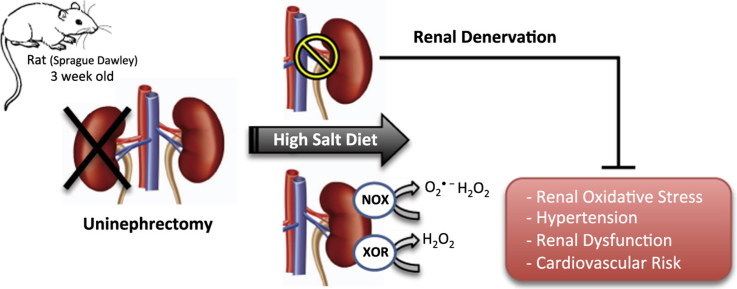
Abstract
Oxidative stress is considered a central pathophysiological event in cardiovascular disease, including hypertension. Early age reduction in renal mass is associated with hypertension and oxidative stress in later life, which is aggravated by increased salt intake. The aim of the present study was to examine if renal sympathetic denervation can exert blood pressure lowering effects in uninephrectomized (UNX) rats (3-week old) fed with high salt (HS, 4%; w/w) diet for 4 weeks. Moreover, we investigated if renal denervation is associated with changes in NADPH and xanthine oxidase-derived reactive oxygen species.
Rats with UNX + HS had reduced renal function, elevated systolic and diastolic arterial pressures, which was accompanied by increased heart weight, and cardiac superoxide production compared to sham operated Controls. UNX + HS was also associated with higher expression and activity of NADPH and xanthine oxidase in the kidney. Renal denervation in rats with UNX + HS attenuated the development of hypertension and cardiac hypertrophy, but also improved glomerular filtration rate and reduced proteinuria. Mechanistically, renal denervation was associated with lower expression and activity of both NADPH oxidase and xanthine oxidase in the kidney, but also reduced superoxide production in the heart.
In conclusion, our study shows for the first time that renal denervation has anti-hypertensive, cardio- and reno-protective effects in the UNX + HS model, which can be associated with decreased NADPH oxidase- and xanthine oxidase-derived reactive oxygen species (i.e., superoxide and hydrogen peroxide) in the kidney.
Abbreviations: HS, high salt; UNX, uninephrectomy; eGFR, estimated glomerular filtration rate; NADPH, nicotinamide adenine dinucleotide phosphate; RDN, renal denervation; ROS, reactive oxygen species; TXB2, 11-dehydro-thromboxane B2; 8,12-iso-iPF2a-VI, 12-iso−5(R), 6E, 14Z-prostagladin F2a; 5-iPF2a-VI, 5-prostagladin F2a-VI; ADMA, asymmetrical dimethylarginine; SDMA, symmetrical dimethylarginine; MDA, malondialdehyde; O2•−, superoxide; H2O2, hydrogen peroxide; phox, phagocytic oxidase; NOX, NADPH oxidase; XOR, xanthine oxidoreductase; TBP, TATA-binding protein; Crea, creatinine; bpm, beats per minute
Keywords: Renal denervation, Xanthine oxidase, NADPH oxidase, Hypertension, Renal dysfunction, Cardiovascular disease
Graphical abstract
Highlights
-
•
Uninephrectomy + high salt intake (UNX + HS) is linked with hypertension and renal dysfunction.
-
•
UNX + HS increases renal NADPH oxidase-mediated O2•− and H2O2 production.
-
•
UNX + HS increases renal xanthine oxidase-mediated H2O2 production.
-
•
Renal denervation attenuates development of hypertension and renal dysfunction.
-
•
Renal denervation is associated with lower NADPH and xanthine oxidase activity.
1. Introduction
Previous studies have shown that reduction of the total nephron number by uninephrectomy (UNX), after the completion of nephrogenesis, combined with high salt (HS) intake can lead to the development of renal and cardiovascular disease in animals and humans [1], [2], [3], [4], [5]. Mechanistically, the progression of hypertension in this setting has been associated with oxidative stress and nitric oxide deficiency in the renal cortex, and pharmacological interventions limiting reactive oxygen species (ROS) have been shown to have therapeutic effects [6], [7].
NADPH oxidase and xanthine oxidase are two major enzymes capable of producing large amounts of ROS in the kidney, and their over-activation has been linked to development or progression of cardiovascular disease including hypertension [8], [9], [10], [11]. Increased sympathetic nerve activity and noradrenaline-mediated signaling activates renal NADPH oxidase and vice versa, thus creating a vicious cycle with detrimental effects in the cardiovascular system [12], [13], [14]. As far the interplay between xanthine oxidase and sympathetic nerves is concerned, there are currently few studies showing that xanthine oxidase-derived ROS may promote sympathetic innervation or conversely that sympathetic stimulation can activate xanthine oxidase [15], [16].
Considering the important role of the sympathetic nervous system in the regulation of cardiovascular and renal functions [17], this has been an important target for the therapeutic interventions. Although there are controversies regarding the therapeutic value of renal sympathetic denervation (RDN) in patients with resistant hypertension [18], [19], there are numerous experimental studies demonstrating anti-hypertensive and cardioprotective effects, as well as renoprotection [20], [21]. However, the underlying mechanism and the importance of renal sympathetic nerves as modulators of ROS producing enzymes is not fully understood [18].
Therefore, in the present study we aimed to investigate the hypothesis that RDN may attenuate hypertension and modulate the major ROS-producing enzymes NADPH and xanthine oxidase, by using a clinically relevant model of cardiovascular and renal disease (i.e., combination of uninephrectomy and high salt diet).
2. Material and methods
2.1. Animal protocol
This study was approved by the institutional ethics review board in Stockholm (N139/15). All animal procedures were performed according to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes or National Institutes of Health guidelines. A detailed description of experimental protocol is given in the Supplement (Fig. S1).
2.2. Uninephrectomy and RDN
The surgical procedures were performed as previously described [1], [6], [7], [22]. After surgery, all rats were left to grow with free access to either regular chow or HS diet. The efficiency of RDN was validated by measuring the protein expression of tyrosine hydroxylase (data not shown), which is an established marker of sympathetic nerve activity [23].
2.3. Blood pressure and heart rate measurements
Blood pressure and heart rate were measured as previously described [24] by using the Coda High Throughput Non-invasive Tail Monitoring System (Kent Scientific, Torrington, CT, USA). After proper training period to minimize stress due to the restraining procedure, systolic, diastolic, and mean arterial pressure (MAP) together with heart rate were collected over 5 consecutive measurements. Averaged data of all values accepted by the software was used for analysis.
2.4. Glomerular filtration, proteinuria and urinary markers associated with oxidative stress
Rats were housed individually in metabolism cages for 12 h and water was given ad libitum. Urine production was measured gravimetrically and subsequently stored at −80 °C for later analysis. Estimated glomerular filtration rate (eGFR) was measured by using creatinine clearance in matched urine and plasma samples. Urine protein content was measured with the Bradford assay (Protein Assay Dye Reagent Concentrate, Biorad) in undiluted urine samples centrifuged at 1000g, 15 min, 4 °C. Protein concentration was normalized by the total urine volume and the animals’ body weight. Urinary markers (11-dehydro TXB2, 8,12-iso-iPF2a-VI, 5-iPF2a-VI) were measured by liquid chromatography tandem mass spectrometry (LC-MS) [7] and normalized by urine creatinine.
2.5. Plasma markers associated with oxidative stress
Plasma asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and N-monomethylarginine (MNMA) were measure by HPLC as previously described [7], [25]. Spectrophotometric (Absorbance at 532 nm) analysis of thiobarbituric acid-reactive substances was used to measure content of malondialdehyde (MDA) [26], [27].
2.6. NADPH oxidase and xanthine oxidase activity
NADPH or xanthine oxidase-mediated superoxide (O2•−) production was measured in renal tissue by lucigenin-based chemiluminescence [9], [24]. Cardiac O2•− production was measured in lysed left ventricle by lucigenin-based chemiluminescence with NADPH as a substrate [24]. NADPH or xanthine oxidase-mediated hydrogen peroxide (H2O2) production was measured by an Amplex Red mediated-fluorescence method [28]. Relevant modifications of our previously published methods are given in the supplement.
2.7. mRNA expression
mRNA expression of NADPH oxidase subunits and of xanthine oxidase was measured by SYBR Green based Real Time PCR [22], [24]. All measured genes were normalized by the expression of TATA-binding protein (TBP) using the ΔΔCt method, and expressed as relative changes compared to Controls. Information about the primers sequences is given in the supplement.
2.8. Protein expression
Protein expression of NADPH oxidase subunits and xanthine oxidase was measured by western blot [28], [29]. All measured proteins were normalized by the expression of β-actin, and expressed as relative changes compared to Controls. Information about the primary antibodies and all the blots are provided as supplement (Table S1 & Fig. S3).
2.9. Statistical analysis
Statistical analysis was performed by using one-way ANOVA followed by appropriate posthoc test for comparisons among three or more groups. Single comparisons between normally distributed parameters between two groups were made by using unpaired Student's t-test as appropriate. All statistical calculations were made by using Graphpad Prism 6 (La Jolla, CA, USA). Statistical significance was denoted as p < 0.05 and all the values are presented as Mean ± SE.
3. Results and discussion
At the end of the experimental protocol (i.e., time of termination) there were no differences in body weight among the groups (Supplemental Information).
3.1. RDN attenuates hypertension and cardiovascular complications
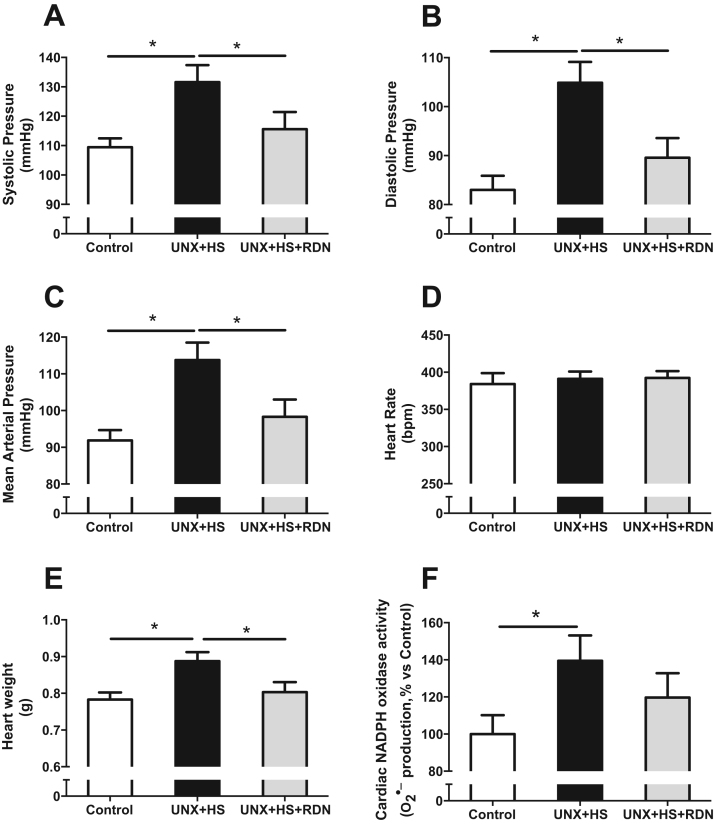
We have previously shown that UNX + HS, after the completion of nephrogenesis, leads to elevated blood pressure in later life in both rats and mice [1], [6], [7], [30]. The present study supports previous findings, and show that UNX + HS is associated with elevated systolic, diastolic and mean arterial pressure (Fig. 1A-C), compared with Control rats, but without any effect on heart rate (Fig. 1D). In addition, UNX + HS was linked with cardiovascular abnormalities, as manifested by cardiac hypertrophy and increased O2•− production in the cardiac tissue (Fig. 1E-F). Importantly, RDN in the UNX + HS group was associated with similar blood pressure, heart weight and cardiac O2•− production as that observed in Control rats, and had no effect on heart rate (Fig. 1A-F). We have recently demonstrated that RDN is able to attenuate hypertension, cardiac hypertrophy and oxidative stress in a model of renal disease (i.e., experimentally induced hydronephrosis) [22]. In agreement with previous findings in normal healthy animals, we did not observe any significant effects of RDN alone on cardiovascular parameters, i.e., blood pressure, heart rate, heart weight (Fig. S5A-C). However, this is the first study demonstrating protective effects of RDN in rats with UNX + HS, which a clinically relevant model of compromised kidney function and cardiovascular disease.
Fig. 1.
Systolic (A), diastolic (B), mean arterial pressure (C) and heart rate (D) in Control, UNX + HS and UNX + HS + RDN rats. Cardiac hypertrophy estimated as the total heart weight (E) and cardiac O2•− production (F). n = 7–10/group, *p < 0.05 vs. indicated group.
3.2. RDN improves kidney function, reduces proteinuria and oxidative stress
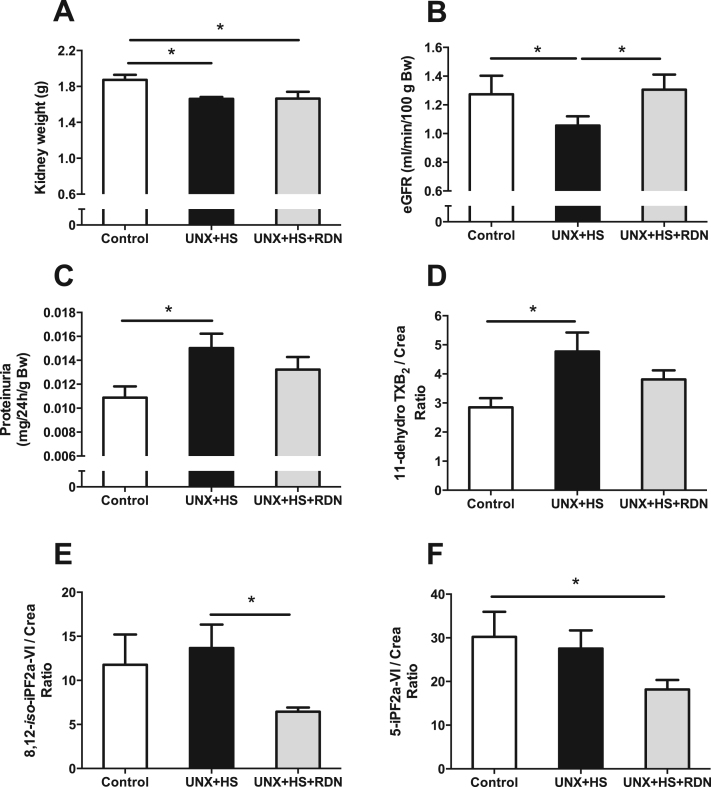
UNX + HS rats had reduced total kidney weight, which was not affected by RDN (Fig. 2A). In agreement with previous findings [1], renal function, as determined by eGFR, was reduced in UNX + HS rats and accompanied by proteinuria and higher urinary 11-dehydro TXB2 levels (Fig. 2B-D). 11-dehydro TXB2 is positively associated with the risk of cardiovascular events, stroke and mortality in humans [31], [32], thus elevated 11-dehydro TXB2 / creatinine ratio in the UNX + HS group could be indicative of cardiovascular pathologies. Importantly, RDN prevented all abovementioned pathologies associated with UNX + HS (Fig. 2B-D), and also lowered the oxidative stress markers 8,12-iso-iPF2a-VI and 5-iPF2a-VI (Fig. 2E-F). Previous studies, monitoring animals for a longer period of time, showed increased plasma MDA, ADMA and SDMA levels following UNX + HS, but this was not observed in this study using a shorter duration with UNX + HS (Fig. S4A-D). This finding suggests a vicious cycle with UNX + HS-induced renal and cardiovascular abnormalities progressing over time. RDN alone had no significant effects on estimated renal function or kidney weight (1.87 ± 0.05 vs. 1.80 ± 0.06 g; P > 0.05) in the control rats (Fig. S5E) (Fig. 3).
Fig. 2.
Total kidney weight (A), kidney function determined as eGFR (B), proteinuria (C) and urinary markers of oxidative stress, i.e., 11-dehydro TXB2/Creatinine (D), 8,12-iso-iPF2a-VI/ Creatinine (E) and 5-iPF2a-VI/Creatinine Ratio (F) in Control, UNX + HS and UNX + HS + RDN rats. n = 7–10/group for (A), n = 9–10/group for (B), n = 5–8/group for (C-E), *p < 0.05 vs. indicated group.
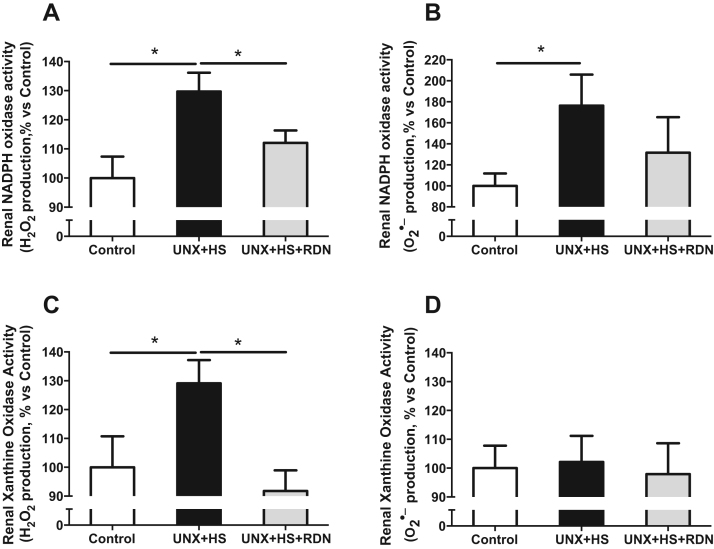
Fig. 3.
Renal NADPH oxidase-mediated H2O2 (A) and O2•− (B) production, as well as renal xanthine oxidase-mediated H2O2 (C) and O2•− (D) production in Control, UNX + HS and UNX + HS + RDN rats. n = 6–10/group, *p < 0.05 vs. indicated group.
3.3. RDN attenuates expression and activity of NADPH and xanthine oxidase
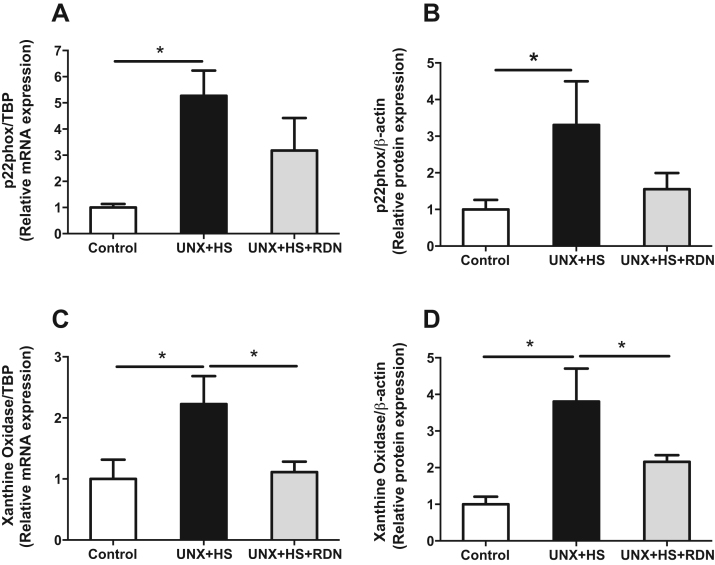
Our previous findings suggested that increased renal NADPH oxidase-derived O2•− production is a causative factor for the development of hypertension in the UNX + HS model [6]. However, there is lack of knowledge regarding the specific NADPH oxidase subunits, and the contribution of xanthine oxidase and potential changes in H2O2 production. Our findings show that UNX + HS led to significant elevation of mRNA expression of p22phox (Fig. 4A) and NOX4 (Fig. S2B) as well as xanthine oxidase (Fig. 4C), which were all normalized by RDN. In addition, UNX + HS animals had higher protein expression of p22phox (Fig. 4B) and p47phox (Fig. S2E) as well as xanthine oxidase (Fig. 4D). At the protein level, RDN normalized the expression of p22phox and xanthine oxidase (Fig. 4B, D) and suppressed also p67phox (Fig. S2F). Since both NADPH oxidase and xanthine oxidase are capable of producing large amounts of O2•− and H2O2, we also evaluated the production of these oxidative molecules. As shown in Fig. 3, UNX + HS rats presented significantly higher NADPH oxidase-derived O2•− and H2O2 production (Fig. 3A,B), and increased xanthine oxidase-derived H2O2 levels (Fig. 3C). Finally, RDN alone had no significant effects on cardiac or renal NADPH oxidase and xanthine oxidase activities in the control rats (Fig. S5D,F,G,H).
Fig. 4.
Relative changes in p22phox mRNA (A) and protein (B) expression, as well as relative changes in xanthine oxidase mRNA (C) and protein (D) expression in kidneys from Control, UNX + HS and UNX + HS + RDN rats. n = 6–10/group for (A & C), n = 5–8/group for (B & D), *p < 0.05 vs. indicated group.
We have previously shown that tempol, which acts as a superoxide dismutase and also as a multifunctional antioxidant [33], is able to attenuate hypertension in the UNX + HS animals, indicating that superoxide and oxidative stress is a causative factor that leads to the progression of hypertension in this model [6]. In a subset of rats we included in vivo treatment with tempol in their drinking water, which protected the UNX + HS rats from developing cardiac hypertrophy and NADPH or xanthine oxidase-mediated oxidative stress (Fig. S6). Importantly, the UNX + HS + RDN treated rats that also received tempol in their drinking water did not differ significantly from the UNX + HS treated animals (Fig. S6). Although we cannot be sure that the reduced NADPH and xanthine oxidase activity observed here is the only causative factor leading to the cardiac and renal protection exerted by RDN, our data from tempol treated rats indicate that attenuation of oxidative stress is a possible mechanism, contributing to the observed protective effects following RDN. However, the presence of other simultaneous protective mechanisms mediated by RDN e.g., attenuated activity of the renin-angiotensin-aldosterone system and reduced renal water and sodium reabsorption, as well as modulation of central sympathetic nerve activity, cardiac angiogenesis and immune cell function cannot be excluded [21], [34], [35].
The prevalence of hypertension is increasing worldwide, and is one of the main risk factors for developing adverse renal and cardiovascular complications. Emerging evidence suggests that increased sympathetic nerve activity can promote or accelerate the development of hypertension and cardiac hypertrophy, and increase the risk of adverse complications [36]. Several experimental and clinical studies have demonstrated favorable effects of renal denervation (RDN) in hypertension and renal disease [20], [21]. However, findings obtained from patients with drug-resistant hypertension (SYMPLICITY HTN 1–3) have started a global debate regarding the therapeutic value of RDN in patients with resistant hypertension, but also other chronic disorders [21], [37], [38]. Further evaluation in rigorously designed clinical trials are needed, together with additional experimental studies to investigate the series of events, and also to further characterize the proposed interaction between sympathetic nerves and NADPH oxidase and xanthine oxidase regulation in renal and cardiovascular disease.
In conclusion, reduced renal mass combined with a high salt intake (UNX + HS) is associated with cardiac and renal abnormalities in later life, which is linked with increased oxidative stress. RDN prevents the development of hypertension, cardiac hypertrophy, and renal dysfunction and is associated with an attenuation of NADPH oxidase and xanthine oxidase-mediated O2•− and H2O2 production in the kidney.
Authors' contributions
M.P. and M.C. designed experiments, performed experiments and wrote the paper. P.F., Z.Z. and C.G. conceived or designed aspects of the research work, acquired data, and played important role in interpreting the results. C.E.W. and A.G.P. offered insight into the design and data analysis, and edited the manuscript. M.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
The authors declare no conflict of interest.
Sources of funding
This work was supported by grants from the Swedish Heart and Lung Foundation (Dnr: 20140448), the Swedish Research Council (Dnr: 2016-01381), and by KID-funding from the Karolinska Institutet (Dnr 2415/2012-225 and Dnr 2-3707/2013).
Acknowledgements
We thank Carina Nihlén, Annika Olsson and Margareta Stensdotter (Karolinska Institutet, Stockholm) for their technical assistance.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.06.013.
Appendix A. Supplementary material
Supplementary material
References
- 1.Carlstrom M., Sallstrom J., Skott O., Larsson E., Persson A.E. Uninephrectomy in young age or chronic salt loading causes salt-sensitive hypertension in adult rats. Hypertension. 2007;49:1342–1350. doi: 10.1161/HYPERTENSIONAHA.107.087213. [DOI] [PubMed] [Google Scholar]
- 2.Gossmann J., Wilhelm A., Kachel H.G., Jordan J., Sann U., Geiger H., Kramer W., Scheuermann E.H. Long-term consequences of live kidney donation follow-up in 93% of living kidney donors in a single transplant center. Am. J. Transplant. 2005;5:2417–2424. doi: 10.1111/j.1600-6143.2005.01037.x. [DOI] [PubMed] [Google Scholar]
- 3.Zoccali C., Mallamaci F. The salt epidemic: old and new concerns. Nutr. Metab. Cardiovasc. Dis. 2000;10:168–171. [PubMed] [Google Scholar]
- 4.Boudville N., Prasad G.V., Knoll G., Muirhead N., Thiessen-Philbrook H., Yang R.C., Rosas-Arellano M.P., Housawi A., Garg A.X., Donor N. Nephrectomy outcomes research, meta-analysis: risk for hypertension in living kidney donors. Ann. Intern. Med. 2006;145:185–196. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Meneton P., Jeunemaitre X., de Wardener H.E., MacGregor G.A. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol. Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 6.Carlstrom M., Brown R.D., Yang T., Hezel M., Larsson E., Scheffer P.G., Teerlink T., Lundberg J.O., Persson A.E. l-arginine or tempol supplementation improves renal and cardiovascular function in rats with reduced renal mass and chronic high salt intake. Acta Physiol. 2013;207:732–741. doi: 10.1111/apha.12079. [DOI] [PubMed] [Google Scholar]
- 7.Carlstrom M., Persson A.E., Larsson E., Hezel M., Scheffer P.G., Teerlink T., Weitzberg E., Lundberg J.O. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc. Res. 2011;89:574–585. doi: 10.1093/cvr/cvq366. [DOI] [PubMed] [Google Scholar]
- 8.Datla S.R., Griendling K.K. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension. 2010;56:325–330. doi: 10.1161/HYPERTENSIONAHA.109.142422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao X., Yang T., Liu M., Peleli M., Zollbrecht C., Weitzberg E., Lundberg J.O., Persson A.E., Carlstrom M. NADPH oxidase in the renal microvasculature is a primary target for blood pressure-lowering effects by inorganic nitrate and nitrite. Hypertension. 2015;65:161–170. doi: 10.1161/HYPERTENSIONAHA.114.04222. [DOI] [PubMed] [Google Scholar]
- 10.Laakso J., Mervaala E., Himberg J.J., Teravainen T.L., Karppanen H., Vapaatalo H., Lapatto R. Increased kidney xanthine oxidoreductase activity in salt-induced experimental hypertension. Hypertension. 1998;32:902–906. doi: 10.1161/01.hyp.32.5.902. [DOI] [PubMed] [Google Scholar]
- 11.Laakso J.T., Teravainen T.L., Martelin E., Vaskonen T., Lapatto R. Renal xanthine oxidoreductase activity during development of hypertension in spontaneously hypertensive rats. J. Hypertens. 2004;22:1333–1340. doi: 10.1097/01.hjh.0000125441.28861.9f. [DOI] [PubMed] [Google Scholar]
- 12.Harrison D.G., Gongora M.C. Oxidative stress and hypertension. Med. Clin. N. Am. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Rafiq K., Noma T., Fujisawa Y., Ishihara Y., Arai Y., Nabi A.H., Suzuki F., Nagai Y., Nakano D., Hitomi H., Kitada K., Urushihara M., Kobori H., Kohno M., Nishiyama A. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation. 2012;125:1402–1413. doi: 10.1161/CIRCULATIONAHA.111.064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagasu H., Satoh M., Kuwabara A., Yorimitsu D., Sakuta T., Tomita N., Kashihara N. Renal denervation reduces glomerular injury by suppressing NAD(P)H oxidase activity in Dahl salt-sensitive rats. Nephrol. Dial. Transplant. 2010;25:2889–2898. doi: 10.1093/ndt/gfq139. [DOI] [PubMed] [Google Scholar]
- 15.Lee T.M., Chen C.C., Hsu Y.J. Differential effects of NADPH oxidase and xanthine oxidase inhibition on sympathetic reinnervation in postinfarct rat hearts. Free Radic. Biol. Med. 2011;50:1461–1470. doi: 10.1016/j.freeradbiomed.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 16.Puschel G.P., Nath A., Jungermann K. Increase of urate formation by stimulation of sympathetic hepatic nerves, circulating noradrenaline and glucagon in the perfused rat liver. FEBS Lett. 1987;219:145–150. doi: 10.1016/0014-5793(87)81207-3. [DOI] [PubMed] [Google Scholar]
- 17.DiBona G.F., Kopp U.C. Neural control of renal function. Physiol. Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 18.Bohm M., Linz D., Urban D., Mahfoud F., Ukena C. Renal sympathetic denervation: applications in hypertension and beyond. Nat. Rev. Cardiol. 2013;10:465–476. doi: 10.1038/nrcardio.2013.89. [DOI] [PubMed] [Google Scholar]
- 19.Krum H., Schlaich M., Whitbourn R., Sobotka P.A., Sadowski J., Bartus K., Kapelak B., Walton A., Sievert H., Thambar S., Abraham W.T., Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 20.Bohm M., Ukena C., Ewen S., Linz D., Zivanovic I., Hoppe U., Narkiewicz K., Ruilope L., Schlaich M., Negoita M., Schmieder R., Williams B., Zeymer U., Zirlik A., Mancia G., Mahfoud F. S.R.I. Global, Renal denervation reduces office and ambulatory heart rate in patients with uncontrolled hypertension: 12-month outcomes from the global SYMPLICITY registry. J. Hypertens. 2016;34:2480–2486. doi: 10.1097/HJH.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 21.Carlstrom M. Therapeutic value of renal denervation in cardiovascular disease? Acta Physiol. 2017;220:11–13. doi: 10.1111/apha.12816. [DOI] [PubMed] [Google Scholar]
- 22.Peleli M., Al-Mashhadi A., Yang T., Larsson E., Wahlin N., Jensen B.L., AE G.P., Carlstrom M. Renal denervation attenuates NADPH oxidase-mediated oxidative stress and hypertension in rats with hydronephrosis. Am. J. Physiol. Ren. Physiol. 2016;310:F43–F56. doi: 10.1152/ajprenal.00345.2015. [DOI] [PubMed] [Google Scholar]
- 23.Burgi K., Cavalleri M.T., Alves A.S., Britto L.R., Antunes V.R., Michelini L.C. Tyrosine hydroxylase immunoreactivity as indicator of sympathetic activity: simultaneous evaluation in different tissues of hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R264–R271. doi: 10.1152/ajpregu.00687.2009. [DOI] [PubMed] [Google Scholar]
- 24.Hezel M., Peleli M., Liu M., Zollbrecht C., Jensen B.L., Checa A., Giulietti A., Wheelock C.E., Lundberg J.O., Weitzberg E., Carlstrom M. Dietary nitrate improves age-related hypertension and metabolic abnormalities in rats via modulation of angiotensin II receptor signaling and inhibition of superoxide generation. Free Radic. Biol. Med. 2016;99:87–98. doi: 10.1016/j.freeradbiomed.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Teerlink T., Nijveldt R.J., de Jong S., van Leeuwen P.A. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal. Biochem. 2002;303:131–137. doi: 10.1006/abio.2001.5575. [DOI] [PubMed] [Google Scholar]
- 26.Lapenna D., Ciofani G., Pierdomenico S.D., Giamberardino M.A., Cuccurullo F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic. Biol. Med. 2001;31:331–335. doi: 10.1016/s0891-5849(01)00584-6. [DOI] [PubMed] [Google Scholar]
- 27.Montenegro M.F., Amaral J.H., Pinheiro L.C., Sakamoto E.K., Ferreira G.C., Reis R.I., Marcal D.M., Pereira R.P., Tanus-Santos J.E. Sodium nitrite downregulates vascular NADPH oxidase and exerts antihypertensive effects in hypertension. Free Radic. Biol. Med. 2011;51:144–152. doi: 10.1016/j.freeradbiomed.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Peleli M., Zollbrecht C., Montenegro M.F., Hezel M., Zhong J., Persson E.G., Holmdahl R., Weitzberg E., Lundberg J.O., Carlstrom M. Enhanced XOR activity in eNOS-deficient mice: effects on the nitrate-nitrite-NO pathway and ROS homeostasis. Free Radic. Biol. Med. 2016;99:472–484. doi: 10.1016/j.freeradbiomed.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Zollbrecht C., Persson A.E., Lundberg J.O., Weitzberg E., Carlstrom M. Nitrite-mediated reduction of macrophage NADPH oxidase activity is dependent on xanthine oxidoreductase-derived nitric oxide but independent of S-nitrosation. Redox Biol. 2016;10:119–127. doi: 10.1016/j.redox.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T., Zollbrecht C., Winerdal M.E., Zhuge Z., Zhang X.M., Terrando N., Checa A., Sallstrom J., Wheelock C.E., Winqvist O., Harris R.A., Larsson E., Persson A.E., Fredholm B.B., Carlstrom M. Genetic abrogation of adenosine A3 receptor prevents uninephrectomy and high salt-induced hypertension. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eikelboom J.W., Hankey G.J., Thom J., Bhatt D.L., Steg P.G., Montalescot G., Johnston S.C., Steinhubl S.R., Mak K.H., Easton J.D., Hamm C., Hu T., Fox K.A., Topol E.J., R. Clopidogrel for High Atherothrombotic, M. Ischemic Stabilization, I. Avoidance Incomplete inhibition of thromboxane biosynthesis by acetylsalicylic acid: determinants and effect on cardiovascular risk. Circulation. 2008;118:1705–1712. doi: 10.1161/CIRCULATIONAHA.108.768283. [DOI] [PubMed] [Google Scholar]
- 32.Pastori D., Pignatelli P., Farcomeni A., Cangemi R., Hiatt W.R., Bartimoccia S., Nocella C., Vicario T., Bucci T., Carnevale R., Lip G.Y., Violi F. Urinary 11-dehydro-thromboxane B2 is associated with cardiovascular events and mortality in patients with atrial fibrillation. Am. Heart J. 2015;170:490–497. doi: 10.1016/j.ahj.2015.05.011. (e1) [DOI] [PubMed] [Google Scholar]
- 33.Dornas W.C., Silva M., Tavares R., de Lima W.G., dos Santos R.C., Pedrosa M.L., Silva M.E. Efficacy of the superoxide dismutase mimetic tempol in animal hypertension models: a meta-analysis. J. Hypertens. 2015;33:14–23. doi: 10.1097/HJH.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 34.Denker M.G., Cohen D.L. Resistant hypertension and renal nerve denervation. Methodist Debakey Cardiovasc. J. 2015;11:240–244. doi: 10.14797/mdcj-11-4-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu D., Wang K., Wang S., Zhang B., Liu Q., Zhang Q., Geng J., Shan Q. Beneficial effects of renal denervation on cardiac angiogenesis in rats with prolonged pressure overload. Acta Physiol. 2017;220:47–57. doi: 10.1111/apha.12793. [DOI] [PubMed] [Google Scholar]
- 36.Grassi G., Mark A., Esler M. The sympathetic nervous system alterations in human hypertension. Circ. Res. 2015;116:976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhave P.D. Renal denervation therapy for the treatment of arrhythmias: is the sky the limit? J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulati R., Raphael C.E., Negoita M., Pocock S.J., Gersh B.J. The rise, fall, and possible resurrection of renal denervation. Nat. Rev. Cardiol. 2016;13:238–244. doi: 10.1038/nrcardio.2016.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material