Abstract
Background
Sudden death is a leading cause of death in patients on maintenance hemodialysis (HD). During HD sessions, the gradient between serum and dialysate levels results in rapid electrolytes shifts, which may contribute to arrhythmias and sudden death. Controversies exist on the optimal electrolyte concentration in the dialysate; specifically, it is unclear whether patient outcomes differ among those treated with dialysate potassium (DK) concentration of 3 mEq/L compared to 2 mEq/L.
Study Design
Prospective cohort study
Setting & Participants
55,183 patients from 20 countries in the Dialysis Outcomes and Practice Patterns Study phases 1–5 (1996–2015).
Predictor
DK at study entry.
Outcomes
Cox regression was used to estimate the association between DK and both all-cause mortality and an arrhythmia composite outcome (arrhythmia-related hospitalization or sudden death), adjusting for potential confounders.
Results
During a median follow-up of 16.5 months, 24% of patients died and 7% had an arrhythmia composite outcome. No meaningful difference in clinical outcomes were observed for patients treated with DK 3 vs. 2 mEq/L; the adjusted hazard ratio (95% CI) was 0.96 (0.91, 1.01) for mortality and 0.98 (0.88, 1.08) for the arrhythmia composite. Results were similar across pre-dialysis serum potassium (SK) levels. As in prior studies, higher SK was associated with adverse outcomes. However, DK only had minimal impact on SK measured pre-dialysis (+0.09 mEq/L SK per 1 mEq/L DK; 95% CI: 0.05, 0.14).
Limitations
Data were not available on delivered (vs. prescribed) DK and post-dialysis SK; possible unmeasured confounding.
Conclusions
In combination, these results suggest that approaches other than altering DK concentration (e.g., education on dietary K sources, prescription of K-binding medications) may merit further attention to reduce risks associated with high SK.
Index words: dialysate potassium, hemodialysis, hyperkalemia, mortality, serum potassium
Introduction
Sudden death is a leading cause of death in patients requiring hemodialysis (HD), with 27% of all deaths attributable to arrhythmic mechanisms.1 In thrice weekly maintenance HD, these events tend to cluster in the period just prior to the first dialysis session of the week, when fluid overload and the level of various uremic toxins are highest, and in the period during and immediately following HD sessions.2–4 While a multiplicity of factors contribute to sudden death, it is speculated that the increased risk during and immediately following the HD session is associated with large fluid and electrolyte shifts that occur during this time.3
Hyperkalemia is common in patients with kidney failure due to diminished renal potassium excretion causing disturbances in heart rhythm and cardiac arrest in extreme cases.5,6 High pre-dialysis serum potassium (SK) is recognized as a risk factor for sudden death and all-cause mortality in HD patients.7,8 Potassium has the potential to move freely across the dialyzer membrane during the HD session, typically being transferred from a patient’s blood into the dialysate.9 The dialysate potassium (DK) concentration is a modifiable factor that can alter SK concentrations throughout the HD session and thus potentially impacts the risk for arrhythmias and cardiac arrest. 10 Results of studies examining DK effects on sudden death and all-cause mortality have been mixed. Kovesdy et al.7 advised that hyperkalemic patients with a lower DK bath may have better survival, while two large case-control studies investigated sudden death events occurring during dialysis and concluded there was an increased risk of sudden death for patients dialyzing with DK=1 or even DK=0.11,12 While no recommendation on DK levels has been provided in the Kidney Disease Outcomes Quality Initiative (KDOQI) cardiovascular disease guidelines,13 several recent reviews are in agreement that DK < 2 mEq/L should be avoided, particularly in patients with high pre-dialysis potassium, to avoid a rapid decrease in plasma potassium.6,14–18 Accordingly, anecdotal reports indicate that the use of DK < 2 mEq/L has become increasingly rare. This prompted us to investigate whether DK = 2 mEq/L was still too low, in comparison to a higher DK of 3 mEq/L.
In the absence of conclusive results, many clinicians’ DK prescription often aims to keep SK within an “acceptable” range. Some nephrologists make decisions qualitatively based on clinical judgment and experience, others anecdotally use the “rule of seven” and prescribe DK to make the sum of DK and pre-dialysis SK approximately seven,19 while some facilities’ medical staff elect to provide a uniform DK to all patients. However, optimal prescription practices remain unknown: treating with lower DK promotes greater intradialytic K flux and increases the likelihood of hypokalemia; conversely, treating with a higher DK may predispose patients to hyperkalemia. The former may be worsened among patients with already low pre-dialysis SK and the latter among patients with high pre-dialysis SK. Further, treating high pre-dialysis SK patients with a low DK may cause a rapid intradialytic shift in potassium during the first hour of dialysis. Thus, there is reason to speculate that the effect of DK may be modified based on patients’ pre-dialysis SK.
A previous Dialysis Outcomes and Practice Patterns Study (DOPPS) analysis20 observed associations between sudden death and various modifiable practices: low treatment time, low Kt/V, high ultrafiltration volume, and low DK. Given more recent trends towards higher DK and the lack of clear evidence across studies comparing the two most commonly used DK prescriptions (2 vs. 3 mEq/L), we revisited the issue of DK and clinical outcomes using a larger and more contemporary cohort of patients. In this study, we leveraged data from the international cohort of in-center HD patients in the DOPPS to assess the risks of different DK prescriptions overall and among patients with different SK levels. We also examined the associations between pre-dialysis SK levels and outcomes as well as the association between DK and SK.
Methods
Data source
The DOPPS (http://www.dopps.org) is an international prospective cohort study of patients age 18 or older treated with in-center HD. The study is designed to observe patients over time and correlate practices and outcomes in different medical settings around the world to help researchers and clinicians identify best practices and other modifiable characteristics that improve dialysis patient lives. Patients were randomly selected from national samples of dialysis facilities within each country.21,22 In this analysis, data from participants in the DOPPS phase 1 (1996–2001), phase 2 (2002–2004), phase 3 (2005–2008), phase 4 (2009–2011), and phase 5 (2012–2015) were used. Participating countries included France, Germany, Italy, Japan, Spain, United Kingdom, and the US in phases 1–5, Australia and New Zealand (ANZ), Belgium, Canada, and Sweden in phases 2–5, and the Gulf Cooperation Council (GCC, including Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates), China, Russia, and Turkey in phase 5. US Study approval was obtained by a central Institutional Review Board (study number 98004-19). Additional study approval and patient consent were obtained as required by national and local ethics committee regulations.
Data on demographics, comorbid conditions, laboratory values (single most recent value), and prescriptions were abstracted from medical records using uniform and standardized data collection tools. Mortality and hospitalization events and the primary causes of these events were collected during study follow-up. Patients with pre-dialysis SK and DK data available at DOPPS entry (baseline: single most recent value at study entry) were eligible for this analysis. Patients in Japan were excluded from analyses of clinical outcomes due to lack of variation in the primary exposure variable (DK=2 mEq/L in all patients), but were included in descriptive figures to illustrate the within- and across-country variation. See Figure 1 for eligibility criteria details.
Figure 1. Flow chart of DOPPS patients eligible for analysis.
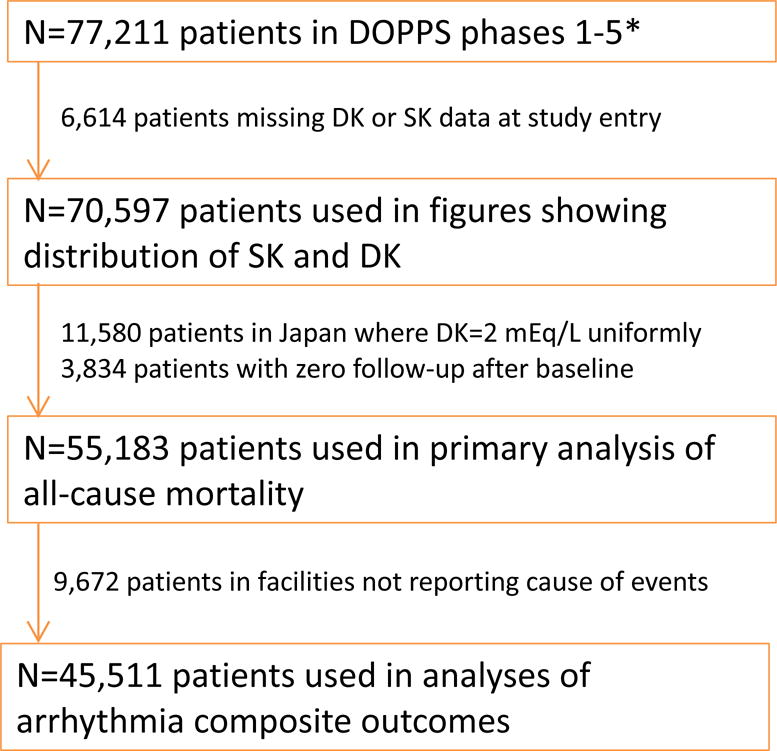
*Current as of September 29, 2015. SK=serum potassium; DK=dialysate potassium.
Statistical analysis
To estimate the association between baseline pre-dialysis SK and all-cause mortality, we used Cox regression stratified by DOPPS phase and country, and by US large dialysis organization when applicable. Proportional hazards were confirmed by examination of log-log survival plots and by testing the interaction between log-time and the exposure of interest. We accounted for facility clustering using robust sandwich covariance estimators. We categorized SK, with 4.0–5.0 mEq/L (50% of patients) as the reference group. Models were analyzed both unadjusted and adjusted for the following baseline covariates: age, sex, vintage, the 13 comorbid conditions listed in Table 1, vascular access, body mass index (BMI), nPCR, serum albumin, calcium, bicarbonate, phosphorus (linear and quadratic term to account for U-shaped association), dialysate bicarbonate, hemoglobin, treatment time, and Kt/V. Time at risk started at study enrollment and ended at the time of death, seven days after leaving the facility due to transfer or change in kidney replacement therapy modality, loss to follow-up, transplantation, or end of study phase (whichever event occurred first). The secondary study outcome was an arrhythmia composite defined as time to the first of any of the following events: death due to either hyperkalemia, hypokalemia, cardiac arrhythmia, or cardiac arrest (cause unknown), or inpatient hospitalization due to atrial fibrillation or other arrhythmia, or a procedure for cardioversion, AICD (defibrillator) or pacemaker placement. We excluded from analyses of the composite outcome facilities lacking information on causes of death and hospitalization.
Table 1a.
Patient characteristics by pre-dialysis serum K
| Serum K (mEq/L) | ||||
|---|---|---|---|---|
| < 4.0 | 4.0 – 5.0 | 5.1 – 6.0 | > 6.0 | |
| N patients (%) | 6300 (11%) | 27525 (50%) | 16959 (31%) | 4399 (8%) |
| Demographics | ||||
| Age (years) | 63.8 ± 15.2 | 63.0 ± 15.2 | 61.8 ± 15.3 | 60.4 ± 15.3 |
| Sex (% men) | 56% | 58% | 59% | 56% |
| Black race (%) | 20% | 17% | 11% | 7% |
| Vintage (years) | 2.0 ± 3.7 | 2.9 ± 4.4 | 4.0 ± 5.2 | 4.5 ± 5.4 |
| Hemodialysis-related characteristics | ||||
| Central venous catheter use (%) | 47% | 34% | 26% | 25% |
| Single Pool Kt/V | 1.41 ± 0.35 | 1.46 ± 0.34 | 1.46 ± 0.33 | 1.44 ± 0.34 |
| Treatment time (min) | 219 ± 37 | 225 ± 38 | 230 ± 39 | 233 ± 39 |
| Dialysate Potassium (mEq/L) | 2.4 ± 0.7 | 2.2 ± 0.6 | 2.1 ± 0.6 | 2.0 ± 0.6 |
| Laboratory and biometric measurements | ||||
| Body mass index (kg/m2) | 26.6 ± 6.4 | 26.6 ± 6.3 | 26.0 ± 6.0 | 25.2 ± 5.5 |
| Pre-dialysis SBP (mm Hg) | 144 ± 24 | 145 ± 23 | 145 ± 23 | 145 ± 23 |
| Hemoglobin (g/dL) | 10.7 ± 1.6 | 11.0 ± 1.5 | 11.2 ± 1.6 | 11.2 ± 1.7 |
| Normalized PCR (g/kg/day) | 0.85 ± 0.24 | 0.95 ± 0.25 | 1.04 ± 0.26 | 1.10 ± 0.27 |
| Serum Creatinine (mg/dL) | 6.6 ± 2.8 | 7.7 ± 3.0 | 8.9 ± 2.9 | 9.7 ± 3.0 |
| Serum Albumin (g/dL) | 3.46 ± 0.61 | 3.65 ± 0.53 | 3.76 ± 0.51 | 3.81 ± 0.52 |
| WBC count (103 cells/mm3) | 7.6 ± 2.9 | 7.3 ± 2.5 | 7.2 ± 2.4 | 7.4 ± 2.5 |
| Serum Bicarbonate (mEq/L) | 23.9 ± 4.0 | 22.9 ± 3.6 | 22.1 ± 3.6 | 21.3 ± 3.8 |
| Serum Calcium (mg/dL) | 8.8 ± 0.9 | 9.0 ± 0.9 | 9.1 ± 0.9 | 9.2 ± 0.9 |
| Serum Phosphorus (mg/dL) | 4.7 ± 1.7 | 5.2 ± 1.7 | 5.6 ± 1.8 | 6.0 ± 2.0 |
| Serum Potassium (mEq/L) | 3.6 ± 0.3 | 4.5 ± 0.3 | 5.5 ± 0.3 | 6.5 ± 0.4 |
| Medications (%) | ||||
| ACEi | 19% | 23% | 25% | 23% |
| ARB | 12% | 13% | 15% | 15% |
| Diuretic | 35% | 30% | 24% | 18% |
| Potassium-binding resin | 4% | 6% | 11% | 17% |
| Comorbid conditions (%) | ||||
| Coronary artery disease | 43% | 42% | 41% | 38% |
| Cancer (non-skin) | 13% | 13% | 12% | 11% |
| Other cardiovascular disease | 31% | 30% | 32% | 31% |
| Cerebrovascular disease | 17% | 16% | 15% | 14% |
| Heart failure | 36% | 33% | 32% | 30% |
| Diabetes | 48% | 46% | 40% | 34% |
| Gastrointestinal bleeding | 7% | 5% | 5% | 5% |
| Hypertension | 84% | 84% | 84% | 82% |
| Lung disease | 13% | 13% | 13% | 13% |
| Neurologic disease | 11% | 10% | 10% | 10% |
| Psychiatric disorder | 21% | 18% | 18% | 19% |
| Peripheral vascular disease | 27% | 26% | 26% | 25% |
| Recurrent cellulitis, gangrene | 9% | 9% | 9% | 9% |
Mean ± SD or % shown; N=55,183 patients who were included in all-cause mortality analyses.
ACEi=angiotensin-converting-enzyme inhibitor; ARB=Angiotensin II receptor antagonist; nPCR=normalized protein catabolic rate; SBP=systolic blood pressure; WBC=white blood cell.
Similarly, we used Cox regression to estimate the associations of baseline DK with all-cause mortality and the arrhythmia composite outcome, both unadjusted and adjusted as above. DK was categorized into three groups, with 2.0–2.5 mEq/L as the reference. We also estimated the effect of DK as a continuous variable using instrumental variable analyses, performed as described below, but modified to use a Cox model in the second stage. We then assessed whether the association between DK and clinical outcomes was modified by level of pre-dialysis SK. Because DK=2.0 and DK=3.0 mEq/L are by far the most common prescriptions in use, we restricted this comparison to patients with either DK=2.0 (reference) or 3.0 mEq/L and tested the association among four subgroups of patients based on pre-dialysis SK level.
To assess the association between DK and pre-dialysis SK, we first modeled SK as the outcome variable in a linear mixed model to account for facility clustering, treating DK as a continuous exposure variable. We modeled the relationship adjusting for DOPPS phase and country only, and subsequently adjusting as above. Because we suspected a large degree of confounding by indication (i.e., patients with lower pre-dialysis SK being individualized to receive a higher DK), we performed an instrumental variable two-stage least squares (2SLS) analysis to account for this bias. While violations of other assumptions of instrumental variable analyses cannot be formally assessed, we demonstrated the strength of the DOPPS facility as the instrument23: F = 13.6. Additionally, we observed better balance across patient characteristics by quartile of facility mean DK than by patient DK (Table S2). While using facility as the instrument can be an effective strategy to address unmeasured patient-level confounding, group-level confounding can also be a concern.24 We thus additionally adjusted for six dialysis unit practices: the percentage of patients in a facility with a catheter, with spKt/V < 1.2, with albumin < 3.5 g/dL, and with phosphorus ≥ 5.5 mg/dL, and mean within-facility levels of hemoglobin and dialysate bicarbonate. Because K-binding resins are also likely prescribed on the basis of SK, we treated K resins as an additional endogenous variable to simultaneously account for the confounding by indication caused by DK and potassium-binding resin use.25 To test the robustness of the instrumental variable findings, sensitivity analyses were conducted using linear mixed models with: (1) crude facility mean DK as the exposure, and (2) restricting to facilities prescribing a uniform DK to ≥ 90% of patients, a strategy similar to that employed by Hecking et al. for dialysate sodium studies.26
We assumed the data were missing at random; missing covariate values were addressed by multiple imputation using the chained equation method by IVEware.27,28 Missing values were sequentially updated using the bootstrap or Markov Chain Monte Carlo method, based on multiple regression models with other variables as covariates. This procedure was carried out for 10 cycles, thereby constructing an imputed data set. Results from five such imputed data sets were combined for the final analysis using Rubin’s formula.29 Largely due to the high number of model covariates, 67% of patients were missing data for at least one adjustment covariate. The proportion of missing data was below 10% for all variables used for covariate adjustment, with the exception of nPCR (35%), Kt/V (32%), serum bicarbonate (22%), and dialysate bicarbonate (18%). All analyses were conducted using SAS software, version 9.4 (SAS institute, Cary, NC).
Results
Pre-dialysis serum K levels
The distribution of SK, by country, in DOPPS phase 5 is shown in Figure 2a. Mean SK was highest in Russia (5.3 mEq/L) and lowest in the United States (US) (4.6 mEq/L). Trend analyses (Figure 2b) demonstrated that SK has decreased over the past 20 years in Europe, Australia, New Zealand (Europe/ANZ) and Japan. In North America, mean SK remained fairly constant. In each country, SK levels collected at the first HD session of the week (Monday/Tuesday) were slightly higher than levels collected midweek (Wednesday/Thursday); the difference ranged from 0.01 mEq/L in China to 0.19 mEq/L in Germany.
Figure 2a. Pre-dialysis serum K distribution by country in DOPPS phase 5 (2012–2015).
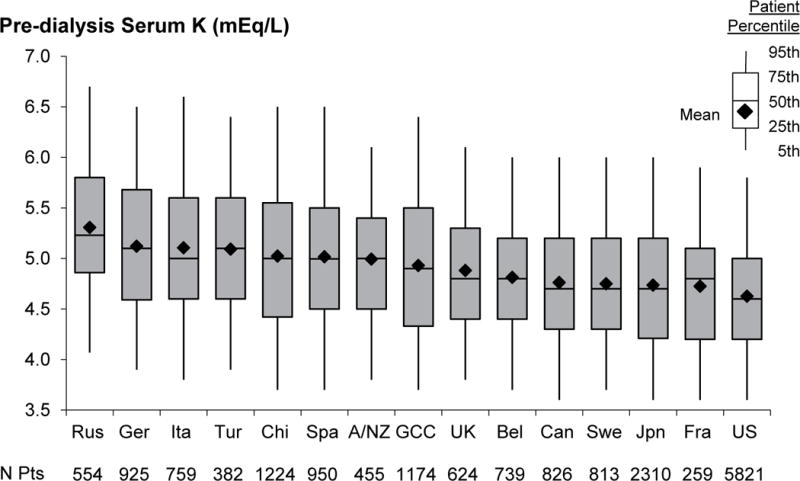
N=17,815 patients. Country abbreviations: A/NZ=Australia and New Zealand, Bel=Belgium, Can=Canada, Chi=China, Fra=France, GCC=Gulf Cooperation Council (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates), Ger=Germany, Ita=Italy, Jpn=Japan, Rus=Russia, Spa=Spain, Swe=Sweden, Tur=Turkey, UK=United Kingdom, US=United States.
Figure 2b. Temporal trends in pre-dialysis serum K by DOPPS region (1996–2015).
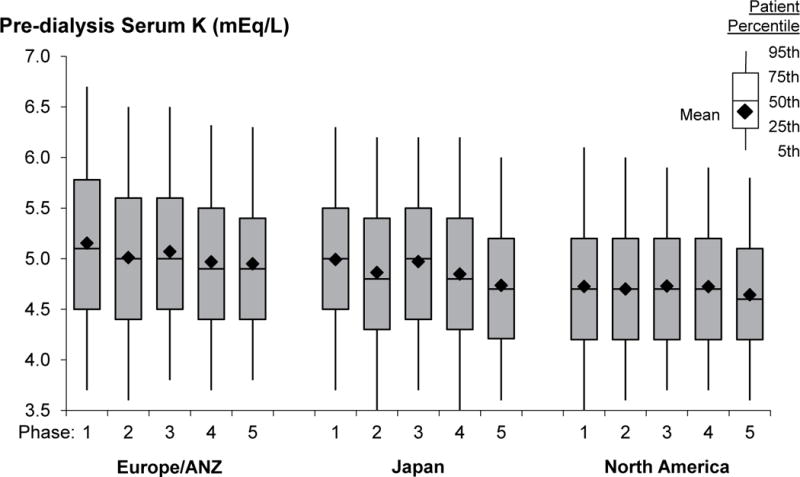
N=67,263 patients. DOPPS phase 1: 1996–2001, phase 2: 2002–2004, phase 3: 2005–2008, phase 4: 2009–2011, phase 5: 2012–2015. A/NZ=Australia and New Zealand. Note that countries recently joining the DOPPS in phase 5 (N=3,334 patients) are not represented in this figure.
Dialysate K prescription
Figure 3a shows DK prescription patterns in each DOPPS country during phase 5 (2012–15). DK of 2.0–2.5 mEq/L was the most common prescription worldwide, prescribed to 75% of patients in the US and >99% of patients in Japan. DK was highest in Germany, where DK of 3.0 mEq/L or greater was used in 75% of patients. Prescription of DK of 1.0–1.5 mEq/L was primarily concentrated in Spain. The practice pattern of prescribing a uniform DK to ≥ 90% of patients in the facility varied widely across countries (table insert below Figure 3a). In addition to Japan, uniform DK prescription of 2.0 mEq/L was common in China (84% of facilities) and Turkey (79%). In contrast, uniform DK prescription was less common in the US (27% of facilities) and rarely used in Germany (5%) and Canada (5%). The trend analyses (Figure 3b) show that DK has been steadily increasing in Europe/ANZ across the DOPPS phases. In North America, recent trends show a decline in the proportion of patients prescribed DK < 2 mEq/L, down to 5% in phase 5.
Figure 3a. Dialysate K distribution by country in DOPPS phase 5 (2012–2015).
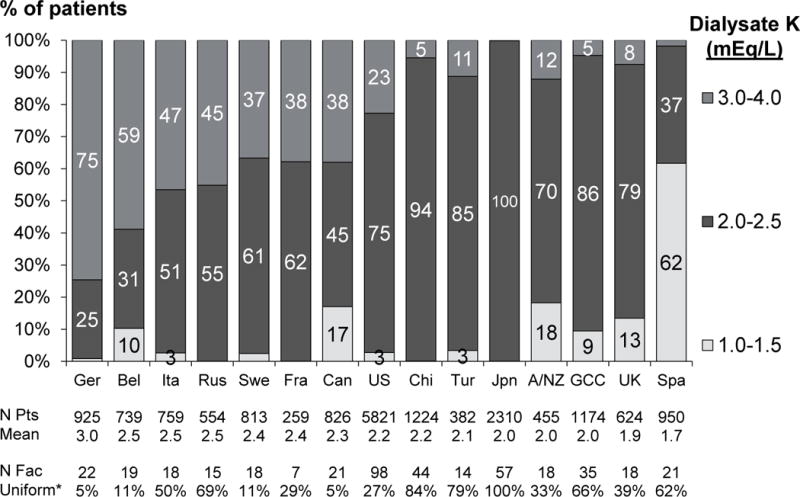
*Indicates proportion of facilities prescribing a uniform dialysate K to ≥90% of patients. N=17,815 patients. Country abbreviations: A/NZ=Australia and New Zealand, Bel=Belgium, Can=Canada, Chi=China, Fra=France, GCC=Gulf Cooperation Council (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates), Ger=Germany, Ita=Italy, Jpn=Japan, Rus=Russia, Spa=Spain, Swe=Sweden, Tur=Turkey, UK=United Kingdom, US=United States. In the DK 3.0–4.0 group, 89% of patients had DK=3.0 mEq/L; in the DK 2.0–2.5 group, 98% of patients had DK=2.0 mEq/L; the DK 1.0–1.5 group was primarily concentrated in Spain, where 98% of patients prescribed DK 1.0–1.5 had DK=1.5 mEq/L; elsewhere, 75% of patients in the 1.0–1.5 group had DK 1.0 mEq/L.
Figure 3b. Temporal trends in dialysate K by DOPPS region (1996–2015).
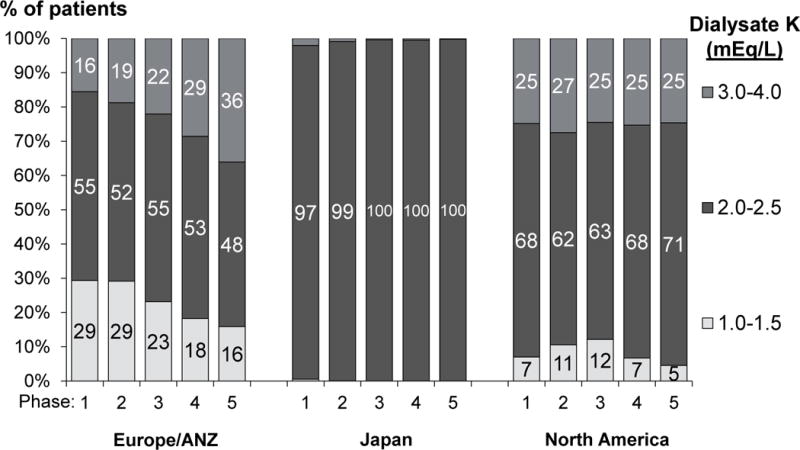
N=67,263 patients. DOPPS phase 1: 1996–2001, phase 2: 2002–2004, phase 3: 2005–2008, phase 4: 2009–2011, phase 5: 2012–2015. A/NZ=Australia and New Zealand. Note that countries recently joining the DOPPS in phase 5 (N=3,334 patients) are not represented in this figure.
Study sample characteristics
Descriptive patient characteristics, by SK and DK categories, are shown in Tables 1a and 1b. Patients in the lowest SK group differed from patients in the highest SK group in many ways; for instance they were older, had shorter dialysis vintage, more catheter use, lower normalized protein catabolic rate (nPCR), and lower creatinine, albumin, and phosphorus concentrations. Patient characteristics also differed across DK prescriptions; for instance, those in the highest DK group were older, had shorter dialysis vintage, more catheter use, lower nPCR, lower creatinine and albumin concentrations, lower SK, and were more likely to have been prescribed a diuretic.
Table 1b.
Patient characteristics by dialysate K
| All | Dialysate K (mEq/L) | |||
|---|---|---|---|---|
| 1.0 – 1.5 | 2.0 – 2.5 | 3.0 – 4.0 | ||
| N patients (%) | 55183 | 8109 (15%) | 33497 (61%) | 13577 (25%) |
| Demographics | ||||
| Age (years) | 62.5 ± 15.3 | 61.4 ± 15.4 | 61.6 ± 15.4 | 65.5 ± 14.5 |
| Sex (% men) | 58% | 60% | 58% | 56% |
| Black race (%) | 15% | 6% | 18% | 11% |
| Vintage (years) | 3.3 ± 4.7 | 4.3 ± 5.5 | 3.5 ± 4.8 | 2.1 ± 3.7 |
| Hemodialysis-related characteristics | ||||
| Central venous catheter use (%) | 32% | 26% | 30% | 41% |
| Single Pool Kt/V | 1.45 ± 0.34 | 1.46 ± 0.33 | 1.46 ± 0.33 | 1.42 ± 0.35 |
| Treatment time (min) | 226 ± 39 | 227 ± 39 | 227 ± 37 | 225 ± 43 |
| Dialysate Potassium (mEq/L) | 2.2 ± 0.6 | 1.3 ± 0.3 | 2.0 ± 0.1 | 3.1 ± 0.3 |
| Laboratory and biometric measurements | ||||
| Body mass index (kg/m2) | 26.3 ± 6.2 | 25.5 ± 5.5 | 26.4 ± 6.3 | 26.6 ± 6.1 |
| Pre-dialysis SBP (mm Hg) | 145 ± 23 | 144 ± 23 | 146 ± 23 | 142 ± 23 |
| Hemoglobin (g/dL) | 11.1 ± 1.6 | 11.3 ± 1.6 | 11.0 ± 1.6 | 10.9 ± 1.6 |
| Normalized PCR (g/kg/day) | 0.98 ± 0.26 | 1.07 ± 0.26 | 0.98 ± 0.26 | 0.91 ± 0.25 |
| Serum Creatinine (mg/dL) | 8.1 ± 3.1 | 8.7 ± 2.9 | 8.5 ± 3.1 | 6.9 ± 2.8 |
| Serum Albumin (g/dL) | 3.68 ± 0.54 | 3.74 ± 0.51 | 3.69 ± 0.53 | 3.59 ± 0.58 |
| WBC count (103 cells/mm3) | 7.3 ± 2.5 | 7.2 ± 2.4 | 7.3 ± 2.6 | 7.4 ± 2.6 |
| Serum Bicarbonate (mEq/L) | 22.7 ± 3.7 | 22.7 ± 3.5 | 22.7 ± 3.7 | 22.8 ± 3.8 |
| Serum Calcium (mg/dL) | 9.0 ± 0.9 | 9.2 ± 0.9 | 9.0 ± 0.9 | 8.9 ± 0.9 |
| Serum Phosphorus (mg/dL) | 5.3 ± 1.8 | 5.4 ± 1.8 | 5.4 ± 1.8 | 5.1 ± 1.7 |
| Serum Potassium (mEq/L) | 4.9 ± 0.8 | 5.1 ± 0.8 | 4.9 ± 0.8 | 4.7 ± 0.8 |
| Medications (%) | ||||
| ACEi | 23% | 21% | 23% | 24% |
| ARB | 14% | 14% | 13% | 14% |
| Diuretic | 28% | 16% | 24% | 43% |
| Potassium-binding resin | 8% | 11% | 9% | 6% |
| Comorbid conditions (%) | ||||
| Coronary artery disease | 42% | 39% | 41% | 46% |
| Cancer (non-skin) | 12% | 12% | 12% | 15% |
| Other cardiovascular disease | 31% | 33% | 28% | 35% |
| Cerebrovascular disease | 15% | 15% | 15% | 17% |
| Heart failure | 33% | 31% | 33% | 33% |
| Diabetes | 43% | 35% | 44% | 47% |
| Gastrointestinal bleeding | 6% | 5% | 5% | 6% |
| Hypertension | 84% | 83% | 83% | 85% |
| Lung disease | 13% | 14% | 12% | 15% |
| Neurologic disease | 10% | 10% | 10% | 11% |
| Psychiatric disorder | 18% | 19% | 18% | 19% |
| Peripheral vascular disease | 26% | 27% | 25% | 29% |
| Recurrent cellulitis, gangrene | 9% | 9% | 8% | 9% |
Mean ± SD or % shown; N=55,183 patients who were included in all-cause mortality analyses.
ACEi=angiotensin-converting-enzyme inhibitor; ARB=Angiotensin II receptor antagonist; nPCR=normalized protein catabolic rate; SBP=systolic blood pressure; WBC=white blood cell.
In the DK 3.0–4.0 group, 89% of patients had DK=3.0 mEq/L; in the DK 2.0–2.5 group, 98% of patients had DK=2.0 mEq/L; the DK 1.0–1.5 group was primarily concentrated in Spain, where 98% of patients prescribed DK 1.0–1.5 had DK=1.5 mEq/L; elsewhere, 75% of patients in the 1.0–1.5 group had DK 1.0 mEq/L.
Serum K and clinical outcomes
Among the 55,183 patients from DOPPS phases 1–5 included in outcome analyses, median (interquartile range) follow-up was 16.5 (8.1, 25.5) months and 13,114 (24%) died during follow-up, resulting in a mortality rate of 16.1 per 100 patient-years. Table 2 shows that compared with the reference group of SK 4.0–5.0 mEq/L, lower SK but not higher SK was associated with mortality in unadjusted analysis; however, after comprehensive multivariable adjustment, particularly for nutritional indicators (Table S1), the shape of the association changed, now with higher SK but not lower SK being associated with mortality. Among the 45,511 patients eligible for the cause-specific outcome analysis, 3300 (7%) had an arrhythmia composite event during follow-up. The adjusted association between SK and the composite arrhythmia outcome appeared approximately monotonic, with increased risk for patients with higher SK levels (Table 2).
Table 2.
Associations between pre-dialysis serum K and clinical outcomes
| HR (95% CI), All-cause mortality | HR (95% CI), Arrhythmia composite^ | ||||
|---|---|---|---|---|---|
| Serum K (mEq/L) |
N patients (%) | Unadjusted | Adjusted* | Unadjusted | Adjusted* |
| < 4.0 | 6153 (11%) | 1.18 (1.12–1.24) | 1.03 (0.97–1.09) | 0.99 (0.88–1.11) | 0.94 (0.83–1.05) |
| 4.0 – 5.0 | 27107 (50%) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 5.1 – 5.5 | 10635 (20%) | 0.95 (0.91–0.99) | 1.02 (0.97–1.07) | 0.97 (0.89–1.07) | 1.00 (0.91–1.10) |
| 5.6 – 6.0 | 6238 (11%) | 1.02 (0.96–1.08) | 1.13 (1.06–1.20) | 1.05 (0.95–1.17) | 1.07 (0.96–1.20) |
| > 6.0 | 4403 (8%) | 1.00 (0.93–1.07) | 1.12 (1.04–1.21) | 1.16 (1.02–1.32) | 1.21 (1.05–1.38) |
HR: Hazard ratio; Cox models stratified by DOPPS phase, country, US large dialysis organization (all-cause mortality only), and accounted for facility clustering.
Arrhythmia composite includes sudden death or arrhythmia-related hospitalizations.
For the arrhythmia composite outcome, 45,511 patients (3300 events, 7%) were eligible for the analysis after excluding facilities where cause of death and/or hospitalization was not obtained.
Adjustments: age, sex, vintage, 13 comorbidities, vascular access, BMI, albumin, nPCR, serum Ca, serum phosphorus, serum phosphorus squared, serum bicarbonate, dialysate bicarbonate, hemoglobin, treatment time, Kt/V.
Dialysate K and clinical outcomes
We observed higher unadjusted mortality for patients with high DK (Table 3). After comprehensive adjustment for potential confounders, the hazard ratio (HR) (95% CI) for mortality was 0.95 (0.90, 1.00) for patients treated with DK 3.0–4.0, and 1.04 (0.97–1.11) for patients treated with DK 1.0–1.5 mEq/L, compared to the reference group of 2.0–2.5 mEq/L. Analysis of the arrhythmia composite outcome is also shown in Table 3. Using instrumental variable methods, the HR (95% CI) per 1 mEq/L higher DK was 0.99 (0.92, 1.07) for all-cause mortality and 0.96 (0.82, 1.12) for the arrhythmia composite outcome.
Table 3.
Associations between dialysate K and clinical outcomes
| HR (95% CI), All-cause mortality |
HR (95% CI), Arrhythmia composite^ |
||||
|---|---|---|---|---|---|
| Dialysate K (mEq/L) | N patients (%) | Unadjusted | Adjusted* | Unadjusted | Adjusted* |
| 1.0 – 1.5 | 8114 (15%) | 0.96 (0.90–1.03) | 1.04 (0.97–1.11) | 1.09 (0.95–1.24) | 1.14 (1.00–1.30) |
| 2.0 – 2.5 | 33017 (61%) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 3.0 – 4.0 | 13405 (25%) | 1.13 (1.07–1.18) | 0.95 (0.90–1.00) | 1.05 (0.96–1.15) | 0.95 (0.86–1.04) |
HR: Hazard ratio; Cox models stratified by DOPPS phase, country, US large dialysis organization (all-cause mortality only), and accounted for facility clustering.
Arrhythmia composite includes sudden death or arrhythmia-related hospitalizations.
For the arrhythmia composite outcome, 45,511 patients (3300 events, 7%) were eligible for the analysis after excluding facilities where cause of death and/or hospitalization was not obtained.
Adjustments: age, sex, vintage, 13 comorbidities, vascular access, BMI, albumin, nPCR, serum Ca, serum phosphorus, serum phosphorus squared, serum bicarbonate, dialysate bicarbonate, hemoglobin, treatment time, Kt/V.
In Table 4, we show the associations with clinical outcomes of DK 3.0 mEq/L vs. 2.0 mEq/L at various levels of pre-dialysis SK. Again, the estimated differences in mortality risk were minimal with HRs ranging from 0.94 to 1.03 across the four SK subgroups, with no discernible pattern. Similarly, we found no associations between DK and the arrhythmia composite outcome in any of the SK strata. Further, there was no evidence for effect modification of DK by SK for all-cause mortality (p for interaction = 0.7) or the arrhythmia composite outcome (p for interaction = 0.7).
Table 4.
Associations between dialysate K and clinical outcomes, by pre-dialysis serum K concentration
| All patients | Pre-dialysis serum K (mEq/L) | ||||
|---|---|---|---|---|---|
| Dialysate K (mEq/L) | < 4.0 | 4.0 – 5.0 | 5.1 – 6.0 | > 6.0 | |
| All-cause mortality | |||||
| DK 3 vs. DK 2 (Ref.) |
0.96 (0.91–1.01) | 1.03 (0.91–1.17) | 0.96 (0.90–1.03) | 0.94 (0.85–1.03) | 0.97 (0.78–1.20) |
| Arrhythmia composite | |||||
| DK 3 vs. DK 2 (Ref.) |
0.98 (0.88–1.08) | 1.13 (0.86–1.47) | 0.91 (0.80–1.04) | 1.06 (0.89–1.27) | 1.15 (0.81–1.62) |
Hazard ratio (95% CI) shown.
Cox models stratified by DOPPS phase, country, US large dialysis organization (all-cause mortality only), and accounted for facility clustering.
Arrhythmia composite includes sudden death or arrhythmia-related hospitalizations.
N patients (events) in all-cause mortality analyses: N=6300 (1642) for SK < 4.0, N=27525 (6473) for SK 4.0–5.0, 16959 (3985) for SK 5.0–6.0, 4399 (1014) for SK > 6.0.
N patients (events) in arrhythmia composite analyses: N=5105 (350) for SK < 4.0, N=21997 (1607) for SK 4.0–5.0, 14428 (1039) for SK 5.0–6.0, 3981 (304) for SK > 6.0.
Adjustments: age, sex, vintage, 13 comorbidities, vascular access, BMI, albumin, nPCR, serum Ca, serum phosphorus, serum phosphorus squared, serum bicarbonate, dialysate bicarbonate, hemoglobin, treatment time, Kt/V.
Association between dialysate and serum K
In a linear regression model adjusted only for DOPPS phase and country, we observed an inverse association between DK and pre-dialysis SK (−0.35 mEq/L SK per 1 mEq/L DK; 95% CI: −0.37, −0.34). After multivariate adjustment for confounders, the inverse association remained (−0.25, 95% CI: −0.26, −0.24). In an instrumental variable analysis, however, we observed a weak positive association between DK and SK (+0.09 mEq/L SK per 1 mEq/L DK, 95% CI: +0.05, +0.14). Sensitivity analyses using (1) facility mean DK as the exposure and (2) restricting to facilities that prescribed a uniform DK to ≥90% of patients resulted in findings consistent with the instrumental variable analysis.
Discussion
In the DOPPS, a large international prospective cohort study of HD patients, where there were considerable variations in pre-dialysis SK levels and practice patterns of DK prescription, high SK was associated with increased all-cause mortality and arrhythmia/sudden death after multivariable adjustment. When comparing the two most common DK prescriptions (DK=3 vs. DK=2 mEq/L), we did not find evidence of differential risk of adverse events, overall or at any level of pre-dialysis SK. We also observed only a minimal impact of DK on pre-dialysis SK in an instrumental variable analysis designed to minimize confounding by indication.
Consistent with prior studies, we observed an elevated risk of both all-cause mortality and an arrhythmia composite outcome in patients with high levels of SK. Similar to Kovesdy et al.,7 the strong unadjusted association between hypokalemia and adverse events was substantially confounded and mostly attenuated by adjustment for case-mix and indicators of malnutrition, as very low pre-dialysis SK is more characteristic of patients in generally poor health.
The use of DK < 2 mEq/L has declined across study phases in North America and Europe; in the most recent DOPPS phase (2012–2015), the proportion of patients with DK < 2 mEq/L was down to 5% in North America and 16% in Europe/ANZ (only 6% in Europe/ANZ outside of Spain). Thus, analyses of DK 1.0–1.5 mEq/L compared to 2 mEq/L may be limited in scope and generalizability. Since they are the most frequent prescriptions and given the lack of data on their comparative impact of clinical outcomes, we focused analyses on the practice of DK=3 vs. 2 mEq/L.
Prior results linking DK level to clinical outcomes have been mixed,7, 11, 12 although recent editorials recommend avoiding DK < 2 mEq/L, particularly in patients with high pre-dialysis SK.6,14–18 The potential hazards of very low (< 2 mEq/L) DK are triggered by a larger removal of potassium during HD causing intradialytic and post-dialysis hypokalemia, and often a rapid rebound of potassium levels post-dialysis, contributing to cardiac instability.30 In our primary analysis comparing more common DK prescriptions, we found a similar risk of adverse events; any differences were modest and unlikely to be clinically important. The comparison of all-cause mortality risk using DK ≥ 3.0 vs. 2.0–2.5 mEq/L was qualitatively similar to a previous DOPPS publication, 20 which found an 8% increased risk of mortality for the lower DK. Jadoul et al.20 observed a stronger association using instrumental variable analyses, but a larger sample size of more contemporary patients and small differences in methodology used in the current study combined to result in instrumental variable analyses more consistent with the standard methods.
Rather than considering serum and dialysate K as independent risk factors, minimizing the potassium gradient, defined as the difference between the patient’s pre-dialysis SK and DK, is often recommended.7,31, 32 However, a naïve analysis of gradient K and mortality would be driven by SK because variability in gradient K is driven largely by SK and high SK is associated with worse outcomes. Further, an analysis of gradient K adjusted for SK is identical to an analysis of DK adjusted for SK. To more precisely test whether a large K gradient is associated with adverse events, we instead investigated whether a lower DK (2.0 mEq/L) would be particularly harmful in combination with a high SK (> 6.0 mEq/L, resulting in a gradient K > 4.0 mEq/L), but found minimal association among this subgroup of potentially high-risk patients (Table 4).
After accounting for confounding by indication, we observed only a minimal effect of DK on pre-dialysis SK. While lower DK leads to lower SK throughout and immediately following the HD session,10,30,33 we would not expect to observe a strong association with SK levels measured pre-dialysis, 2–3 days after exposure to DK. Thus interventions to avoid chronic hyperkalemia, such as prescription of K-binding medications and/or education to reduce dietary K intake, may be more effective than lowering DK.
Strengths of our study include a very large sample size, capture of representative patients in typical care settings, detailed data collection of potential confounders, causes of death and hospitalization, and considerable variation in practice patterns which facilitated analyses.
Our study also has several limitations. First, because of its observational design, this study cannot estimate the causal impact of SK and DK on the risk of adverse events. While adjustment for a comprehensive set of potential confounders in both standard Cox regression and instrumental variable analyses helps to mitigate bias, residual confounding may remain. Second, our analysis of the arrhythmia composite outcome is limited by missingness and potential misclassification of the causes of death and hospitalization; our large sample is generally considered a strength but in this case smaller studies may be able to characterize these causes more uniformly. Third, we did not have data on post-dialysis SK. Because the association between potassium and adverse events may be mediated by post-dialysis SK level, these measurements could have informed these analyses. Finally, only prescribed DK information was available; in cases where the patient may be individualized to a DK that differs from the prescribed DK for each HD session based on pre-dialysis SK measurement, our data do not capture the precise DK administered. This may be especially problematic in cases where the DK administered during the treatment directly preceding an arrhythmic event differed from the standing prescription. Similarly, DK profiling, the varying of DK during a single HD session,31,32,34 was not captured in the DOPPS and thus we cannot calculate its prevalence nor speculate on its effect on clinical outcomes.
Despite the limitations, these findings have important implications for DK prescribing practices and future research. We did not find evidence supporting a clinically meaningful difference in mortality or arrhythmias comparing DK of 3 vs. 2 mEq/L at any level of pre-dialysis SK, and thus cannot provide a recommendation for any immediate changes in practice. Long-term, our results support equipoise for future research of an easily modifiable practice pattern in a randomized setting. As previously reported, high pre-dialysis SK was associated with increased risk of adverse events. However, we observed minimal association between DK and SK measured before dialysis. In combination, these results suggest that approaches other than altering DK concentration (e.g., education on dietary K sources, prescription of K-binding medications) may merit further attention to reduce risks associated with high SK.
Supplementary Material
Table S1. Association between serum K and all-cause mortality, by level of adjustment. Hazard ratio (95% CI) shown; Cox models stratified by DOPPS phase, country, US large dialysis organization, and accounted for facility clustering. Median (interquartile range) follow-up was 16.5 (8.1, 25.5) months and 13,114 patients (24%) died during follow-up. Level 1: BMI, albumin, nPCR, serum calcium, serum phosphorus, serum phosphorus squared, serum bicarbonate. Level 2: dialysate bicarbonate, hemoglobin, treatment time, single pool Kt/V.
Table S2. Patient characteristics by quartile of facility mean dialysate K. Mean ± SD or % shown; N=55,158 of 55,183 patients who were included in all-cause mortality analyses; N=25 excluded due to small number of eligible patients (N < 7) in facility. ACEi=angiotensin-converting-enzyme inhibitor; ARB=Angiotensin II receptor antagonist; nPCR=normalized protein catabolic rate; SBP=systolic blood pressure; WBC=white blood cell.
Acknowledgments
The DOPPS program is supported by Amgen, Kyowa Hakko Kirin, AbbVie, Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma. Additional support for specific projects and countries is provided by Keryx Biopharmaceuticals, Merck Sharp & Dohme Corp., Proteon Therapeutics, Relypsa, and F. Hoffmann-LaRoche; in Canada by Amgen, BHC Medical, Janssen, Takeda, and the Kidney Foundation of Canada (for logistics support); in Germany by Hexal, DGfN, Shire, and the WiNe Institute; and for PDOPPS in Japan by the Japanese Society for Peritoneal Dialysis (JSPD). All support is provided without restrictions on publications.
SMB is employed by DaVita has been on advisory boards for Keryx, Otsuka, Bayer, AstraZeneca. SMB’s wife is employed by AstraZeneca. LAU is employed by and owns stock in Fresenius Medical Care. DEW receives salary support for research projects from Dialysis Clinic Inc. FWM is employed by and owns stock in Fresenius Medical Care. ARN is employed by DaVita. Dr. Jadoul has been a speaker for Fresenius Pharma (marketing Sorbisterit = K binder) and participated in an Ad Board organized by ZS Pharma (other K binder under development). WCW reports having received honoraria for service on scientific advisory, event adjudication, or data safety monitoring boards for Akebia, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Medtronic, Relypsa, Vifor Fresenius Medical Care Renal Pharma, and Zoll. BMR has received speaker fees for Kyowa Hakko Kirin. FT has received honorarium from DSI and is a member of the AAKP medical advisory board. The other authors declare that they have no relevant financial interests.
Footnotes
Contributions: research idea and study design: AK, JZ, SMB, LAU, DEW, FKP, BMR, FT; data acquisition: AK, BMR, FT; data analysis/interpretation: AK, JZ, SMB, LAU, DEW, FWM, ARN, MJ, FL, WCW, FKP, BMR, FT; statistical analysis: AK, JZ, SMB, LAU, DEW, WCW, FKP, BMR, FT; supervision or mentorship: SMB, LAU, DEW, FWM, ARN, MJ, FL, WCW, FKP, BMR, FT. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. AK takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1.Herzog CA, Mangrum JM, Passman R. Sudden cardiac death and dialysis patients. Semin Dial. 2008;21(4):300–307. doi: 10.1111/j.1525-139X.2008.00455.x. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365(12):1099–1107. doi: 10.1056/NEJMoa1103313. [DOI] [PubMed] [Google Scholar]
- 3.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney Int. 2006;69(12):2268–2273. doi: 10.1038/sj.ki.5000446. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Schaubel DE, Kalbfleisch JD, et al. Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule affects day-of-week mortality. Kidney Int. 2012;81(11):1108–15. doi: 10.1038/ki.2011.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perazella MA, Mahnensmith RL. Hyperkalemia in the elderly: drugs exacerbate impaired potassium homeostasis. J Gen Intern Med. 1997;12(10):646–656. doi: 10.1046/j.1525-1497.1997.07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labriola L, Jadoul M. Sailing between Scylla and charybdis: the high serum K-low dialysate K quandary. Semin Dial. 2014;27(5):463–471. doi: 10.1111/sdi.12252. [DOI] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Regidor DL, Mehrotra R, et al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2(5):999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 8.Genovesi S, Valsecchi MG, Rossi E, et al. Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24(8):2529–2536. doi: 10.1093/ndt/gfp104. [DOI] [PubMed] [Google Scholar]
- 9.Agar BU, Culleton BF, Fluck R, Leypoldt JK. Potassium kinetics during hemodialysis. Hemodial Int. 2015;19(1):23–32. doi: 10.1111/hdi.12195. [DOI] [PubMed] [Google Scholar]
- 10.Hou S, McElroy PA, Nootens J, Beach M. Safety and efficacy of low-potassium dialysate. Am J Kidney Dis. 1989;13(2):137–143. doi: 10.1016/s0272-6386(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 11.Karnik JA, Young BS, Lew NL, et al. Cardiac arrest and sudden death in dialysis units. Kidney Int. 2001;60(1):350–357. doi: 10.1046/j.1523-1755.2001.00806.x. [DOI] [PubMed] [Google Scholar]
- 12.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79(2):218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. Am J Kidney Dis. 2005;45(suppl 3):S1–S154. [PubMed] [Google Scholar]
- 14.Basile C, Lomonte C. A Neglected Issue in Dialysis Practice: Haemodialysate. Clin Kidney J. 2015;8(4):393–399. doi: 10.1093/ckj/sfv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locatelli F, La Milia V, Violo L, Del Vecchio L, Di Filippo S. Optimizing hemodialysate composition. Clin Kidney J. 2015;8(5):580–589. doi: 10.1093/ckj/sfv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Gomez MV, Gonzalez-Parra E, Ortiz A. Haemodialysate: long neglected, difficult to optimize, may modify hard outcomes. Clin Kidney J. 2015;8(5):576–9. doi: 10.1093/ckj/sfv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung AM, Hakim RM. Dialysate and Serum Potassium in Hemodialysis. Am J Kidney Dis. 2015;66(1):125–132. doi: 10.1053/j.ajkd.2015.02.322. [DOI] [PubMed] [Google Scholar]
- 18.Thornley-Brown D, Saha M. Dialysate content and risk of sudden cardiac death. Curr Opin Nephrol Hypertens. 2015;24(6):557–62. doi: 10.1097/MNH.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 19.Al-Ghamdi G, Hemmelgarn H, Klarenbach S, et al. Dialysate potassium and risk of death in chronic hemodialysis patients. J Nephrol. 2010;23(1):33–40. [PubMed] [Google Scholar]
- 20.Jadoul M, Thumma J, Fuller DS, et al. Modifiable Practices Associated with Sudden Death among Hemodialysis Patients in the Dialysis Outcomes and Practice Patterns Study. Clin J Am Soc Nephrol. 2012;7(5):765–774. doi: 10.2215/CJN.08850811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int. 2000;57(suppl 74):S-74–S-81. [Google Scholar]
- 22.Pisoni RL, Gillespie BW, Dickinson DM, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): Design, data elements, and methodology. Am J Kidney Dis. 2004;44(Suppl 2):7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Staiger D, Stock J. Instrumental variables regression with weak instruments. Econometrica. 1997;65:557–86. [Google Scholar]
- 24.Li Y, Lee Y, Wolfe RA, et al. On a preference-based instrumental variable approach in reducing unmeasured confounding-by-indication. Stat Med. 2015;34(7):1150–68. doi: 10.1002/sim.6404. [DOI] [PubMed] [Google Scholar]
- 25.Jadoul M, Karaboyas A, Goodkin DA, et al. Potassium-binding resins: Associations with serum chemistries and interdialytic weight gain in hemodialysis patients. Am J Nephrol. 2014;39(3):252–259. doi: 10.1159/000360094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hecking M, Karaboyas A, Saran R, et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol. 2012;7(1):92–100. doi: 10.2215/CJN.05440611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: Imputation and Variance Estimation Software: User Guide. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2002. [Google Scholar]
- 29.Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York, NY: Wiley; 1987. [Google Scholar]
- 30.Blumberg A, Roser HW, Zehnder C, Müller-Brand J. Plasma potassium in patients with terminal renal failure during and after haemodialysis: relationship with dialytic potassium removal and total body potassium. Nephrol Dial Transplant. 1997;12(8):1629–1634. doi: 10.1093/ndt/12.8.1629. [DOI] [PubMed] [Google Scholar]
- 31.Redaelli B, Locatelli F, Limido D, et al. Effect of new model of hemodialysis potassium removal on the control of ventricular arrhythmias. Kidney Int. 1996;50(2):609–617. doi: 10.1038/ki.1996.356. [DOI] [PubMed] [Google Scholar]
- 32.Santoro A, Mancini E, London G, et al. Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrol Dial Transplant. 2008;23(4):1415–1421. doi: 10.1093/ndt/gfm730. [DOI] [PubMed] [Google Scholar]
- 33.Robinson BM, Karaboyas A, Sen A, et al. Variation in dialysate potassium use and resultant post-dialysis potassium levels: Results from the DOPPS. NDT Plus. 2011;4(suppl 2):#F391. [Abstract] [Google Scholar]
- 34.Gabutti L, Salvadé I, Lucchini B, et al. Haemodynamic consequences of changing potassium concentrations in haemodialysis fluids. BMC Nephrol. 2011;12:14. doi: 10.1186/1471-2369-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association between serum K and all-cause mortality, by level of adjustment. Hazard ratio (95% CI) shown; Cox models stratified by DOPPS phase, country, US large dialysis organization, and accounted for facility clustering. Median (interquartile range) follow-up was 16.5 (8.1, 25.5) months and 13,114 patients (24%) died during follow-up. Level 1: BMI, albumin, nPCR, serum calcium, serum phosphorus, serum phosphorus squared, serum bicarbonate. Level 2: dialysate bicarbonate, hemoglobin, treatment time, single pool Kt/V.
Table S2. Patient characteristics by quartile of facility mean dialysate K. Mean ± SD or % shown; N=55,158 of 55,183 patients who were included in all-cause mortality analyses; N=25 excluded due to small number of eligible patients (N < 7) in facility. ACEi=angiotensin-converting-enzyme inhibitor; ARB=Angiotensin II receptor antagonist; nPCR=normalized protein catabolic rate; SBP=systolic blood pressure; WBC=white blood cell.


