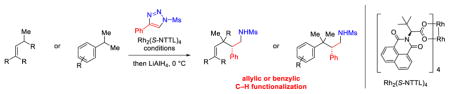
Abstract
The enantioselective intermolecular sp3 C–H functionalization at allylic and benzylic positions was achieved using rhodium-catalyzed reactions with 4-phenyl-N-methanesulfonyl-1,2,3-triazole. The optimum dirhodium tetracarboxylate catalyst for these reactions was Rh2(S-NTTL)4. The rhodium-bound α-imino carbene intermediates preferentially reacted with tertiary over primary C–H bonds in good yields and moderate levels of enantioselectivity (66-82% ee). This work demonstrates that N-sulfonyltriazoles can be applied to the effective C–H functionalization at sp3 C–H bonds of substrates containing additional functionality.
Graphical Abstract
The selective functionalization of C–H bonds is becoming a powerful approach for the construction of various organic compounds of academic and medicinal interest.1–3 Of the many new methods4 to achieve selective C–H functionalization, donor-acceptor metallocarbenes have emerged as privileged reactive intermediates for the functionalization of sp3 C–H bonds because their reactions are often highly site-selective, diasteroselective, and enantioselective.5 Donor-acceptor metallocarbenes are typically generated by extrusion of dinitrogen from aryldiazoacetates in the presence of dirhodium(II)-tertracarboxylate catalysts, and these intermediates insert into C–H bonds through a concerted mechanism where site-selectivity is determined by the competing influences of the steric bulk of the dirhodium carbene complex and the ability of the insertion site carbon to stabilize developing positive charge.6
As an alternative to aryldiazoacetates, 4-aryl-N-sulfonyl-1,2,3-triazoles have become valuable synthons in Rh(II)-catalyzed reactions in recent years because these heterocycles are in equilibrium with their open-chain α-diazo imine forms in solution.7 Consequently, N-sulfonyl-1,2,3-triazoles undergo many similar Rh(II)-catalyzed transformations known to their diazo ester congeners such as cyclopropanation, [3+2] dipolar cycloaddition, arylation, and [4+3] cycloaddition reactions.8
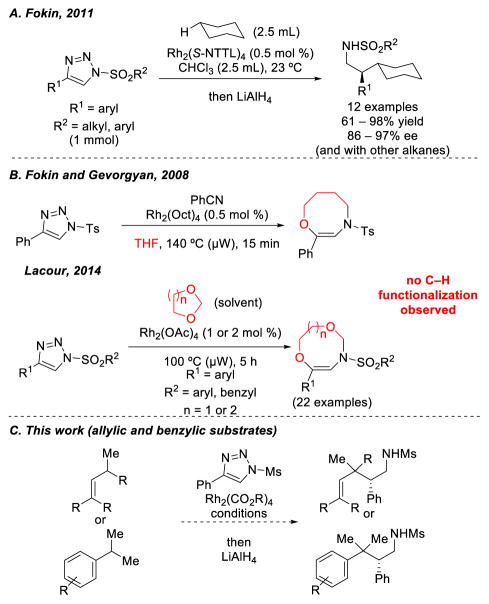
In contrast, the utility of N-sulfonyl-1,2,3-triazoles for selective intermolecular C–H functionalization reactions, to date, has been limited to a single study.9,10 In 2011, Fokin and coworkers showed that α-diazo imines generated from the requisite 4-aryl-N-sulfonyl-1,2,3-triazole precursors undergo intermolecular C–H functionalization reactions with hydrocarbons (as cosolvent) in the presence of Rh2(S-NTTL)4 or Rh2(S-PTAD)4 at room temperature (Scheme 1, A).9 Comparatively, when 4-aryl-N-sulfonyl-1,2,3-triazoles were reacted with tetrahydrofuran or 1,3-dioxolane, both favorable substrates for C–H functionalization reactions with α-diazoesters,11 no observable C–H functionalization products were reported. Instead, ring-expanded products were obtained, derived from rearrangement of oxo-nium ylide intermediates (Scheme 1, B).12 Presumably because of these results, there are no further reports on intermolecular sp3 C–H functionalization with N-sulfonyltriazoles. Thus, we decided to explore whether N-sulfonyl triazoles could be used for C–H functionalization of other activated C–H bonds. Herein we describe our initial evaluation of C–H functionalization of allylic and benzylic C–H bonds (Scheme 1, C).
Scheme 1.
Previous work on intermolecular C–H functionalization using N-sulfonyl-1,2,3-triazoles.
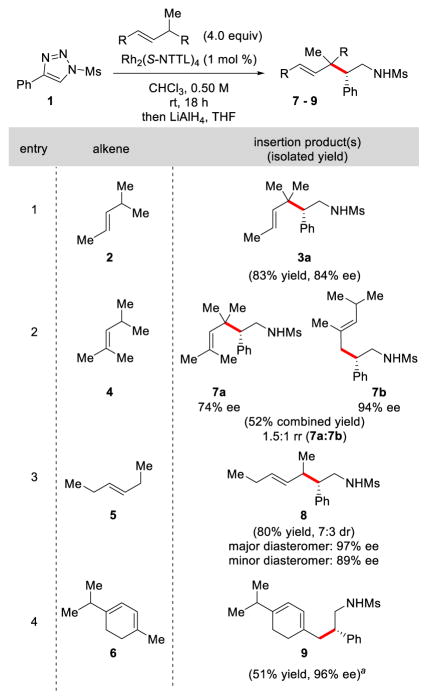
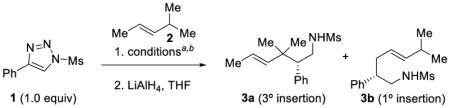
We began our studies by optimizing the reaction of 4-phenyl-1-methanesulfonyl-1,2,3-triazole (1) with trans-4-methyl-2-pentene (2) (Table 1). After a brief survey of dirhodium tetracarboxylate catalysts (see the Supporting Information for de- was identified as the optimal catalyst for tails), Rh2(S-NTTL)4 this transformation.13 Thus, taking compound 1 and 2.0 equiv of 2 in CHCl3 (0.5 M with respect to 1) and stirring the reaction mixture for 18 h at ambient temperature with 1 mol % Rh2(S-NTTL)4 led to the formation of C–H insertion product 3a (after in situ reduction of the intermediate sulfonyl imine) in 74% isolated yield with a >30:1 regioselective preference for the tertiary C–H insertion over the primary C–H insertion product 3b in 77% ee (Table 1, entry 1). Increasing the amount of alkene 2 from 2.0 to 4.0 equiv resulted in a slight improvement in the isolated yield (83%) and enantioselectivity (84% ee) (Table 1, entries 2–3). Changes to the concentration of triazole 1 (Table 1, entries 4–5) had little effect on the isolated yield and enantioselectivity. Increasing the reaction temperature to 40 ºC (Table 1, entry 6) resulted in a decrease in yield of 3a, while lowering the reaction temperature to 0 ºC resulted in no product formation (Table 1, entry 7). Shorter reaction times resulted in slightly diminished yield and no advantage was observed when using molecular sieves (Table 1, entries 8–10). Using other chlorinated solvents such as CH2Cl2 and 1,2-dichloroethane (Table 1, entries 11 and 13) typically resulted in slightly diminished isolated yields with modest increases in enantioselectivity to 85% and 86% ee, respectively. Other solvents such as α,α,α-trifluorotoluene14 (Table 1, entry 16) gave lower isolated yields and enantioselectivity, and coordinating solvents, such as ethyl acetate, (Table 1, entry 18) were ineffective for this transformation. The absolute configuration of 3a was determined to be (S)- by X-ray crystallographic analysis.15 The absolute configurations of the other C–H functionalization products are tentatively assigned assuming the same face selectivity during the approach of the substrate to the rhodium-bound carbene.
Table 1.
| |||||||
|---|---|---|---|---|---|---|---|
| entry | solvent | equiv of 2 | t (°C) | concn of 1 (M) | 3°:1° insertion | yield (%) of 3a | % ee of 3a |
| 1 | CHCl3 | 2 | rt | 0.50 | >30:1 | 74 | 77 |
| 2 | CHCl3 | 4 | rt | 0.50 | >30:1 | 83 | 84 |
| 3 | CHCl3 | 8 | rt | 0.50 | >30:1 | 77 | 84 |
| 4 | CHCl3 | 4 | rt | 1.0 | >30:1 | 77 | 83 |
| 5 | CHCl3 | 4 | rt | 0.25 | >30:1 | 76 | 83 |
| 6 | CHCl3 | 4 | 40 | 0.50 | >30:1 | 78 | 83 |
| 7 | CHCl3 | 4 | 0 | 0.50 | n/a | 0 | n/a |
| 8c | CHCl3 | 4 | rt | 0.50 | >30:1 | 74 | 84 |
| 9d | CHCl3 | 4 | rt | 0.50 | >30:1 | 82 | 83 |
| 10e | CHCl3 | 4 | rt | 0.50 | >30:1 | 80 | 83 |
| 11 | CH2Cl2 | 4 | rt | 0.50 | >30:1 | 73 | 85 |
| 12 | CH2Cl2 | 4 | reflux | 0.50 | >30:1 | 70 | 82 |
| 13 | 1,2-DCE | 4 | rt | 0.50 | >30:1 | 63 | 86 |
| 14 | 1,2-DCE | 4 | 40 | 0.50 | >30:1 | 72 | 67 |
| 15 | TFT | 4 | rt | 0.50 | n/a | 0 | n/a |
| 16 | TFT | 4 | 40 | 0.50 | >30:1 | 69 | 79 |
| 17 | EtOAc | 4 | rt | 0.50 | n/a | 0 | n/a |
| 18 | EtOAc | 4 | 40 | 0.50 | n/a | 0 | n/a |
Reaction conditions: Rh2(S-NTTL)4 (1.0 mol %), 1.0 mmol triazole 1.
18 h reaction time unless otherwise stated.
4 Å molecular sieves.
6 h reaction time.
12 h reaction time. DCE = dichloroethane, TFT = α,α,α-trifluorotoluene.
With these optimized conditions in hand, we then explored the scope of this transformation with various alkenes (Table 2). A tri-substituted alkene with two potential sites of reactivity, such as trans-(2,4-dimethyl)-2-pentene (4) gave a mixture of the C–H insertion products 7a and 7b in 52% combined yield (1.5:1 rr) under the optimized reaction conditions. Interestingly, we found that 7b, resulting from primary C–H insertion, was obtained in 94% ee whereas 7a, resulting from insertion into the tertiary C–H bond, was obtained in 74% ee. Similar higher enantioselectivity for primary C–H insertion versus tertiary C–H insertion was seen in the rhodium-catalyzed reactions with ar-yldiazoacetates.5b In the case of 3-hexene which has a secondary C–H site, product 8 was furnished in 80% yield with moderate diastereoselectivity (7:3 dr) and high enantioselectivity (97% and 89% ee for the major and minor diastereomer, respectively). When α-terpinene (6) was used as a reaction substrate, a reversal of site-selectivity in the C–H insertion reaction was observed. Specifically, the product resulting from primary insertion (9) was formed as the major product in 51% isolated yield and 96% ee in addition to less than 3% of a mixture of various other insertion products. In all of the presented scenarios, no products derived from cyclopropanation of the olefin were observed.
Table 2.
Substrate scope for allylic C–H functionalization.
Traces (< 3%) of a mixture of other insertion products were observed.
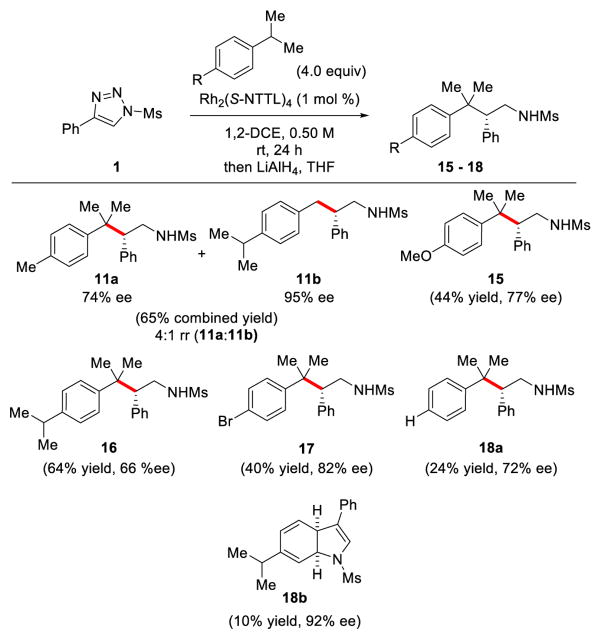
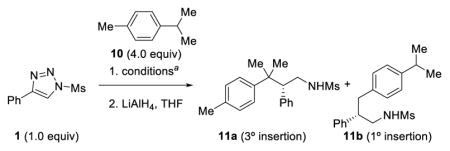
With the allylic C–H functionalization reaction established, we then investigated the selective functionalization of benzylic C–H bonds (Table 3). Using p-cymene 10 as a model substrate, Rh2(S-NTTL)4 again proved to be the best catalyst to produce products 11a and 11b in terms of yields, regioselectivity, and enantioselectivity (Table 3, entry 1). Other catalysts such as Rh2(S-PTAD)4 and Rh2(S-TCPTAD)4 gave diminished yields and/or stereoselectivity (Table 3, entries 4–7). Reducing the reaction time to 3 h resulted in slightly lower isolated yields (Table 3, entry 3). A similar solvent effect was observed between chloroform and 1,2–dichloroethane where the former gave slightly better yields and the latter better levels of enantioselectivity (Table 3, entries 1 and 2). In all cases, the tertiary C–H insertion product 11a was favored over the primary insertion product 11b.
Table 3.
Optimization for benzylic C–H functionalization reaction.a
| ||||||
|---|---|---|---|---|---|---|
| entry | solvent | catalyst | time (h) | 3°:1° insertion | combined yield (%) | % ee of 3a |
| 1 | CHCl3 | Rh2(S-NTTL)4 | 24 | 79:21 | 75 | 68 |
| 2 | 1,2-DCE | Rh2(S-NTTL)4 | 24 | 80:20 | 65 | 74 |
| 3 | 1,2-DCE | Rh2(S-NTTL)4 | 3 | 80:20 | 54 | 77 |
| 4 | 1,2-DCE | Rh2(S-PTAD)4 | 24 | 64:36 | 16 | 70 |
| 5 | 1,2-DCE | Rh2(S-TCPTAD)4 | 24 | 67:33 | 13 | 50 |
| 6 | 1,2-DCE | Rh2(S-PTTL)4 | 24 | 71:29 | 28 | 65 |
| 7 | 1,2-DCE | Rh2(S-NTV)4 | 18 | 83:17 | 64 | 62 |
Reactions were run at ambient temperature at 0.5 M concentration, with 1 mol % of catalyst. See the supporting information for the structures of the catalysts.
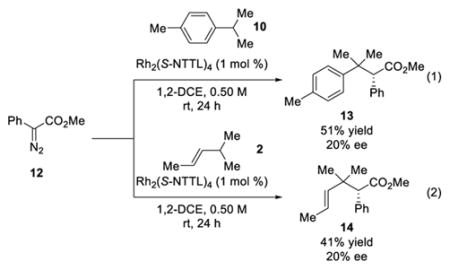
For comparison, when methyl phenyldiazoacetate (12) was used instead of triazole 1 as the C–H insertion partner (Eq 1) in the Rh2(S-NTTL)4-catalyzed reaction with p-cymene, the tertiary C–H insertion product 13 was formed in 51% yield, but with low enantioinduction (20% ee). When the analogous reaction was performed with trans-4-methyl-2-pentene, the C–H insertion product 14 was isolated in 41% yield and 20% ee (Eq 2). These results suggest that even though Rh2(S-NTTL)4 is generally the most effective chiral catalyst to date for the reactions of N-sulfonyltriazoles, its performance is inferior for the reactions of aryldiazoacetates.5e
 |
Further exploration with a variety of isopropylbenzene substrates is presented in Figure 1. Substrates such as p-isopropyl anisole, 1,4-diisopyropylbenzene, cumene, and 4-bromo cumene all afforded their corresponding tertiary C–H insertion products 15 – 18 in moderate yields (24 – 64% yield) and enantioselectivity (66 – 82% ee). Interestingly, when using isopropylbenzene as a substrate which does not feature a 1,4-substitution pattern, we isolated dihydroindole 18b in 10% yield and 92% ee resulting from an intermolecular [3+2] dipolar cycloaddition reaction, in addition to the desired C–H insertion product 18a16
Figure 1.
Substrate scope for benzylic C–H functionalization reaction.
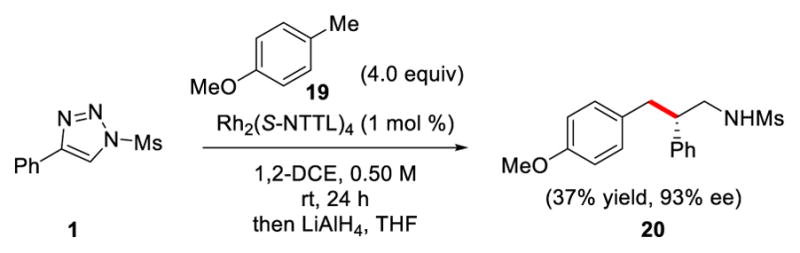
Encouraged by the high levels of enantioselectivity obtained for primary C–H insertion products 9 and 11b (95% ee), we finally investigated the insertion of α-diazo imine intermediates into primary benzylic positions. Specifically, we found that reacting 4-methylanisole (19) with triazole 1 under the optimized reaction conditions, produced the primary insertion product 20 in 37% yield and 93% ee after LiAlH4 reduction. Even though at this stage the yield of the C–H functionalization products at primary benzylic C–H bonds is relatively low, these systems are capable of high levels of enantioinduction with Rh2(S-NTTL)4 as the catalyst, which is promising for future reaction development.
In conclusion, we have demonstrated C–H functionalization reactions of allylic and benzylic sp3 C–H bonds using donor-acceptor carbenes generated from 4-aryl-N-sulfonyl-1,2,3-tria-zoles. These reactions show modest selectivity for tertiary C–H bonds in most cases and proceed with good asymmetric induction. In cases where mixtures of tertiary and primary insertion products are obtained, we observed the highest levels of enantioselectivity for the primary C–H insertion products. Future directions include extending the scope and selectivity of this reaction and developing a mechanistic understanding of the transformation.
Supplementary Material
Scheme 2.
Reaction of triazole 1 with 4-methylanisole
Acknowledgments
We are grateful for the NIH for financial support (project number 2R01GM099142-05). R.W.K., II is grateful for an Emory University graduate diversity fellowship, J.D.M. is grateful for postdoctoral funding from AbbVie Inc. S.M.W.H is grateful for a UNCF-Merck postdoctoral fellowship. TY is grateful for an international exchange funding from ITbM, which is supported by the World Premier International Research Center (WPI) Initiative, Japan. We wish to thank Dr. John Bacsa from the Emory University X-ray Crystallography Center for the X-ray crystallographic structure determination.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors.
Full experimental data for the compounds described in the paper and X-ray crystallographic data for compound 3a. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.For an overview of recent advances in C–H functionalization, see: Davies HML, Morton D. J Org Chem. 2016;81:343–350. doi: 10.1021/acs.joc.5b02818.Farmer ME, Laforteza BN, Yu JQ. Bioorg Med Chem. 2014;22:4445–4452. doi: 10.1016/j.bmc.2014.05.031.Brückl T, Baxter RD, Ishihara Y, Baran PS. Acc Chem Res. 2012;45:826–839. doi: 10.1021/ar200194b.Gutekunst WR, Baran PS. Chem Soc Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a.Newhouse T, Baran PS. Angew Chem Int Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368.
- 2.For C–H functionalization for materials and natural products, see: McMurray L, O’Hara F, Gaunt MJ. Chem Soc Rev. 2011;40:1885–1898. doi: 10.1039/c1cs15013h.Yamaguchi J, Yamaguchi AD, Itami K. Angew Chem Int Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666.Ku-ninobu Y, Sueki S. Synthesis. 2015;47:3823–3845.
- 3.For a review on the use of C–H functionalization in medicinal chemistry, see: Cernak T, Dykstra KD, Tyagarajan S, Vachal P, Krska SW. Chem Soc Rev. 2016;45:546–576. doi: 10.1039/c5cs00628g.
- 4.For recent methods for C–H functionalization using Rh(I)-Rh(III) catalytic cycle review, see: Motevalli S, Sokeirik Y, Ghanem A. Eur J Org Chem. 2016;8:1459–1475.
- 5.For reviews on C–H functionalization using donor-acceptor metalocarbenes, see: Davies HML, Manning JR. Nature. 2008;451:417–424. doi: 10.1038/nature06485.Davies HML, Morton D. Chem Soc Rev. 2011;40:1857–1869. doi: 10.1039/c0cs00217h.Davies HML, Lian Y. Acc Chem Res. 2012;45:923–935. doi: 10.1021/ar300013t.. For recent examples of C–H functionalization using donor-acceptor metalocarbenes, see: Qin C, Davies HML. J Am Chem Soc. 2014;136:9792. doi: 10.1021/ja504797x.Guptill DM, Davies HML. J Am Chem Soc. 2014;136:17718–17721. doi: 10.1021/ja5107404.Fu L, Guptill DM, Davies HML. J Am Chem Soc. 2016;138:5761–5764. doi: 10.1021/jacs.6b01941.Liao K, Negretti S, Musaev DG, Bacsa J, Davies HML. Nature. 2016;533:230–234. doi: 10.1038/nature17651.
- 6.For mechanistic studies of Rh(II)-tetracarboxylate-catalyzed C–H insertion reactions, see: Hansen J, Autschbach J, Davies HML. J Org Chem. 2009;74:6555–6563. doi: 10.1021/jo9009968.Bess EN, Guptill DM, Davies HML, Sigman MS. Chem Sci. 2015;6:3057–3062. doi: 10.1039/c5sc00357a.
- 7.The ring-opening and rearrangement is known as the Dimroth rearrangement Dimroth O. Liebigs Ann Chem. 1909;364:183–226.Gilchrist TL, Gymer GE. Adv Heterocycl Chem. 1974;16:33–85.
- 8.For a review on the utility of N-sulfonyl triazoles, see: Gulevich AV, Gevorgyan V. Angew Chem Int Ed. 2013;52:1371–1373. doi: 10.1002/anie.201209338.Davies HML, Alford JS. Chem Soc Rev. 2014;43:5151–5162. doi: 10.1039/c4cs00072b.. For cyclopropanation reactions using N-sulfonyl-1,2,3-triazoles, see: Grimster N, Zhang L, Fokin VV. J Am Chem Soc. 2010;132:2510–2511. doi: 10.1021/ja910187s.Chuprakov S, Kwok SW, Zhang L, Lercher L, Fokin VV. J Am Chem Soc. 2009;131:18034–18035. doi: 10.1021/ja908075u.Shultz EE, Lindsay VNG, Sarpong R. Angew Chem Int Ed. 2014;53:9904–9908. doi: 10.1002/anie.201405356.Tian Y, Wang Y, Shang H, Xu X, Tang Y. Org Biomol Chem. 2015;13:612–619. doi: 10.1039/c4ob01910e.. For arylation reactions, see: Selander N, Worrell BT, Chuprakov S, Velaparthi S, Fokin VV. J Am Chem Soc. 2012;134:14670–14673. doi: 10.1021/ja3062453.. For dipolar cycloadditions with indoles, see: Spangler JE, Davies HML. J Am Chem Soc. 2013;135:6802–6805. doi: 10.1021/ja4025337.
- 9.Chuprakov S, Malik JA, Zibinsky M, Fokin VV. J Am Chem Soc. 2011;133:10352–10355. doi: 10.1021/ja202969z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For intramolecular C–H insertion reactions using N-sulfonyl-1,2,3-triazole substrates, see: Shen MH, Pan YP, Jia ZH, Ren XT, Zhang P, Xu HD. Org Biomol Chem. 2015;13:4851–4854. doi: 10.1039/c5ob00085h.Lindsay VNG, Viart HMF, Sarpong R. J Am Chem Soc. 2015;137:8368–8371. doi: 10.1021/jacs.5b04295.Senoo M, Furukawa A, Hata T, Urabe H. Chem Eur J. 2016;22:890–895. doi: 10.1002/chem.201503823.. For the formal sp2 C–H insertion into electron-rich arenes, see Yadagiri D, Anbarasan P. Org Lett. 2014;16:2510–2513. doi: 10.1021/ol500874p.Shin S, Park Y, Kim CE, Son JY, Lee PH. J Org Chem. 2015;80:5859–5869. doi: 10.1021/acs.joc.5b00891.
- 11.For the C–H functionalization of tetrahydrofuran using α-diazo esters, see: Davies HML, Hansen T, Churchill RM. J Am Chem Soc. 2000;122:3063–3070.Davies HML, Tore H. J Am Chem Soc. 1997;119:9075–9076.Suematsu H, Tsutomu K. J Am Chem Soc. 2009;131:14128–14129.Li Y, Huang JS, Zhou ZY, Che CM, You XZ. J Am Chem Soc. 2002;124:13185–13193. doi: 10.1021/ja020391c.Fraile JM, Garcia JI, Mayoral JA, Roldán M. Org Lett. 2007;9:731–733. doi: 10.1021/ol070092y.Lovely CJ, Flores JA, Meng X, Dias HVR. Synlett. 2009:129–132.Fraile JM, López-Ram-de-Viu P, Mayoral JA, Roldán M, Santafé-Valero J. Org Bi-omol Chem. 2011;9:6075–6081. doi: 10.1039/c1ob05499f.Thrun F, Butschke B, Graening T. Chem Eur J. 2012;18:4854–4858. doi: 10.1002/chem.201102427.. For 1,3-di-oxolanes, see: Jimenez-Osés G, Vispe E, Roldán M, Rodríguez-Rodríguez S, López-Ram-de-Viu P, Salvatella L, Mayoral JA, Fraile JM. J Org Chem. 2013;78:5851–5857. doi: 10.1021/jo400415b.
- 12.For the seminal report of this reactivity, see footnote 11 in Horneff T, Chuprakov S, Chernyak N, Gevorgyan V, Fokin VV. J Am Chem Soc. 2008;130:14972–14974. doi: 10.1021/ja805079v.Medina F, Besnard C, Lacour J. Org Lett. 2014;16:3232–3235. doi: 10.1021/ol5012532.Pospech J, Ferraccioli R, Neumann H, Beller M. Chem Asian J. 2015;10:2460–2630. doi: 10.1002/asia.201500493.
- 13.For the seminal publication of synthesis of amino acid-derived Rh(II)-tetracarboxylate catalysts, see: Hashimoto S, Watanabe N, Ikegami S. Tetrahedron Lett. 1990;31:5173–5174.Hashimoto S, Watanabe N, Ikegami S. Synlett. 1994:353–355.. For the synthesis of tert-leucine derived Rh(II)-carboxylate catalysts see: Anada MM, Watanabe N. Chem Commun. 1998:1517–1518.. For the naphthyl derivatives see: Adly FG, Gardiner MG, Ghanem A. Chem Eur J. 2016;22:3447–3461. doi: 10.1002/chem.201504817.
- 14.(a) Ogawa A, Curran DP. J Org Chem. 1997;62:450–451. doi: 10.1021/jo9620324. [DOI] [PubMed] [Google Scholar]; (b) Maul JJ, Ostrowski PJ, Ublacker GA, Linclau B, Curran DP. Top Curr Chem. 1999;206:79–105. [Google Scholar]
- 15.The crystal structure for 3a has been deposited in the Cambridge Database (1475827).
- 16.Murakami and coworkers have reported an intramolecular version of this transformation; however, the authors state that they were unable to observe the intermolecular formation of dihydroindoles even up to 140 ºC with microwave heating. See: Miura T, Funakoshi Y, Murakami M. J Am Chem Soc. 2014;136:2272–2275. doi: 10.1021/ja412663a.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.