Abstract
Introduction
High levels of amyloid β (Aβ) are associated with cognitive decline in cognitively normal (CN) older adults. This study investigated the nature of cognitive decline in healthy individuals who did not progress to mild cognitive impairment or dementia.
Method
Cognition was measured over 72 months and compared between low (Aβ−) and high (Aβ+) CN older adults (n = 335) who did not progress to mild cognitive impairment or dementia and who remained free of severe or uncontrolled systemic illness.
Results
Compared to the Aβ− group, the Aβ+ group showed no cognitive impairment at baseline but showed substantial decline in verbal learning, episodic memory, and attention over 72 months.
Discussion
Moderate cognitive decline, particularly for learning and memory, was associated with Aβ+ in CN older adults in the absence of clinical disease progression and uncontrolled or serious comorbid illness.
Keywords: Alzheimer disease, Amyloid, Cognitive aging, Normal aging, Memory
1. Background
The preclinical phase of Alzheimer's disease (AD) is characterized by the presence of abnormal levels of amyloid β (Aβ+) and progressive cognitive decline in otherwise healthy older adults [1]. There is growing emphasis on the preclinical phase of AD as a starting point for clinical trials of anti-amyloid agents with the goal of halting the disease before the development of clinical symptoms [2]. Although it is recognized that cognitive decline in preclinical AD most likely reflects multiple biological changes, studies and clinical trials often require only Aβ+ for the classification of preclinical AD. In this context, developing accurate estimates of Aβ+-related cognitive change in cognitively normal (CN) older adults is essential. However, the precise nature and magnitude of Aβ+-related cognitive decline differs between studies [3], [4], [5], [6]. One possible reason for this difference is that neuropsychological study of preclinical AD has more in common with the study of aging than the study of dementia. Thus, issues known to influence or inflate estimates of age-related cognitive change in CN older adults might also increase the estimates of Aβ+-related cognitive decline in preclinical AD [7], [8].
One factor that may influence estimates of Aβ+-related cognitive decline is progression from preclinical AD to a clinical disease stage (i.e., mild cognitive impairment [MCI] or dementia). Although studies of preclinical AD exclude individuals who meet clinical criteria for MCI or dementia at enrollment or baseline assessment, rates of clinical disease progression during prospective studies are consistently large (e.g., 10.8% in Australian Imaging Biomarkers and Lifestyle [AIBL] [3], 10% in Knight Alzheimer's Disease Research Centre [Knight ADRC] [9], 7.7% in Alzheimer's Disease Neuroimaging Initiative [ADNI] [10], and 6.1% in Mayo Clinic Study of Aging samples [11]). Estimates of Aβ+-related cognitive decline derived from samples that include high rates of clinical disease progression might therefore be inflated. When assessed on a single occasion, CN Aβ+ adults generally show no cognitive impairment relative to matched Aβ− adults [12], [13], [14]. Thus, it is possible that in the absence of any clinical disease progression, Aβ+ might be associated with only small, or even no, cognitive decline in CN older adults.
The inclusion of individuals with severe or uncontrolled systemic illnesses in study samples may also inflate estimates of Aβ+-related cognitive decline. In older adults, systemic illnesses, such as diabetes mellitus or hypertension, show increased prevalence and are associated with subtle cognitive impairment [15], [16]. Given their high prevalence in aging, epidemiological studies of preclinical AD include large proportions of individuals with these systemic illnesses [17]. In addition, the published criteria for most experimental samples do not specify whether such conditions have been excluded or state these are excluded only if they prevent longitudinal assessment (e.g., ADNI or Knight ADRC [5], [10], [18]). As such, inclusion of older adults with systemic illness in studies of preclinical AD may have led to inflated estimates of Aβ+-related cognitive decline.
Finally, the accuracy of estimates of Aβ+-related cognitive decline might be reduced by the use of neuropsychological tests that are not appropriate for use with CN older adults. Many studies of preclinical AD use neuropsychological tests selected for their sensitivity to the substantial cognitive impairment associated with dementia (e.g., Clock Drawing, Boston Naming Test, or Mini–Mental Status Examination [MMSE] [4], [10], [19], [20]). When such tests are applied in CN samples, the resulting data distributions are often characterized by negative skew, ceiling effects, and restriction of range [21], [22], [23]. Consequently, random error is biased toward the negative end of measurement scales inflating estimates of cognitive decline [24].
To obtain unbiased estimates of Aβ+-related cognitive decline, appropriate neuropsychological tests should be applied prospectively to Aβ+ CN adults, who do not progress to clinical AD during the study period, and who do not have severe systemic illness. In other neuropsychological contexts, samples that exclude data from individuals who later meet clinical criteria for cognitive impairment or dementia are defined as robust samples [25]. Group mean performance on neuropsychological tests in robust samples is generally superior and has reduced variability compared with estimates from conventional normative samples [26], [27]. Therefore, application of the neuropsychological robust sample method may provide more accurate estimates of the nature and magnitude of Aβ+-related cognitive decline in preclinical AD.
The aim of this study was to determine the nature and magnitude of Aβ+-related cognitive decline and impairment, in the absence of clinical disease progression and other illnesses, such as systemic illness, that are associated with older age. Although Aβ+ is unlikely to be the sole causative factor for cognitive decline and likely interacts with other markers of AD, such as tau and neurodegeneration, it is necessary for the classification of preclinical AD and has been shown to be a strong predictor of cognitive decline. By developing accurate estimates of Aβ+-related cognitive decline, a solid framework can be established to then further investigate the contribution of other factors. It was hypothesized that the Aβ+ CN older adults would show subtle cognitive decline relative to the Aβ− CN older adults. The second hypothesis was that Aβ+ CN older adults would show no cognitive impairment relative to the Aβ− CN older adults.
2. Method
2.1. Participants
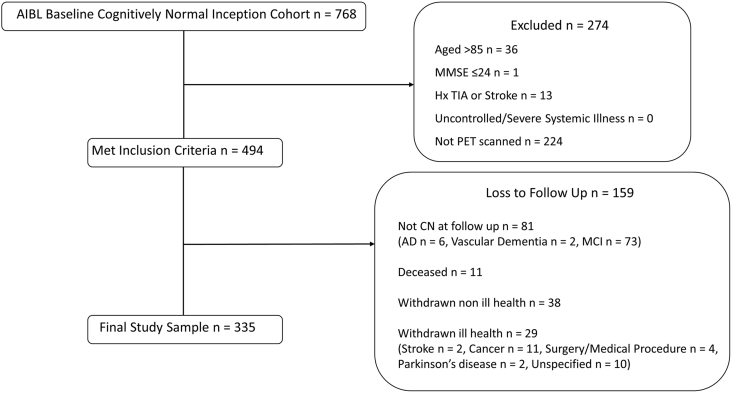
Participants were recruited from the AIBL study inception cohort (Fig. 1 summarizes the method used to select participants). The AIBL sample and methodology have been described in detail previously [28]. The AIBL study was approved by three institutional research and ethics committees (St Vincent's Health, Austin Health, and Edith Cowan University). All participants provided written informed consent before participating in the study. Exclusion criteria included a history of non-AD dementia, Parkinson's disease, schizophrenia, bipolar disorder, cancer (other than basal skin carcinoma) within the last 2 years, uncontrolled diabetes, current depression (indicated by a Geriatric Depression Scale score >5), or current regular alcohol consumption exceeding two standard drinks per day for women or four per day for men according to recommended consumption levels from the Australian Alcohol Guidelines [29].
Fig. 1.
Sample screening process. Abbreviation: MCI, mild cognitive impairment.
The current health status of participants at baseline was also reviewed. All participants were determined to have no, or only subclinical and medically well controlled, systemic or neurological illness on the basis of a medical examination including measurement of vital signs (height, weight, blood pressure, and abdominal circumference), blood pathology, and medical history. Participants who later withdrew from the AIBL study for reasons of ill health (e.g., cancer or stroke) were also excluded from the current analyses.
The sample included 335 CN older adults aged between 60 and 85 years. All participants had undergone positron emission tomography neuroimaging with an Aβ tracer and at least two clinical and neuropsychological assessments (mean number of follow-up assessments 4.81, with 88.1% of participants completing all 5 assessments) within 72 months. Included participants also scored above 24 on the MMSE at baseline and did not meet criteria for MCI [30] or dementia [31] at any follow-up assessment.
2.2. Measures
2.2.1. Neuroimaging
Participants underwent positron emission tomography neuroimaging on a separate day to their clinical and neuropsychological assessment. Where multiple scan results were available, Aβ status was determined from the first scan (n = 109 at baseline, n = 17 at 18 months, n = 134 at 36 months, n = 63 at 54 months, and n = 12 at 72 months).
Participants were administered either 11C-Pittsburgh compound-B (PiB; n = 174), 18F-florbetapir (FBP; n = 72), or 18F-flutemetamol (FLUTE; n = 89), and then standardized uptake value (SUV) data were acquired 40 to 70, 50 to 70, or 90 to 110 minutes after injection, respectively. Data were then summed and normalized to obtain the SUV ratio (SUVR) using the cerebellar cortex as reference region for PiB, the whole cerebellum for FBP, and the pons for FLUTE [32], [33]. A linear regression transformation was then applied to the SUVR scores for FBP and FLUTE to transform them into a “PiB-like” SUVR unit termed “Before the Centiloid Kernel Transformation” [33]. These procedures have been described in greater detail previously [32], [33].
2.2.2. Demographics
Age and gender were self-reported. Depression and anxiety symptoms were assessed by the Hospital Anxiety and Depression Scale (HADS [34]). The Wechsler Test of Adult Reading (WTAR [35]) was administered to estimate intelligence quotient.
2.2.3. Neuropsychological assessment
The AIBL neuropsychological test battery and procedures for administration have been described previously [28]. The present study used a subset of the AIBL battery selected specifically because they provided normal data distributions, had a high test-retest reliability, and were sensitive to cognitive changes associated with healthy aging [23] (Table 1). The selected tests included measures of learning, immediate and delayed recall aspects of episodic memory, psychomotor speed, attention, and executive function.
Table 1.
Summary of the neuropsychological test battery and their outcome measures
| Cognitive domain | Neuropsychological test | Outcome | Reference |
|---|---|---|---|
| Processing speed and attention | Digit Symbol Coding∗ | Number of items correctly coded within 2 minutes | [36] |
| Stroop Words | Seconds to complete trial | [37] | |
| Detection | Speed (log10 milliseconds) | [38] | |
| Identification | Speed (log10 milliseconds) | [38] | |
| Executive Function | Digit Span∗ | Total correct trials (forwards and backwards) | [36] |
| Letter Fluency (FAS) | Number of words produced | [39] | |
| Category Fluency (animals and boys names) | Number of words produced | [39] | |
| Category Fluency (fruit and furniture) | Number of words produced while switching categories | [39] | |
| One Back | Speed (log10 milliseconds) | [38] | |
| Learning and Immediate Recall | Logical Memory 1† | Number of elements correctly recalled | [40] |
| CVLT-II trials 1–5 | Number of words correctly recalled | [41] | |
| RCFT Short Delay | Number of elements correctly drawn | [42] | |
| One Card Learning | Accuracy (arcsine proportion correct) | [38] | |
| Delayed Recall | Logical Memory 2† | Number of elements correctly recalled | [40] |
| CVLT-II Long Delay | Number of words correctly recalled | [41] | |
| RCFT Long Delay | Number of elements correctly drawn | [42] |
Abbreviations: CVLT-II, California Verbal Learning Test-Second edition; RCFT, Rey Complex Figure Test; WAIS-III, Wechsler Adult Intelligence Scale–Third Edition.
Subtest of the WAIS-III.
Story A only, subtest of the Wechsler Memory Scale–Revised.
3. Procedure
Participants fasted overnight before attending the research facility in the morning. Written consent was obtained. An 80-ml blood sample was then drawn and sent for genotyping and clinical pathology testing. Participants were provided breakfast while they completed self-report questionnaires on demographics, mood, and medical history. Finally, trained research assistants conducted a clinical interview and neuropsychological assessment and assessed vital signs in a single 120-minute session according to standardized protocols.
4. Data coding
Neuroimaging results were classified as Aβ+ or Aβ− to create a single-dichotomous categorical variable. Aβ+ was classified according to an SUVR/Before the Centiloid Kernel Transformation threshold of ≥1.5 [33]. Individuals were also classified as to whether they carried an apolipoprotein E ε4 (APOE) ε4 allele, and this was also coded as a single-dichotomous categorical variable. Only three APOE ε4 homozygotes were included in this group.
5. Data screening and analysis
All analyses were conducted using Statistical Package for Social Sciences (SPSS), version 23.0 and R statistical computing packages.
Data frequency distributions were generated for each outcome measure and inspected to identify outlier data points beyond 1.5 times the interquartile range. These data points were excluded from analyses. Visual inspection of data frequency distributions indicated diversion from normality for MMSE, Wechsler Test of Adult Reading, HADS-D, and HADS-A. Nonparametric testing was used for these variables.
First, independent-sample t-tests were used to compare the Aβ− and Aβ+ groups on demographic and clinical characteristics. Where outcome data were not normally distributed, a Mann-Whitney U test was used to compare the groups, or where data were categorical, a χ2 test was used.
Second, a series of linear mixed model analyses were conducted to model the rate of change over time in cognitive function for each of the Aβ groups. Aβ group, time (baseline, 18, 36, 54, and 72 months), and the time × Aβ group interaction were entered as fixed factors; participants and time as random factors; and performance on each of the neuropsychological tests as the dependent variable. Age and APOE ε4 status were included as covariates to account for their potential to influence cognitive performance in CN older adults. Cohen's d effect size measure of standardized mean difference was then calculated to determine the magnitude of the difference in rate of change in cognitive function between Aβ groups.
Third, a series of one-way analysis of covariance were used to determine the impact of Aβ on each cognitive test at the baseline assessment. Aβ group was entered as a factor, age and APOE ε4 status as covariates and the baseline performance on each of the neuropsychological tests as the dependent variable. Cohen's d effect size measure of standardized mean difference was then calculated to determine the magnitude of the difference in performance between Aβ groups.
Statistical significance for all comparisons was set to P < .05 level. As performance on the cognitive measures used in the present study was likely to be correlated, strict adjustment of error rates using Bonferroni criteria would be overly conservative. In addition, inspection of estimates of effect size that were generated for each comparison was used to guide interpretation of results and minimize Type I error. Group differences or associations were only interpreted where they were statistically significant, and estimates of effect sizes were nontrivial (i.e., d > 0.2 [43]).
6. Results
6.1. Sample characteristics
Demographic and clinical characteristics of the total sample and each Aβ group are summarized in Table 2. Overall, the total sample had slightly more females than males, median-estimated intelligence quotient was in the average range, symptoms of depression and anxiety were reported to be low, and vital signs were within subclinical ranges. The Aβ+ group was slightly older and had a higher proportion of APOE ε4 carriers than the Aβ− group. No other differences between groups were observed.
Table 2.
Demographic and clinical assessment means (SD) for total sample and each Aβ group at baseline assessment
| Demographic and clinical characteristic | Total |
Aβ− |
Aβ+ |
P |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| N | 335 | 277 | 58 | |
| %Female | 57.9 | 58.5 | 55.2 | .64 |
| %APOE ε4 carrier | 25.1 | 19.5 | 35.7 | .00 |
| Age | 68.28 (5.71) | 67.62 (5.36) | 71.43 (6.26) | .00 |
| MMSE∗ | 29 (5) | 29 (5) | 29 (4) | .49 |
| Estimated IQ (WTAR)∗ | 114 (35) | 114 (35) | 114 (27) | .13 |
| HADS Depression∗ | 2 (14) | 2 (11) | 2 (14) | .87 |
| HADS Anxiety∗ | 4 (15) | 4 (12) | 4 (15) | .98 |
| Systolic BP | 136.52 (14.24) | 136.11 (14.56) | 138.58 (12.39) | .20 |
| Diastolic BP | 78.42 (9.23) | 78.13 (9.52) | 79.89 (7.51) | .21 |
| Heart Rate | 67.05 (9.26) | 66.84 (9.33) | 68.06 (8.97) | .38 |
| Abdominal Circumference | 91.40 (12.56) | 91.45 (12.81) | 91.15 (11.29) | .88 |
| Body Mass Index | 26.36 (3.93) | 26.47 (3.954) | 25.84 (3.81) | .28 |
Abbreviations: APOE, apolipoprotein E; BP, blood pressure; HADS, Hospital Anxiety and Depression Scale; IQ, intelligence quotient; MMSE, Mini–Mental State Examination; SD, standard deviation; WTAR, Wechsler Test of Adult Reading.
NOTE. Bold data indicates statistical significance (P <.05).
Non-normal distribution–Mann-Whitney test, median and range reported.
6.2. Effect of Aβ on cognitive decline
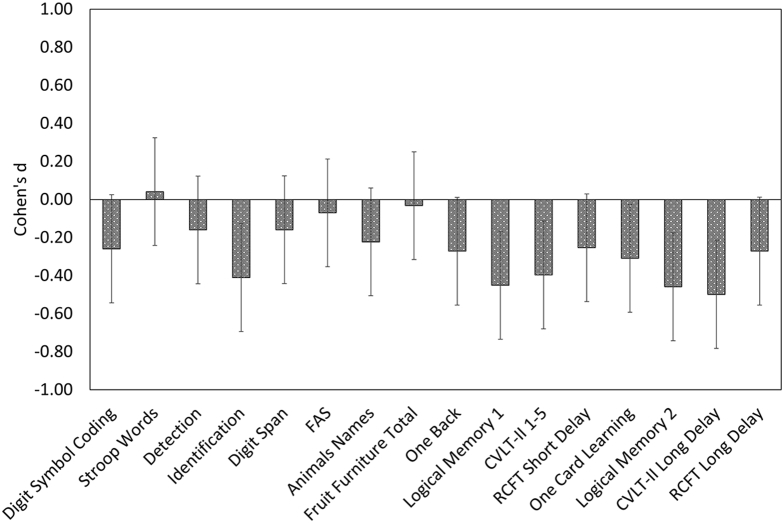
Mean slopes and standard deviation of performance over 72 months for each Aβ group are shown in Table 3. Linear mixed model analysis indicated significant Aβ group × time interactions for performance on the identification, Logical Memory 1 and 2, California Verbal Learning Test-Second edition trials 1–5 and Long Delay, and One Card Learning tasks (Table 3). Each of these analyses indicated that the Aβ+ group declined faster over 72 months than the Aβ− group, and differences in rate of change were small to moderate in magnitude (Cohen's d = −0.31 to −0.50; Fig. 2). No difference in rate of change of cognitive performance was observed between groups for Digit Symbol, Stroop Words, Detection, Digit Span, Letter Fluency (FAS), Category Fluency (animals and boys names), Category Fluency (fruit and furniture), One Back, and Rey Complex Figure Test Short or Long Delay.
Table 3.
Results of linear mixed model analyses covaried for age and APOE ε4 with mean (SD) slopes for each Aβ group
| Neuropsychological measure | Age |
APOE ε4 |
Aβ status |
Time |
Aβ status × time |
Aβ− |
Aβ+ |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | Slope (SD) | Slope (SD) | |
| Digit Symbol Coding | 73.85 | .00 | 2.86 | .09 | 1.42 | .23 | 3.36 | .07 | 2.73 | .10 | 0.54 (7.15) | −1.22 (4.57) |
| Stroop Words | 40.61 | .00 | 1.36 | .25 | 0.16 | .69 | 0.01 | .92 | 0.07 | .79 | 0.12 (2.42) | 0.02 (1.60) |
| Detection | 3.78 | .05 | 0.86 | .35 | 1.99 | .16 | 8.54 | .00 | 1.04 | .31 | 0.01 (0.10) | 0.03 (0.06) |
| Identification | 18.19 | .00 | 3.73 | .05 | 9.42 | .00 | 23.89 | .00 | 6.83 | .01 | 0.00 (0.05) | 0.02 (0.03) |
| Digit Span | 2.18 | .14 | 2.49 | .12 | 1.76 | .19 | 4.93 | .03 | 1.03 | .31 | −0.12 (2.13) | −0.44 (1.42) |
| FAS | 4.85 | .03 | 2.25 | .13 | 4.33 | .04 | 0.10 | .76 | 0.20 | .66 | 0.60 (6.25) | 0.18 (3.91) |
| Animals Names | 20.41 | .00 | 0.09 | .76 | 3.07 | .08 | 4.70 | .03 | 2.03 | .16 | 0.05 (4.99) | −1.01 (3.26) |
| Fruit Furniture Total | 36.22 | .00 | 0.04 | .85 | 0.01 | .94 | 2.39 | .12 | 0.04 | .84 | −0.24 (2.09) | −0.30 (1.41) |
| One Back | 24.03 | .00 | 5.32 | .02 | 5.33 | .02 | 2.41 | .12 | 2.97 | .09 | −0.01 (0.06) | 0.01 (0.04) |
| Logical Memory 1 | 7.53 | .01 | 0.61 | .43 | 0.65 | .42 | 5.40 | .02 | 8.29 | .00 | 0.54 (2.56) | −0.55 (1.68) |
| CVLT-II 1–5 | 23.65 | .00 | 1.58 | .21 | 0.11 | .74 | 2.85 | .09 | 6.29 | .01 | 1.64 (7.59) | −1.19 (4.46) |
| RCFT Short Delay | 16.64 | .00 | 0.68 | .41 | 1.04 | .31 | 0.30 | .58 | 2.62 | .11 | 0.70 (3.67) | −0.19 (2.41) |
| One Card Learning | 9.20 | .00 | 0.38 | .54 | 6.66 | .01 | 3.73 | .05 | 3.88 | .05 | 0.01 (0.10) | −0.02 (0.06) |
| Logical Memory 2 | 6.39 | .01 | 0.86 | .36 | 1.11 | .29 | 4.31 | .04 | 8.54 | .00 | 0.64 (2.64) | −0.51 (1.67) |
| CVLT-II Long Delay | 13.85 | .00 | 1.48 | .22 | 0.54 | .46 | 10.32 | .00 | 10.14 | .00 | 0.31 (1.79) | −0.53 (1.17) |
| RCFT Long Delay | 20.51 | .00 | 0.94 | .33 | 2.51 | .11 | 0.57 | .45 | 3.02 | .08 | 0.67 (3.53) | −0.25 (2.43) |
Abbreviations: Animals Names, category fluency for animals and boys names; CVLT-II, California Verbal Learning Test-Second edition; FAS, letter fluency; Fruit Furniture Total, total Correct Responses for switching category fluency for fruit and furniture; RCFT, Rey Complex Figure Test; SD, standard deviation.
NOTE. Bold data indicates statistical significance (P <.05).
Fig. 2.
Aβ-associated cognitive decline—magnitude of difference (Cohen's d) between the mean slope of the Aβ+ group relative to the Aβ− group over 72 months. Negative Cohen's d values indicate greater decline in function over time for that measure in the Aβ+ group compared with the Aβ− group. Abbreviations: Animals Names, category fluency for animals and boys names; CVLT-II, California Verbal Learning Test-Second edition; FAS, letter fluency; Fruit Furniture Total, total Correct Responses for switching category fluency for fruit and furniture; RCFT, Rey Complex Figure Test.
6.3. Effect of Aβ on cognitive impairment
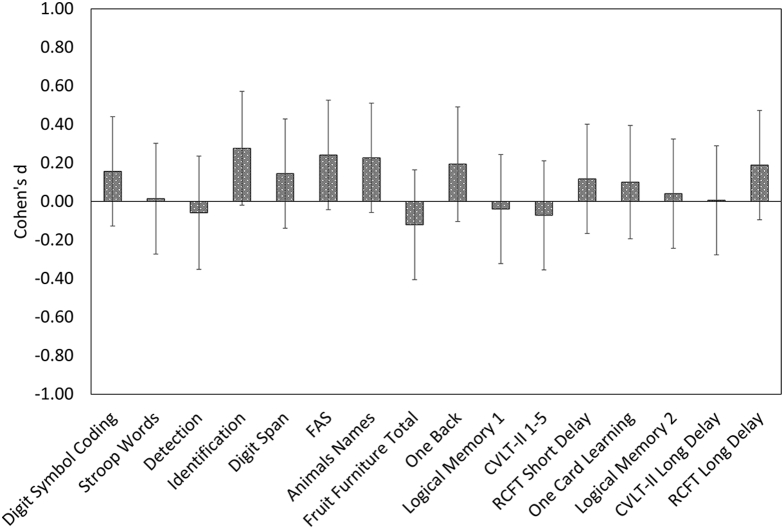
Group means and standard deviations for baseline performance on the neuropsychological tests are shown in Table 4. There was a small difference (d = 0.28) in performance between the Aβ groups on the Identification task, with the Aβ+ group showing faster mean response times than the Aβ− group. There were no significant differences in performance between the Aβ groups on any other neuropsychological test. Fig. 3 shows the magnitude of the difference in performance between groups on each test, which was uniformly trivial to small (Cohen's d = 0.01 to 0.28).
Table 4.
Baseline mean (SD) performance of each Aβ group on the neuropsychological tests and results of analysis of covariance with age and APOE ε4 as covariates
| Neuropsychological measure | Aβ− |
Aβ+ |
F | P |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Digit Symbol Coding | 64.28 (12.53) | 61.91 (15.60) | 1.52 | .22 |
| Stroop Words | 17.53 (4.08) | 17.60 (5.07) | 0.01 | .91 |
| Detection | 2.52 (0.12) | 2.51 (0.14) | 0.17 | .68 |
| Identification | 2.69 (0.06) | 2.72 (0.08) | 4.89 | .03 |
| Digit Span | 18.64 (4.20) | 17.91 (5.21) | 1.31 | .25 |
| FAS | 45.32 (12.19) | 41.77 (15.17) | 3.62 | .06 |
| Animals Names | 43.47 (8.70) | 41.09 (10.81) | 3.18 | .08 |
| Fruit Furniture Total | 13.91 (2.79) | 14.32 (3.47) | 0.89 | .35 |
| One Back | 2.92 (0.09) | 2.94 (0.11) | 2.18 | .14 |
| Logical Memory 1 | 13.59 (3.90) | 13.78 (4.85) | 0.09 | .76 |
| CVLT-II 1–5 | 53.37 (10.24) | 54.25 (12.74) | 0.32 | .57 |
| RCFT Short Delay | 18.10 (5.77) | 17.28 (7.18) | 0.85 | .36 |
| One Card Learning | 1.04 (0.12) | 1.03 (0.14) | 0.55 | .46 |
| Logical Memory 2 | 12.55 (4.00) | 12.36 (4.97) | 0.10 | .75 |
| CVLT-II Long Delay | 12.42 (3.00) | 12.40 (3.74) | 0.00 | .96 |
| RCFT Long Delay | 18.47 (5.91) | 17.12 (7.36) | 2.22 | .14 |
Abbreviations: Animals Names, category fluency for animals and boys names; CVLT-II, California Verbal Learning Test-Second edition; FAS, letter fluency; Fruit Furniture Total, total Correct Responses for switching category fluency for fruit and furniture; RCFT, Rey Complex Figure Test; SD, standard deviation.
NOTE. Bold data indicates statistical significance (P <.05).
Fig. 3.
Aβ-associated cognitive impairment–magnitude of difference (Cohen's d) between performance of Aβ+ group relative to the Aβ− group at baseline. Negative Cohen's d values indicate worse performance on that measure in the Aβ+ group compared with the Aβ− group at the baseline assessment. Abbreviations: Animals Names, category fluency for animals and boys names; CVLT-II, California Verbal Learning Test-Second edition; FAS, letter fluency; Fruit Furniture Total, total Correct Responses for switching category fluency for fruit and furniture; RCFT, Rey Complex Figure Test.
To account for the potential influence of baseline difference in performance on the Identification task in rate of change on the task, the longitudinal model was rerun adding baseline performance as a covariate. After adjusting for baseline performance, the Aβ group × time interaction remained statistically significant and the magnitude of difference in rate of change remained small (d = 0.38).
7. Discussion
The hypothesis that in Aβ+ CN older adults, who do not progress to MCI or dementia over 72 months and who have no severe or uncontrolled systemic illness, would show subtle cognitive decline relative to Aβ− CN adults was supported partially. Relative to the Aβ− group, the Aβ+ group showed decline over 72 months in verbal learning, episodic memory, and attention. Despite the exclusion of individuals whose disease had progressed to MCI or dementia, Aβ+-associated cognitive decline was small to moderate in magnitude (e.g., Cohen's d between −0.31 and −0.50; summarized in Fig. 2) with the greatest decline observed for verbal episodic memory. These estimates of Aβ+-related memory decline accord with estimates from recent meta-analyses of cognitive decline in preclinical AD, which found Aβ+ was associated with small to moderate cognitive decline (Cohen's d between −0.24 and −0.30) over an average of 5 years [6]. They also support brain-behavior models of early AD, which propose that disruption to episodic memory, due to the early predilection for Aβ-related cell death in the medial temporal lobes, is the earliest clinical manifestation of AD [1].
Although the magnitude of the cognitive decline observed in the current robust sample is consistent with that observed previously [4], [6], [44], the nature of Aβ+-related decline observed here was qualitatively different. For example, in the present study, Aβ+-related decline was observed for aspects of episodic memory, including verbal list learning, visual pattern separation, and paragraph learning, as well as for aspects of attention (Table 3). However, previous studies report Aβ+ to also be associated with decline in global cognition, visuospatial function, and executive function [4], [6], [45], [46]. Notably, the sample size of the present study is similar or larger than that of previous studies (excluding meta-analyses), and the effect sizes for those tests where there was an absence of significant Aβ-related cognitive decline were predominantly trivial and small at best. This suggests that a failure to detect Aβ-related cognitive decline on these measures was not due to insufficient statistical power. One possible reason for the absence of decline in these same domains in this study may be that the neuropsychological tests used had optimal psychometric properties for the assessment of CN older adults. For example, in previous studies, the use of tests that generate data distributions characterized by skew or restricted range (e.g., Clock Drawing, Boston Naming Test, or MMSE) may have resulted in random error in the data distribution that is biased to the negative end of the measurement scale and consequently increased group estimates of Aβ+-related decline for those measures [21], [23], [24].
Qualitative differences in Aβ+-related cognitive decline between this and previous studies may also be due to the absence of any clinical disease progression in the current sample. Previous studies include a relatively high proportion (e.g., ∼10%) of individuals who progressed to clinical disease during the study period. Pathological studies indicate that during the earliest stages of AD, the hippocampus and entorhinal cortex, which subserve episodic memory function, are most affected [47]. However, as the disease progresses, the limbic system and neocortex become involved [47]. Hence, the absence of decline in executive and visuospatial function may reflect the very early stage of disease in the current sample.
A third possibility is that the more diverse Aβ+-related cognitive decline observed in previous studies reflects the presence of uncontrolled or severe systemic illness in these samples. For example, epidemiological studies, such as the Mayo Clinic Study of Aging, deliberately include high proportions of individuals who have one or more systemic illnesses due to their high prevalence in older adults [17]. Other experimental or natural history samples often do not specify whether such conditions were excluded (e.g., ADNI or Knight ADRC [5], [10]). Systemic illnesses are prevalent in older adults and have been shown to be associated with reduced cognitive function [15]. Given this, it is possible that in prior studies such illnesses influenced the nature of cognitive decline in preclinical AD. The results of the present study highlight the potential of the robust sample method for enhancing estimates of cognitive decline and for examining changes occurring in the earliest disease stages.
The second hypothesis that Aβ+ CN older adults would show no cognitive impairment relative to the Aβ− CN older adults was partially supported. Consistent with prior studies, there were no, or only small, significant differences in performance observed between the Aβ groups on the neuropsychological tests [12], [13], [14]. Furthermore, effect sizes for the group differences were uniformly trivial to small (Fig. 3), indicating that the absence of statistically significant group differences was not due to low statistical power. Although meta-analytic investigations do suggest that Aβ+ is associated with a small to moderate cognitive impairment (e.g., Cohen's d = 0.26 to 0.47) of global cognition, visuospatial function, processing speed, executive function, and episodic memory in CN older adults, none of the samples submitted to meta-analyses could be considered robust [6], [48]. Thus, data from the present study suggest that Aβ+-associated cognitive impairment in CN older adults may have been overestimated previously.
Although the results of the present study provide an indication of the effect of Aβ+ on cognitive function for CN older adults, independent of systemic illness or progression to clinical AD, they should be interpreted in the context of several limitations. The Aβ status of a large proportion of the sample (67.5%) was not able to be determined at baseline, as those participants did not undergo neuroimaging until a later follow-up assessment. However, given the relatively slow rate of accumulation of Aβ in CN individuals (e.g., mean increase of 0.02 in SUVR per year [49]), it is unlikely that any individual who was identified at Aβ+ on the basis of a scan at follow-up would not have also been Aβ+ at baseline. In addition, the AIBL sample is a convenience sample and as such may not be representative of the broader population of older adults in Australia specifically, or developed countries more generally. This is especially the case after exclusion of CN older adults with severe or uncontrolled systemic illness. Related to this, the application of the robust sampling method and rigorous health criteria applied in the present study raises the issue of whether such samples, particularly Aβ− CN older adults, should be considered as representative of super aging or successful aging rather than normal aging [7], [50]. However, in the absence of any clear neuropsychological criteria for super or successful aging, this is not currently able to be determined. Although the representativeness of this sample is limited, the data from the study do show clearly that in the absence of more advanced disease or comorbid illnesses, high Aβ is associated with moderate cognitive decline, but not impairment, in CN older adults.
The cognitive decline observed in the present study may be reflective of the influence of Aβ on cognition, however, may also be indicative of the interaction of Aβ and other factors. There is increasing emphasis on the importance of the role of tau and other indicators of neurodegeneration for predicting clinical disease progression and cognitive decline in AD [3], [11], [51]. The aim of this study was not to investigate these relationships, but rather to provide a foundation for future investigations, by first establishing accurate estimates of the relationship between Aβ and cognitive decline independent of clinical disease progression and comorbid illness. It would be useful for future studies to now consider the potential for tau or neurodegeration markers to modify these estimates of Aβ+-related cognitive decline in robust samples of healthy older adults.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using sources such as PubMed, MEDLINE, and PsycINFO. There is consensus that elevated amyloid β (Aβ+) is associated with cognitive decline in healthy older adults, the nature and magnitude of this varies between studies. This may be because preclinical Alzheimer's disease (AD) samples often include individuals with comorbid medical illness or whose disease progresses during the study period; both factors will increase estimates of cognitive decline. These relevant studies are appropriately cited.
-
2.
Interpretation: Moderate decline in attention, learning, and memory occurred in the robust sample of Aβ+ cognitively normal older adults. This finding confirms that Aβ+ is sufficient to cause cognitive decline in older adults.
-
3.
Future directions: Future studies should investigate Aβ+-related cognitive decline in robust samples of preclinical AD with specific comorbid systemic illnesses, as well as interaction with other AD biomarkers.
Acknowledgments
K.D.H. received a PhD scholarship cofunded by the Alzheimer's Australia Dementia Research Foundation and the Florey Institute of Neuroscience and Mental Health. K.D.H. also received top-up scholarships funded by the Cooperative Research Centre (CRC) for Mental Health and the Yulgilbar Alzheimer's Research Program. The authors acknowledge the financial support of the CRC for Mental Health. The CRC program is an Australian Government Initiative.
The AIBL study received partial financial support provided by the Alzheimer's Association (US), the Alzheimer's Drug Discovery Foundation, the Science and Industry Endowment Fund, the Dementia Collaborative Research Centres, the Victorian Government's Operational Infrastructure Support Program, the McCusker Alzheimer's Research Foundation, the National Health and Medical Research Council, and the Yulgilbar Foundation. Numerous commercial interactions have supported data collection and analysis. In-kind support has also been provided by Sir Charles Gairdner Hospital, CogState Ltd., Hollywood Private Hospital, the University of Melbourne, and St Vincent's Hospital.
The AIBL study would like to thank all of the participants who took part in the study and the clinicians who referred participants. The AIBL study (www.AIBL.csiro.au) is a consortium between Austin Health, CSIRO, Edith Cowan University, the Florey Institute (The University of Melbourne), and the National Ageing Research Institute.
References
- 1.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M., Salmon D.P. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnham S.C., Bourgeat P., Doré V., Savage G., Brown B., Laws S. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer's disease pathophysiology (SNAP) or Alzheimer's disease pathology: a longitudinal study. Lancet Neurol. 2016;15:1044–1053. doi: 10.1016/S1474-4422(16)30125-9. [DOI] [PubMed] [Google Scholar]
- 4.Doraiswamy P.M., Sperling R.A., Johnson K., Reiman E.M., Wong T.Z., Sabbagh M.N. Florbetapir F 18 amyloid PET and 36-month cognitive decline:a prospective multicenter study. Mol Psychiatry. 2014;19:1044–1051. doi: 10.1038/mp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roe C.M., Fagan A.M., Grant E.A., Hassenstab J., Moulder K.L., Maue Dreyfus D. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker J., Lim Y., Pietrzak R., Hassenstab J., Snyder P., Masters C. Cognitive impairment and decline in cognitively normal older adults with high amyloid-β: a meta-analysis. Alzheimers Dement (Amst) 2016;6:108–121. doi: 10.1016/j.dadm.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daffner K.R. Promoting successful cognitive aging: a comprehensive review. J Alzheimers Dis. 2010;19:1101–1122. doi: 10.3233/JAD-2010-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salthouse T.A. What and When of Cognitive Aging. Curr Dir Psychol Sci. 2004;13:140–144. [Google Scholar]
- 9.Vos S.J., Xiong C., Visser P., Jasielec M., Hassenstab J., Grant E. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edmonds E.C., Delano-Wood L., Galasko D.R., Salmon D.P., Bondi M.W. Subtle cognitive decline and biomarker staging in preclinical Alzheimer's disease. J Alzheimers Dis. 2015;47:231–242. doi: 10.3233/JAD-150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack C., Therneau T.M., Wiste H.J., Weigand S.D., Knopman D.S., Lowe V.J. Transition rates between amyloid and neurodegeneration biomarker states and to dementia: a population-based, longitudinal cohort study. Lancet Neurol. 2016;15:56–64. doi: 10.1016/S1474-4422(15)00323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim Y.Y., Ellis K., Harrington K., Kamer A., Pietrzak R., Bush A. Cognitive consequences of high beta-amyloid levels in people with mild cognitive impairment and healthy older adults: Implications for early detection of Alzheimer's disease. Alzheimers Dement. 2013;9:P446. doi: 10.1037/a0032321. [DOI] [PubMed] [Google Scholar]
- 13.Amariglio R.E., Becker J.A., Carmasin J., Wadsworth L.P., Lorius N., Sullivan C. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington K.D., Lim Y.Y., Ellis K.A., Copolov C., Darby D., Weinborn M. The association of Aβ amyloid and composite cognitive measures in healthy older adults and MCI. Int Psychogeriatr. 2013;25:1667–1677. doi: 10.1017/S1041610213001087. [DOI] [PubMed] [Google Scholar]
- 15.Bergman I., Blomberg M., Almkvist O. The importance of impaired physical health and age in normal cognitive aging. Scand J Psychol. 2007;48:115–125. doi: 10.1111/j.1467-9450.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 16.Melis R., Marengoni A., Angleman S., Fratiglioni L. Incidence and Predictors of Multimorbidity in the Elderly: A Population-Based Longitudinal Study. PLoS One. 2014;9:e103120. doi: 10.1371/journal.pone.0103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts R.O., Geda Y.E., Knopman D.S., Cha R.H., Pankratz V.S., Boeve B.F. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim Y.Y., Maruff P., Pietrzak R.H., Ames D., Ellis K.A., Harrington K. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer's disease. Brain. 2014;137:221–231. doi: 10.1093/brain/awt286. [DOI] [PubMed] [Google Scholar]
- 20.Mormino E.C., Betensky R.A., Hedden T., Schultz A.P., Ward A., Huijbers W. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–1767. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storandt M., Morris J.C. Ascertainment bias in the clinical diagnosis of Alzheimer's disease. Arch Neurol. 2010;67:1364–1369. doi: 10.1001/archneurol.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim Y.Y., Snyder P.J., Pietrzak R.H., Ukiqi A., Villemagne V.L., Ames D. Sensitivity of composite scores to amyloid burden in preclinical Alzheimer's disease: Introducing the Z-scores of Attention, Verbal fluency, and Episodic memory for Nondemented older adults composite score. Alzheimers Dement (Amst) 2015;2:19–26. doi: 10.1016/j.dadm.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington K.D., Lim Y.Y., Ames D., Hassenstab J., Rainey-Smith S.R., Robertson J. Using robust normative data to investigate the neuropsychology of cognitive aging. Arch Clin Neuropsychol. 2017;32:142–154. doi: 10.1093/arclin/acw106. [DOI] [PubMed] [Google Scholar]
- 24.Wang L., Zhang Z., McArdle J.J., Salthouse T.A. Investigating ceiling effects in longitudinal data analysis. Multivariate Behav Res. 2009;43:476–496. doi: 10.1080/00273170802285941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sliwinski M., Lipton R.B., Buschke H., Stewart W. The effects of preclinical dementia on estimates of normal cognitive functioning in aging. J Gerontol B Psychol Sci Soc Sci. 1996;51:P217–P225. doi: 10.1093/geronb/51b.4.p217. [DOI] [PubMed] [Google Scholar]
- 26.Clark L.R., Koscik R.L., Nicholas C.R., Okonkwo O.C., Engelman C.D., Bratzke L.C. Mild cognitive impairment in late middle age in the wisconsin registry for Alzheimer's prevention study: prevalence and characteristics using robust and standard neuropsychological normative data. Arch Clin Neuropsychol. 2016;31:675–688. doi: 10.1093/arclin/acw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grober E., Mowrey W., Katz M., Derby C., Lipton R.B. Conventional and robust norming in identifying preclinical dementia. J Clin Exp Neuropsychol. 2015;37:1098. doi: 10.1080/13803395.2015.1078779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis K.A., Bush A.I., Darby D., De Fazio D., Foster J., Hudson P. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr. 2009;21:672–687. doi: 10.1017/S1041610209009405. [DOI] [PubMed] [Google Scholar]
- 29.National Health and Medical Research Council . National Health and Medical Research Council; Canberra: 2001. Australian Alcohol Guidelines: Health Risks and Benefits. p. 2001. [Google Scholar]
- 30.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 31.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 32.Rowe C.C., Ellis K.A., Rimajova M., Bourgeat P., Pike K.E., Jones G. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Villemagne V.L., Dore V., Yates P., Brown B., Mulligan R.S., Bourgeat P. En attendant centiloid. Adv Res. 2014;2:723–729. [Google Scholar]
- 34.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 35.Wechsler D. Psychological Corporation; San Antonio, TX: 2001. Wechsler Test of Adult Reading: Examiner's Manual. [Google Scholar]
- 36.Wechsler D. 3rd ed. Psychological Corporation; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale (WAIS-III) [Google Scholar]
- 37.Strauss E., Sherman E.M.S., Spreen O. 3rd ed. Oxford University Press; New York: 2006. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. [Google Scholar]
- 38.Lim Y.Y., Ellis K.A., Harrington K., Ames D., Martins R.N., Masters C.L. Use of the CogState Brief Battery in the assessment of Alzheimer's disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Clin Exp Neuropsychol. 2012;34:345–358. doi: 10.1080/13803395.2011.643227. [DOI] [PubMed] [Google Scholar]
- 39.Delis D., Kaplan E., Kramer J.H. Psychological Corporation; San Antonio TX: 2001. The Delis-Kaplan Executive Function System (D-KEFS) [Google Scholar]
- 40.Wechsler D. Psychological Corporation; New York: 1987. Wechsler Memory Scale-Revised. [Google Scholar]
- 41.Delis D., Kramer J., Kaplan E., Ober B. 2nd ed. Psychological Corporation; San Antonio, TX: 2000. California Verbal Learning Test. [Google Scholar]
- 42.Meyers J.E., Meyers K.R. Psychological Assessment Resource, Inc.; Odessa, FL: 1995. Rey Complex Figure Test and Recognition Trial: Professional Manual. [Google Scholar]
- 43.Cohen J. Elsevier Science; Saint Louis: 2013. Statistical Power Analysis for the Behavioral Sciences. [electronic resource] p. 2013. [Google Scholar]
- 44.Lim Y.Y., Pietrzak R.H., Ellis K.A., Jaeger J., Harrington K., Ashwood T. Rapid decline in episodic memory in healthy older adults with high amyloid-β. J Alzheimers Dis. 2013;33:675–679. doi: 10.3233/JAD-2012-121516. [DOI] [PubMed] [Google Scholar]
- 45.Papp K.V., Mormino E.C., Amariglio R.E., Munro C., Dagley A., Schultz A.P. Biomarker validation of a decline in semantic processing in preclinical Alzheimer's disease. Neuropsychology. 2016;30:624–630. doi: 10.1037/neu0000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Storandt M., Mintun M.A., Head D., Morris J.C. Cognitive decline and brain volume loss are signatures of cerebral Aβ deposition identified with PIB. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 48.Hedden T., Oh H., Younger A.P., Patel T.A. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 50.Harada C.N., Natelson Love M.C., Triebel K. Normal cognitive aging. Clin Geriatr Med. 2013;29:737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Feldman H.H., Frisoni G.B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]