Abstract
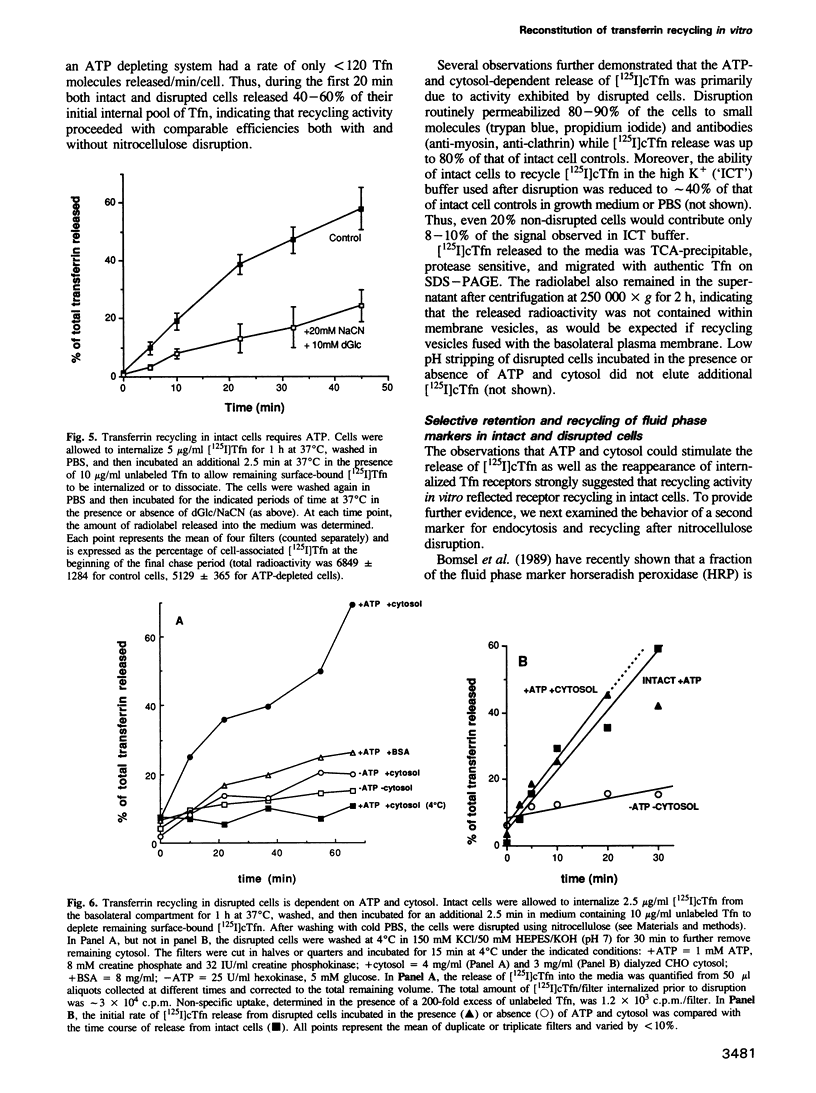
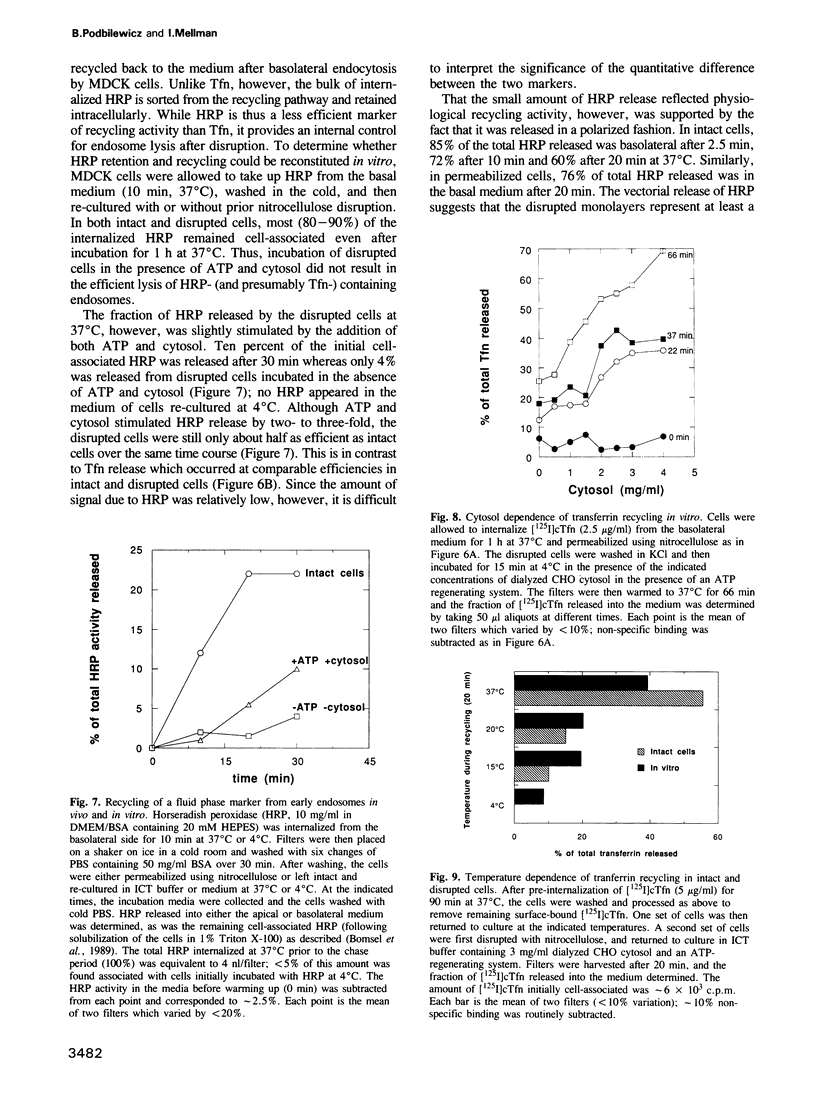
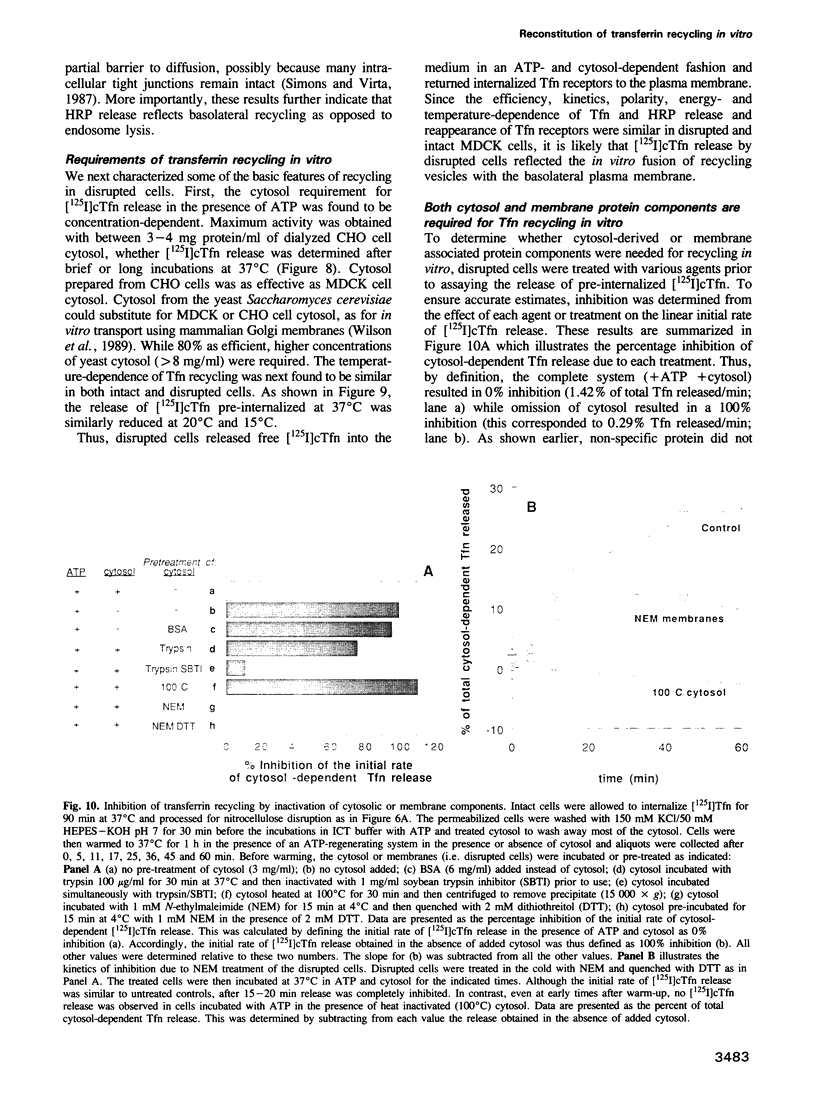
We have developed an in vitro system for studying membrane transport during receptor-mediated endocytosis. Using nitrocellulose disruption to permeabilize selectively the apical domain of filter-grown MDCK cells, the recycling of receptor-bound transferrin (Tfn) from an intracellular pool was reconstituted in vitro with a rate and efficiency similar to that of intact cells. Tfn and Tfn receptor recycling from endosomes back to the cell surface was dependent on added ATP and cytosol-derived proteins. Thus, incubation of intact cells under conditions of ATP depletion resulted in the clearance of Tfn receptors from the basolateral membrane, this was reversible upon removal of the energy poisons. Reappearance of previously internalized receptors could also be obtained in disrupted cells but required the addition of both ATP and cytosol to the assay mixture. Similarly, when intact cells were allowed to internalize labeled Tfn prior to disruption, efficient and rapid release of ligand back into the medium was markedly stimulated by ATP and cytosol. Recycling was judged to be both selective and vectorial since only the expected small fraction of a previously internalized horseradish peroxidase was released after addition of ATP and cytosol, and release was primarily into the basal medium. While the cytosol contributed one or more protein factors, none was sensitive to N-ethylmaleimide. Alkylation of the disrupted cells, however, did inactivate recycling.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajioka R. S., Kaplan J. Intracellular pools of transferrin receptors result from constitutive internalization of unoccupied receptors. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6445–6449. doi: 10.1073/pnas.83.17.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon R. A., Salminen A., Ruohola H., Novick P., Ferro-Novick S. The GTP-binding protein Ypt1 is required for transport in vitro: the Golgi apparatus is defective in ypt1 mutants. J Cell Biol. 1989 Sep;109(3):1015–1022. doi: 10.1083/jcb.109.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984 Dec;39(2 Pt 1):405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Beckers C. J., Balch W. E. Calcium and GTP: essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989 Apr;108(4):1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Wandinger-Ness A., Simons K. Release of putative exocytic transport vesicles from perforated MDCK cells. EMBO J. 1988 Dec 20;7(13):4075–4085. doi: 10.1002/j.1460-2075.1988.tb03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. R., Glick B. S., Wilcox C. A., Wieland F. T., Rothman J. E. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M., Prydz K., Parton R. G., Gruenberg J., Simons K. Endocytosis in filter-grown Madin-Darby canine kidney cells. J Cell Biol. 1989 Dec;109(6 Pt 2):3243–3258. doi: 10.1083/jcb.109.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braell W. A. Fusion between endocytic vesicles in a cell-free system. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1137–1141. doi: 10.1073/pnas.84.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Gerace L. A cell free system to study reassembly of the nuclear envelope at the end of mitosis. Cell. 1986 Feb 28;44(4):639–652. doi: 10.1016/0092-8674(86)90273-4. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Dautry-Varsat A., Lodish H. F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983 Aug 25;258(16):9681–9689. [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J., Hurtley S. M., Warren G. Reconstitution of an endocytic fusion event in a cell-free system. Cell. 1985 Dec;43(3 Pt 2):643–652. doi: 10.1016/0092-8674(85)90236-3. [DOI] [PubMed] [Google Scholar]
- Davis R. J., Faucher M., Racaniello L. K., Carruthers A., Czech M. P. Insulin-like growth factor I and epidermal growth factor regulate the expression of transferrin receptors at the cell surface by distinct mechanisms. J Biol Chem. 1987 Sep 25;262(27):13126–13134. [PubMed] [Google Scholar]
- Diaz R., Mayorga L. S., Weidman P. J., Rothman J. E., Stahl P. D. Vesicle fusion following receptor-mediated endocytosis requires a protein active in Golgi transport. Nature. 1989 Jun 1;339(6223):398–400. doi: 10.1038/339398a0. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Schmid S., Mellman I. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc Natl Acad Sci U S A. 1989 Jan;86(2):539–543. doi: 10.1073/pnas.86.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
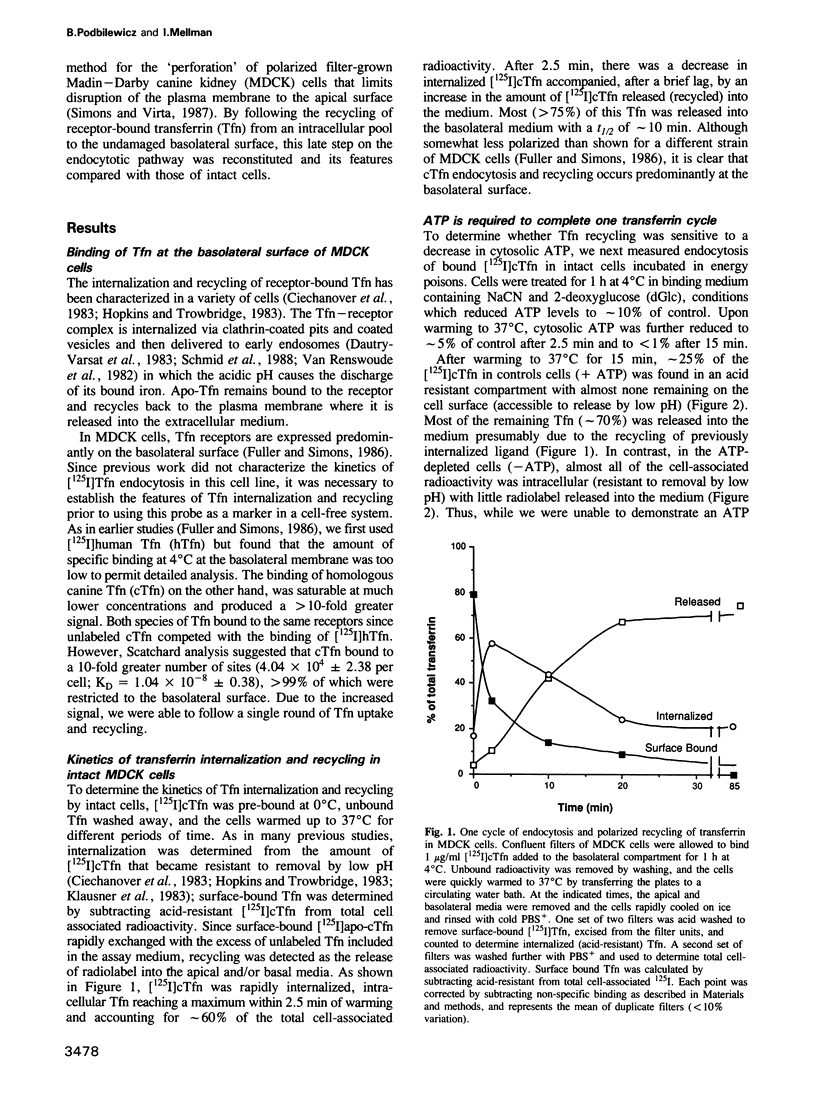
- Fuller S. D., Simons K. Transferrin receptor polarity and recycling accuracy in "tight" and "leaky" strains of Madin-Darby canine kidney cells. J Cell Biol. 1986 Nov;103(5):1767–1779. doi: 10.1083/jcb.103.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y., Pfeffer S. R. Selective recycling of the mannose 6-phosphate/IGF-II receptor to the trans Golgi network in vitro. Cell. 1988 Oct 21;55(2):309–320. doi: 10.1016/0092-8674(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. E., Howell K. E. Reconstitution of vesicle fusions occurring in endocytosis with a cell-free system. EMBO J. 1986 Dec 1;5(12):3091–3101. doi: 10.1002/j.1460-2075.1986.tb04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson E. J., Hopkins C. R. Endocytic pathways in polarized Caco-2 cells: identification of an endosomal compartment accessible from both apical and basolateral surfaces. J Cell Biol. 1990 Feb;110(2):337–348. doi: 10.1083/jcb.110.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
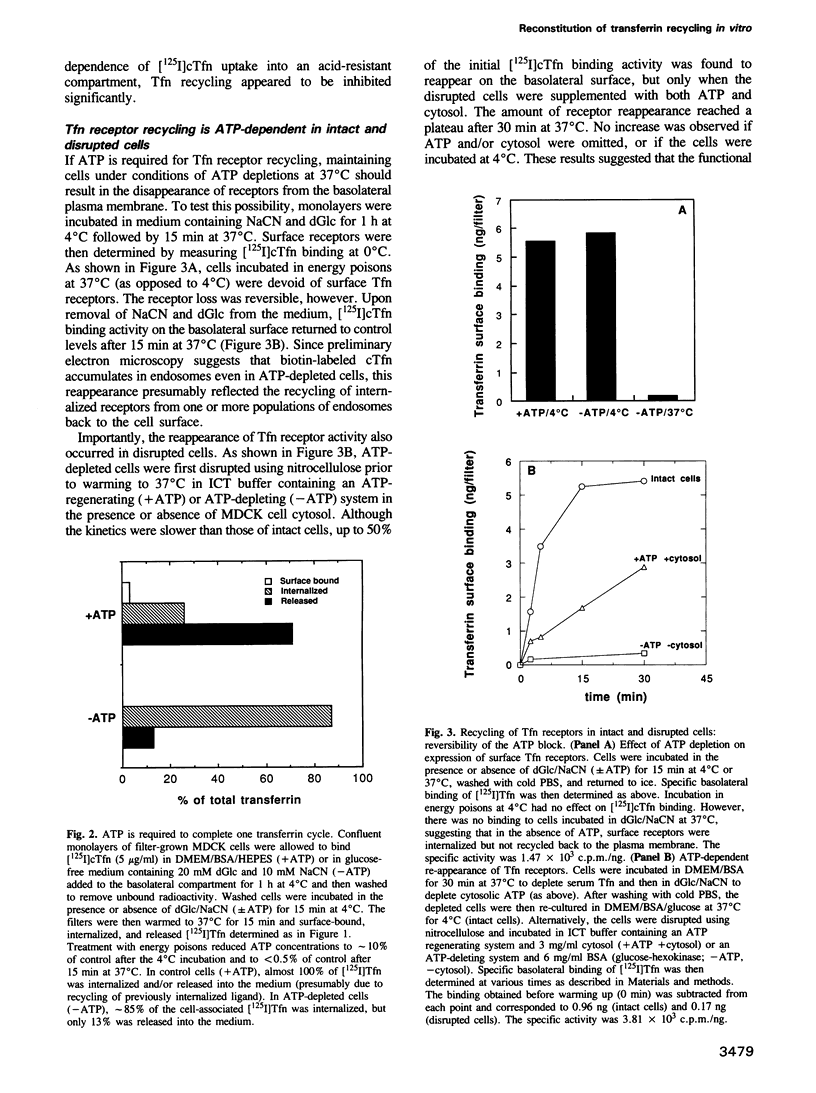
- Mahaffey D. T., Moore M. S., Brodsky F. M., Anderson R. G. Coat proteins isolated from clathrin coated vesicles can assemble into coated pits. J Cell Biol. 1989 May;108(5):1615–1624. doi: 10.1083/jcb.108.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Stahl P. D. Regulatory role for GTP-binding proteins in endocytosis. Science. 1989 Jun 23;244(4911):1475–1477. doi: 10.1126/science.2499930. [DOI] [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Mellman I. S., Plutner H., Steinman R. M., Unkeless J. C., Cohn Z. A. Internalization and degradation of macrophage Fc receptors during receptor-mediated phagocytosis. J Cell Biol. 1983 Mar;96(3):887–895. doi: 10.1083/jcb.96.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Mahaffey D. T., Brodsky F. M., Anderson R. G. Assembly of clathrin-coated pits onto purified plasma membranes. Science. 1987 May 1;236(4801):558–563. doi: 10.1126/science.2883727. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Branch W. J., van Schaik M., Gilbert L. K., Luzio J. P. Reconstitution of an endosome-lysosome interaction in a cell-free system. J Cell Biol. 1989 Jun;108(6):2093–2099. doi: 10.1083/jcb.108.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G., Prydz K., Bomsel M., Simons K., Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby canine kidney cell in late endosomes. J Cell Biol. 1989 Dec;109(6 Pt 2):3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Schmid S. L. Enzymatic recycling of clathrin from coated vesicles. Cell. 1986 Jul 4;46(1):5–9. doi: 10.1016/0092-8674(86)90852-4. [DOI] [PubMed] [Google Scholar]
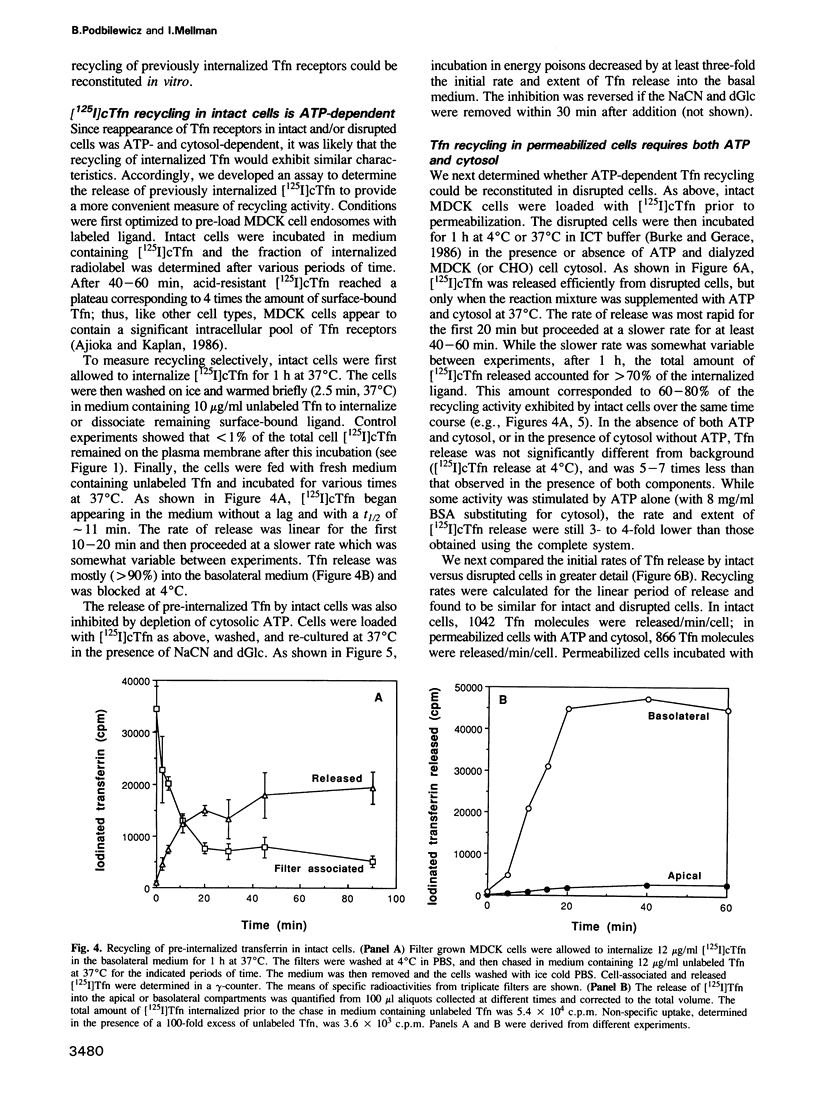
- Ruohola H., Kabcenell A. K., Ferro-Novick S. Reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex in yeast: the acceptor Golgi compartment is defective in the sec23 mutant. J Cell Biol. 1988 Oct;107(4):1465–1476. doi: 10.1083/jcb.107.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Fuchs R., Male P., Mellman I. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988 Jan 15;52(1):73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Simons K., Virta H. Perforated MDCK cells support intracellular transport. EMBO J. 1987 Aug;6(8):2241–2247. doi: 10.1002/j.1460-2075.1987.tb02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe E., Pypaert M., Lucocq J., Warren G. Formation of coated vesicles from coated pits in broken A431 cells. J Cell Biol. 1989 Mar;108(3):843–853. doi: 10.1083/jcb.108.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Davoust J., Cockcroft A. Recycling of transferrin receptors in A431 cells is inhibited during mitosis. EMBO J. 1984 Oct;3(10):2217–2225. doi: 10.1002/j.1460-2075.1984.tb02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Englund P. T. Inhibition of DNA replication in Escherichia coli by cyanide and carbon monoxide. J Biol Chem. 1975 Nov 10;250(21):8536–8542. [PubMed] [Google Scholar]
- Wilson D. W., Wilcox C. A., Flynn G. C., Chen E., Kuang W. J., Henzel W. J., Block M. R., Ullrich A., Rothman J. E. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989 Jun 1;339(6223):355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]