Abstract
Pediatric lymphoma is common in sub-Saharan Africa, where survival estimates are often based on limited follow-up with incomplete retention, introducing potential for bias. We compared follow-up and overall survival (OS) between passive and active tracing within a prospective cohort of children with lymphoma in Malawi. Median follow-up times were 4.4 months (interquartile range [IQR] 2.0–9.4) and 10.8 months (IQR 6.2–20.6) in passive and active follow-up, respectively. Twelve-month overall survival (OS) was 69% (95% confidence interval [CI] 54–80) in passive and 44% (95% CI 34–54) in active follow-up. Passive follow-up significantly overestimated the OS and underestimated the mortality. Efforts to improve retention in regional studies are needed.
Keywords: loss to follow-up, lymphoma, Malawi, pediatric cancer, sub-Saharan Africa
1 INTRODUCTION
Lymphoma is the commonest pediatric cancer in sub-Saharan Africa (SSA).1,2 Long-term survival of >90% is achievable in developed countries,3,4 but outcomes are worse in SSA.5,6 Moreover, survival estimates from SSA are often limited by short observation times and loss to follow-up (LTFU). This is problematic, since patients contribute follow-up time in survival analyses until LTFU, with deaths being unascertained when patients are no longer under observation. This poses particular challenges in SSA, where retaining patients is difficult, deaths often occur at home, and vital registries are absent.
In human immunodeficiency virus (HIV) literature from the region, bias introduced by LTFU repeatedly leads to underestimated mortality, since death is a frequent cause of LTFU.7,8 In SSA cancer populations, similar findings have been noted for HIV-associated Kaposi sarcoma.9,10 For pediatric lymphoma, LTFU can occur among children who are deceased or cured. If the former is more common, LTFU would underestimate mortality similar to regional HIV literature. If the latter is more common, with families reluctant to spend time and effort to return for follow-up when intervention is no longer required, mortality could be overestimated. Since bias introduced by LTFU has not been evaluated for pediatric lymphoma in SSA, we sought to quantify this within a prospective pediatric lymphoma cohort in Lilongwe.
2 RESULTS
We studied children <18 years of age with newly diagnosed lymphoma between June 1, 2013 and March 31, 2016 enrolled in a prospective observational cohort.5,11 Kaplan–Meier methods were used to estimate the overall survival (OS) from enrollment until death, LTFU, or administrative censoring on June 30, 2016. OS differences were assessed using the log-rank test.
Children were seen at each chemotherapy visit during active treatment, every 3 months after treatment completion for up to 2 years, and for any interim illness. Transportation reimbursement was provided to promote retention. For missed visits during active treatment, and monthly after treatment completion, cellphone contact was attempted by nonmedical study staff using up to three cellphone numbers provided by the guardian at the time of informed consent for study participation. During phone contact, the navigator determined vital status, health status, and encouraged the family to return to clinic with a reminder that transportation costs would be reimbursed. If cellphone contact was unsuccessful on three consecutive attempts, physical tracing was attempted using the home address provided by the guardian at the time of informed consent. For these analyses, we did not distinguish between LTFU during or after treatment. To assess potential bias, we compared observation times and OS estimates using two approaches. In passive follow-up, patients were considered lost to follow-up at the last clinic visit. For active follow-up, analyses were repeated with inclusion of data generated by cellphone and physical tracing. The study was approved by the University of North Carolina at Chapel Hill Institutional Review Board and Malawi National Health Sciences Research Committee.
During the study period, 121 children were enrolled with lymphoma, all of whom were included in these analyses. Of these, 100 (83%) had Burkitt lymphoma (BL) and 21 (27%) Hodgkin lymphoma (HL), the two commonest pediatric lymphoma subtypes in SSA. However, we acknowledge that less frequent non-Hodgkin lymphoma subtypes might be diagnosed locally as BL given limited immunohistochemistry and absent molecular diagnostic tools, despite major efforts by our group to improve diagnostic pathology in Lilongwe.12,13 Baseline characteristics are shown in Table 1. Median age was 10.1 years (interquartile range [IQR] 7.1–12.8) for the entire cohort, 9.4 years (IQR 7.0–12.8) for BL, and 11.5 years (IQR 8.3–13.2) for HL. Eighty-four (69%) were male, 85 (70%) had stage III/IV disease, 112 (93%) had B-symptoms, and 94 (78%) had poor performance status.
TABLE 1.
Baseline characteristics of children with lymphoma in Lilongwe, Malawi, June 2013 to March 2016
| All (n = 121) | BL (n = 100) | HL (n = 21) | |
|---|---|---|---|
| Male, n (%) | 84 (69) | 66 (66) | 18 (86) |
| HIV status, n (%) | |||
| Negative | 113 (93) | 94 (94) | 19 (91) |
| Positive | 2 (2) | 2 (2) | 0 |
| Unknown | 6 (5) | 4 (4) | 2 (10) |
| Clinical stage, n (%) | |||
| Stage I/II | 36 (30) | 23 (23) | 13 (62) |
| Stage III | 49 (40) | 47 (47) | 2 (10) |
| Stage IV | 36 (30) | 30 (30) | 6 (29) |
| Age in years, median (IQR) | 10.1 (7.1–13) | 9.4 (7–13) | 11.5 (8.3–13.2) |
| Primary site, n (%) | |||
| Abdominal only | 56 (46) | 48 (48) | 8 (38) |
| Facial only | 28 (23) | 28 (28) | 0 |
| Cervical only | 7 (5.8) | 2 (2) | 5 (24) |
| Multiple sites | 20 (17) | 16 (16) | 4 (19) |
| Other | 10 (8.3) | 6 (6) | 4 (19) |
| Diagnostic specimen, n (%) | |||
| Cytology | 76 (63) | 71 (71) | 5 (24) |
| Histology | 45 (37) | 29 (29) | 16 (76) |
| B symptoms, n (%) | 112 (93) | 95 (95) | 17 (81) |
| Duration of symptomatic swelling, n (%) | |||
| < 1 month | 34 (28) | 31 (31) | 3 (14) |
| ≥ 1 month | 87 (72) | 69 (69) | 18 (86) |
| Lansky performance status ≤70, n (%) | 94 (78) | 82 (82) | 12 (57) |
BL, Burkitt lymphoma; HL, Hodgkin lymphoma; IQR, interquartile range
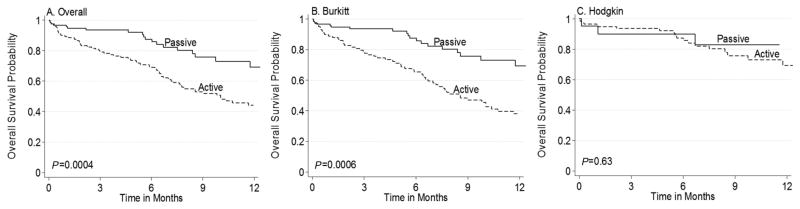
Among children not known to have died on June 30, 2016, median observation time in passive follow-up was 4.4 months (IQR 2.0–9.4) for the entire cohort, 3.9 months (IQR 1.9–8.0) for BL, and 9.4 months (IQR 6.1–12.5) for HL. With active follow-up, the median observation time was 10.8 months (IQR 6.2–20.6) for the entire cohort, 10.4 months (IQR 5.3–18.4) for BL, and 11.6 months (IQR 9.4–20.8) for HL. Kaplan–Meier 12-month OS is shown in Figure 1. With passive follow-up, 12-month OS for the entire cohort was 69% (95% confidence interval [CI] 54–80], 63% (95% CI 44–77) for BL, and 83% (95% CI 55–94) for HL group. With active follow-up, 12-month OS decreased to 44% (95% CI 34–54) for the entire cohort, 38% (95% CI 27–48) for BL, and 74% (95% CI 48–89) for HL. OS differences between passive and active follow-up were significant for the entire cohort (P = 0.0004) and BL (P = 0.0006), but not for HL (P = 0.63).
FIGURE 1.
Kaplan–Meier OS with passive versus active follow-up for (A) overall cohort, (B) Burkitt lymphoma, and (C) Hodkin lymphoma
On June 30, 2016, vital status was known for 63 (52%) of the cohort with passive follow-up and 116 (96%) with active follow-up. Of 65 deaths, 22 (34%) were ascertained during passive follow-up and 43 (66%) during active follow-up. Among 53 children for whom additional information was obtained through active follow-up, including updated vital status or additional observation time, cellphone tracing was sufficient for 42 (79%) and physical tracing was required for 11 (21%). Of five children with unknown vital status despite active follow-up on June 30, 2016, three were from neighboring Mozambique, had come to Malawi for care and subsequently returned home, and could not be reached by any method.
3 DISCUSSION
We evaluated bias in OS estimates introduced by LTFU in a prospective pediatric lymphoma cohort in Malawi by comparing results from passive versus active follow-up. With active tracing, median follow-up times increased and OS estimates were corrected downward. Differences between passive and active follow-up were greater for BL than for HL, with passive follow-up leading to many unascertained deaths.
There are various approaches to address bias from LTFU in resource-limited settings, where retaining patients in longitudinal studies is difficult. One approach is to exclude LTFU cases from survival analyses, but this may increase bias if LTFU is not independent of the risk of death.14–16 Another approach is to assume all patients with LTFU have died.14 However, we found many children in remission whose families no longer felt time and expense were worthwhile to simply return to clinic to document ongoing event-free survival. Loss-adjusted survival methods have also been used, where LTFU is considered nonrandom, but this may be limited if missing data are associated with the risk of death or other prognostic factors.14
Study strengths include a prospective longitudinal cohort with relatively long observation times compared with other SSA reports and ascertainment of vital status for nearly all children. Study limitations include referral bias at a national teaching hospital.
To summarize, passive follow-up relying on clinic and hospital encounters overestimated OS for pediatric lymphoma in Malawi, even when families were reminded about missed visits and provided transportation reimbursements to return. We found regular cellphone contact to be an effective method to address this, with substantially increased observation times and vital status ascertainment, and significant correction of OS estimates. Low-cost, implementable methods to improve retention are needed to generate accurate pediatric lymphoma outcome data in the region, which is an essential first step to improve outcomes further. Additionally, greater transparency and standardization are needed in reporting pediatric cancer outcomes in SSA to increase comparability across studies. Finally, we are undertaking qualitative interviews to examine LTFU reasons in Malawi, both during and after treatment, to inform targeted interventions for improving retention throughout the continuum of care.
Acknowledgments
The authors would like to thank the patients and their families for agreeing to participate in the study, and Dr. Peter Kazembe for his leadership in initiating pediatric cancer care in Lilongwe over the last several decades. They are also grateful to leadership of Kamuzu Central Hospital, Malawi Ministry of Health, UNC Project-Malawi, Lineberger Comprehensive Cancer Center, and Baylor College of Medicine Children’s Foundation Malawi for support of this study. This work is supported by grants from the National Institutes of Health (K01TW009488, R21CA180815, and U54CA190152 to S.G.), the Lineberger Comprehensive Cancer Center (P30CA016086), and Fogarty Global Health Fellows Program (R25TW009340).
Abbreviations
- BL
Burkitt lymphoma
- CI
confidence interval
- HIV
human immunodeficiency virus
- HL
Hodgkin lymphoma
- IQR
interquartile range
- LTFU
loss to follow-up
- OS
overall survival
- SSA
sub-Saharan Africa
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- 1.Msyamboza KP, Dzamalala C, Mdokwe C, et al. Burden of cancer in Malawi: common types, incidence and trends: national population-based cancer registry. BMC Res Notes. 2012;5:149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mwanda WO, Rochford R, Rainey J, Wilson ML. Challenges in the epidemiological and clinical aspects of Burkitt’s lymphoma in Kenya: linking evidence and experience. East Afr Med J. 2004;81(Suppl 8):S111–S116. doi: 10.4314/eamj.v81i8.9215. [DOI] [PubMed] [Google Scholar]
- 3.Ferry JA. Burkitt’s lymphoma: clinicopathologic features and differential diagnosis. Oncologist. 2006;11(4):375–383. doi: 10.1634/theoncologist.11-4-375. [DOI] [PubMed] [Google Scholar]
- 4.Magrath I. Lessons from clinical trials in African Burkitt lymphoma. Curr Opin Oncol. 2009;21:462–468. doi: 10.1097/CCO.0b013e32832f3dcd. [DOI] [PubMed] [Google Scholar]
- 5.Stanley CC, Westmoreland KD, Heimlich BJ, et al. Outcomes for paediatric Burkitt lymphoma treated with anthracycline-based therapy in Malawi. Br J Haematol. 2016;173(5):705–712. doi: 10.1111/bjh.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Togo B, Keita M, Medefo D, Traore F, Sidibe T. Maxillofacial location of Burkitt’s lymphoma in children treated at the University Hospital Center in Bamako, Mali: a 24-case series. Med Trop (Mars) 2008;68(6):600–602. [PubMed] [Google Scholar]
- 7.Egger M, Spycher BD, Sidle J, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8(1):e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkhof MW, Spycher BD, Yiannoutsos C, et al. Adjusting mortality for loss to follow-up: analysis of five ART programmes in sub-Saharan Africa. PLoS ONE. 2010;5(11):e14149. doi: 10.1371/journal.pone.0014149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semeere A, Freeman E, Busakhala N, Asirwa FC, Mwebesa B, Martin J. Mortality after diagnosis of Kaposi’s sarcoma among HIV infected Adults in East Africa in the era of Antiretroviral Therapy. AORTIC 2015: 10th International Conference on Cancer in Africa; Marrakech, Morocco. 18–22 November 2015; [Accessed 5 October 2016]. Available at http://aorticconference.org/programme/ [Google Scholar]
- 10.Freeman E, Semeere A, Wenger M, et al. Pitfalls of practicing cancer epidemiology in resource-limited settings: the case of survival and loss to follow-up after a diagnosis of Kaposi’s sarcoma in five countries across sub-Saharan Africa. BMC Cancer. 2016;16:65. doi: 10.1186/s12885-016-2080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westmoreland KD, Stanley CC, Montgomery ND, et al. Hodgkin Lymphoma, HIV, and Epstein–Barr virus in Malawi: longitudinal results from the Kamuzu Central Hospital Lymphoma Study. Pediatr Blood Cancer. 2017;64:e26302. doi: 10.1002/pbc.26302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopal S, Krysiak R, Liomba NG, et al. Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PLoS ONE. 2013;8(8):e70361. doi: 10.1371/journal.pone.0070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery ND, Liomba NG, Kampani C, et al. Accurate real-time diagnosis of lymphoproliferative disorders in Malawi through clinicopathologic teleconferences: a model for pathology services in Sub-Saharan Africa. Am J Clin Pathol. 2016;146(4):423–430. doi: 10.1093/ajcp/aqw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swaminathan R, Rama R, Shanta V. Lack of active follow-up of cancer patients in Chennai, India: implications for population-based survival estimates. Bull World Health Organ. 2008;86(7):509–515. doi: 10.2471/BLT.07.046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sankaranarayanan R, Black RJ, Swaminathan R, Parkin DM. An overview of cancer survival in developing countries. IARC Sci Publ. 1998;(145):135–173. [PubMed] [Google Scholar]
- 16.Gondos A, Brenner H, Wabinga H, Parkin DM. Cancer survival in Kampala, Uganda. Br J Cancer. 2005;92(9):1808–1812. doi: 10.1038/sj.bjc.6602540. [DOI] [PMC free article] [PubMed] [Google Scholar]