Abstract
Purpose
Vismodegib is a Hedgehog pathway inhibitor approved for the treatment of advanced basal cell carcinoma. Currently, the pharmacokinetics (PK) and safety of vismodegib in patients with hepatic dysfunction are unknown and are the objective of this study.
Methods
Patients with advanced solid malignancies and hepatic impairment were enrolled into one of four cohorts: normal [bilirubin (bili) < upper limit of normal (ULN) ], mild (ULN < bili ≤ 1.5 × ULN), moderate (1.5 × ULN < bili ≤ 3×ULN), and severe (3 × ULN < bili < 10 × ULN) dysfunction. Patients received oral vismodegib 150 mg daily. Plasma PK samples on days 1, 3, 5, and 8 were collected. Vismodegib therapy was continued until disease progression, intolerable toxicity, or withdrawal of consent.
Results
Thirty-one patients were accrued: nine normal, eight mild, eight moderate, and six severe. Four patients experienced dose-limiting toxicity of hyperbilirubinemia on study: one in the moderate cohort and three in the severe cohort. Six patients died within 30 days after the last dose of vismodegib. All deaths were attributed to disease progression. Observed maximal and average steady state concentrations and AUC of vismodegib at steady state (day 8) were similar across cohorts. Average AAG concentrations in patients with hepatic impairment were comparable to those of patients with normal hepatic function.
Conclusions
Hepatic impairment does not appear to impact vismodegib PK, and therefore, dose adjustment is not necessary in this special population. The study was influenced by the high number of patients with hepatocellular carcinoma with advanced cirrhosis; rendering it difficult to draw any causal relationships between vismodegib exposure and the serious adverse events.
Keywords: Vismodegib, Hepatic, Impairment, Safety, Pharmacokinetics
Introduction
The Hedgehog (Hh) signaling pathway, whose activation has been implicated in several types of cancer, is a novel and proven beneficial target for cancer therapy [1]. The Hh ligand in the extracellular space binds to patched protein 1 (PTCH1), a 12-pass transmembrane receptor on the surface of cells. Hh binding relieves the inhibitory effect of PTCH1 on smoothened protein (SMO), a 7-pass transmembrane domain protein with homology to the G-protein-coupled receptor superfamily [2]. Signal transduction by SMO then leads to the activation and nuclear localization of GLI transcription factors and induction of Hh target gene transcription, many of which are involved in proliferation, survival, and differentiation of cells [3]. Alterations in the Hh receptor components, PTCH1 or SMO, result in constitutive pathway activation and have been identified in basal cell carcinoma, medulloblastoma, and other tumors [2, 4, 5].
Vismodegib is a small-molecule inhibitor of the Hh signaling pathway that binds to and inhibits SMO, resulting in the blockade of Hh pathway signaling. The efficacy and pharmacokinetics (PK) of vismodegib were evaluated in multiple clinical studies, including those involving patients with advanced basal cell carcinoma [5, 15]. The number of cancer patients with associated or pre-existing liver or kidney dysfunction is likely to increase because of the aging of the population and the prevalence of pre-disposing conditions, including those associated with liver failure [e.g., hepatocellular carcinoma (HCC) ] and renal dysfunction (e.g., diabetes and hypertension) [6]. Frequently, patients with significantly impaired liver or kidney function are excluded from clinical trials; therefore, the amount of PK and safety data on investigational drugs is relatively scarce in these patient populations [6]. Vismodegib has been shown to be slowly eliminated by a combination of metabolism and excretion of parent drug, most of which was recovered in feces (i.e., hepatic metabolism). Metabolic pathways of vismodegib in humans included oxidation, glucuronidation, and uncommon pyridine ring cleavage [7]. Therefore, hepatic impairment could theoretically alter vismodegib disposition, resulting in an increase in plasma exposure (i.e., due to decreased hepatic clearance) [8]. On the other hand, renal elimination appears to be a minor route as it accounts for excretion of approximately 4% of the vismodegib oral dose [7]. The impact of hepatic impairment on the PK and safety of vismodegib was evaluated in the current study to provide dosing recommendations in this special population.
Materials and methods
Study population
Patients with histologically or cytologically confirmed advanced solid malignancies with hepatic impairment were offered to participate in the study, contingent upon the presence of metastatic or unresectable disease for which there were no standard curative or palliative therapies available. Subjects were aged ≥18 years, had an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and had acceptable bone marrow functions of absolute neutrophil count 1.2 • 109/L, platelet count 75 • 109/L, and hemoglobin count 9 g/dL. Patients with gliomas or brain metastases had stable or on stable steroid regimen and have completed any radiation therapy at least 4 weeks before starting on therapy. Subjects completed any prior therapy 4 weeks (6 weeks for nitrosoureas or mitomycin C) prior to entering the study. Pregnant or lactating women and subjects with active concurrent illnesses were excluded. Patients receiving medications that were known inhibitors of CYP2C8/9 or CYP3A4 were excluded, or discontinued these for at least 28 days prior to initiating vismodegib therapy. The study was approved by the institutional review board of participating institution, and informed consent was obtained from all individual participants included in the study.
Study cohorts
Subjects were enrolled into four cohorts as defined by National Cancer Institute Organ Dysfunction Working Group criteria: normal [bilirubin (bili) < upper limit of normal (ULN); cohort 1], mild (ULN < bili < 1.5 × ULN; cohort 2), moderate (1.5 × ULN < bili < 3 × ULN; cohort 3), and severe (3 × ULN < bili < 10 × ULN; cohort 4) dysfunction. Subjects needed to have stable hepatic function for 2 weeks prior to starting therapy.
Therapeutic intervention and outcome
Subjects received 150 mg of oral vismodegib daily. No dose modification or interruptions were planned or permitted during this period. Vismodegib was continued until disease progression, intolerable toxicity, or withdrawal of consent. Response assessment data using Response Evaluation Criteria in Solid Tumors, version 1.1 [9], progression-free survival, and duration of response were all studied by cohort.
Pharmacokinetic sampling schedule and analysis
On days 1, 3, 5, and 8, the predose plasma PK samples were collected. In addition, on day 8, serial plasma and urine samples were collected for up to 24 h postdosing. The steady state plasma PK parameters for total vismodegib, maximum plasma concentration (Cmax), area under the curve (AUC0–24 h), and average steady state plasma concentration on day 8 (Css) were calculated from the serial plasma samples collected at pre-dose and at 0.5, 1, 2, 4, 8, and 24 h post-dose using non-compartmental methods; in addition to PK parameters for total vismodegib, unbound vismodegib Css was also calculated. Renal clearance (CLr) of vismodegib was calculated as the ratio of amount of vismodegib excreted in urine over a 24-h period on day 8 (Ae0–24 h) and plasma AUC0–24 h on day 8. Total plasma concentrations of vismodegib [10] were determined using validated high-performance liquid chromatography with tandem mass spectrometric detection (HPLC–MS/MS) analytical procedures (Covance Laboratories Madison, WI). Unbound plasma concentrations of vismodegib were determined using a validated HPLC–MS/MS analytical procedure following equilibrium dialysis [11] (Covance Laboratories Madison, WI). Plasma concentrations of AAG were determined using a using a validated quantitative immunoturbidimetric method (Covance CCLS, Indianapolis, IN) with confirmed sample stability of up to 12 months. In an immunochemical reaction, the AAG in the human serum sample forms immune complexes with specific antibodies. These complexes scatter a beam of light passed through the sample. The intensity of scattered light in the nephelometer depends on the AAG content of the sample, and therefore, the AAG concentration can be determined versus dilutions of a standard of known concentration.
Definition of dose-limiting toxicities
Safety and tolerability were assessed throughout the study and up to 45 days after the last dose of vismodegib. Dose-limiting toxicities (DLTs) are defined as treatment-related AEs that occur during treatment days 1–8, according to the following criteria: an increase in aspartate aminotransferase (AST) or alkaline phosphatase 2.5 baseline or an increase in total bilirubin ≥1.5 × baseline, any treatment-related Common Terminology Criteria for Adverse Events (CTCAE version 4.03) grade 3 non-hematologic toxicities that are likely to be life threatening or irreversible, and any treatment-related CTCAE grade 4 hematologic toxicity.
Statistical methods
The study was designed as an open-label PK and safety trial with a sample size of approximately 30 patients. Six evaluable patients in each cohort were estimated to provide 84 and 88% power for evaluating the relative differences in maximum plasma concentration (Cmax) and area under the curve from 0 to 24 h (AUC0–24 h) between the hepatic dysfunction cohorts and the normal hepatic function (control) cohort, considering boundaries of 0.5–2.0 (95% CI of GMR), and a vismodegib Cmax and steady state AUC0–24 h interpatient coefficient of variation ∼37.4 and ∼35.8%, respectively, with a two-sided α-level of 0.10 [14]. These boundaries were selected because an increase or decrease of up to twofold was not deemed to be clinically meaningful, based on the PK, safety, and efficacy profiles of vismodegib. A comparison of the Cmax and AUC0–24 h post-day 8 dose between the control cohort (cohort 1) and the hepatic impairment cohorts (cohorts 2–4) was made to test whether the ratio of median Cmax or AUC0–24 h between an impairment cohort and control cohort (cohort 1) is 2 or 0.5. The data were analyzed on the natural log scale using two onesided t tests for differences (of >ln2 or -ln2) in mean at the 5% significance level for each test. Relationships between hepatic function [total bilirubin levels and aspartate aminotransferase (AST) levels] or AAG and plasma Css of vismodegib were explored graphically. All other data analyses were descriptive.
Study approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Demographics
A total of 31 patients were enrolled into the study: nine in the normal hepatic function cohort, eight in the mild, eight in the moderate, and six in the severe hepatic dysfunction cohorts. Patient demographics were balanced among the treatment groups. The median age of all patients on study was 66.0 years (range 36–82), and the majority of patients were male (77.4%). Only one patient was of childbearing potential. The majority of patients on study were white (61.3% overall), with the highest percentage of white patients in the moderate hepatic impairment group (75.0%) and the lowest in the mild and severe hepatic impairment groups (50% each). Median age, sex, ECOG performance status, and cancer diagnosis are as described in Table 1.
Table 1. Summary of patient demographics and disease characteristics by hepatic impairment status.
| Parameter | Normal* n = 9 | Mild* n = 8 | Moderate* n = 8 | Severe* n = 6 |
|---|---|---|---|---|
| Median age, years (range) | 67 (37–79) | 64.5 (36–82) | 68 (47–77) | 65 (51–75) |
| Male, n (%) | 8 (88.9) | 5 (62.5) | 6 (75) | 5 (83.3) |
| ECOG 0/1/2, n (%) | 3 (33.3)/6 (66.7)/0 | 2 (25)/4 (50)/2 (25) | 0/8 (100)/0 | 0/5 (83.3)/1 (16.7) |
| Cancer diagnosis | ||||
| Basal cell carcinoma, n (%) | 1 (11.1) | 0 | 0 | 0 |
| Colorectal cancer, n (%) | 2 (22.2) | 0 | 0 | 1 (16.7) |
| Hepatocellular carcinoma, n (%) | 5 (55.6) | 5 (62.5) | 6 (75) | 5 (83.3) |
| Other cancer, n (%) | 1 (11.1) (oropharyngeal) | 3 (37.5) (renal cell carcinoma, ovarian and breast, and ocular melanoma) | 2 (25) (appendiceal and cholangiocarcinoma) | 0 |
ECOG Eastern Cooperative Oncology Group performance status.
Normal (bilirubin [bili] < upper limit of normal [ULN]) ], mild (ULN < bili ≤ 1.5 × ULN), moderate (1.5 × ULN < bili ≤ 3 × ULN), and severe (3 × ULN < bili < 10 × ULN) liver dysfunction.
Patient disposition
The median duration of vismodegib treatment during the study was 35 days (range 2–225), with the highest in cohort 1 (65 days) and lowest in the severe cohort (20 days). The median total dose of vismodegib was 5250 mg for all patients; the highest dose was in the normal cohort (9750 mg), and the lowest was in the severe cohort (3000 mg) (Table 2). The median dose intensity for vismodegib during the PK collection period was 100% for all treatment groups. During the PK collection period, the median duration of exposure was 8 days (range 2–8) and was similar among the treatment groups. One subject in the moderate cohort and three subjects in the severe cohort did not complete treatment during the 8-day PK collection period. These subjects were excluded from the PK analysis.
Table 2. Summary of disposition, safety, and tolerability by hepatic impairment status.
| Parameter | Normal* n = 9 | Mild* n = 8 | Moderate* n = 8 | Severe* n = 6 |
|---|---|---|---|---|
| Median (range) duration of therapy, days | 65 (43–225) | 28.5 (8–64) | 27.5 (7–56) | 20 (2–103) |
| Total cumulative dose, mg (range) | 9750 (5400–33,750) | 4050 (1200–8100) | 3975 (1050–8100) | 3000 (300–15,450) |
| Dose-limiting toxicity, hyperbilirubinemia | 0 | 0 | 1 | 2 |
| Deaths due to progression of disease | 0 | 1 | 3 | 1 |
| Adverse events leading to withdrawal of vismodegib | 0 | 1 (intracranial bleed) | 1 (hyperbilirubinemia), 2 (GI bleeds) | 2 (hyperbilirubinemia), 1 (leukocytosis), 1 (fatigue), 1 (hyponatremia) |
GI gastrointestinal
Normal (bilirubin [bili] < upper limit of normal [ULN]) ], mild (ULN < bili ≤ 1.5 × ULN), moderate (1.5 × ULN < bili ≤ 3 × ULN), and severe (3 × ULN < bili < 10 × ULN) liver dysfunction
Safety and tolerability
Almost all patients (96.8% in all groups) experienced at least one AE. Adverse events that were reported most frequently (i.e., 20% in all patients) were those typically associated with vismodegib [12, 13], including fatigue (67.7%), nausea (35.5%), and abdominal pain, vomiting, dysgeusia, decreased appetite, and muscle spasms (each 22.6%). A total of 67.7% of all AEs reported were grade 3 or 4 in severity (44.4% control cohort, 75% mild cohort, 87.5% moderate cohort, and 66.7% severe cohort). A total of 16 patients (51.6%) experienced serious AEs, which were highest in the moderate cohort (6 [75%] vs 3 [33.3%] in the normal cohort, 4 [50%] in the mild cohort, and 3 [50%] in the severe cohort]. Serious AEs occurring in at least two patients were abdominal pain, upper gastrointestinal hemorrhage, and hyperbilirubinemia (2 patients each, 6.5%). A total of three patients experienced DLTs of hyperbilirubinemia: one in the moderate cohort and three in the severe cohort. Five patients died during the study; all deaths occurred ≤30 days after the last dose of study treatment and all were attributable to disease progression.
Pharmacokinetic data
The plasma pharmacokinetics of vismodegib (total and unbound) and AAG concentrations are described in Tables 3a and 3b. The primary PK measure was total vismodegib in this study; therefore, “vismodegib” means “total vismodegib” throughout the text and corresponding figures/tables. When referring to unbound vismodegib, it is stated explicitly in the text and in the title of Table 3b. The observed vismodegib concentrations following administration of oral vismodegib 150 mg QD were relatively constant over the 24-h dosing interval at steady state as summarized in Figure 1. The observed Css in all patients, regardless of hepatic function, were within the 5th and 95th percentile steady state trough range (7.6 - 53 μM) as derived from a population PK analysis [14]. Plasma PK exposure of vismodegib at steady state (AUC0–24 h, Cmax, and Css) appeared comparable across all cohorts (Figure 2). Statistical analysis revealed that the 90% confidence intervals of the geometric mean ratios were contained within the pre-specified boundaries 0.5 and 2 for vismodegib and unbound vismodegib (Tables 3a, 3b), implying that there were no statistically significant differences in PK parameters between patients with normal and impaired hepatic function.
Table 3a. Summary of vismodegib PK parameters in plasma in patients with normal or impaired hepatic function after oral administration of vismodegib (150 mg QD).
| Parameter | Normal* n = 9 | Mild* n = 8 | Moderate* n = 6 | Severe* n = 3 | |
|---|---|---|---|---|---|
| AUC0–24 h (μM h) | Median | 409 | 605 | 562 | 368 |
| GMR (90% CI) | Comparator | 1.24 (0.9–1.7) | 1.31 (0.9–1.9) | 0.86 (0.5– 1.4) | |
| Cmax, μM | Median | 18.1 | 27.4 | 26.9 | 17.1 |
| GMR (90% CI) | Comparator | 1.3 (0.95–1.8) | 1.3 (0.9–1.8) | 0.87 (0.5– 1.4) | |
| Css, μM | Median | 17 | 24.3 | 24.5 | 15.7 |
| GMR (90% CI) | Comparator | 1.24 (0.9–1.7) | 1.27 (0.9–1.8) | 0.88 (0.6– 1.4) | |
AAG alpha-1-acid glycoprotein, AUC area under the curve, CI confidence interval, Cmax peak serum concentration, Css average steady state concentration, GMR geometric mean ratio.
Normal (bilirubin [bili] < upper limit of normal [ULN]) ], mild (ULN < bili ≤ 1.5× ULN), moderate (1.5× ULN < bili ≤3 × ULN), and severe (3 × ULN < bili < 10 × ULN) liver dysfunction
Table 3b. Summary of unbound plasma vismodegib Css in patients with normal or impaired hepatic function after oral administration of vismodegib (150 mg QD).
| Unbound vismodegib, Css (μM) | AAG concentration (μM) | |||
|---|---|---|---|---|
| Hepatic function | Mean (±SD) | Median (range) | GMR (90% CI) | Mean (±SD) |
| Normal* (n = 9) | 0.233 (±0.098) | 0.223 (0.100–0.441) | Comparator | 23.2 (±14.8) |
| Mild* (n = 8) | 0.221 (±0.063) | 0.218 (0.157–0.356) | 0.99 (0.7–1.3) | 25.4 (±12.5) |
| Moderate* (n = 6) | 0.246 (±0.084) | 0.217 (0.145–0.376) | 1.09 (0.8–1.6) | 27.1 (±14.0) |
| Severe* (n = 3) | 0.227 (±0.085) | 0.232 (0.140–0.310) | 1 (0.6–1.7) | 19.5 (±11.6) |
AAG alpha-1 acid glycoprotein, Css average steady state concentration, QD once daily/
Normal (bilirubin [bili] < upper limit of normal [ULN]) ], mild (ULN < bili ≤ 1.5 × ULN), moderate (1.5 × ULN < bili ≤ 3 > ULN), and severe (3 × ULN < bili < 10 × ULN) liver dysfunction
Figure 1.
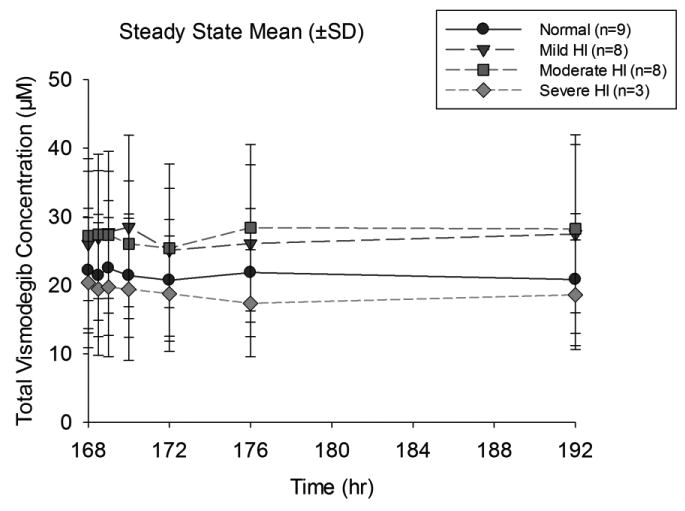
Mean (±SD) concentration–time profiles of vismodegib at steady state in subjects with normal or impaired hepatic function after oral administration of vismodegib (150 mg QD)
QD, once daily; SD, standard deviation.
Figure 2.
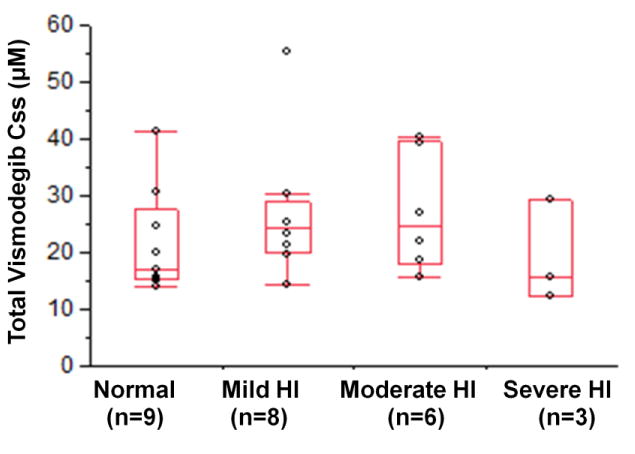
Plasma vismodegib Css in subjects with normal or impaired hepatic function after oral administration of vismodegib (150 mg QD).
Css average steady state concentration; QD once daily.
Renal clearance (CLR) of vismodegib appeared to be lower in patients with hepatic impairment compared to those with normal hepatic function. However, the percentage of dose excreted in urine over a 24-h interval was not considered to be significant because it is <1% in all patients, regardless of their hepatic function.
Alpha-1-acid glycoprotein concentrations
Average AAG concentrations in patients with mild or moderate hepatic impairment were comparable to those with normal hepatic function. However, in patients with severe hepatic impairment, AAG levels appeared lower than those in all other cohorts in the study. In addition, a strong correlation was observed between AAG and vismodegib concentrations in this study (Figure 3).
Figure 3.
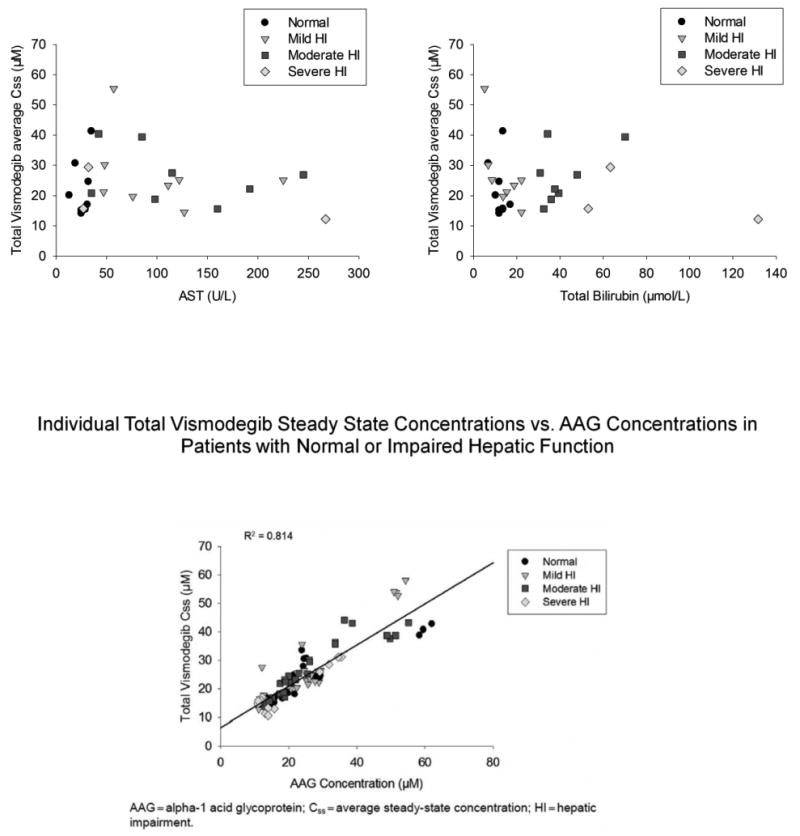
Individual vismodegib (Figure 3a total, Figure 3b unbound) steady state Css versus AAG concentrations in patients with normal or impaired hepatic function.
Css average steady state concentration
Hepatocellular carcinoma group
The study accrued a total 21 patients with HCC as detailed in Table 2. The diagnosis undoubtedly infuenced outcomes, considering the complex nature of the disease. There were seven subjects with diagnosed hepatitis B and another seven with hepatitis C. Two of these 14 patients had cirrhosis. However, vismodegib Css was not correlated with AST or total bilirubin values.
Effects on tumor burden
Responses were only assessed in the patients with HCC considering the relatively larger number of patients with this cancer subtype enrolled into the current study. Of 11 HCC patients, nine patients had progressive disease (3 were defined as having progressive disease based solely on symptomatic deterioration) and two had stable disease (both in the control cohort).
Discussion
Hepatic impairment studies are conducted routinely during small-molecule drug development. Clinically meaningful changes in PK and resulting AEs observed in such special populations can result in product label revisions and dose adjustments. The impact of hepatic impairment on the PK and safety of vismodegib was evaluated in the current study to provide dosing recommendations in this special population.
The route of elimination and extent of vismodegib metabolism were established in a human mass balance study conducted in healthy female subjects [7]. Vismodegib was slowly eliminated by a combination of metabolism and excretion of parent drug, most of which was recovered in feces. The estimated excretion of the orally administered dose (i.e., parent drug) was 86.6% on average, with 82.2 and 4.4% recovered in the feces and urine, respectively [7]. These results indicated that vismodegib and any associated metabolic products are mainly eliminated through the hepatobiliary pathway after oral administration [7]. Interestingly, the results of our study suggest that impairment of the liver (the major clearing organ for vismodegib) does not appear to impact the PK of vismodegib.
Considering the primary route of vismodegib elimination is via the liver, it is plausible that the PK of vismodegib could be impacted in patients with hepatic impairment. However, taking results of previous vismodegib clinical pharmacology studies into consideration, the lack of impact of hepatic impairment on PK can be reconciled. When circulating in plasma, vismodegib is greater than 98% protein bound to both AAG and albumin [7, 10, 12, 13]. The influence of AAG on vismodegib PK has been established, with AAG levels explaining >70% of the PK variability [14]. In a food effect study conducted in patients with cancer, a high-fat meal increased single-dose vismodegib exposure relative to the fasted state (up to 38%), with no corresponding change in Css upon multiple dosing [15]. Vismodegib absorption, and hence exposure, was increased by food under single-dose conditions when AAG binding is not saturated; however, with continual daily dosing, steady state vismodegib exposure was highly correlated with AAG concentrations [16]. In a recently published drug–drug interaction study [17], it was shown that when a single dose of vismodegib was co-administered with the proton pump inhibitor rabeprazole, a 42% decrease in mean vismodegib AUC0–24 h was observed (AAG binding not saturated under single-dose conditions). However, in the same study, after multiple doses of vismodegib, only a 14% decrease in vismodegib AUC0–24 h was observed (AAG binding saturated at steady state) [17]. Taken together, these results provide evidence that with continuous dosing, steady state vismodegib exposure is highly infuenced by levels of AAG in plasma and not infuenced by extrinsic factors that may be anticipated to alter vismodegib PK. The PK data from the patients in this study suggested no impact of mild, moderate, or severe hepatic impairment on vismodegib PK parameters, when compared to patients with normal hepatic function. The limited number of patients in the severe group may limit the pertinence of this conclusion to this specific group. As demonstrated in previous studies, a strong correlation between AAG and vismodegib concentrations was observed and explained most of the variability in the vismodegib concentration data in the current study. As expected, AAG levels did not influence the concentration of unbound vismodegib (Table 3b). Taken together, results from this study suggest that vismodegib exposure in plasma appears to be impacted by AAG levels and not hepatic impairment.
The study is infuenced by the high number of patients with HCC with advanced cirrhosis, rendering it challenging to draw any causal relationships between DLT and serious AEs associated with vismodegib. This is corroborated by the lack of correlation between day 8 vismodegib Css values and AST values or total bilirubin concentrations. Administration of vismodegib in patients with severe hepatic impairment and HCC should be carefully monitored within the context of the DLT and serious AEs reported herein. Considering that vismodegib is not considered a narrow therapeutic index drug and that the PK of vismodegib was not infuenced by mild, moderate, or severe hepatic impairment, results of this study suggest that no dose adjustments are warranted in this special population.
Acknowledgments
We wish to thank the patients and their families without whom this study would not have been possible.
Funding This study was funded by Roche and Genentech.
Footnotes
Previously presented at the AACR 2015 Annual Meeting: Abou-Alfa GK, Lewis LD, LoRusso P, Maitlant M, Cheeti S, Colburn D, Williams S, Simmons B, Graham R, Chand P (2015) Pharmacokinetics and safety of vismodegib in patients with advanced solid malignancies and hepatic dysfunction [abstract]. Cancer Res. 75(15 suppl):CT308.
Compliance with ethical standards: Conflict of interest All the investigators' institutions received a grant from Roche Genentech to cover the costs of participation in this clinical study.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Ingham PW, McMahon PW. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 2.Di Magliano P, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 3.Romer J, Curran T. Targeting medulloblastoma: small-molecule inhibitors of the Sonic Hedgehog pathway as potential cancer therapeutics. Cancer Res. 2005;65:4975–4978. doi: 10.1158/0008-5472.CAN-05-0481. [DOI] [PubMed] [Google Scholar]
- 4.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kot-kow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signaling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 5.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hains-worth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, Marmur E, Rudin CM, Chang AL, Low JA, Mackey HM, Yauch RL, Graham RA, Reddy JC, Hauschild A. Effcacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abou-Alfa GK, Mulkerin D, Huang SM. Developing drugs in special populations: organ dysfunction studies. Poster presented at American society of clinical oncology annual meeting; June 4-8, 2010; Chicago, Illinois. 2010. [Google Scholar]
- 7.Graham RA, Lum BL, Morrison G, Chang I, Jorga K, Dean B, Shin YG, Yue Q, Mulder T, Malhi V, Xie M, Low JA, Hop CE. A single dose mass balance study of the Hedgehog pathway inhibitor vismodegib (GDC-0449) in humans using accelerator mass spectrometry. Drug Metab Dispos. 2011;39:1460–1467. doi: 10.1124/dmd.111.039339. [DOI] [PubMed] [Google Scholar]
- 8.Superfin D, Iannucci AA, Davies AM. Commentary: oncologic drugs in patients with organ dysfunction: a summary. Oncologist. 2007;12:1070–1083. doi: 10.1634/theoncologist.12-9-1070. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Ding X, Chou B, Graham RA, Cheeti S, Percey S, Matassa LC, Reuschel SA, Meng M, Liu S, Voelker T, Lum BL, Rudewicz PJ, Hop CE. Determination of GDC-0449, a small-molecule inhibitor of the hedgehog signaling pathway, in human plasma by solid phase extraction-liquid chromatographic-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878:785–790. doi: 10.1016/j.jchromb.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Deng Y, Wong H, Graham RA, Liu W, Shen HS, Shi Y, Wang L, Meng M, Malhi V, Ding X, Dean B. Determination of unbound vismodegib (GDC-0449) concentration in human plasma using rapid equilibrium dialysis followed by solid phase extraction and high-performance liquid chromatography coupled to mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2011;879:2119–2126. doi: 10.1016/j.jchromb.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 12.Erivedge [package insert] South San Francisco, CA: Genentech USA Inc; 2015. [Google Scholar]
- 13.Erivedge [summary of product characteristics] Welwyn Garden City, UK: Roche Registration Ltd; 2015. [Google Scholar]
- 14.Lu T, Wang B, Gao Y, Dresser M, Graham RA, Jin JY. Semi-mechanism-based population pharmacokinetic modeling of the Hedgehog pathway inhibitor vismodegib. CPT Pharmacomet Syst Pharmacol. 2015;4:680–689. doi: 10.1002/psp4.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma MR, Karrison TG, Kell B, Wu K, Turcich M, Geary D, Kang SP, Takebe N, Graham RA, Maitland ML, Schilsky RL, Ratain MJ, Cohen EE. Evaluation of food effect on pharmacokinetics of vismodegib in advanced solid tumor patients. Clin Cancer Res. 2013;19:3059–3067. doi: 10.1158/1078-0432.CCR-12-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, Graham RA, Zerivitz KL, Low JA, Von Hoff DD. Phase 1 trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhi V, Colburn D, Williams SJ, Hop CE, Dresser MJ, Chandra P, Graham RA. A clinical drug-drug interaction study to evaluate the effect of a proton-pump inhibitor, a combined P-glycoprotein/cytochrome 450 enzyme (CYP)3A4 inhibitor, and a CYP2C9 inhibitor on the pharmacokinetics of vismodegib. Cancer Chemother Pharmacol. 2016;78(1):41–49. doi: 10.1007/s00280-016-3020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


