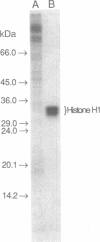
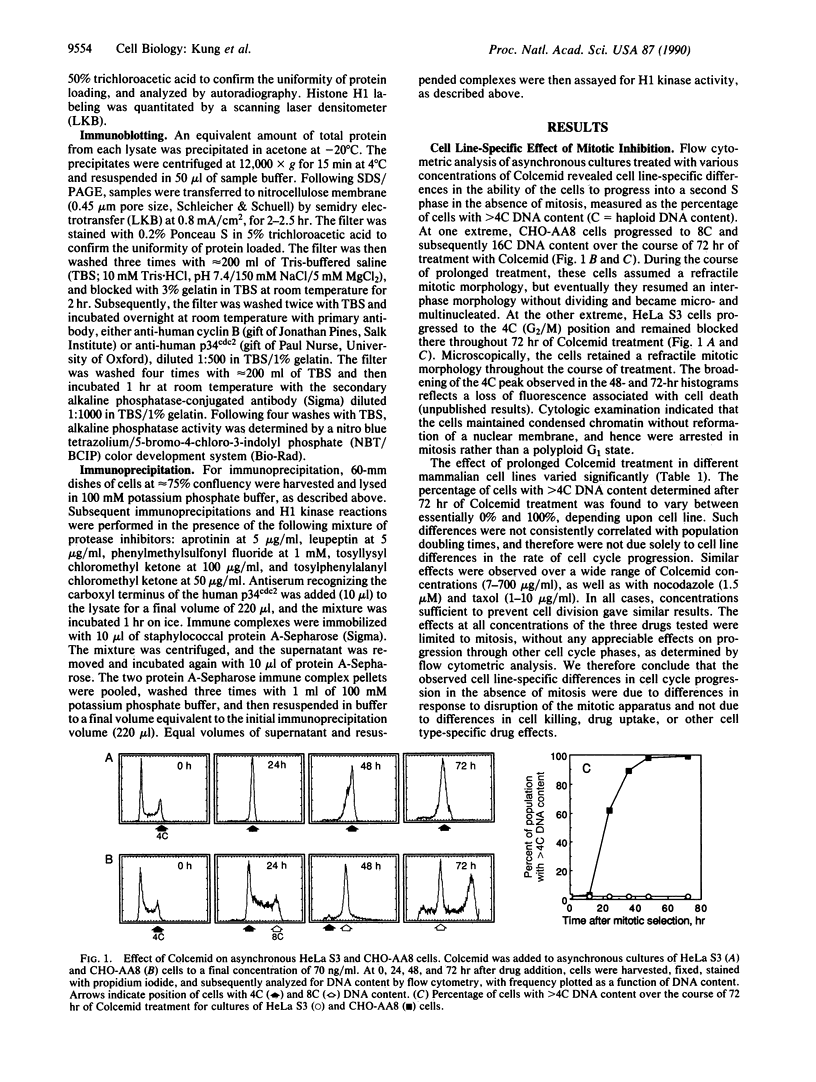
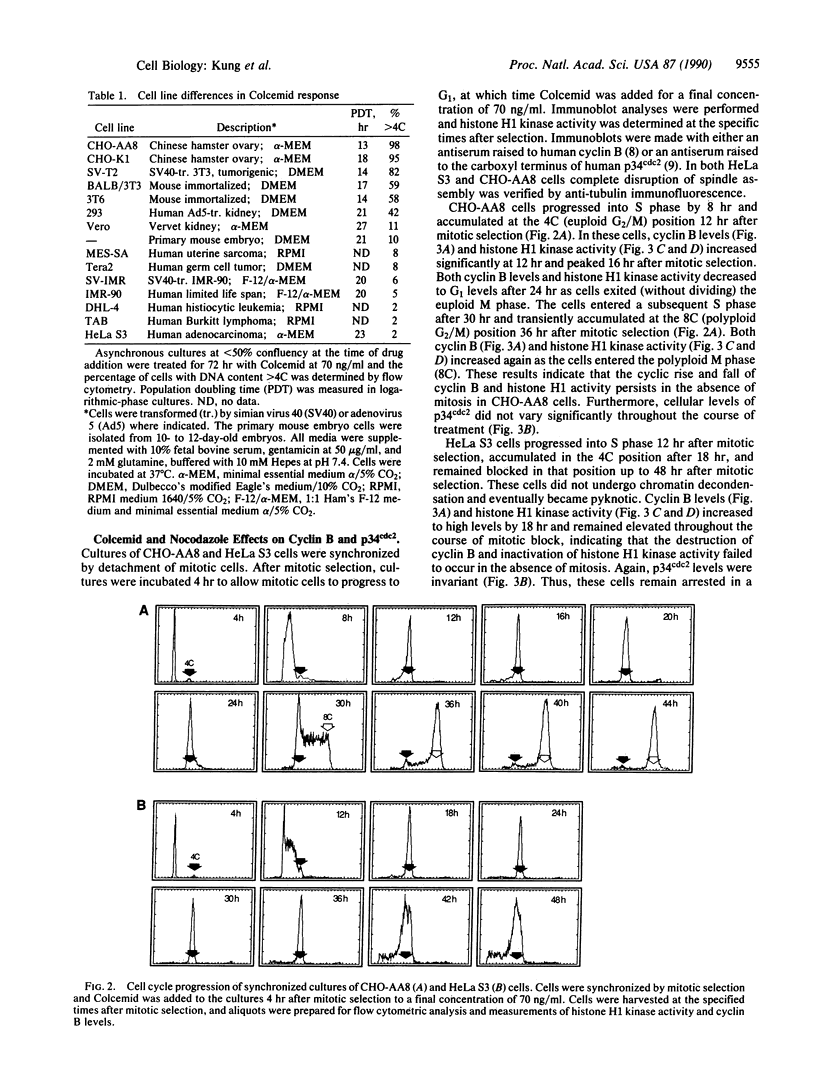
Abstract
This paper reports that there are major differences between mammalian cell lines in the propensity to progress into subsequent cell cycles when mitosis is inhibited with agents that disrupt the assembly of the mitotic spindle apparatus (Colcemid, nocodazole, and taxol). Human HeLa S3 cells, which represent one extreme, remain arrested in mitosis, with elevated levels of cyclin B and p34cdc2 kinase activity. In Chinese hamster ovary cells, at the other extreme, the periodic rise and fall of cyclin B levels and p34cdc2 kinase activity is only transiently inhibited in the absence of mitosis. The cells progress into subsequent cell cycles, without dividing, resulting in serial doublings of cellular DNA content. In general, the propensity to progress into subsequent cell cycles in the absence of mitosis appears to be species related, such that human cell lines remain permanently blocked in a mitotic state, whereas rodent cell lines are only transiently inhibited when spindle assembly is disrupted. We interpret these results to indicate that in mammalian cell lines there exists a checkpoint which serves to couple cell cycle progression to the completion of certain karyokinetic events. Furthermore, either such a checkpoint exists in some cell lines but not in others or the stringency of the control mechanism varies among different cell lines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham I., Marcus M., Cabral F., Gottesman M. M. Mutations in alpha- and beta-tubulin affect spindle formation in Chinese hamster ovary cells. J Cell Biol. 1983 Oct;97(4):1055–1061. doi: 10.1083/jcb.97.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly E., Dorée M., Nurse P., Bornens M. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989 Dec 20;8(13):3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch T., Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990 Feb 23;60(4):665–673. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990 Jun 1;61(5):743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- Mariani B. D., Slate D. L., Schimke R. T. S phase-specific synthesis of dihydrofolate reductase in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4985–4989. doi: 10.1073/pnas.78.8.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Dominoes and clocks: the union of two views of the cell cycle. Science. 1989 Nov 3;246(4930):614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J. Identifying tumor suppressor genes in human colorectal cancer. Science. 1990 Jan 5;247(4938):12–13. doi: 10.1126/science.2403692. [DOI] [PubMed] [Google Scholar]