Abstract
Objective
To assess in-vitro effects of monocyte-derived macrophage (MDM) polarization into M1 and M2a cells on HIV-1 replication and transmission and obtain new insights into the potential importance of macrophage polarization in vivo.
Design
Human peripheral blood monocytes were differentiated into MDM for 7 days. Control and MDM polarized into M1 or M2a cells were exposed to different strains of HIV-1 and assessed for their ability to bind and transmit virus to CD4+ T lymphocytes.
Methods
MDM were incubated with either tumour necrosis factor-alpha (TNF-α) along with interferon-gamma (IFN-γ) or with interleukin-4 (IL-4) for 18 h to obtain M1 or M2a cells, respectively. Expression of cell surface antigens, including CD4 and dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN), was evaluated by flow cytometry. C-C chemokine receptor type 5 (CCR5)-dependent (R5) HIV-1 binding, DNA synthesis and viral replication were assessed in the presence or absence of anti-DC-SIGN blocking mAbs. Transmission of C-X-C chemokine receptor type 4 (CXCR4)-dependent (X4) and R5 HIV-1 from MDM to IL-2 activated CD4+ T cells was also investigated.
Results
DC-SIGN was strongly upregulated on M2a-MDM and downregulated on M1-MDM compared with control MDM. DC-SIGN facilitated HIV-1 entry and DNA synthesis in M2a-MDM, compensating for their low levels of CD4 cell expression. M2a-MDM efficiently transmitted both R5 and X4 HIV-1 to CD4+ T cells in a DC-SIGN-dependent manner.
Conclusion
DC-SIGN facilitates HIV-1 infection of M2a-MDM, and HIV-1 transfer from M2a-MDM to CD4+ T cells. M2a-polarized tissue macrophages may play an important role in the capture and spread of HIV-1 in mucosal tissues and placenta.
Keywords: dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin, HIV transmission, HIV-1, macrophage polarization, T cells
Introduction
Mononuclear phagocytes, including monocytes, tissue macrophages and myeloid dendritic cells (mDCs), play a pivotal role in the establishment of HIV-1 infection in mucosal tissues [1–6]. mDCs capture HIV-1 at sites of mucosal entry and migrate to lymph nodes, where they transmit virions to CD4+ T cells [4,7]. C-type lectins, such as dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN), play an important role in this process [8–10]. In contrast, macrophages are nonmigratory residential cells that play a local role in HIV-1 propagation. Unlike mDCs, macrophages are susceptible to productive HIV-1 infection by CCR5-dependent (R5) viruses [11] and, upon infection, accumulate newly synthesized virus in subcellular compartments. These virions can be transferred to susceptible CD4+ T cells following formation of virological synapses [6,12,13].
Macrophages are characterized by extensive diversity and plasticity, and can be transiently polarized into functionally distinct subsets including M1 (via toll-like receptor ligands or IFN-γ) and M2a (via IL-4 or IL-13) cells. M1 macrophages produce proinflammatory cytokines, promote Th1 responses and display microbicidal and tumouricidal activity. M2a produce IL-10, express scavenger and mannose receptors, promote Th2 responses and tissue repair, and have immunoregulatory functions [14–16]. Functional polarization of macrophages plays an important role in the maintenance of tissue microenvironments and resolution of inflammation [16]. Under pathological conditions, M1 polarization has been associated with viral infections, steatosis, arthritis, atherosclerosis, diabetes and antitumour effects, whereas M2a polarization has been linked to asthma, helminth infections and cancer progression [15,16]. Therapeutic strategies exploiting functional macrophage polarization into M1 or M2a cells are currently being explored [17].
We have previously shown that HIV-1 replication is inhibited in M1 and M2a macrophages compared with control MDM [18]. In the current study, we report that M2a-MDM, in contrast to M1-MDM, express high levels of DC-SIGN. Furthermore, we show that upregulation of DC-SIGN is associated with an increased ability of M2a cells to bind and be infected by HIV-1, and transmit virus to autologous CD4+ T cells despite of low CD4 cell expression.
Materials and methods
Reagents
Human recombinant tumour necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ) and interleukin-4 (IL-4) were from R&D Systems (Minneapolis, Minnesota, USA); IL-2 from Boehringer (Mannheim, Germany); D-MEM, PBS, foetal bovine serum (FBS), normal human serum (NHS), penicillin, streptomycin and glutamine from Cambrex (Verviers, Belgium). mAbs were from the following sources: anti-CD18 FITC clone MEM-48, anti-HLA-DR PE clone MEM-12, anti-CD14 PE clone MEM-18 and anti-CD4 FITC clone MEM-115 from Immunotools (Friesoythe, Germany); CD209 anti-DC-SIGN PE clone 120507 and anti-CD163 PE clone 215927 from R&D Systems; anti-CD16 PE-Cy5.5 clone 3G8 and anti-CD206 PE clone 3.29B1.10 from Beckman Coulter (Miami, Florida, USA); anti-CD80 FITC clone 2D10.4 and anti-CD86 PE clone IT2.2 from eBioscience (San Diego, Caifornia, USA); blocking Leu3a anti-CD4 mAb from BD Biosciences, (San Jose, California, USA). RETROtek P24 ELISA was from ZeptoMetrix (Buffalo, New York, USA).
Isolation and differentiation of monocytes into monocyte-derived macrophage
Monocytes were differentiated as previously reported [18]. Peripheral blood mononuclear cells (PBMCs) were isolated from HIV-1 seronegative donors by Ficoll-Hypaque density gradient centrifugation. Monocytes were separated by adherence to plastic at 37°C and differentiated in a complete medium (D-MEM along with 10% FBS and 5% NHS) for 7 days at 37°C in 5% CO2 (≥95% CD14+ cells). MDMs were stimulated with TNF-α along with IFN-γ (2 and 20 ng/ml, respectively) or IL-4 (20 ng/ml) for 18 h to obtain M1 or M2a polarized cells, respectively. Following polarization, M1, M2a, and control MDM were washed and incubated in cytokine-free complete medium as described [18].
Phenotypic analysis
Polarized and control MDMs were detached by incubation with cold EDTA/PBS for 30 min at 4°C and scraping with a rubber policeman. Following detachment, MDMs were preincubated for 15 min at 4°C in a complete medium (10% FCS and 5% NHS) to prevent nonspecific binding, incubated for 30 min at 4°C with specific mAb, washed and fixed with 2% paraformaldehyde (PFA) for flow cytometric analysis. MDMs were identified on the basis of forward and side scatter characteristics followed by one-colour or two-colour immunofluorescence analysis using a CYAN ADP flow cytometer (DakoCytomation). Immunophenotyping of control, M1 and M2a cells was performed 18 h after polarization and at days 3 and 7 post-polarization. Results were analysed using FlowJo version 8.4.6 (Tree Star Inc., Ashland, Oregon, USA) and reported as the percentage of positive cells.
HIV-1 binding to monocyte-derived macrophage
After polarization, MDMs were washed and incubated for 2 h at 4°C with an R5 reference strain HIV-1BaL at a multiplicity of infection of 1 (moi = 1) or with replication-competent recombinant NL4-3 viruses expressing macrophage-tropic R5 primary HIV-1 envelopes (Env) from ADA and YU2 clones or brain-derived UK7BR1 and UK1BR15 clones using 100 μl of virus stock corresponding to 10 000 3H cpm of reverse transcriptase activity (equivalent to ~10 ng HIV p24) per 2.5 × 105 cells [19,20]. The cells were then washed to remove unbound virus and lysed in 50 μl of lysis buffer (ZeptoMatrix); HIV-1 p24 Gag was quantified using the RETROtek P24 antigen ELISA. To measure the relative amount of virus bound to CD4 and DC-SIGN, MDMs (0.25 × 106 cells/well in quadruplicate) were preincubated in complete media to prevent nonspecific antibody binding and then with either Leu3a (20 μg/ml) and/or anti-CD209 (20 μg/ml) [21] for 20 min at room temperature prior to HIV-1 exposure.
Quantification of HIV-1 DNA
Control, M1-MDM and M2a-MDM were polarized for 18 h, washed and infected with HIV-1BaL (moi = 0.1) that was previously treated with RNAse-free DNAse. An aliquot of cells were immediately washed five times and nucleic acid carry over with the viral stock was verified and excluded by PCR. After 48 h incubation in complete medium [22], MDMs (1 ×106 cells) were harvested, washed, resuspended in lysis buffer containing polyoxyethylene (0.1%), lauryl ether 10 mol/l (Sigma) along with proteinase K (0.1 mg/ml) and digested for 2 h at 65°C; proteinase K was heat-inactivated for 15 min at 95°C [23]. Quantitative real-time PCR amplification of HIV-1 gag DNA was performed using a primer/probe combination that detects all viral DNA synthesized after second-strand transfer including both unintegrated and integrated DNA species [18]. To determine the relative contribution of CD4 and DC-SIGN to virus entry, MDMs were preincubated with 15% serum (10% FCS, 5% NHS) for 15 min at 4°C and subsequently with Leu3a or CD209 mAb (20 μg/ml each) for 20 min at room temperature.
Transmission of CXCR4 or CCR5-dependent HIV-1 to activated T cells
To evaluate the effects of functional polarization on the ability of MDM to transmit HIV-1 to autologous CD4+ T cells, MDMs were incubated for 2 h at 37°C with either HIV-1LAI/IIB, an X4 viral strain that does not replicate efficiently in MDM [24,25], or with replication-competent recombinant NL4-3 viruses expressing R5 primary Envs that efficiently replicate in MDM. After washing, MDMs were cocultured with 7-day-old autologous monocyte-depleted PBMC (predominantly T cells) at a 1 : 1 ratio. After 6 h coculture in medium enriched in IL-2 (20 U/ml), PBMCs were removed, seeded in RPMI 1640 containing 10% FBS and IL-2, and maintained in culture for 12 additional days. Supernatants were collected every 3 days and stored at −20°C for analysis of Mg2+-dependent reverse transcriptase activity or HIV-1 p24 Gag levels [18].
Statistical analysis
Statistical analyses were performed using Prism 5 from GraphPad Software (La Jolla, California, USA). Results are reported as means + SD. Multivariate analyses comparing control, M1-MDM and M2a-MDM were conducted using one-way analysis of variance (ANOVA) and Tukey post-test or paired t-test. P values less than 0.05 (P < 0.05) were considered significant. To control for interdonor variability, all assays were performed in triplicate using MDM derived from four to eight independent donors.
Results
Differential expression of activation, differentiation and T-cell costimulatory markers on the surface of M1-MDM, M2a-MDM and control monocyte-derived macrophage
We previously reported that M1 and M2a polarization has no effect on CCR5 expression and leads to downregulation of CD4 and CXCR4 [18]. Here, we investigated the effects of polarization on expression of markers of macrophage differentiation (CD14, CD16, CD18, CD163), cellular activation (HLA-DR), T-cell costimulation (B7.1/CD80, B7.2/CD86) and HIV-1 transmission to CD4+ T cells [DC-SIGN (CD209), mannose receptor (CD206)]. As previously reported [18], M1, and to a lesser extent M2a, polarization resulted in a significant reduction in the percentage of CD4-positive cells (Fig. 1a). In contrast, the percentage of CD16 (FcγRIII) and CD163-positive cells was significantly lower in M1-MDM than in control and M2a-MDM (Table 1). CD14, CD18, CD80, CD86 and HLA-DR were not significantly affected by MDM polarization.
Fig. 1. Regulation of dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin and CD4 expression on the surface of human M1- and M2a-MDM.
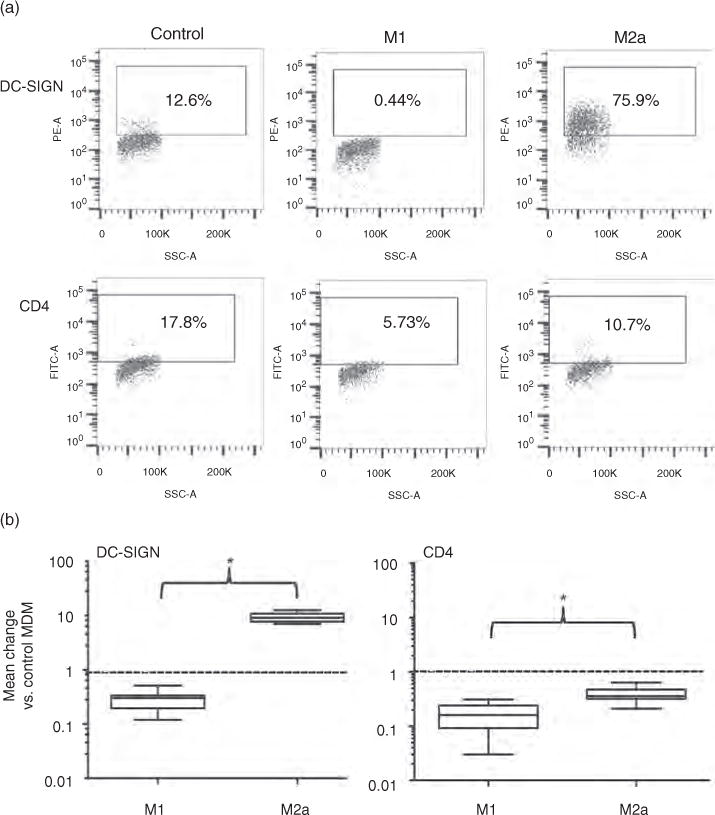
(a) MDM populations of a single donor were labelled with anti-DC-SIGN or anti-CD4 mAbs 18 h after polarizing or control (unpolarized) conditions and were analysed by flow cytometry. (b) These results were confirmed in six independent MDM donors; downregulation of CD4 from the cell surface of both M1 and M2a-MDM is shown in the right panel. DC-SIGN and CD4 expression levels in polarized cells were normalized to those of autologous control MDM indicated as 1 (dashed line). DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin; MDM, monocyte-derived macrophage.
Table 1.
Percentage of positive cells expressed as a receptor expression ratio by M1-MDM and M2a-MDM vs. control MDM.
| Marker | No.° | Expression by control MDM (Range) | M1 vs. control ratio (Mean ± SD) | M2a vs. control ratio (Mean ± SD) | M1 vs. control | M2a vs. control | M1 vs. M2a |
|---|---|---|---|---|---|---|---|
| CD4 | 6 | 33–59 | 0.14 ± 0.08 | 0.32 ± 0.08 | ** | ** | * |
| CD14 | 4 | 73–97 | 1.00 ± 0.24 | 0.89 ± 0.29 | |||
| CD16 | 4 | 36–59 | 0.09 ± 0.11 | 0.92 ± 0.13 | ** | * | |
| CD18 | 4 | 91–95 | 0.87 ± 0.39 | 1.14 ± 0.05 | |||
| CD80 | 4 | 25–36 | 1.06 ± 0.06 | 1.13 ± 0.12 | |||
| CD86 | 4 | 60–76 | 0.99 ±0.03 | 1.10 ± 0.12 | |||
| CD163 | 4 | 59–72 | 0.57 ±0.10 | 0.98 ± 0.02 | * | * | |
| CD206 | 4 | 50–57 | 0.57 ± 0.10 | 0.98 ± 0.02 | * | * | |
| HLA-DR | 4 | 83–97 | 1.07 ± 0.06 | 1.00 ± 0.72 | |||
| DC-SIGN | 6 | 5–17 | 0.34 ± 0.11 | 9.08 ± 2.93 | * | ** | ** |
The percentage of positive cells was determined by flow cytometry after 18 h of culture in polarizing (M1 and M2a) or nonpolarizing conditions (control). ANOVA, analysis of variance; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin; MDM, monocyte-derived macrophage.
P ≤ 0.05,
P ≤ 0.001 as assessed by one-way ANOVA and Tukey post-test. No. identifies the number of independent monocyte donors.
The expression of DC-SIGN and CD206, molecules implicated in cell-mediated transmission of HIV-1 from monocytes, MDM and mDC to T cells [8,9,26,27], was differentially affected by macrophage polarization. CD206, similar to CD16 and CD163, was expressed on the majority of control and M2a MDM and minimally expressed on M1-MDM. In contrast, DC-SIGN was expressed on 5–17% of control MDMs; it was almost undetectable on the surface of M1-MDM and was expressed by most M2a-MDM (nine-fold increase in the percentage of cells expressing DC-SIGN above background, Table 1, Fig. 1a and b). Thus, among cell surface molecules investigated in this study, DC-SIGN (and not CD206) was the best marker for discriminating among control, M1-MDM and M2a-MDM.
Anti-dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin mAb reduces HIV-1 binding to M2a-MDM, but not to M1-MDM or control monocyte-derived macrophage
DC-SIGN, when expressed on dendritic cell, serves as a high-affinity attachment molecule for various pathogens including HIV-1 [9,27]. In order to investigate whether MDM can also bind HIV-1 via DC-SIGN, we incubated MDM with HIV-1BaL (moi = 1) at 4°C to prevent virus entry; we then measured the amount of HIV-1 p24 Gag antigen attached to the cell surface. Both control and polarized MDM bound HIV-1BaL (Fig. 2a), with control and M2a-MDM exhibiting higher mean levels of binding than M1-MDM (35. ± 3.7 vs. 34.9 ± 5.8 vs. 29.6 ± 3.9 pg/ml of HIV-1 p24 Gag, respectively, P < 0.05; n = 8). Incubation of these MDM populations with Leu3a, an anti-CD4 mAb that interferes with HIV-1 binding CD4, led to a mean 47% decrease (interdonor range: 36–67%) of virus binding to control MDM, but had no effect on HIV-1 binding to either M1-MDM and M2a-MDM, presumably due to the low-level expression of CD4 on both polarized MDM (Fig. 2b, as reported) [15]. In contrast, preincubation with anti-DC-SIGN mAb led to a mean 52.5% (interdonor range: 29–74%) reduction in the amount of HIV-1 bound to M2a-MDM, but had no effect on virus binding to both control and M1-MDM (Fig. 2b). Preincubation with a combination of anti-DC-SIGN and anti-CD4 mAbs failed to induce any further decrease in HIV-1 binding to control, M1-MDM or M2a-MDM, when compared with MDM subsets preincubated with only anti-CD4 or anti-DC-SIGN mAbs (Fig. 2b). This finding is consistent with the paucity of DC-SIGN on control and M1-MDM and of CD4 on M2a-MDM (Fig. 1a) [28].
Fig. 2. Differential binding of R5 HIV-1 strains to control (unpolarized) and polarized monocyte-derived macrophage.
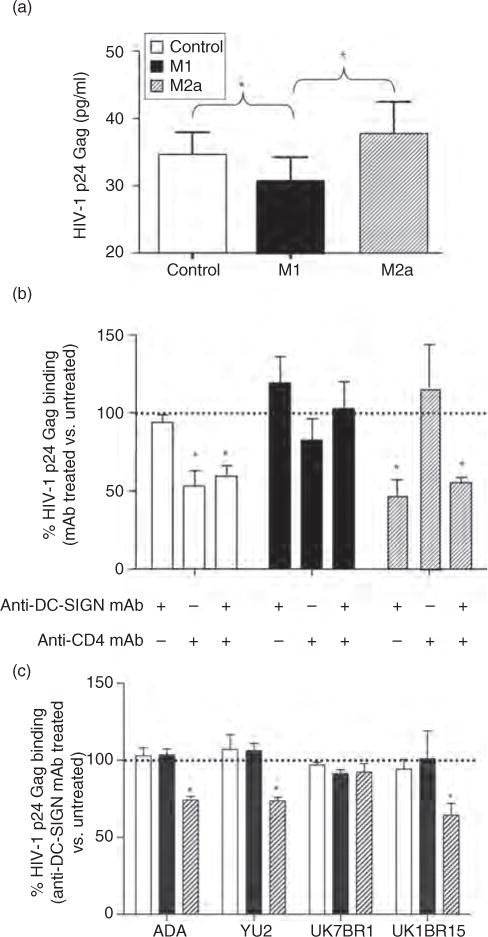
(a) HIV-1 binding to control (CD4highDC-SIGNlow), M1-MDM (CD4lowDC-SIGNneg) and M2a-MDM (CD4lowDC-SIGNhigh) was measured 18 h after polarizing or control conditions as the amount of HIV-1 p24 Gag antigen bound to the cell surface after removal of excess viral inoculums and extensive washing. The results are presented as the average cell surface bound p24 Gag antigen detected in MDM cultures established from eight individual donors. Significantly higher levels of binding of HIV-1 were observed in control and M2a-MDM vs. M1 cells. Differences were assessed using one-way ANOVA and Tukey post-tests. *P ≤ 0.05. (b) Anti-DC-SIGN mAb reduce HIV-1BaL binding to M2a, but not to control or M1-MDM, independently of CD4. Values represent the percentage positivity for p24 Gag antigen vs. control and polarized populations that were not incubated with the indicated mAb (dotted line). The results show the mean + SD of three replicates per each MDM culture established from eight independent donors. The statistical difference in p24 Gag binding between cultures incubated in the presence or absence of the indicated mAb was evaluated by a paired t-test. *P ≤ 0.05. (c) Anti-DC-SIGN mAb reduced binding of NL4-3 viruses expressing macrophage-tropic envelopes from HIV-1ADA, HIV-1YU2 and HIV-1UK1BR15, but not HIV-1UK7BR1, in M2a. As discussed above, values represent the percentage of bound p24 Gag on MDM preincubated with anti-DC-SIGN mAb compared with untreated cells. Shown is the mean + SD of experiments performed in triplicate from two independent donors. The statistical difference in p24 Gag binding between cultures incubated in the presence or absence of the indicated mAb was evaluated by a paired t-test. ANOVA, analysis of variance; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin; MDM, monocyte-derived macrophage. *P ≤ 0.05.
To determine whether DC-SIGN expressed on M2a-MDM could bind R5 viruses other than HIV-1BaL, we incubated control, M1-MDM and M2a-MDM with a panel of NL4-3 viruses expressing macrophage-tropic R5 primary Envs (ADA, YU2, UK7BR1 and UK1BR15) [19,20]. Control, M1-MDM and M2a-MDM bound equal levels of these viruses. Preincubation with anti-DC-SIGN mAb led to a mean 29%, 35% and 45% reduction in HIV-1 binding to M2a-MDM by ADA, YU2 and UK1BR15 envelopes, respectively, whereas binding of UK7BR1 was not affected by pretreatment with anti-DC-SIGN mAb (Fig. 2c).
Dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin facilitates accumulation of HIV-1 DNA in M2a-MDM, but not in M1-MDM or control MDM
As DC-SIGN has been shown to enhance cis- and trans-mediated infection of dendritic cell and cell lines [27,28], we investigated whether DC-SIGN could also facilitate entry of HIV-1 into M2a cells. Polarized and control MDM were infected with HIV-1BaL and levels of HIV-1 DNA synthesized were quantified by real-time PCR after 48 h of infection, an estimated time required to complete a single round of virus replication in these cells [22].
MDM polarization inhibited HIV-1 replication in polarized vs. control cells (Fig. 3a), as previously reported [18]. Blockade of DC-SIGN in control MDM led to an average 40% increase in HIV-1 DNA (Fig. 3b). As expected, anti-DC-SIGN mAb did not affect HIV-1 DNA synthesis in M1-MDMs, which express only negligible amounts of DC-SIGN and CD4. As reported [18], HIV-1 DNA levels in M2a-MDM were equivalent to those of control MDM, indicating that there was no impairment of viral entry or DNA synthesis in spite of lower levels of CD4 expression than those of control MDM. Incubation of M2a-MDM with anti-DC-SIGN mAb prior to infection resulted in a mean 24% decrease in HIV-1 DNA (Fig. 3b), whereas pretreatment with anti-CD4 mAb completely blocked infection. These findings are consistent with previous studies demonstrating that DC-SIGN alone cannot mediate HIV-1 infection but rather facilitates entry via a CD4-dependent mechanism [27].
Fig. 3. Effect of dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin expression on viral DNA synthesis in polarized and control MDM.
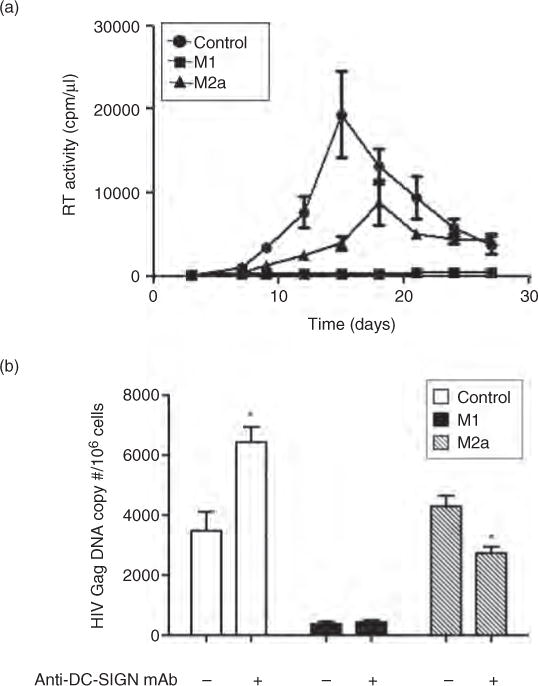
(a) HIV-1 replication kinetics in control, M1-MDM and M2a-MDM quantified by measuring RT activity of culture supernatants over a 28-day infection period. Results are representative of the average replication (±SD) in MDM cultures established from a representative donor. (b) Dichotomous effect of anti-DC-SIGN mAb on HIV-1 DNA accumulation in control vs. M1-MDM and M2a-MDM. Following MDM activation in 18 h of polarizing or control conditions, cells were washed and preincubated with anti-DC-SIGN mAb for 20 min at room temperature before infection with R5 HIV-1BaL. The results shown (48 h post-infection) were obtained from MDM cultures established from a single donor out of eight independent donors tested in duplicate. The statistical difference between cultures incubated in the presence or absence of the indicated mAb was evaluated by a paired t-test. DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin. *P ≤ 0.05.
Dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin expression on M2a-MDM correlates with rapid and efficient transfer of X4 and R5 HIV-1 to CD4+ T cells
Several studies have shown that DC-SIGN expressed on the surface of dendritic cell, breast milk macrophages, activated B-lymphocytes and transfected cell lines (DC-SIGN+/CD4+ cells) can capture HIV-1 and trans-infect CD4+ T lymphocytes [8,29]. Therefore, we investigated whether M2a-MDMs also possess an increased capacity to transfer HIV-1 to CD4+ T cells independent of their productive infection. To investigate this possibility, MDM were incubated for 2 h at 37°C with HIV-1LAI/IIIB, an X4 strain incapable of establishing productive infection in MDM [24,25]. Cells were then thoroughly washed and cocultivated with autologous monocyte-depleted, IL-2-activated PBMC (mainly T cells, referred to hereafter as ‘T cells’). After 6 h of coculture, the nonadherent T cells were removed and incubated an additional 12 days in a medium enriched with IL-2. As expected, no X4 virus replication was detected in control, M1-MDM or M2a-MDM (Fig. 4a). However, M2a-MDM exposed to X4 HIV-1 could mediate trans-infection and establishment of productive infection in T cells more efficiently than either control or M1-MDM (Fig. 4a). Preincubation with anti-DC-SIGN mAb before exposure to HIV-1 resulted in a near complete inhibition of the ability of M2a cells to trans-infect T cells, but had no significant effect on virus transmission by control or M1-MDM (Fig. 4b). However, given the limits of sensitivity of the reverse transcriptase assay, we cannot exclude the possibility of low levels of viral replication that were not blocked by anti-DC-SIGN mAb in these M2a cell transfer experiments.
Fig. 4. DC-SIGN+ M2a transfer of X4 and R5 HIV-1 to activated CD4+ T cells.
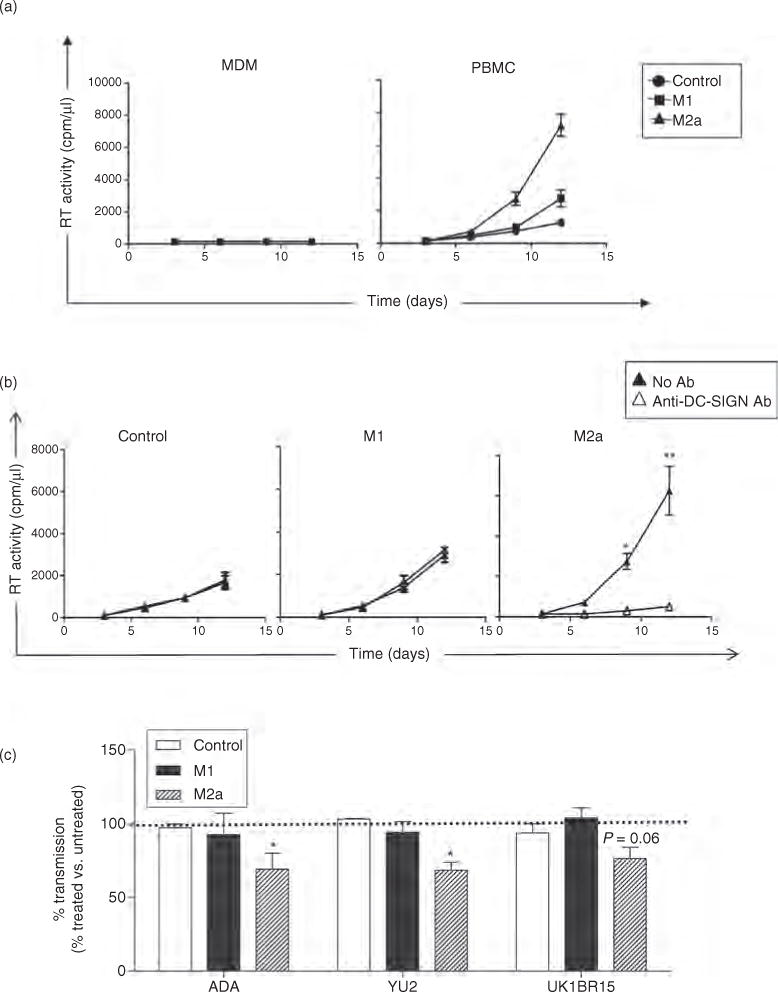
(a) After 18 h in polarizing or control conditions, control, M1-MDM and M2a-MDM were washed and incubated with X4 HIV-1LAI/IIIB for 2 h at 37° C. MDM were then washed and cocultured for 6 h with IL-2 activated, monocyte-depleted PBMC (T cells). HIV-1 transfer was determined by the productive infection of T cells, as measured by RT activity in culture supernatants. Results represent the average ± SD from MDM established from four independent donors. Neither control nor polarized MDMs were productively infected by X4 HIV-1 (left panel), whereas M2a-MDM transferred infectious X4 HIV-1 to T cells (right panel) with much greater efficiency than control or M1-MDM (*P > 0.05 by ANOVA and Tukey post-test). (b) Preincubation of MDM with anti-DC-SIGN mAb prior to exposure to HIV-1LAI/IIIB prevented the ability of these cells, but not that or M1-MDM, to transmit infectious HIV-1 to IL-2 lymphocytes (mean ± SD of MDM cultures established from four individual donors; paired t-test. *P ≤ 0.05, **P ≤ 0.01). (c) Preincubation of MDM with anti-DC-SIGN mAb prior to exposure to viruses expressing Env from HIV-1ADA, HIV-1YU2 and HIV-1UK1BR15 blocked 23–32% of transmission from M2a-MDM to IL-2-stimulated lymphocytes. Results represent average p24 Gag levels ± SD from MDM from two independent donors at day 12 of coculture. We detected an average of 300, 281 and 274 ng/ml of p24 Gag in supernatants from PBMC cocultured with untreated MDM (no anti-DC-SIGN Ab) exposed to NL4-3 viruses with ADA, YU2 and UK1BR15 Envs, respectively. The difference in p24 Gag in culture supernatant between MDM incubated in the presence or absence of the indicated mAb was evaluated by a paired t-test. DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin; MDM, monocyte-derived macrophage; PBMC, peripheral blood mononuclear cell. *P ≤ 0.05.
Finally, we tested whether control, M1-MDM and M2a-MDM could transfer R5 HIV-1 to activated T cells in a DC-SIGN-dependent manner using a panel of macrophage-tropic viruses (AD8, YU2 and UK1BR15) shown to bind to DC-SIGN in the preceding experiments (Fig. 2c). Unlike HIV-1LAI/IIIB, control, M1-MDM and M2a-MDM transferred similar levels of these R5 viruses, resulting in the establishment of low-level R5 virus replication in activated T cells. As observed with the X4 virus, anti-DC-SIGN mAb partially blocked HIV-1 transmission from M2a-MDM, but not from control or M1 cells, to T cells (range: 23–32%; Fig. 4c).
Discussion
In this study, we examined the effects of M1 and M2a polarization on the expression of membrane receptors that play a role in propagation and pathogenesis of HIV-1 in mucosal tissues. Of the molecules examined, only DC-SIGN, a C-type lectin involved in the attachment and transfer of infectious virions from dendritic cell to CD4+ T cells [8,9,21], exhibited a strong differential response to M1 vs. M2a polarization. DC-SIGN was markedly upregulated on the surface of M2a-MDM and downregulated to near-undetectable levels on that of M1-MDM, a pattern that was maintained for at least 7 days post-polarization. The upregulation of DC-SIGN was associated with increased accumulation of HIV-1 DNA in M2a-MDM, potentially compensating for low levels of CD4 on the surface of these cells. In addition, DC-SIGN+ M2a-MDM showed a superior efficiency in transmitting either R5 or X4 viruses compared with control or M1-MDM, thus mimicking mDC in their capacity to bind and transmit HIV-1 to CD4+ T cells [27]. Together, these findings suggest that M2a macrophages may play an important role in local propagation of HIV-1 in tissues favouring TH2 microenvironments.
There has been limited success in identifying phenotype-restricted markers of human macrophage polarization that can be used as research and clinical tools [15]. Here, we identified several markers, including CD16, CD163 and CD206, which showed modest differential expression in M1 vs. M2a cells. However, DC-SIGN (CD209) was the only receptor significantly and differentially affected by M1 and M2a polarization compared with unpolarized control cells. The strong upregulation of DC-SIGN on the surface of M2a-MDM is consistent with an independent study showing that long-term (5 days) stimulation of MDM with IL-4 is associated with increased DC-SIGN mRNA and protein expression [30]. Similarly, exposure of breast milk macrophages to IL-4 leads to increased DC-SIGN expression [31]. Other investigators reported that DC-SIGN expression on monocytes and macrophages is regulated by specific cytokines and dependent on activation of signal transducer activator of transcription 6 (STAT6), typically induced by IL-4 and IL-13 stimulation [32–34]. Conversely, IFN-α and IFN-γ have been shown to suppress DC-SIGN expression via inhibition of tyrosine phosphorylation and nuclear translocation of STAT6 [33,34]. Thus, a better understanding of mechanisms regulating DC-SIGN expression on MDM may lead to new insights for modulation of M2a responses.
We have previously shown that M1 and M2a polarization inhibits productive R5 HIV-1 infection (whereas X4 infection was not observed in either polarized or unpolarized MDM) [18]. M1 restriction occurs at an early, preintegration level and is associated with strong downregulation of CD4, whereas M2a-associated restriction occurs later without affecting HIV-1 DNA synthesis and accumulation [18]. Here, we found that blockade of DC-SIGN was associated with decreased HIV-1 DNA accumulation in M2a-MDM and increased accumulation in control MDM. The mechanisms facilitating DC-SIGN-mediated entry into MDM are poorly understood, but may reflect differences in abundance of DC-SIGN vs. CD4 on these cells (with M2a-MDM expressing low CD4 and high DC-SIGN, whereas control MDM express high CD4 and low DC-SIGN) [27]. Upregulation of DC-SIGN on M2a-MDM may compensate for low CD4 by facilitating binding and receptor/coreceptor-mediated virus entry) [28]. This interpretation is supported by a study [35] showing that gp120 Env binding to DC-SIGN enhances access to the CD4-binding site. Increased expression of DC-SIGN on M2a-MDM may also promote oligomerization of DC-SIGN, or changes in the configuration of CD4/CCR5/DC-SIGN complexes [28].
In addition to enhancing HIV-1 DNA accumulation via cis-infection and/or trans-infection of adjacent M2a-MDM, DC-SIGN+ M2a cells rapidly (6 h) transferred X4 HIV-1 to T cells. M2-MDM also efficiently transmitted R5 HIVBaL and recombinant NL4-3 viruses expressing both ADA and YU2 Env, but not R5 brain-derived Env. These differences likely reflect heterogeneous N-linked glycosylation of primary HIV Env glycoproteins, which effects lectin receptor binding [36]. Unlike X4 HIV-1IIIB, anti-DC-SIGN Abs only partially blocked R5 virus transmission. This finding is consistent with a study by Chehimi et al. [30], indicating that anti-DC-SIGN Ab neutralized 39–48% of MDM-T cell transmission. In addition, Chehimi’s study showed that mannin blocked transmission by 67–75%, suggesting that other C-type lectins including the macrophage mannose receptor likely contribute to HIV-1 transmission to T cells [30]. In long-term coculture (72 h), these authors did not observe a significant increase in X4 HIV-1 transmission from IL-4-stimulated MDM to Sup-T1 T-cell lines. This discrepancy may reflect either the use of Sup-T1 cells vs. IL-2-stimulated PBMC in our study, differences in duration of coculture or technical variants in the MDM differentiation protocol. In fact, Saidi et al. [37], using long-term (6-day) coculture conditions, demonstrated DC-SIGN-dependent transfer of R5 HIV-1 from IL-4-stimulated MDM to both activated and nonactivated T cells. Thus, M2a macrophages may mimic dendritic cell in their ability to transmit HIV-1 to CD4+ T via DC-SIGN.
Additional investigations are required to determine the in-vivo significance of our findings. Upregulation of DC-SIGN on M2a-MDM raises the possibility that this receptor may play a role in the M2/TH2 axis of immunity [30,38]. In vivo, DC-SIGN has been detected on dendritic cell and on a select subset of specialized macrophages in the lung and placenta, organs with TH2-biased microenvironments [21,39]. Consistent with this idea, a central feature of pathogens that interact with DC-SIGN, such as Schistosoma mansoni, Leishmania parasites and Mycobacterium tuberculosis, is their capacity to modulate the TH1/TH2 cell balance, leading to establishment of persistent infections [40].
In summary, our study demonstrates in-vitro conditions that induce persistent upregulation of DC-SIGN on MDM and the capacity of DC-SIGN+ M2a-MDM to bind and facilitate HIV-1 infection in M2a cells and primary T-cells, respectively. Macrophages are well represented in TH2-inducing environments such as the lung and gastrointestinal tract [41,42] and may play an important role not only in transmission of HIV-1 but also in the establishment or expansion of viral reservoirs. Thus, targeting of DC-SIGN may provide an important tool for prevention and treatment of HIV-1 and other infectious agents that exploit DC-SIGN for survival and spread in humans.
Acknowledgments
E.C. performed this study as partial fulfilment of her joint PhD in ‘Molecular and Cellular Biology’ of the Vita-Salute University of Milan (Milan, Italy) and the Open University of London, UK. L.C. performed some aspects of the study as partial fulfilment of his PhD in ‘Molecular Medicine-Section of Basic and Applied Immunology’ of the Vita-Salute University of Milan (Milan, Italy).
E.C. conceived and performed most of the experiments and significantly contributed to writing of the manuscript; L.C. performed some of the experiments with DC-SIGN and contributed to writing of the manuscript; C.R. optimized and performed PCR-based experiments for quantification of HIV-1 DNA; M.A. contributed to the experimental design, supervised all the initial results and contributed to writing of the manuscript; D.G and G.P. contributed to the design, supervised some of the experimental results and contributed to writing of the manuscript. All authors contributed equally to interpretation of data.
We thank Anna Mondino and Marika Falcone from the San Raffaele Scientific Institute for critical reading of the manuscript and helpful suggestions.
This study was supported in part by the Fondation Dormeur, by Europrise (grant no. LSHP CT-2006-037611 to G.P.) and by the Italian Ministry of Health Grant Program of AIDS Research 2009–2010 (to M.A. and G.P.). E.C. was supported in part by a CIHR fellowship. D.G. was supported by NIH R01 MH83588.
Footnotes
Conflicts of interest
The authors have no financial conflicts of interest.
References
- 1.van’t Wout AB, Kootstra NA, Mulder-Kampinga GA, Albrecht-van Lent N, Scherpbier HJ, Veenstra J, et al. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J Clin Invest. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JC, Bandres JC. Cells of the monocyte-macrophage lineage and pathogenesis of HIV-1 infection. J Acquir Immune Defic Syndr. 1999;22:413–429. doi: 10.1097/00126334-199912150-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6:20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson J, Cunningham AL. Mucosal transmission of HIV-1: first stop dendritic cells. Curr Drug Targets. 2006;7:1563–1569. doi: 10.2174/138945006779025482. [DOI] [PubMed] [Google Scholar]
- 5.Morrow G, Vachot L, Vagenas P, Robbiani M. Current concepts of HIV transmission. Curr HIV/AIDS Rep. 2007;4:29–35. doi: 10.1007/s11904-007-0005-x. [DOI] [PubMed] [Google Scholar]
- 6.Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008;111:4660–4663. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- 7.de Witte L, Nabatov A, Geijtenbeek TB. Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission. Trends Mol Med. 2008;14:12–19. doi: 10.1016/j.molmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 9.Baribaud F, Pohlmann S, Doms RW. The role of DC-SIGN and DC-SIGNR in HIV and SIV attachment, infection, and transmission. Virology. 2001;286:1–6. doi: 10.1006/viro.2001.0975. [DOI] [PubMed] [Google Scholar]
- 10.Lekkerkerker AN, van Kooyk Y, Geijtenbeek TB. Viral piracy: HIV-1 targets dendritic cells for transmission. Curr HIV Res. 2006;4:169–176. doi: 10.2174/157016206776055020. [DOI] [PubMed] [Google Scholar]
- 11.Carter CA, Ehrlich LS. Cell biology of HIV-1 infection of macrophages. Annu Rev Microbiol. 2008;62:425–443. doi: 10.1146/annurev.micro.62.081307.162758. [DOI] [PubMed] [Google Scholar]
- 12.Sharova N, Swingler C, Sharkey M, Stevenson M. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 2005;24:2481–2489. doi: 10.1038/sj.emboj.7600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr JM, Hocking H, Li P, Burrell CJ. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology. 1999;265:319–329. doi: 10.1006/viro.1999.0047. [DOI] [PubMed] [Google Scholar]
- 14.Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53:11–24. doi: 10.1007/s12026-012-8291-9. [DOI] [PubMed] [Google Scholar]
- 15.Cassetta L, Cassol E, Poli G. Macrophage polarization in health and disease. ScientificWorldJournal. 2011;11:2391–2402. doi: 10.1100/2011/213962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baay M, Brouwer A, Pauwels P, Peeters M, Lardon F. Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin Dev Immunol. 2011;2011:565187. doi: 10.1155/2011/565187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassol E, Cassetta L, Rizzi C, Alfano M, Poli G. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J Immunol. 2009;182:6237–6246. doi: 10.4049/jimmunol.0803447. [DOI] [PubMed] [Google Scholar]
- 19.Dunfee RL, Thomas ER, Gabuzda D. Enhanced macrophage tropism of HIV in brain and lymphoid tissues is associated with sensitivity to the broadly neutralizing CD4 binding site antibody b12. Retrovirology. 2009;6:69. doi: 10.1186/1742-4690-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunfee RL, Thomas ER, Wang J, Kunstman K, Wolinsky SM, Gabuzda D. Loss of the N-linked glycosylation site at position 386 in the HIV envelope V4 region enhances macrophage tropism and is associated with dementia. Virology. 2007;367:222–234. doi: 10.1016/j.virol.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurney KB, Elliott J, Nassanian H, Song C, Soilleux E, McGowan I, et al. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J Virol. 2005;79:5762–5773. doi: 10.1128/JVI.79.9.5762-5773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collin M, Gordon S. The kinetics of human immunodeficiency virus reverse transcription are slower in primary human macrophages than in a lymphoid cell line. Virology. 1994;200:114–120. doi: 10.1006/viro.1994.1169. [DOI] [PubMed] [Google Scholar]
- 23.Monini P, Colombini S, Sturzl M, Goletti D, Cafaro A, Sgadari C, et al. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi’s sarcoma. Blood. 1999;93:4044–4058. [PubMed] [Google Scholar]
- 24.Collman R, Hassan NF, Walker R, Godfrey B, Cutilli J, Hastings JC, et al. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989;170:1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuitemaker H, Kootstra NA, de Goede RE, de Wolf F, Miedema F, Tersmette M. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991;65:356–363. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turville S, Wilkinson J, Cameron P, Dable J, Cunningham AL. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J Leukoc Biol. 2003;74:710–718. doi: 10.1189/jlb.0503208. [DOI] [PubMed] [Google Scholar]
- 27.van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 28.Lee B, Leslie G, Soilleux E, O’Doherty U, Baik S, Levroney E, et al. cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J Virol. 2001;75:12028–12038. doi: 10.1128/JVI.75.24.12028-12038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang JH, Janas AM, Olson WJ, KewalRamani VN, Wu L. CD4 coexpression regulates DC-SIGN-mediated transmission of human immunodeficiency virus type 1. J Virol. 2007;81:2497–2507. doi: 10.1128/JVI.01970-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chehimi J, Luo Q, Azzoni L, Shawver L, Ngoubilly N, June R, et al. HIV-1 transmission and cytokine-induced expression of DC-SIGN in human monocyte-derived macrophages. J Leukoc Biol. 2003;74:757–763. doi: 10.1189/jlb.0503231. [DOI] [PubMed] [Google Scholar]
- 31.Satomi M, Shimizu M, Shinya E, Watari E, Owaki A, Hidaka C, et al. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. J Infect Dis. 2005;191:174–181. doi: 10.1086/426829. [DOI] [PubMed] [Google Scholar]
- 32.Relloso M, Puig-Kroger A, Pello OM, Rodriguez-Fernandez JL, de la Rosa G, Longo N, et al. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol. 2002;168:2634–2643. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- 33.Dickensheets HL, Donnelly RP. IFN-gamma and IL-10 inhibit induction of IL-1 receptor type I and type II gene expression by IL-4 and IL-13 in human monocytes. J Immunol. 1997;159:6226–6233. [PubMed] [Google Scholar]
- 34.Dickensheets HL, Venkataraman C, Schindler U, Donnelly RP. Interferons inhibit activation of STAT6 by interleukin 4 in human monocytes by inducing SOCS-1 gene expression. Proc Natl Acad Sci U S A. 1999;96:10800–10805. doi: 10.1073/pnas.96.19.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du T, Hu K, Yang J, Jin J, Li C, Stieh D, et al. Bifunctional CD4-DC-SIGN fusion proteins demonstrate enhanced avidity to gp120 and inhibit HIV-1 infection and dissemination. Antimicrob Agents Chemother. 2012;56:4640–4649. doi: 10.1128/AAC.00623-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eggink D, Melchers M, Wuhrer M, van Montfort T, Dey AK, Naaijkens BA, et al. Lack of complex N-glycans on HIV-1 envelope glycoproteins preserves protein conformation and entry function. Virology. 2010;401:236–247. doi: 10.1016/j.virol.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saidi H, Carbonneil C, Magri G, Eslahpazire J, Sekaly RP, Belec L. Differential modulation of R5-tropic HIV-1 transfer from macrophages towards T cells under IL-4/IL-13 microenvironment. Hum Immunol. 2009;71:1–13. doi: 10.1016/j.humimm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 39.Soilleux EJ, Morris LS, Lee B, Pohlmann S, Trowsdale J, Doms RW, Coleman N. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J Pathol. 2001;195:586–592. doi: 10.1002/path.1026. [DOI] [PubMed] [Google Scholar]
- 40.den Dunnen J, Gringhuis SI, Geijtenbeek TB. Innate signaling by the C-type lectin DC-SIGN dictates immune responses. Cancer Immunol Immunother. 2009;58:1149–1157. doi: 10.1007/s00262-008-0615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 42.Platt AM, Mowat AM. Mucosal macrophages and the regulation of immune responses in the intestine. Immunol Lett. 2008;119:22–31. doi: 10.1016/j.imlet.2008.05.009. [DOI] [PubMed] [Google Scholar]


