediates disruption of Ca(2+) homeostasis by carbon monoxide via Ca(2+) ATPase degradation. Antioxid Redox Signal 2012; 17: 744–755. [DOI] [PubMed] [Google Scholar]
- Dallas ML, Yang Z, Boyle JP, Boycott HE, Scragg JL, Milligan CJ et al. Carbon monoxide induces cardiac arrhythmia via induction of the late Na+ current. Am J Respir Crit Care Med 2012; 186: 648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IF, Boyle JP, Plant LD, Pearson HA, Peers C. Hypoxic remodeling of Ca2+ stores in type I cortical astrocytes. J Biol Chem 2003; 278: 4875–4881. [DOI] [PubMed] [Google Scholar]
- Dallas ML, Boycott HE, Atkinson L, Miller A, Boyle JP, Pearson HA et al. Hypoxia suppresses glutamate transport in astrocytes. J Neurosci 2007; 27: 3946–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.