Abstract
Introduction
The blood-brain barrier (BBB) is a dynamic biological interface which actively controls the passage of substances between the blood and the central nervous system (CNS). From a biological and functional standpoint, the BBB plays a crucial role in maintaining brain homeostasis inasmuch that deterioration of BBB functions are prodromal to many CNS disorders. Conversely, the BBB hinders the delivery of drugs targeting the brain to treat a variety of neurological diseases.
Area covered
This article reviews recent technological improvements and innovation in the field of BBB modeling including static and dynamic cell-based platforms, microfluidic systems and the use of stem cells and 3D printing technologies. Additionally, the authors laid out a roadmap for the integration of microfluidics and stem cell biology as a holistic approach for the development of novel in vitro BBB platforms.
Expert opinion
Development of effective CNS drugs has been hindered by the lack of reliable strategies to mimic the BBB and cerebrovascular impairments in vitro. Technological advancements in BBB modeling have fostered the development of highly integrative and quasi- physiological in vitro platforms to support the process of drug discovery. These advanced in vitro tools are likely to further current understanding of the cerebrovascular modulatory mechanisms.
Keywords: In vitro, Alternative, Technology, Cell culture, Brain, Cerebrovascular, Drug testing, Permeability, Brain disorders
1. Introduction
1.1. Vascular differentiation: The Blood-Brain Barrier
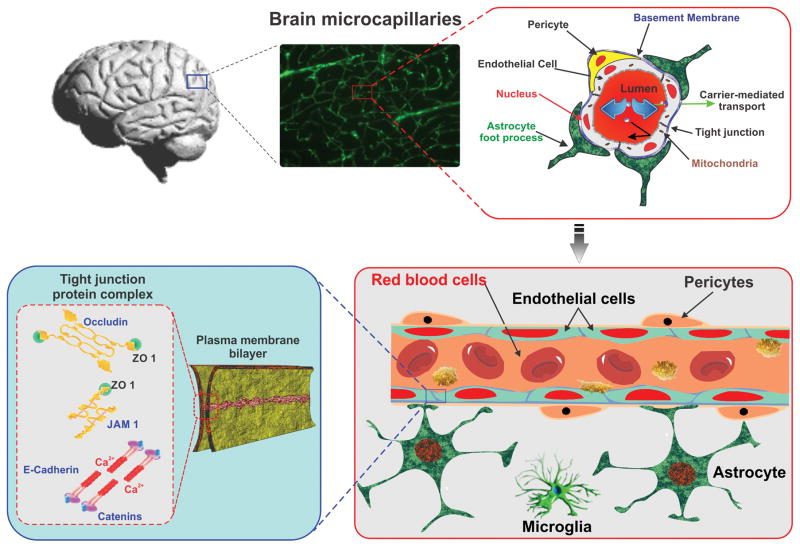
The structural and functional integrity of the blood-brain barrier (BBB) is vital to maintain the homeostasis of the central nervous system (CNS). The BBB endothelial cell (EC) are highly polarized (evidenced by an asymmetrical distribution of transporters between the luminal and the basolateral membranes) and their cytoplasm is of uniform thickness with few pinocytotic vesicles, absence of fenestrations and a very high density of mitochondria (almost 5 times higher than other endothelial phenotypes) denoting high metabolic activity. Inter-endothelial tight junctions (TJ) consisting of three main integral protein types [claudins, occludins, and junctional adhesion molecules (JAM)] connect adjacent ECs and form a diffusion barrier, which selectively excludes polar molecules (including blood-borne and xenobiotics) from entering the brain [1, 2]. A number of cytoplasmic accessory proteins such as zonulae occludentes (ZO) and cingulin crosslink these transmembrane proteins to the cytoskeleton (see Figure 1). Electrolytes and polar molecules in general, cross the barrier with great difficulty whereas lipid-soluble substances such as alcohol, narcotics and anticonvulsants pass with relative ease. Specific transport systems are responsible for the passage of biologically important substances such as D-Glucose, Phenylamine, etc. The most important of them are summarized below in table 1.
Figure 1. Schematic illustration of BBB anatomy.
A cross-section of brain microcapillary representing luminal compartment composed of basal lamina, endothelial cells and pericytes tightly ensheathed by the astrocytic end-feet. Tight junctions (TJs), present between the cerebral endothelial cells selectively excludes paracellular trafficking of substances from entering into brain.
Table 1.
Transport systems at the BBB
| Transport systems | Substrates |
|---|---|
| 1. Amine | Choline |
| 2. Nucleoside | Adenosine |
| 3. Hexose | D-Glucose |
| 4. Mono carboxylic acid | Lactate |
| 5. Neutral amino acids | Phenylamine |
| 6. Basic amino acids | Arginine |
| 7. Purine | Adenine |
The periendothelial accessory structures of the BBB include the basal membrane, pericytes and the astrocytes end-feet which envelop > 99% of the BBB endothelium. The basal lamina is produced by perivascular astrocytes; it is approximately 40–50 nm thick and composed of type IV collagen, heparin sulfate proteoglycan, laminin, fibronectin, and tenascin. It has several functions including mechanical support for cell adhesion and migration with a mechanism involving transmembrane receptors (integrins) bridging the cytoskeletal elements of a cell to the extracellular matrix [3]. This lamina also regulates the communication between cells and embodies an additional barrier to the passage of macromolecules between the vascular system and the brain [4]. The Astrocytes interaction with the BBB ECs modulates the differentiation of the cerebrovascular endothelium, regulates protein expression, and appears to be critical for the induction and maintenance of the tight junctions and BBB properties [5].
2. Modeling the BBB in vitro: general scope and limitations
The broad scope of in vitro modeling is to develop a highly controlled environment outside a living organism to assess the physiological and pathological responses to specific experimental stimuli otherwise difficult to reproduce, dissect out, modify and/or characterize in vivo. From a translational and pharmacological point of view BBB models should be considered as tools to facilitate CNS drug discovery (e.g., drug permeability screening) and/or the development of strategic solutions to bypass the intrinsic resistance of the BBB to the passage of CNS therapeutics. Further, from a practicality stand point, these platforms should be user friendly, scalable, cost effective and capable of high throughput [6].
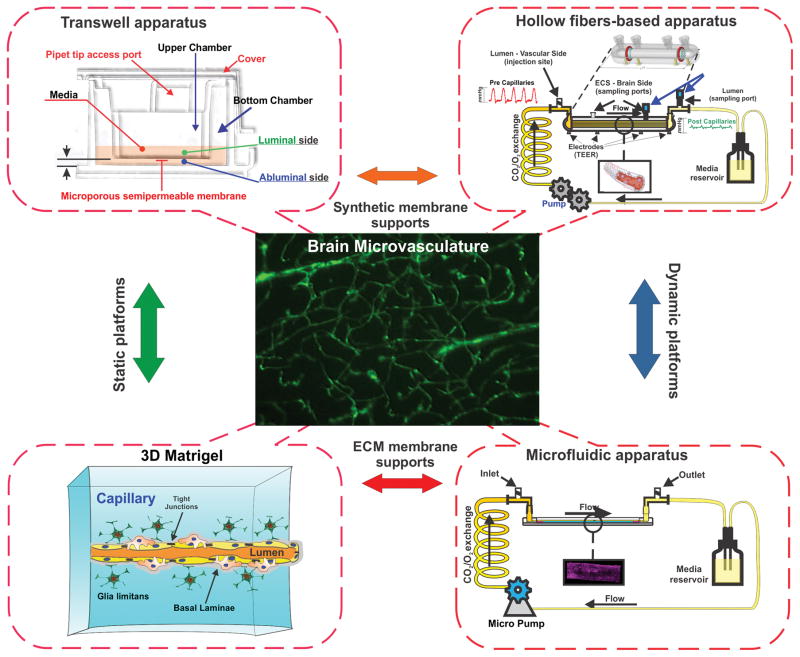
Current technological barriers do not allow to fully reproduce the physiological functions and responses of the BBB in vivo in a single platform, however, recent advancements in the biotechnology field, materials engineering and understanding of BBB biology have enabled the development of innovative and highly integrated “quasi-physiological” (e.g., capable of reproducing a number of environmental complexities and biological features of the BBB including vascular hemodynamics and physiological/pathological responses to stimuli) in vitro BBB models (see Figure 2) which are discussed herein.
Figure 2. Schematic diagram of currently available in vitro BBB models simulating in vivo NVU milieu based on two distinct principles- static vs dynamic culture.
Static models include transwell and 3D ECM platform while dynamic models utilize hollow fiber based apparatus or micro fluidic devices.
3. Cell culture-based in vitro BBB models: Static systems
3.1. Mono, Co- and Triple culture platforms
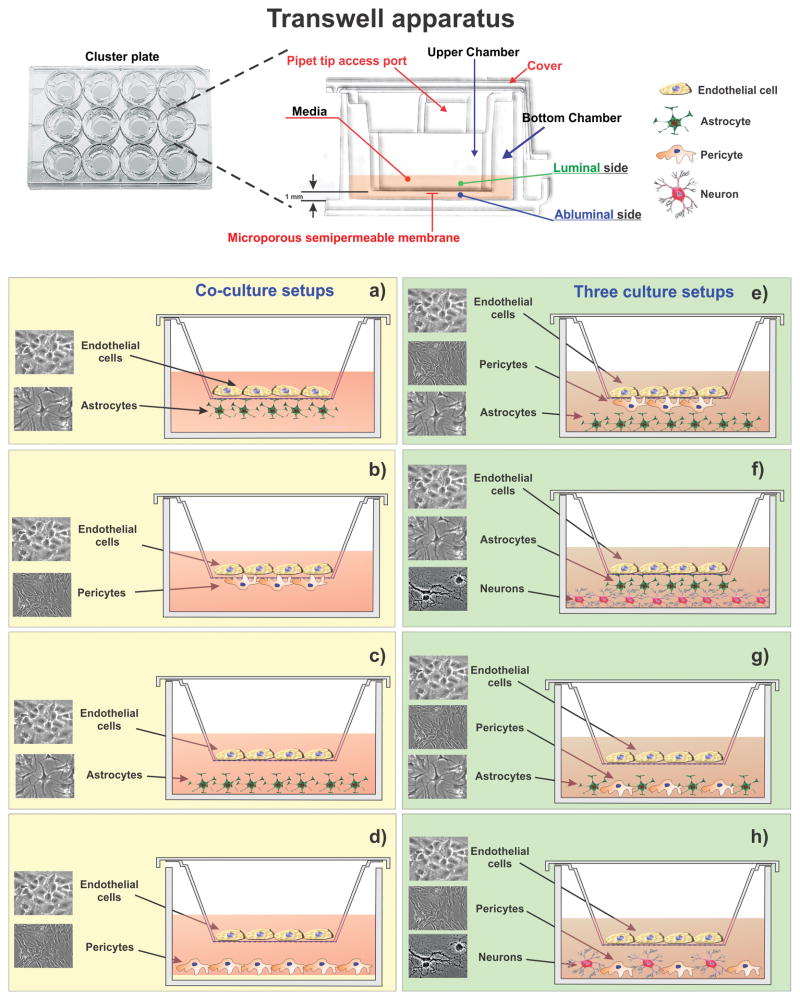
The simplest and most feasible in vitro BBB model consists of a monolayes of brain capillary endothelial cells seeded on a permeable support under static culture conditions. In the Transwell apparatus cells are grown on microporous semipermeable inserts which allows the passage of solutes (including cell derived factors) from and to the growth medium or between the apical (luminal) and basolateral (abluminal) compartments which are separated by the insert itself (see Figure 3).
Figure 3. In vitro BBB models on transwell platform using co (left panel) or triple (right panel) culture.
BMECs are cultured on top of semipermeable microporous inserts while astrocytes or pericytes are seeded at the bottom (a & b) of the insert or bottom of the wells (c & d) in co-culture conditions. Triple cultures using three different cells pericytes, astrocytes, and/or neurons in different arrangements (e,f,g,h) have also been investigated. Key: a-[129, 130]; b-[129]; c-[14]; d-[13]; e-[21]; f-[9]; g- [131]; h- [10]
This BBB model allows performing cell migration and drug transport assays across the BBB endothelium. The cerebrovascular endothelial cells obtained from various origins (see Table 2 & 3) such as mouse, rat, bovine, porcine, monkey or human have been utilized in these experimental setups. From a practical perspective this model is user friendly and cost effective. The drawback is that cells lack the barriergenic modulatory stimuli afforded by neighboring cell signaling (astrocytes and pericytes) and mechanical stimuli (e.g., shear stress). This limit the ability of these cells to maintain their BBB properties over a long term period [7, 8] and as a result more sophisticated models including co- and triple culture systems have been developed. Owing to the close spatial relationship, co-cultures of cerebrovascular endothelial cells with astrocytes are widely used since astrocytes play a crucial role in the development of the paracellular tightness of the BBB. Different experimental systems were developed to mimic the astrocytic influence on the BBB endothelium. One of the most commonly used configurations include endothelial cells seeded on the apical surface of a microporous membrane (Lumen) and juxtaposed astrocytes loaded on its basal surface (Ablumen). Although this arrangement allows for the direct contact between endothelial cells and astrocytes; the relative higher thickness of the artificial membrane compared to the basal lamina in vivo limits cell-cell interaction. An alternative approach is to culture astrocytes at the bottom of the wells and allow for the diffusible released factors to reach the BBB endothelium on the other side of the membrane (see Figure 3). Although pericytes are in close contact with ECs they are relatively less characterized. However, their role in modulating endothelial functions has been well established in recent years and is not surprising that their use in BBB modeling has also been exploited. Pericytes from different origins have been incorporated in a range of co and triple cultures BBB setups in Transwell platforms using various configurations (see Figure 3). A common triple culture setup consists of ECs monolayers laid on the top of a microporous insert with juxtaposed pericytes on the basal side of the membrane and astrocytes seeded at the bottom of the well. Astrocyte and pericytes culture mixes at the bottom of the well have also been experimented with in few occasions [9, 10]. Triple cell culture models using BMECs, neurons and astrocytes/pericytes have also been reported. Neuron based triple cultures recreate the basic structure, function and cell-cell interaction of the neurovascular unit (NVU) in vivo. Therefore, NVU models incorporating neurons can also be used to assess (to some extent) the effect of the drug treatment on the CNS. However, the complexity of the culture environment increases significantly, drastically reducing the manageability of these systems.
Table 2.
BBB Models based on Primary BMECs
| Origin | Cell | Culture | Model design | Approx TEER (ohm.cm2) | Ref |
|---|---|---|---|---|---|
| Mouse | Mouse primary BMEC | Monoculture | EC on top of transwell insert | ~50 ~150 |
[13] [14] |
| Co-culture with murine pericyte | EC on top of transwell insert and pericyte on bottom well | ~150 | [13] | ||
| Co-culture with mouse astrocyte cell lines | EC on top & astrocytes on the bottom of transwell insert | 80 | [129] | ||
| Co-culture with rat astrocyte | EC on top of transwell insert and astrocyte on bottom well | ~200 | [14] | ||
| Rat | Rat primary BMEC | Co-culture with rat astrocyte | EC on top of transwell insert and astrocyte on bottom well | 300 >600 |
[131] [17] |
| Triple culture with rat astrocytes and rat pericytes | EC on top, pericyte on bottom of transwell insert and astrocyte on bottom well | 400 | [18] | ||
| Bovine | Bovine primary BMEC | Co-culture with rat astrocytes | - | 600–1800 | [19] |
| Porcine | Porcine primary BMEC | Co-culture with rat astrocytes | EC on top of transwell insert and astrocyte on bottom well | 800 | [20] |
| Triple culture with rat/porcine astrocyte and pericyte | EC on top, pericyte on bottom of transwell insert and astrocyte on bottom well | >1000 | [21] |
Table 3.
BBB Models based immortalized endothelial cell lines
| Origin | Cell | Model Type | Approx TEER (ohm.cm2) | Ref |
|---|---|---|---|---|
| Mouse | bEnd.3 | Monocuture | 60–70 | [132] |
| bEnd.5 | Monoculture | 40–50 | [133] | |
| Immortalized mouse cerebral endothelial cells (cEND) | Monoculture | 400 | [134] | |
| Rat | RBE4 | Monoculture | >100 | [135] |
| Coculture with rat glial cells | - | [136] | ||
| Human | Immortalized human brain endothelial cells (hCMEC/D3) | Monoculture | 40–50 | [24] [137] |
| Coculture with astrocytes or pericytes | 50–60 | [24] | ||
| Triculture with astrocytes & pericytes | >40 | [24] |
3.2. Primary cultures
Since mice are extensively used in preclinical brain cancer research [11] as well as many CNS neurodegenerative and neuroinflammatory disorders, mouse BBB models have been developed in parallel to improve data reproducibility when transitioning from in vitro to in vivo. BBB integrity and tightness in vitro is commonly assessed by measuring the electrical impedance posed by the barrier. A functional parameter defined as trans-endothelial electrical resistance (TEER) which is measured in Ohms (Ω) × cm2. TEER is used to determine the integrity and tightness of the BBB in vitro and to compare it to that in vivo (usually in the range of 1800 to 2000 Ω·cm2 [12]). BBB models have been developed from freshly prepared mouse BMEC monocultures or in combination with pericytes. The reported TEER values were in the range of ~50 and ~150 Ω·cm2 respectively [13] and rising up to ~200 Ω·cm2 when astrocytes are incorporated into the culture system (see Table 2) [14, 15]. Although primary BMECs closely mimic the BBB phenotype in vivo, presence of non-endothelial cell contaminants (e.g. pericytes and fibroblasts) is one of the major issues researchers have to deal with during the extraction process. These cells can disrupt the intactness and uniformity of the endothelial culture by forming holes or void spaces in the monolayer. This can impact the reliability and accuracy of permeability testing. Although, in some instances, (e.g., primary rat BMEC) it is possible to exploit the intrinsic biological features of the cells (e.g., high expression level P-glycoprotein) to purify the cell cultures with toxic levels of a specific compound (e.g., Puromycin; a P-glycoprotein substrate) to kills the contaminants without affecting ECs viability [16]. Primary rat BMECs exhibit higher TEER values (ranging from 300 to more than 600 Ω·cm2- see Table 2) then mouse derived ECs either in mono, co- or triple cultures with astrocytes, pericytes, or both [16, 17, 18]. However, primary bovine and porcine BMECs exhibit even higher electrical impedance (TEER >1000 Ω·cm2 see Table 2) and expression levels of TJ proteins ZO-1 and Claudin-5 [19, 20, 21].
3.3. Immortalized endothelial cell lines
To reduce cost and labor associated with the procurement of primary ECs, several immortalized endothelial cell lines from diverse origin have been developed. But only a few of them express the desired barrier properties and in vivo BBB functionality (e.g., TJ formation and expression of distinct endothelial markers and transporters) and are currently used in BBB research. Recently, Rahman et al., in a systematic narrative review, listed a total of 36 immortalized endothelial cell lines being used in BBB modeling. The authors ranked human brain microvascular endothelial cell line hCMEC/D3, rat endothelial cell line RBE4 and mouse brain microvascular endothelial cell line bEnd.3 as the top three based on the frequency of their use [22]. The more frequent use of a human-derived brain microvascular cell line is rationalized by the fact that it represents a more realistic match of the human NVU. However, the use of endothelial cell lines from nonhuman origin such as mouse, rat or porcine can prove to be a relevant and perhaps better surrogate if the in vitro results obtained are correlated with the same preclinical model.
Since the introduction of hCMEC/D3 cell line to academic and industrial research, more than a hundred articles have been published investigating diverse aspects of CNS pathology, drug discovery and development [23]. Although the hCMEC/D3 monolayer displays moderate TEER values (~50 Ω·cm2 see Table 3) [24], it seems to recapitulate quite effectively a considerable number of BBB EC characteristics and became the unique and commonly used model for “humanized” in vitro BBB studies. This cell line is able to preserve the in vivo endothelial phenotype till 35th passages including the expression and topographical distribution of a number of TJ proteins and BBB endothelial transporters and receptors (e.g., MDR1, BCRP, MRP4, transferrin receptor, insulin receptor, Glut-1, metabolizing enzymes) which made them feasible for routine use [23]. One of the common strategies used to improve the barrier tightness when using hCMEC/D3 cells is to co-culture them with pericytes or (more effectively) astrocytes [24] in juxtaposed configuration. The soluble factors released by the astrocytes are relatively less effective in vitro than astrocyte in direct contact (via astrocytic end-feet) with the endothelial cells [24].
bEnd.3 and bEnd.5 are commercially available and widely used mouse-derived immortalized cell lines although of the two bEnd.3 cells seems to develop a tighter barrier than bEnd.5 (see Table 3) due to higher expression levels of TJ proteins- claudin-5, occludin and ZO-1 [25]. bEnd.3 cells grow rapidly and retain the endothelial phenotype over several passages. Low paracellular permeability and in-vivo like endothelial phenotype made this cell line convenient for developing functional BBB model with suitable responsiveness to stimuli [26].
RBE4 is one of the best characterized and commonly used immortalized rat brain endothelial cell lines in BBB modeling despite the fact that it falls short of forming TJ complexes, thus resulting in high paracellular permeability, thus hindering its use in CNS drug distribution assays [27].
4. Shear stress and cell differentiation
Various types of hemodynamic forces regulate the blood vessel function and tone including the regional tissue-blood barrier homeostasis [28]. By virtue of their anatomical location vascular endothelial cells are directly exposed to blood-flow induced frictional forces (shear stress) which play a critical role in vascular homeostasis and remodeling [28] [29]. The pattern of shear stress is complex as it varies from 1 to 60 dynes/cm2 depending on vessel type and size. In brain microvascular capillaries ranging 10 μm in diameter and a flow rate typically from 6 to 12 nL min−1 the corresponding shear stress can range from 10 to 20 dynes cm−2 [30]. Shear stress differentially regulates the structural and functional phenotype [28, 31, 32] of the endothelium. For example, normal and disturbed flow patterns (shear stress) induce differential molecular and functional responses in the local endothelium [28]. Importantly, pathological shear stress causes endothelial dysfunction and perturbs the delicate balance between pro- and anti-atherogenic, thrombogenic and inflammatory states resulting in various vascular pathologies including ischemic stroke [28, 33].
Given the trophic signaling mechanisms between BBB endothelial cells and other cellular elements within the NVU, a functional neurovascular coupling dynamically tethers the regional blood flow to suit the local metabolic demands and thus, the BBB exposed to constant pulsatile flow regulates the CNS microenvironment [34]. Previously, it was shown that intraluminal flow is critical for development and maintenance of BBB phenotype in vivo and in vitro resulting in high barrier tightness [8, 35, 36]. Various in vitro dynamic models (DIV-BBB) were developed to understand the role of laminar or pulsatile shear stress on BBB endothelial function and dysfunction in various pathological conditions including inflammation and stroke [31, 37, 38, 39, 40]. For instance, exposure of immortalized hCMEC/D3 cell line to pulsatile flow strongly potentiates the barrier integrity, with 10-fold increase in TEER values compared to static cultures [41]. Cucullo, Desai and colleagues demonstrated flow-induced down-regulation of cell cycle related genes and cytoskeletal rearrangement including induction of antioxidant gene responses in BBB endothelium [8, 35]. Shear stress was also shown to effectively induce TJ gene expression in primary BMECs of various species (including human), thus enhancing the BBB tightness [29, 42]. Notably, high to low shear stress fluctuations mimicking the pathology of cerebral ischemia was shown to affect the BBB ion transport prodromal to cerebral edema [43], and disturbed flow also results in inflammatory activation of BBB endothelium [44].
5. Microfluidic devices
Microfluidic tissue-on-a-chip approaches have emerged as promising techniques to establish in vitro barrier tissues as potential tools in discovery biology and to study BBB endothelial responses to shear stress [46, 47]. These systems combine microengineering techniques with living cell populations to recapitulate salient organizational features of the in vivo environment including spatially defined co-cultures and polarized cell architectures [48]. Favorable scaling effects (e.g. short diffusion distances, laminar flows, and surface tension effects) are leveraged to replicate in vivo biophysical/biochemical cues by enabling controlled dosing with test compounds, introduction of physiological flows and shear stresses, and exposure to well-defined soluble factor gradients [49, 50]. The incorporation of custom designed electrodes, precise sample handling, and imaging capabilities also facilitate real-time barrier integrity assessment using TEER and small molecule transport studies [51, 52].
In a typical tissue-on-a-chip embodiment, microfluidic channels are fabricated using “soft lithography” techniques by molding an elastomeric material, polydimethylsiloxane (PDMS), against a photo-defined master mold (see review by Weibel et al [53]). A porous cell culture substrate (typically a polyester or polycarbonate membrane or thin perforated PDMS membrane) is then sandwiched and sealed between the channel networks [54]. These channels enable either side of the membrane to be independently accessed, and appropriate cell populations are sequentially introduced into the channels and allowed to attach. Cells are maintained under flow to support cell proliferation, impose shear stresses, induce junction formation, and establish polarized tissues. These techniques have been used to successfully establish several barrier models including the lung, gut, and vasculature over the past decade [55, 56]. Given the vital importance of the BBB in biomedical research, several microfluidic models incorporating closely apposed microvascular endothelium and neuronal populations have also been established. [57, 58, 59, 60, 61]. To support the growing trend of 3D cultures, we focus our discussion here on emerging techniques published within the past year where 3D gels were directly incorporated into microfluidic architectures to create NVUs. These approaches support the overall hypothesis that replication of in vivo structure and function can provide more representative in vitro assays [47].
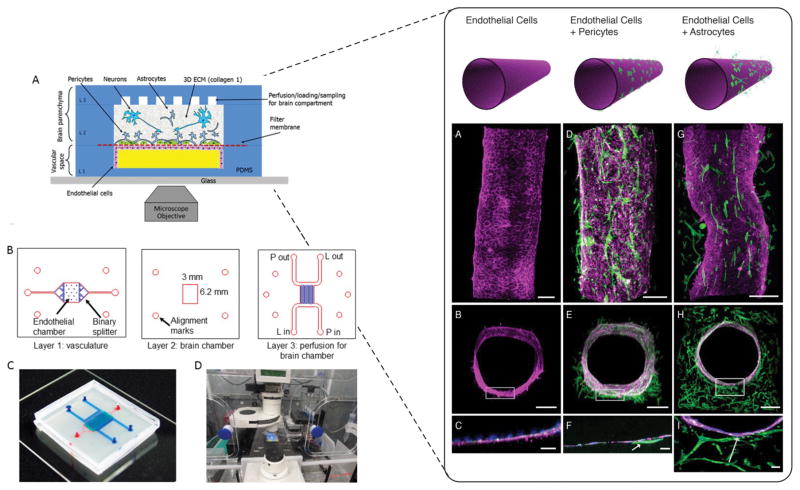
As shown in Figure 4 (left panel) Brown and colleagues [62] reported a NVU comprised of a bottom perfusion channel, a brain chamber, and a top perfusion channel. The bottom perfusion channel was separated from the brain chamber with a porous membrane onto which endothelial cells were cultured on one side and astrocytes and pericytes on the other. The brain compartment was then filled with 3D Collagen I gel embedded with human induced pluripotent stem cell (hiPSC) derived neurons. The top perfusion channels maintain cell viability in the brain compartment. TEER measurements were reported to increase over a 12-day culture period in the endothelial population. ZO-1 staining for endothelial tight junctions, actin alignment under shear flow, and reduced transport of FITC-conjugated dextrans were used to verify barrier function. As expected, permeability of the barrier increases upon exposure to glutamate due to disrupted tight junctions. The authors anticipate significant relevance in long-term studies exploring CNS neurotoxicity and drug delivery.
Figure 4.
Left panel A) Schematic representation of three compartments NVU. B) Microfluidic design layouts with details of each layer C) assembled device with top (blue) and bottom (red) channels. D) Experimental setup with microengineered NVU within environmental chamber for long term imaging. Reproduced from [62] with permission of AIP publishing. Right panel- Confocal imaging of various cell population within the cylindrical collagen lumen. Endothelial cells alone (A–C), with pre-added pericytes (D–F), or astrocytes in the bulk gel (G–I). Magenta is VE-Cadherin, green is F-actin, blue are nuclei. Arrows indicate contact points between cell populations. Reproduced from [63] with permission of PLOS.
Herland and colleagues [63] adapt a technique called “viscous fingering” [64] to establish a cylindrical collagen gel within a microfluidic channel. In this method, channels were filled with liquid collagen and a controlled fluid flow used to remove a central region of the collagen bulk prior to self-polymerization. The NVU was developed by embedding human cortical astrocytes in the bulk gel and sequentially lining the lumen surface with human cortical pericytes and microvascular endothelial cells. With this approach, the desired physiological structure was achieved through integration of all three cell types (see Figure 4, right panel). Endothelial monolayer formation and presence of TJ was verified with VE-Cadherin and ZO-1 staining. BBB permeability was assessed by flowing low molecular weight dextran through the cell-lined collagen gel and observing diffusion into the bulk. The authors also exposed the BBB to TNF-α and found that the secretion profiles for granulocyte colony-stimulating factor (G-CSF) and interleukin-6 (IL-6) were higher in the 3D model than in Transwells, thus anticipating that their platform can be used to explore inflammation of the NVU.
6. Technology advancements in BBB modeling
6.1. 3D extracellular matrices (ECM) for BBB modeling
2D cell cultures using one of the extracellular matrix (ECM) components and a homogenous population of cells (such as microvascular brain endothelial cells, astrocytes, pericytes) has been extensively used to determine the individual cellular responses and cell signaling pathways. However, 2D BBB cultures limit cells to a planar environment, whereas in vivo these cells are embedded into a 3D environment containing multiple ECM components, co-exist with other cell populations and are nurtured by a variety of NVU-secreted factors. Until recently, 3D cultures techniques were limited to 3D spheroids, 3D hydrogel cultures, extracellular matrices [69] and solid scaffolds [67, 70]. Chrobak et al in 2005 established a 3D cell culture method to produce microvascular tubes for studying endothelial and epithelial physiology apart from evaluating inflammatory responses. It consisted of cylindrical channels in collagen gels lined with confluent monolayers of human endothelial cells. Perivascular cells were added to the setup, either by directly embedding within the gels or by adding it after endothelial cells grew to confluence [71]. However, the model did not enable shear stress (as the media was manually recirculated as needed) and other external stimuli such as growth factors and other diffusible elements. Microvascular modeling in the form of microfluidic devices in recent years has addressed these concerns by enabling the formation of pro-angiogenic interstitial gradients of growth factors (VEGF, b-FGF and PMA supplemented media) [72] as well as continuous endothelial exposure to shear stress [72, 73, 74]. In most of these in vitro models, collagen type I has been used as the ECM of choice [72, 74, 75]. Collagen is a naturally derived hydrogel isolated from various biological sources including bovine skin and rat tail tendon [76]. Collagen I can support 3D cell growth and differentiation besides interacting with integrin receptors to modulate gene expression [77]. It has been heavily used in studies involving cell proliferation, cell migration [72, 78], inflammation [71], drug toxicity [79] and tumor cell invasion [80]. Other models have also used poly-d-lysine [75] or matrigel [81] as ECM. Matrigel forms thick, loosely cross-linked gels which can promote 3D tissue organization. By contrast, collagens and laminins have an inherent capacity to polymerize and form a 3D gel spontaneously resulting in tighter packing and marked resistance to proteolysis [78]. The choice of the ECM especially among natural hydrogels depends upon the desired morphology, growth and functionality of cells as well as physical properties such as permeability and matrix stiffness required [68], thereby enabling the ability to reproduce the ordered biological makeup of brain microvessels and/or NVUs.
On the contrary, culturing endothelial cells in these 3D microenvironments is quite a complex and challenging process. First, oxygen, nutrient and soluble growth factors availability (which influences cellular differentiation) is not uniform across the culture layers and gradients arise as the medium diffuses through the gel. Second, imaging and western blot techniques to assess cell function and protein distribution become significantly more challenging due to: a) difficulties in accessing cells for immunostaining or protein and RNA/DNA extraction and b) light scattering, refraction, and attenuation caused by the 3D biological environment. Finally, the addition of a third dimension amplifies the heterogeneities of the cultures, thus affecting the study reproducibility and data comparison [67]. Natural hydrogels also present some shortcomings such as batch-to-batch variability in composition due to their isolation from animal-derived sources. Also, not all natural hydrogels can be used uniformly in all models and one has to be careful to choose a gel best suited to the purpose of the experiment. Alternatively, synthetic hydrogels are comprised of purely non-natural molecules that are biologically inert and are capable of creating defined 3D microenvironment. Such inert gels are simple to manufacture, adapts to the mechanical forces conveniently and are highly reproducible. An example of a synthetic hydrogel is Corning PuraMatrix™ peptide hydrogel which is composed of 99% water and 1% w/v standard amino acids [46, 67]. However, in future there will be a need for ECM with combined properties of natural and synthetic hydrogels as it seems likely that different 3D matrices will induce different cellular responses which will in turn foster the development of more sophisticated 3D models.
6.2. 3D printing technology for BBB modeling
3D printing (bioprinting) involves printing of stereolithography files (STL files) created from tomographic reconstruction (layer by layer) of 2D images captured by computed tomography and/or magnetic resonance imaging [82]. 3D printing has been used to reproduce several human organs such skin, bone, ears, liver [82] and has also been used to create perfused functional vascular channels [83]. However, its application in cerebrovascular research is limited due to the inherent complexities of the NVU and inability of current technology to create such multifaceted environments.
7. The Near Future: Stem cell based BBB models
While there has been a wide spread use of animal derived (rodent, bovine, and porcine) in vitro BBB models, inevitable species differences hinder their translatability to clinical neuroscience and drug development [84, 85, 86, 87, 88]. On the other hand, the limited scalability, phenotypic drift, and high costs of isolated primary human brain endothelial cells (BMEC) hamper the development of widely usable human BBB in vitro models [89, 90, 91]. As an alternative to primary cultures, immortalized human BBB endothelial cell lines such as hCMEC/D3 [92, 93, 94, 95, 96] exhibit suboptimal monolayer integrity and lower expression of tight junction proteins [97, 98, 99]. Therefore, development of robust and physiologically representative in vitro human BBB model of high fidelity remains a critical objective in the field of neurovascular research.
Recent scientific advances in stem cell research have opened new avenues for studying various human diseases and promote drug development in a dish [100, 101, 102, 103, 104]. Both, pluripotent and adult stem cells exhibit significant self-renewal capacity and can acquire any specialized cell genotype/phenotype under cue-directed differentiation for high throughput in vitro disease modeling and toxicity testing [105, 106, 107, 108, 109]. The inherent potential of human induced (iPSC) or embryonic (ESC) pluripotent and progenitor stem cells have been exploited to develop highly functional and physiologically relevant in vitro human BBB models [84, 110, 111]. Here, we provide details on the design and applications of current stem cell BBB modeling (see Table 4) including model-specific limitations. Considerable evidence indicates the utility of cord blood hematopoietic progenitor cells (CD34+ CD45+ CD31+ KDR− vWF− CD14− cells) to develop a reproducible human BBB model in vitro [112, 113] for studying the mechanisms underlying the transmigration of breast cancer cells across the BBB endothelium in brain metastasis [114, 115]. For example, Ponio and colleagues reported that directed differentiation of endothelial progenitor cell colonies derived from cord blood stem cells in the presence of astrocytes induced BBB-like properties with increased monolayer integrity similar to hCMEC/D3 cell line [112]. Similarly, hematopoietic stem cell-derived ECs when co-cultured with pericytes display a mature and functionally responsive BBB EC phenotype with sustained barrier tightness for many days and the ability to predict human brain drug distribution [113]. However, it should be noted that despite relatively low barrier permeability compared to direct differentiation with astrocytes [113] or hCMEC/D3 cell line under static conditions [96], TEER values obtained with hematopoietic stem cell based BBB models are still below 200Ω × cm2 [113, 114] (see Table 4). Thus, the model stability in vitro is outweighed by its relatively poor barrier tightness, compared to other in vitro BBB platforms [112, 116, 117].
Table 4.
| Stem cell source | EC Culture type | Study objective | BBB Integrity | Ref | |
|---|---|---|---|---|---|
| TEER (Ω.cm2) * | Pe (x 10−3 cm/sec) | ||||
| IMR90-c4 hiPSC cell line | Monoculture or co-culture (priming) with human brain pericytes and NPC-derived neuron/astrocyte mixtures; Static; Retinoic acid as inductive factor |
Development of a fully humanized in vitro BBB (neurovascular unit) model and enrichment by retinoic acid | 5160 | Sucrose, <0.006 | [110, 120] |
| Monoculture or co-culture with rat astrocytes; Static; | Development of a robust human BBB model and role of Wnt/β-catenin signaling in BBB development | 1450 | Sucrose, 0.034 Inulin, 0.03 |
[121] | |
| Monoculture; Static; Retinoic acid as inductive factor |
Anticancer drug permeability | >1500 | Sucrose, 0.13 | [124] | |
| Variable effects of ECM and culture medium on stem cell differentiation efficiency | ~1400 | NaF, 0.04 | [123] | ||
| Co-cultured with rat astrocytes; flow-based; Retinoic acid as inductive factor |
Development of BBB-on-a Chip microfluidic model with minimal shear stress for in vitro drug permeability screen | >4300 | Dextran (4–70kDa), 0.0001–0.001 | [122] | |
| H9 hESC cell line DF19-9-11T hiPSC cell line; DF6-9-9T hiPSC cell line |
Monoculture or co-culture with rat astrocytes; Static | BMEC differentiation efficiency of various pluripotent stem cells to model human BBB | >700 | n.a | [121] |
| Monoculture or co-culture with human brain pericytes and neuron/astrocyte; Static; Retinoic acid as inductive factor |
Development of a fully humanized in vitro BBB (neurovascular unit) model and enrichment by retinoic acid | 4740a 1680b |
n.a. | [120] | |
| Embryonic rat/human neural progenitor cells (NPC) | NPC derived neurons + astrocytes mixture co-culture with rat BMECs; Static | Optimization of NPC differentiation to induce in vivo like BBB phenotype in rat BMECs | ~250 | n.a. | [10] |
| Co-culture with rat astrocytes or embryonic NPCs; Static | To understand the embryonic BBB development and BBB-inductive capacity of NPCs including BMEC and NPC cross-talk | 110 | NaF, 0.33 | ||
| CD34+ human umbilical cord blood (hematopoietic) cells | Co-culture with human brain pericytes; Static; VEGF as stimulant |
Breast cancer cells and BMEC interactions in brain metastases | n.a. | LY, 0.53–0.75 | [113] [114] |
| Development and predictive validity of a functional in vitro human BBB model and the role of Wnt signaling in BBB induction | 180 | LY, 0.61 | [112] | ||
| Human cord blood endothelial progenitor cells | Co-cultured with rat astrocytes; Static | Differentiation of circulating endothelial progenitor cells to BBB phenotype and comparison with hCMEC/D3 | n.a. | LY, 1.25 | [111] |
After optimization of several culture conditions including co-cultures, serum%, seeding density, differentiation days and medium, supplementation of enhancer
DF-19-9-11T hiPSC cell line;
H9 hECS cell line;
Additionally, given the tedious procedures and difficulties to isolate pure primary neurons or astrocytes, embryonic or adult brain neuronal progenitor cells (NPC) have served as potential alternative for robust BBB modeling in vitro [10, 84, 118, 119]. Mainly, NPCs are shown to proliferate extensively and differentiate into neuronal and astrocyte lineages that are critically involved in BBB development and maturation in vivo [119]. Differentiating (but not proliferating) rat or human embryonic NPCs significantly elevated the TEER (> 40%) in rat BMEC monolayers possibly by promoting TJ expression and continuity at cell-cell junctions [120]. Although, TEER values in this model reached peak values within 24h, these values remain relatively low (~100Ω × cm2; Table 4) and gradually decreased with the progression of time (24–120h), compared to a sustained increase in BMEC-astrocyte co-culture model [120]. The differentiation protocol was subsequently optimized to improve the model quality and robustness, as tested by previously established BBB-specific gene panel [10]. For instance, embryonic NPCs differentiated in 5% and 10% serum for 12 days elicit both maximal and sustained TEER response in BMEC monolayers (~250Ω.cm2), while low serum concentration did not outperform the tightness exhibited by BMEC monoculture alone [10]. Importantly, this model could have the potential for translatability to a fully humanized and functional BBB model but requires standardization to improve the barrier tightness thus mimicking the in vivo conditions.
Recently, human PSCs have shown a promising potential for robust and scalable BBB modeling in vitro [84, 111]. Human PSC-based BBB modeling also offers the ability to understand the specific signaling mechanisms involved in human BBB development [111, 121, 122]. To this end, Shusta’s laboratory has made significant strides in the development and optimization of a robust humanized BBB model using various hiPSC cell lines [111, 122]. Typically, this process involves the co-differentiation of immature NPCs and EPCs from adherent cultures of hiPSCs followed by EC specification and purification on selective matrix [111, 122]. Extensive characterization of the purified BMECs show abundant expression of TJ proteins enriched along the cell-cell contours and polarized expression of diverse array of functionally active BBB nutrient and efflux transporters [122]. The basic differentiation protocol was extensively optimized in subsequent studies to further enhance the differentiation efficiency of hiPSC cell line and BMEC functional responses to achieve high barrier tightness. For example, co-culture with astrocytes significantly increased the barrier integrity of BMEC monolayers derived from differentiation of IMR90-c4 hiPSC cultures (TEER=1450Ω.cm2; Table 4) when compared to BMEC monocultures [122].
Importantly, conditioning the IMR90-c4 derived BMEC monolayers with human pericytes post retinoic acid treatment and subsequent co-culture with differentiating human NPCs (astrocytes and neuron mixture) resulted in significant enrichment of BBB phenotype with a steep increase in TEER values reaching over 5000Ω × cm2 [121]. These TEER values are unprecedented in vitro and uncertainty prevails regarding the stability of the model in prolonged culture for long-term assessments. Nevertheless, the biochemical phenotype of BMECs differentiated from other cell lines under similar conditions also exhibited a potential improvement of barrier integrity [121]. Interestingly, it was also demonstrated that hiPSC derived BMECs co-cultured with astrocytes in microfluidic platforms can achieve significantly high and sustained TEER values without the necessity for shear stress [123].
In addition to retinoic acid supplementation, the source of matrigel used for early maintenance of stem cells [124] and initial hPSC seeding density before differentiation [122] could significantly affect BMEC yield and BBB tightness. For example, hiPSC differentiation efficiency can be optimized by selecting a moderate seeding density (30,000cells/cm2) that resulted in an improved TEER response [124]. However, this response to initial seeding density appears to be cell line dependent and variations in hPSC seeding density did not affect the biochemical phenotype of BMECs differentiated from IMR90-c4 hiPSC cell line [124]. Importantly, the singularized-cell seeding approach described by the latter study could enhance the scalability and reproducibility of hPSC-derived BBB models. Additionally, BMECs differentiated from reprogrammed iPSCs of a patient can reveal key information of BBB functional disparities in health and disease. Importantly, the TEER responses in stem cell based models are substantially higher as compared to primary human BMEC or hCMEC/D3 cultures [96]. Given a large dynamic range of permeability values (diazepam to sucrose), hPSC derived BBB models could have potential applications as reliable drug screening tools [123, 125], although more functional assays with a diverse array of compounds are required to test the predictive strength of the model [122]. Importantly, stem cells can be integrated with microfluidic approaches towards development of robust BBB-on-chip platforms for high throughput studies [123].
Despite high reproducibility of cord blood stem cell-derived BBB models, their relatively low barrier tightness could be a potential limitation [112, 113, 114]. By contrast, hPSC derived BMEC monolayers suffers from reproducibility and stability deficits. Nevertheless, stem cell based BBB modeling is receiving significant interest as a potential alternative to current in vitro platforms.
8. Conclusion
The BBB phenotype displays unique characteristics anatomically and functionally. Lack of fenestration in the BBB restricts influx of xenobiotics, thus protecting the CNS. The cross-talk between the cells of the NVU is crucial for the formation and maintenance of a functional BBB. Novel biotechnological advancements and better understanding of the processes governing the barriergenesis and barrier functions under normal and pathological conditions have driven the development of more sophisticated and realistic BBB models for studying the pathophysiology of CNS diseases, more effective CNS drugs and helped reduce the needs for inherently complicated and highly variable in vivo studies. The ability to modulate stem cells differentiation into BBB phenotypic cells will further boost the development of clinically relevant NVU models for basic and translational studies and drug development.
9. Expert Opinion
Most of the studies currently available revolve around the use of very cost-effective and user friendly ECs monocultures, which however, offer a scant image of the BBB in vivo and fail to reproduce the cellular and environmental milieu of the NVU. Several alternative approaches such as co- or triple static culture systems and/or incorporation of shear stress [41] into the model have been explored to overcome this limitation. In some instances, improved BBB tightness and selectivity have been observed but the data are not consistent and of limited reproducibility across platforms. Effort should be directed in the standardization of the measurable parameters used to assess the viability of the BBB independently from the platform/system used. Despite these issues, the use of in vitro models as companion tools to further basic and translational research hold great promise in us much the ability to selectively control and manipulate the biological environment (although in its simplest version) allow for testing and experiment conditions that are not easily reproducible in vivo. Use of primary cell cultures (when available) is still advantageous at least from a data reproducibility point of view but not very cost effective. Stem cell technology (although still not fully mature) could indeed provide a breakthrough in BBB modeling (and beyond) allowing for the development of the desired primary cultures within the platform itself, thus reducing the setup cost of the platform and the dedifferentiation issues that originate from having to passage the primary cells multiple times [123]. Further, the ability to strictly control the biological environment inside these platforms could indirect benefit stem cell technology by allowing to study and dissect out the optimal conditions necessary to differentiate and stabilize the cells into their final mature form. Among the various approaches to reproduce the BBB in vitro, microfluidic models have significant advantages over static and previous flow-based platforms including improved experimental flexibility, biomaterial integration, cost effectiveness and possibly high throughput. There is a growing trend in the microfluidics community to develop techniques that can be readily translated from engineering-centric labs to life science laboratories. This is achieved by limiting instrumentation requirements including external pumps [126], developing modular approaches [127], and simplifying experimental workflows [128]. Better accessibility to microfluidic tools for life science studies could help significantly advance BBB research. However, these platforms are still confined to a limited number of laboratories as working prototypes and access to these systems outside the lab of origin remains limited. The high level of technical proficiency required to use these models is also a restrictive factor. From a manufacturing stand point, lots of progresses have been made through the use of bioprinting technology. This process allows to generate spatially-controlled cell patterns where cell function and viability are preserved within the printed construct. At current stage however, the technology is not yet mature for the generation of multipart biological environments where several elements including materials, cell types, growth and differentiation factors pose technical challenges related to the sensitivities of living cells and the construction of more complex tissue structures such as the NVU. Further, lack of high-throughput 3D-bioprinted tissue models for research makes this technology not (yet) suitable for drug discovery and toxicology studies. To achieve these goals, the integration of multiple fields of research including engineering, biomaterials science, cell biology, physics and medicine will be necessary. Concerning the use of stem cells in BBB modeling, although noteworthy progresses have been made toward the differentiation of these cells into viable NVU components there are a number of issues that remain unresolved. Starting with the short term stability of the differentiated cells which at the current technological stage cannot be maintained for more than few days before dedifferentiation occurs. Also there are huge variations in TEER and permeability values reported across the various stem cells-based BBB models developed so far. Differences in the types/source of stem cell model (multipotent vs. pluripotent) used in these studies and the BBB development cues influencing the terminal differentiation process could plausibly explain these remarkable dissimilarities. For example, retinoic acid addition to the differentiated endothelial cell co-cultured with pericytes or differentiating NPC population could elevate the TEER by many-fold. This is attributed to the increased expression of TJ proteins, nutrient and drug transporters. Thus, it should be noted that development of BBB phenotype is stem cell source dependent. While iPSC models could have the potential to generate highly robust BBB with TEERs >3000Ω.cm2, that is several-fold superior to currently used human models, iPSCs may have different properties and differentiation capacities depending on their initial source [84]. Another limitation of the stem cell based models is the lack of comparative quantification studies for the expression of BBB-specific proteins with existing models (including stability). Further, the lack of correlative data between in vivo/human and in vitro permeability studies should be address along with a detailed evaluation of the expression (using QTAP for example) and functionality of major BBB transporters vs in vivo/human.
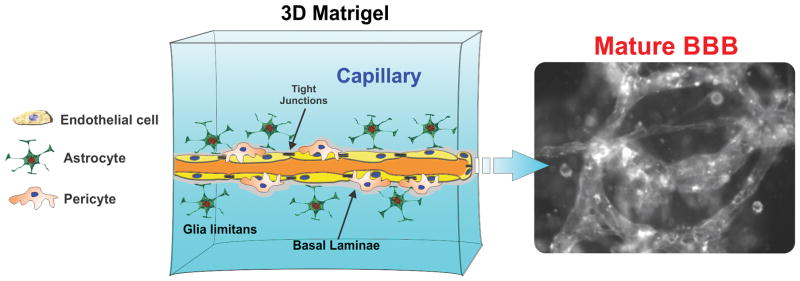
Figure 5.
Schematic representation of a 3D ECM BBB model. This platform allows the cells to co-exist with other cell populations embedded in environment that contains multiple ECM components, and nurtured by a variety of cell-secreted factors necessary for vasculogenesis/angiogenesis and/or cell migration. Thus microcapillary like structures forms within the 3D matrix.
Article highlights box.
Blood-brain barrier models are to be considered companion tools designed to facilitate basic and translational studies in the field of CNS drug discovery and cerebrovascular diseases
Complex co-culture systems may prove effective in the development of quasi-physiological system at the expenses of increased complexity and higher cost.
Microfluidic tissue-on-a-chip approaches have emerged as promising tools to establish in vitro BBB models but the technology is not yet available mainstream.
3D bioprinting technologies are also emerging as tools to develop cerebrovascular models
Cord blood stem cell or hPSC-derived BMEC are valuable alternative sources to generate the BBB cells necessary in the making of in vitro BBB models.
The use of multiple in vitro systems featuring complementary characteristics could help reducing the shortcomings of each platform as standalone systems.
Acknowledgments
Funding: This work was supported by a National Institutes of Health (NIH)/National Institute of Drug Abuse (NIDA) grant (R01DA029121) and by the Alternatives Research and Development Foundation.
Footnotes
Declaration of interest: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest
(**) to readers.
- 1.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–25. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 2.Huber JD, Witt KA, Hom S, et al. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001;280:H1241–8. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- 3.Tilling T, Engelbertz C, Decker S, et al. Expression and adhesive properties of basement membrane proteins in cerebral capillary endothelial cell cultures. Cell Tissue Res. 2002;310:19–29. doi: 10.1007/s00441-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg GA, Kornfeld M, Estrada E, et al. TIMP-2 reduces proteolytic opening of blood-brain barrier by type IV collagenase. Brain Res. 1992;576:203–7. doi: 10.1016/0006-8993(92)90681-x. [DOI] [PubMed] [Google Scholar]
- 5.Liberto CM, Albrecht PJ, Herx LM, et al. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89:1092–100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 6.Alavijeh MS, Chishty M, Qaiser MZ, et al. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx. 2005;2:554–71. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augustine C, Cepinskas G, Fraser DD, et al. Traumatic injury elicits JNK-mediated human astrocyte retraction in vitro. Neuroscience. 2014;274:1–10. doi: 10.1016/j.neuroscience.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 8(**).Cucullo L, Hossain M, Puvenna V, et al. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011;12:40. doi: 10.1186/1471-2202-12-40. This article details the effect of shear stress on BBB endothelial physiology and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue Q, Liu Y, Qi H, et al. A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat. Int J Biol Sci. 2013;9:174–89. doi: 10.7150/ijbs.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippmann ES, Weidenfeller C, Svendsen CN, et al. Blood-brain barrier modeling with co-cultured neural progenitor cell-derived astrocytes and neurons. J Neurochem. 2011;119:507–20. doi: 10.1111/j.1471-4159.2011.07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adkins CE, Nounou MI, Mittapalli RK, et al. A novel preclinical method to quantitatively evaluate early-stage metastatic events at the murine blood-brain barrier. Cancer Prev Res (Phila) 2015;8:68–76. doi: 10.1158/1940-6207.CAPR-14-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12(**).Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood-brain barrier. Neurobiology of disease. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. This review article details the physiological and functional characteristics of the BBB. [DOI] [PubMed] [Google Scholar]
- 13.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shayan G, Choi YS, Shusta EV, et al. Murine in vitro model of the blood-brain barrier for evaluating drug transport. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2011;42:148–55. doi: 10.1016/j.ejps.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Stamatovic SM, Keep RF, Kunkel SL, et al. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. Journal of cell science. 2003;116:4615–28. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- 16.Perriere N, Demeuse P, Garcia E, et al. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J Neurochem. 2005;93:279–89. doi: 10.1111/j.1471-4159.2004.03020.x. [DOI] [PubMed] [Google Scholar]
- 17.Abbott NJ, Dolman DE, Drndarski S, et al. An improved in vitro blood-brain barrier model: rat brain endothelial cells co-cultured with astrocytes. Methods Mol Biol. 2012;814:415–30. doi: 10.1007/978-1-61779-452-0_28. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa S, Deli MA, Kawaguchi H, et al. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int. 2009;54:253–63. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Helms HC, Brodin B. Generation of primary cultures of bovine brain endothelial cells and setup of cocultures with rat astrocytes. Methods Mol Biol. 2014;1135:365–82. doi: 10.1007/978-1-4939-0320-7_30. [DOI] [PubMed] [Google Scholar]
- 20.Patabendige A, Skinner RA, Morgan L, et al. A detailed method for preparation of a functional and flexible blood-brain barrier model using porcine brain endothelial cells. Brain Res. 2013;1521:16–30. doi: 10.1016/j.brainres.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomsen LB, Burkhart A, Moos T. A Triple Culture Model of the Blood-Brain Barrier Using Porcine Brain Endothelial cells, Astrocytes and Pericytes. PLoS One. 2015;10:e0134765. doi: 10.1371/journal.pone.0134765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman NA, Rasil AN, Meyding-Lamade U, et al. Immortalized endothelial cell lines for in vitro blood-brain barrier models: A systematic review. Brain Res. 2016;1642:532–45. doi: 10.1016/j.brainres.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS. 2013;10:16. doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatherell K, Couraud PO, Romero IA, et al. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods. 2011;199:223–9. doi: 10.1016/j.jneumeth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Dohgu S, Takata F, et al. Paracellular barrier and tight junction protein expression in the immortalized brain endothelial cell lines bEND.3, bEND.5 and mouse brain endothelial cell 4. Biol Pharm Bull. 2013;36:492–5. doi: 10.1248/bpb.b12-00915. [DOI] [PubMed] [Google Scholar]
- 26.Brown RC, Morris AP, O’Neil RG. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007;1130:17–30. doi: 10.1016/j.brainres.2006.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux F, Couraud PO. Rat brain endothelial cell lines for the study of blood-brain barrier permeability and transport functions. Cellular and molecular neurobiology. 2005;25:41–58. doi: 10.1007/s10571-004-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colgan OC, Ferguson G, Collins NT, et al. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol. 2007;292:H3190–7. doi: 10.1152/ajpheart.01177.2006. [DOI] [PubMed] [Google Scholar]
- 30.Wong AD, Ye M, Levy AF, et al. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krizanac-Bengez L, Mayberg MR, Janigro D. The cerebral vasculature as a therapeutic target for neurological disorders and the role of shear stress in vascular homeostatis and pathophysiology. Neurol Res. 2004;26:846–53. doi: 10.1179/016164104X3789. [DOI] [PubMed] [Google Scholar]
- 32.Bai K, Wang W. Shear stress-induced redistribution of the glycocalyx on endothelial cells in vitro. Biomech Model Mechanobiol. 2014;13:303–11. doi: 10.1007/s10237-013-0502-3. [DOI] [PubMed] [Google Scholar]
- 33.Balaguru UM, Sundaresan L, Manivannan J, et al. Disturbed flow mediated modulation of shear forces on endothelial plane: A proposed model for studying endothelium around atherosclerotic plaques. Sci Rep. 2016;6:27304. doi: 10.1038/srep27304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Torre JC, Mussivand T. Can disturbed brain microcirculation cause Alzheimer’s disease? Neurol Res. 1993;15:146–53. doi: 10.1080/01616412.1993.11740127. [DOI] [PubMed] [Google Scholar]
- 35.Desai SY, Marroni M, Cucullo L, et al. Mechanisms of endothelial survival under shear stress. Endothelium. 2002;9:89–102. doi: 10.1080/10623320212004. [DOI] [PubMed] [Google Scholar]
- 36.Stanness KA, Westrum LE, Fornaciari E, et al. Morphological and functional characterization of an in vitro blood-brain barrier model. Brain Res. 1997;771:329–42. doi: 10.1016/s0006-8993(97)00829-9. [DOI] [PubMed] [Google Scholar]
- 37.Cerutti C, Soblechero-Martin P, Wu D, et al. MicroRNA-155 contributes to shear-resistant leukocyte adhesion to human brain endothelium in vitro. Fluids Barriers CNS. 2016;13:8. doi: 10.1186/s12987-016-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cucullo L, Hossain M, Tierney W, et al. A new dynamic in vitro modular capillaries-venules modular system: cerebrovascular physiology in a box. BMC Neurosci. 2013;14:18. doi: 10.1186/1471-2202-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cucullo L, Marchi N, Hossain M, et al. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab. 2011;31:767–77. doi: 10.1038/jcbfm.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumpio BJ, Chitragari G, Moriguchi T, et al. African Trypanosome-Induced Blood-Brain Barrier Dysfunction under Shear Stress May Not Require ERK Activation. Int J Angiol. 2015;24:41–6. doi: 10.1055/s-0034-1370890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cucullo L, Couraud PO, Weksler B, et al. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab. 2008;28:312–28. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- 42.Siddharthan V, Kim YV, Liu S, et al. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang E, O’Donnell ME, Barakat AI. Shear stress and 17beta-estradiol modulate cerebral microvascular endothelial Na-K-Cl cotransporter and Na/H exchanger protein levels. Am J Physiol Cell Physiol. 2008;294:C363–71. doi: 10.1152/ajpcell.00045.2007. [DOI] [PubMed] [Google Scholar]
- 44.Xue S, Wang J, Zhang X, et al. Endothelial ATP-binding cassette G1 in mouse endothelium protects against hemodynamic-induced atherosclerosis. Biochem Biophys Res Commun. 2016;477:247–54. doi: 10.1016/j.bbrc.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh C, Gonzalez-Martinez J, Hossain M, et al. Pattern of P450 expression at the human blood-brain barrier: roles of epileptic condition and laminar flow. Epilepsia. 2010;51:1408–17. doi: 10.1111/j.1528-1167.2009.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46(*).Palmiotti CA, Prasad S, Naik P, et al. In vitro cerebrovascular modeling in the 21st century: current and prospective technologies. Pharm Res. 2014;31:3229–50. doi: 10.1007/s11095-014-1464-6. This review article covers a number of BBB models currently used in cerebrovascular research detailing both pros and cons of each approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolff A, Antfolk M, Brodin B, et al. In Vitro Blood-Brain Barrier Models-An Overview of Established Models and New Microfluidic Approaches. J Pharm Sci. 2015;104:2727–46. doi: 10.1002/jps.24329. Epub 2015/01/30. [DOI] [PubMed] [Google Scholar]
- 48.McDonald JC, Duffy DC, Anderson JR, et al. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. Epub 2000/01/14. [DOI] [PubMed] [Google Scholar]
- 49.Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annu Rev Biomed Eng. 2002;4:261–86. doi: 10.1146/annurev.bioeng.4.112601.125916. Epub 2002/07/16. [DOI] [PubMed] [Google Scholar]
- 50.Squires TM, Quake SR. Microfluidics: Fluid physics at the nanoliter scale. Reviews of Modern Physics. 2005;77:977–1026. [Google Scholar]
- 51.Ferrell N, Desai RR, Fleischman AJ, et al. A microfluidic bioreactor with integrated transepithelial electrical resistance (TEER) measurement electrodes for evaluation of renal epithelial cells. Biotechnol Bioeng. 2010;107:707–16. doi: 10.1002/bit.22835. Epub 2010/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young EW, Watson MW, Srigunapalan S, et al. Technique for real-time measurements of endothelial permeability in a microfluidic membrane chip using laser-induced fluorescence detection. Anal Chem. 2010;82:808–16. doi: 10.1021/ac901560w. [DOI] [PubMed] [Google Scholar]
- 53.Weibel DB, Diluzio WR, Whitesides GM. Microfabrication meets microbiology. Nat Rev Microbiol. 2007;5:209–18. doi: 10.1038/nrmicro1616. Epub 2007/02/17. [DOI] [PubMed] [Google Scholar]
- 54.Esch MB, Sung JH, Yang J, et al. On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’ devices. Biomed Microdevices. 2012;14:895–906. doi: 10.1007/s10544-012-9669-0. [DOI] [PubMed] [Google Scholar]
- 55(*).Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nature Reviews Drug Discovery. 2015;14:248–60. doi: 10.1038/nrd4539. This review discusses emerging drug discovery opportunites anabled by orgnas-on-chip and the challenges involved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghaemmaghami AM, Hancock MJ, Harrington H, et al. Biomimetic tissues on a chip for drug discovery. Drug Discov Today. 2012;17:173–81. doi: 10.1016/j.drudis.2011.10.029. Epub 2011/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (muBBB) Lab Chip. 2012;12:1784–92. doi: 10.1039/c2lc40094d. Epub 2012/03/17. [DOI] [PubMed] [Google Scholar]
- 58(**).Griep LM, Wolbers F, de Wagenaar B, et al. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices. 2013;15:145–50. doi: 10.1007/s10544-012-9699-7. Epub 2012/09/08. Article focused on BBB microfluidic systems. [DOI] [PubMed] [Google Scholar]
- 59.Prabhakarpandian B, Shen MC, Nichols JB, et al. SyM-BBB: a microfluidic Blood Brain Barrier model. Lab Chip. 2013;13:1093–101. doi: 10.1039/c2lc41208j. Epub 2013/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Booth R, Kim H. Permeability analysis of neuroactive drugs through a dynamic microfluidic in vitro blood-brain barrier model. Ann Biomed Eng. 2014;42:2379–91. doi: 10.1007/s10439-014-1086-5. Epub 2014/08/15. [DOI] [PubMed] [Google Scholar]
- 61.Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. Journal of laboratory automation. 2015;20:107–26. doi: 10.1177/2211068214561025. Epub 2015/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown JA, Pensabene V, Markov DA, et al. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics. 2015;9:054124. doi: 10.1063/1.4934713. Epub 2015/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herland A, van der Meer AD, FitzGerald EA, et al. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS One. 2016;11:e0150360. doi: 10.1371/journal.pone.0150360. Epub 2016/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bischel LL, Lee SH, Beebe DJ. A practical method for patterning lumens through ECM hydrogels via viscous finger patterning. Journal of laboratory automation. 2012;17:96–103. doi: 10.1177/2211068211426694. Epub 2012/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baeten KM, Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol. 2011;71:1018–39. doi: 10.1002/dneu.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–24. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doyle AD, Yamada KM. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp Cell Res. 2016;343:60–6. doi: 10.1016/j.yexcr.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edmondson R, Broglie JJ, Adcock AF, et al. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12:207–18. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 71.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185–96. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Tourovskaia A, Fauver M, Kramer G, et al. Tissue-engineered microenvironment systems for modeling human vasculature. Exp Biol Med (Maywood ) 2014;239:1264–71. doi: 10.1177/1535370214539228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munson JM, Bellamkonda RV, Swartz MA. Interstitial flow in a 3D microenvironment increases glioma invasion by a CXCR4-dependent mechanism. Cancer Res. 2013;73:1536–46. doi: 10.1158/0008-5472.CAN-12-2838. [DOI] [PubMed] [Google Scholar]
- 74.Zheng Y, Chen J, Craven M, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109:9342–7. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cho H, Seo JH, Wong KH, et al. Three-Dimensional Blood-Brain Barrier Model for in vitro Studies of Neurovascular Pathology. Sci Rep. 2015;5:15222. doi: 10.1038/srep15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Antoine EE, Vlachos PP, Rylander MN. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng Part B Rev. 2014;20:683–96. doi: 10.1089/ten.teb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li W, Zhu B, Strakova Z, et al. Two-way regulation between cells and aligned collagen fibrils: local 3D matrix formation and accelerated neural differentiation of human decidua parietalis placental stem cells. Biochem Biophys Res Commun. 2014;450:1377–82. doi: 10.1016/j.bbrc.2014.06.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–32. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 79.Toh YC, Lim TC, Tai D, et al. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip. 2009;9:2026–35. doi: 10.1039/b900912d. [DOI] [PubMed] [Google Scholar]
- 80.Zervantonakis IK, Hughes-Alford SK, Charest JL, et al. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A. 2012;109:13515–20. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benton G, Arnaoutova I, George J, et al. Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv Drug Deliv Rev. 2014;79–80:3–18. doi: 10.1016/j.addr.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 82.Shafiee A, Atala A. Printing Technologies for Medical Applications. Trends Mol Med. 2016;22:254–65. doi: 10.1016/j.molmed.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 83(*).Lee VK, Kim DY, Ngo H, et al. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials. 2014;35:8092–102. doi: 10.1016/j.biomaterials.2014.05.083. Interesting article focused on the use of biopriting technology to model quasi-physiological vascular segments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aday S, Cecchelli R, Hallier-Vanuxeem D, et al. Stem Cell-Based Human Blood-Brain Barrier Models for Drug Discovery and Delivery. Trends Biotechnol. 2016;34:382–93. doi: 10.1016/j.tibtech.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Shawahna R, Decleves X, Scherrmann JM. Hurdles with using in vitro models to predict human blood-brain barrier drug permeability: a special focus on transporters and metabolizing enzymes. Curr Drug Metab. 2013;14:120–36. [PubMed] [Google Scholar]
- 86.Syvanen S, Lindhe O, Palner M, et al. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab Dispos. 2009;37:635–43. doi: 10.1124/dmd.108.024745. [DOI] [PubMed] [Google Scholar]
- 87.Warren MS, Zerangue N, Woodford K, et al. Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human. Pharmacol Res. 2009;59:404–13. doi: 10.1016/j.phrs.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 88.Wilhelm I, Krizbai IA. In vitro models of the blood-brain barrier for the study of drug delivery to the brain. Mol Pharm. 2014;11:1949–63. doi: 10.1021/mp500046f. [DOI] [PubMed] [Google Scholar]
- 89.Bernas MJ, Cardoso FL, Daley SK, et al. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat Protoc. 2010;5:1265–72. doi: 10.1038/nprot.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cayrol R, Haqqani AS, Ifergan I, et al. Isolation of human brain endothelial cells and characterization of lipid raft-associated proteins by mass spectroscopy. Methods Mol Biol. 2011;686:275–95. doi: 10.1007/978-1-60761-938-3_13. [DOI] [PubMed] [Google Scholar]
- 91.Navone SE, Marfia G, Invernici G, et al. Isolation and expansion of human and mouse brain microvascular endothelial cells. Nat Protoc. 2013;8:1680–93. doi: 10.1038/nprot.2013.107. [DOI] [PubMed] [Google Scholar]
- 92.Artus C, Glacial F, Ganeshamoorthy K, et al. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab. 2014;34:433–40. doi: 10.1038/jcbfm.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eigenmann DE, Xue G, Kim KS, et al. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS. 2013;10:33. doi: 10.1186/2045-8118-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Forster C, Burek M, Romero IA, et al. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol. 2008;586:1937–49. doi: 10.1113/jphysiol.2007.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strazza M, Maubert ME, Pirrone V, et al. Co-culture model consisting of human brain microvascular endothelial and peripheral blood mononuclear cells. J Neurosci Methods. 2016;269:39–45. doi: 10.1016/j.jneumeth.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weksler BB, Subileau EA, Perriere N, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–4. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 97.Ohtsuki S, Ikeda C, Uchida Y, et al. Quantitative targeted absolute proteomic analysis of transporters, receptors and junction proteins for validation of human cerebral microvascular endothelial cell line hCMEC/D3 as a human blood-brain barrier model. Mol Pharm. 2013;10:289–96. doi: 10.1021/mp3004308. [DOI] [PubMed] [Google Scholar]
- 98.Sajja RK, Green KN, Cucullo L. Altered Nrf2 signaling mediates hypoglycemia-induced blood-brain barrier endothelial dysfunction in vitro. PLoS One. 2015;10:e0122358. doi: 10.1371/journal.pone.0122358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Urich E, Lazic SE, Molnos J, et al. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS One. 2012;7:e38149. doi: 10.1371/journal.pone.0038149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bayzigitov DR, Medvedev SP, Dementyeva EV, et al. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Afford New Opportunities in Inherited Cardiovascular Disease Modeling. Cardiol Res Pract. 2016;2016:3582380. doi: 10.1155/2016/3582380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Vos J, Bouckenheimer J, Sansac C, et al. Human induced pluripotent stem cells: A disruptive innovation. Curr Res Transl Med. 2016;64:91–6. doi: 10.1016/j.retram.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 102(**).Kramer N, Rosner M, Kovacic B, et al. Full biological characterization of human pluripotent stem cells will open the door to translational research. Arch Toxicol. 2016;90:2173–86. doi: 10.1007/s00204-016-1763-2. This review article summarizes current knoledge corcening guided differentiation of pluripotent stem cells. [DOI] [PubMed] [Google Scholar]
- 103.Russo FB, Cugola FR, Fernandes IR, et al. Induced pluripotent stem cells for modeling neurological disorders. World J Transplant. 2015;5:209–21. doi: 10.5500/wjt.v5.i4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Siddiqi F, Wolfe JH. Stem Cell Therapy for the Central Nervous System in Lysosomal Storage Diseases. Hum Gene Ther. 2016 doi: 10.1089/hum.2016.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gomez-Lechon MJ, Tolosa L. Human hepatocytes derived from pluripotent stem cells: a promising cell model for drug hepatotoxicity screening. Arch Toxicol. 2016;90:2049–61. doi: 10.1007/s00204-016-1756-1. [DOI] [PubMed] [Google Scholar]
- 106.Kawser Hossain M, Abdal Dayem A, Han J, et al. Recent Advances in Disease Modeling and Drug Discovery for Diabetes Mellitus Using Induced Pluripotent Stem Cells. Int J Mol Sci. 2016;17:256. doi: 10.3390/ijms17020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kelava I, Lancaster MA. Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev Biol. 2016 doi: 10.1016/j.ydbio.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schmidt BZ, Lehmann M, Gutbier S, et al. In vitro acute and developmental neurotoxicity screening: an overview of cellular platforms and high-throughput technical possibilities. Arch Toxicol. 2016 doi: 10.1007/s00204-016-1805-9. [DOI] [PubMed] [Google Scholar]
- 109.Schutgens F, Verhaar MC, Rookmaaker MB. Pluripotent stem cell-derived kidney organoids: An in vivo-like in vitro technology. Eur J Pharmacol. 2016 doi: 10.1016/j.ejphar.2016.06.059. [DOI] [PubMed] [Google Scholar]
- 110(*).Helms HC, Abbott NJ, Burek M, et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab. 2016;36:862–90. doi: 10.1177/0271678X16630991. Interesting review article focused on number of in vitro BBB platforms and guidelines to their use including pros and cons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stebbins MJ, Wilson HK, Canfield SG, et al. Differentiation and characterization of human pluripotent stem cell-derived brain microvascular endothelial cells. Methods. 2016;101:93–102. doi: 10.1016/j.ymeth.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boyer-Di Ponio J, El-Ayoubi F, Glacial F, et al. Instruction of circulating endothelial progenitors in vitro towards specialized blood-brain barrier and arterial phenotypes. PLoS One. 2014;9:e84179. doi: 10.1371/journal.pone.0084179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cecchelli R, Aday S, Sevin E, et al. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS One. 2014;9:e99733. doi: 10.1371/journal.pone.0099733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Drolez A, Vandenhaute E, Julien S, et al. Selection of a Relevant In Vitro Blood-Brain Barrier Model to Investigate Pro-Metastatic Features of Human Breast Cancer Cell Lines. PLoS One. 2016;11:e0151155. doi: 10.1371/journal.pone.0151155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vandenhaute E, Drolez A, Sevin E, et al. Adapting coculture in vitro models of the blood-brain barrier for use in cancer research: maintaining an appropriate endothelial monolayer for the assessment of transendothelial migration. Lab Invest. 2016;96:588–98. doi: 10.1038/labinvest.2016.35. [DOI] [PubMed] [Google Scholar]
- 116.Naik P, Cucullo L. In vitro blood-brain barrier models: current and perspective technologies. J Pharm Sci. 2012;101:1337–54. doi: 10.1002/jps.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wilhelm I, Fazakas C, Krizbai IA. In vitro models of the blood-brain barrier. Acta Neurobiol Exp (Wars) 2011;71:113–28. doi: 10.55782/ane-2011-1828. [DOI] [PubMed] [Google Scholar]
- 118.Chou CH, Sinden JD, Couraud PO, et al. In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells. PLoS One. 2014;9:e106346. doi: 10.1371/journal.pone.0106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Daneman R, Agalliu D, Zhou L, et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–6. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weidenfeller C, Svendsen CN, Shusta EV. Differentiating embryonic neural progenitor cells induce blood-brain barrier properties. J Neurochem. 2007;101:555–65. doi: 10.1111/j.1471-4159.2006.04394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lippmann ES, Al-Ahmad A, Azarin SM, et al. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep. 2014;4:4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lippmann ES, Azarin SM, Kay JE, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol. 2012;30:783–91. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123(*).Wang YI, Abaci HE, Shuler ML. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng. 2016 doi: 10.1002/bit.26045. Interesting reearch article focused on the development of a quasi-physiological BBB microfluidic model characterized by high impedence and tightness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124(*).Patel R, Alahmad AJ. Growth-factor reduced Matrigel source influences stem cell derived brain microvascular endothelial cell barrier properties. Fluids Barriers CNS. 2016;13:6. doi: 10.1186/s12987-016-0030-5. Research article outlined the use of ECM to modulate stem cell differentiation into a BBB EC phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clark PA, Al-Ahmad AJ, Qian T, et al. Analysis of Cancer-Targeting Alkylphosphocholine Analogue Permeability Characteristics Using a Human Induced Pluripotent Stem Cell Blood-Brain Barrier Model. Mol Pharm. 2016 doi: 10.1021/acs.molpharmaceut.6b00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abaci HE, Gledhill K, Guo Z, et al. Pumpless microfluidic platform for drug testing on human skin equivalents. Lab Chip. 2015;15:882–8. doi: 10.1039/c4lc00999a. Epub 2014/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loskill P, Marcus SG, Mathur A, et al. muOrgano: A Lego(R)-Like Plug & Play System for Modular Multi-Organ-Chips. PLoS One. 2015;10:e0139587. doi: 10.1371/journal.pone.0139587. Epub 2015/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abhyankar VV, Wu M, Koh CY, et al. A Reversibly Sealed, Easy Access, Modular (SEAM) Microfluidic Architecture to Establish In Vitro Tissue Interfaces. PLoS One. 2016;11:e0156341. doi: 10.1371/journal.pone.0156341. Epub 2016/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakagawa S, Deli MA, Nakao S, et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cellular and molecular neurobiology. 2007;27:687–94. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang J, Sun L, Si YF, et al. Overexpression of actin-depolymerizing factor blocks oxidized low-density lipoprotein-induced mouse brain microvascular endothelial cell barrier dysfunction. Mol Cell Biochem. 2012;371:1–8. doi: 10.1007/s11010-012-1415-7. [DOI] [PubMed] [Google Scholar]
- 131.Vandenhaute E, Dehouck L, Boucau MC, et al. Modelling the neurovascular unit and the blood-brain barrier with the unique function of pericytes. Curr Neurovasc Res. 2011;8:258–69. doi: 10.2174/156720211798121016. [DOI] [PubMed] [Google Scholar]