Abstract
Objective
Breast cancer survivors report adverse sexual effects (sexual morbidity) such as disrupted sexual function, sexual distress and body dissatisfaction. However, most studies have failed to evaluate the persistence of these effects in long-term survivors. The present study comprehensively assessed the prevalence and predictors of sexual/body image problems among survivors three or more years post diagnosis.
Design/outcome measures
Eighty-three breast cancer survivors completed surveys a median of seven years post diagnosis. Survey items probed demographic, diagnostic and clinical information, in addition to sexual activity, sexual function (Female Sexual Function Index [FSFI]), body image, and distress regarding body changes and sexual problems (Female Sexual Distress Scale-revised; FSDS-R).
Results
Seventy-seven percent of all participants and 60% of sexually active participants qualified for sexual dysfunction based on the FSFI. Between 37 and 51% met criteria for female sexual dysfunction, based on two FSDS-R clinical cut-offs. Body satisfaction was worse than normative values, while body change stress was mid-range. Notable sexual morbidity predictors included mastectomy, which was associated with worse sexual/body change distress, and post-treatment weight gain, which predicted greater body dissatisfaction/body change stress.
Conclusions
Breast cancer survivors report substantial sexual morbidity years after treatment, especially after mastectomy or post-treatment weight gain. Breast cancer patients and their providers should be aware of these potential sexual effects.
Keywords: breast cancer survivors, sexual morbidity, body change stress, mastectomy
The American Cancer Society (ACS) estimates that over 2.9 million women with histories of breast cancer are currently alive in the United States (ACS, 2013). While many survivors report overall quality of life scores comparable to the national average, adverse physical and sexual effects from cancer treatment often linger years after primary treatment (Ganz et al., 2002; Montazeri et al., 2008; Panjari, Bell, & Davis, 2011; Speer et al., 2005). Adverse sexual effects, or sexual morbidity, are characterised by body dissatisfaction, problems in sexual function (i.e. desire, arousal, orgasm and pain), and heightened sexual distress and body change stress (i.e. traumatic-like distress regarding physical changes from treatment; Frierson, Fiel, & Andersen, 2006). These effects have been associated with increased cancer-related distress, depression, greater symptom severity and decreased psychological well-being (Meyerowitz, Desmond, Rowland, Wyatt, & Ganz, 1999; Reese, Shelby, Keefe, Porter, & Abernathy, 2010; Zimmerman, Scott, & Heinrichs, 2010). Nonetheless, long-term sexual morbidity in breast cancer has received less research attention relative to other bio-psychosocial domains. The present study sought to bridge this gap in the literature.
Several predictors of sexual morbidity have been identified in the cancer population. Younger age is an established risk factor for worse sexual function and body change stress in breast and gynecological cancer, among other cancer subtypes (ACS, 2011; Carpenter, Andersen, Fowler, & Maxwell, 2009; Lemieux, Bordeleau, & Goodwin, 2007). Certain cancer treatments may also pose greater relative risk for sexual morbidity. Some research suggests that women undergoing mastectomy procedures, with or without subsequent reconstruction, are significantly more likely to report perceived physical unattractiveness and reduced sexual desire than women undergoing breast-conserving surgery (Alicikus et al., 2009; Ganz et al., 2004; Panjari et al., 2011; Rowland et al., 2000; Sheppard & Ely, 2008).
Chemotherapy may also impose lasting reproductive and other physical damage, such as weight gain, that may instigate or intensify sexual problems (Fobair et al., 2006; Ganz et al., 2002, 2004; Ochsenkuhn et al., 2011). Overweight and weight gain have been linked to greater mood disturbance, reduced satisfaction with physical appearance and loss of self-esteem in the breast cancer population (ACS, 2009; Befort, Austin, & Klemp, 2011; Camoriano et al., 1990; Demark-Wahnefried, Rimer, & Winer, 1997; Rooney & Wald, 2007). In addition, premenopausal women may experience chemotherapy-induced menopause (i.e. premature menopause) marked by dramatic reductions in estrogen and dampened sexual desire (Kuo, Wiggins, & Dizon, 2008; Rogers & Kristjanson, 2001). Finally, important psychosocial correlates of sexual morbidity in this population include partner support/relationship distress (Kinsinger, Laurenceau, Carver, & Antoni, 2011) and depression. These associations appear to be bidirectional, both as causes and consequences of sexual dysfunction and dissatisfaction, and accentuated by impaired partner communication (Carmack Taylor, 2005; Ganz, Desmond, Belin, Meyerowitz, & Rowland, 1999; Levin et al., 2010; Meyerowitz et al., 1999; Reese et al., 2010; Speer et al., 2005; Zimmerman et al., 2010).
Most existing studies have evaluated sexual problems among newly diagnosed breast cancer patients, using single-item or quality-of-life subscales as opposed to measures specific to sexual morbidity (Ganz et al., 2004; Greendale, Peterson, Zibecchi, & Ganz, 2001; Reese et al., 2010). Furthermore, no study has used validated measures of sex-related distress (i.e. sexual distress and body change stress) in combination with sexual function and body satisfaction among long-term survivors. This represents a major shortcoming in the literature, given that distress remains a necessary diagnostic criterion for sexual dysfunction (American Psychiatric Association, 2013; Hendrickx, Gijs, & Enzlin, 2013). Gaining greater perspective on the long-term prevalence and predictors of sexual morbidity, particularly measures of distress, is crucial in light of the associated disturbances described above.
The current study had two objectives: (1) to assess four self-reported sexual morbidity domains, including sexual function, sexual distress, body change stress and body satisfaction, in a sample of long-term breast cancer survivors; and (2) to evaluate the influence of select psychosocial and medical factors based on the extant literature, including age, treatment modality (e.g. mastectomy), specific treatment effects (e.g. weight gain and premature menopause) and psychosocial factors (e.g. depression, marital/relationship status and satisfaction, and quality of life), within and across four sexual morbidity domains. Participants completed an electronic survey a median of seven years following breast cancer diagnosis; question items probed demographic, medical, psychosocial and sexual morbidity information. To the knowledge of the authors, this study represents the first simultaneous evaluation of the four asserted sexual morbidity domains in long-term breast cancer survivors.
Method
Recruitment and data collection
Participants had enrolled in a previously reported parent study that assessed psychiatric disorders in early breast cancer (i.e. within five months of diagnosis); the study was conducted at a National Cancer Institute-designated Comprehensive Cancer Center in Philadelphia between the years 2000 and 2009 (Palmer, Taggi, DeMichele, & Coyne, 2012). Medical and psychosocial data collected by Palmer and colleagues are presented as predictor variables in the secondary analyses reported in this paper.
A subset of participants from the aforementioned parent study was recruited for the current study a median of seven years after cancer diagnosis. Individuals (N = 400) were contacted by telephone by the first and fourth authors, both Master’s level therapists. Unless reached on the initial call attempt (or deceased), all women were called at least two times before being considered ‘not reached’. Those who consented to participate in the current study completed an electronic survey supported by the Qualtrics Research Suite© (2009), though paper copies were mailed on request. The complete survey consisted of 213 items and required 30–40 min to complete. To be eligible for the current study, participants needed to meet the following criteria: (1) age 18–75 years, (2) diagnosed with breast cancer at least three years prior, (3) cancer-free or with stable disease, (4) not currently undergoing cancer treatment (except for hormone therapy), (5) never diagnosed with another form of cancer (except non-invasive skin cancer) and (6) no history of serious mental illness (e.g. psychosis) or significant intellectual deficiency. Preliminary eligibility was assessed in the initial phone contact and confirmed via Qualtrics screening questions that preceded the electronic survey.
Of the 400 call attempts, 225 (56%) were not reached, 128 (7%) were not interested in participating in the current study, 4 (1%) were deceased and 143 (36%) expressed interest in the study. Of those who were reached, 101 (59%) subsequently completed the electronic eligibility screening questions. Ultimately, 85 women were deemed eligible and completed the full survey and, of these, two (2%) were excluded from analyses due either to missing data (i.e. failed to complete 42% of total survey items) or response inconsistency. This left a final participant sample of 83 (see Figure 1). The resulting data were de-identified, password-protected and stored within a secure electronic shared drive designated exclusively for the Drexel University Department of Psychology.
Figure 1.
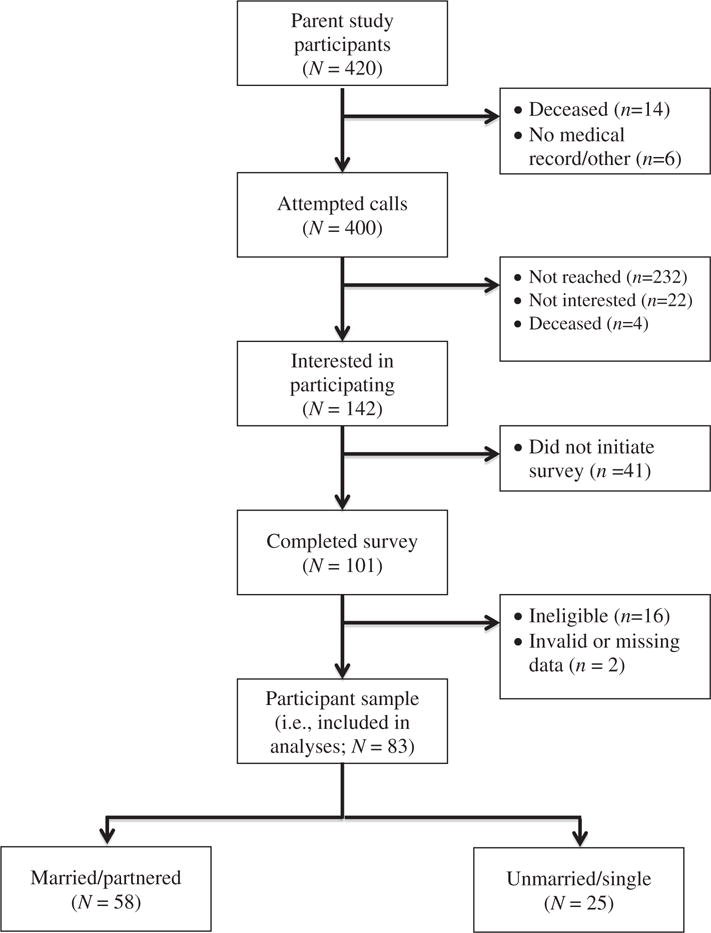
CONSORT flow diagram.
Participants
Based on data gathered by Palmer and colleagues at diagnosis, participants in the current study (N = 83) were younger than those who did not participate in the current study (N = 317; 49 vs. 52 years at diagnosis, respectively; t[397] = 2.86, p = .005), and were more likely to earn at least $60,000 per year (χ2[1, 393] = 8.27, p = .004) and have completed college (χ2[1, 393] = 5.82, p = .016). Of the remaining comparisons (e.g. race/ethnicity, marital status, tumor diagnostic stage and tumor laterality), no other differences were observed.
Measures
Unless otherwise noted, all measures were administered to participants at the time of enrollment in the current study only (i.e. not at diagnosis).
Demographic and cancer characteristics
Demographic variables included age, race/ethnicity, marital/relationship status, employment status, income and education level. Medical variables included AJCC diagnostic Stage (0, I, IIA, IIB, IIIA, IIIB or IV), year of diagnosis, treatment modality (surgery, chemotherapy, radiation and/or hormone/targeted therapy), type of surgery (mastectomy vs. lumpectomy), breast reconstruction (yes/no), breast cancer recurrence (yes/no), treatment-induced menopause failure (yes/no) and treatment-induced weight gain (yes/no; kg [lb]). Other self-report variables included prior cancer history and current hormone therapy. Data were collected from the medical record and surveys completed both at diagnosis and for the present study.
Sexual activity
Sexual activity was quantified using four items: (1) In the past six months, did you engage in any sexual activity (including self-masturbation)? (2) In the past six months, did you engage in any sexual activity with a partner? (3) In the past four weeks, how often did you engage in any sexual activity (including self-masturbation)? and (4) In the past four weeks, how often did you engage in sexual intercourse with a partner? Items 1 and 2 were scored dichotomously (0 = No, 1 = Yes), while items 3 and 4 were scored on a six-point scale (1 = Everyday, 6 = Not at all).
Sexual function
The Female Sexual Function Index (FSFI) assessed sexual function (Rosen et al., 2000). The measure consists of 19 items scored on five- and six-point Likert like scales. The FSFI organises sexual function data across six subscales: desire, arousal, lubrication, orgasm, satisfaction and pain. Total scores range from 2 to 36, with higher scores reflecting better sexual function. A total score of 26.5 differentiates women with and without sexual dysfunction as defined by DSM-IV criteria (Wiegel, Meston, & Rosen, 2006).
Sexual distress
The Female Sexual Distress Scale-revised (FSDS-R) assessed sex-related distress (DeRogatis, Clayton, Lewis-D’Angostino, Wunderlick, & Fu, 2008). The FSDS-R consists of 13 items quantifying the frequency of negative emotions about sexual problems over the prior month. Items are scored on a five-point rating scale (0 = Never, 4 = Always). Total scores range from 0 to 52, with higher scores indicating greater sexual distress. Clinically meaningful information may also be obtained by summing scores across items 1–12 (i.e. FSDS; total score 0–48) or item 13 alone (bothered by low sexual desire). A clinical cut-off score of 11 discriminates women with and without clinically diagnosable female sexual dysfunction (DeRogatis et al., 2008), though a more conservative cut-off of 15 may also be used (DeRogatis, Rosen, Leiblum, Burnett, & Heiman, 2002).
Body change stress
The Breast-impact of Treatment Scale (BITS) assessed survivors’ intrusive thoughts and avoidant behaviours with respect to their bodies since breast cancer treatment (Frierson et al., 2006). The BITS consists of 13 items scored on a four-point rating scale (0 = Not at all, 1 = Rarely, 3 = Sometimes, 5 = Often). Total scores range from 0 to 65, with higher scores suggesting greater body change stress.
Body satisfaction
The Body Areas Satisfaction Scale (BASS), a subscale of the 69-item Multidimensional Body Self-Relations Questionnaire, assessed general body satisfaction (Berscheid, Walster, & Bohrnstedt, 1973; Cash & Henry, 1995). The measure consists of nine items gauging satisfaction with seven body ‘parts’ (body build, stomach, waist, thighs, buttocks, hips and legs), general appearance and weight. Items are rated on a five-point Likert like scale (1 = very dissatisfied, 5 = very satisfied), and scores are averaged across items. Mean scores range from 1 to 5, with higher scores indicating greater body satisfaction.
Marital satisfaction
Eleven items from the Perceived Social Support/Conflict Scale assessed marital/relationship satisfaction among participants who were married or in stable romantic relationships both at diagnosis and follow-up (National Institute on Aging, 2002). Items are scored on a four-point Likert like scale, and an item mean (range, 1–4) is generated. Higher scores indicate greater marital satisfaction. Marital satisfaction data were collected both at diagnosis and for the present study.
Quality of life
The 12-item Medical Outcomes Study-Short Form (SF-12, V.1) assessed health-related quality of life (Ware, Kosinski, & Keller, 1996). The measure yields two subscores: the mental health component summary (MCS) and the physical health component summary (PCS). Scores range from 0 to 100 on each subscore, with higher scores indicating better quality of life. Mean PCS and MCS scores for middle-aged individuals (i.e. 55–64 years) are 47 and 54, respectively (Office of Public Health Assessment, 2004). SF-12 data were collected at diagnosis, while only general health (i.e. item 1) is reported for the present study.
Depression
The Iowa short form of the Center for Epidemiological Studies Depression Scale (CES-D) assessed clinical depression (Radloff, 1977). The CES-D consists of 20 items rating depressive symptoms on a four-point scale (0 = rarely or none of the time, 3 = most or all of the time). Total scores range from 0 to 60, with higher scores indicating more severe depression. Scores of 16 or greater suggest clinically significant depression.
Statistical analysis
Data were analysed using SPSS Statistics 20.0. As there was no discernible pattern to non-responses, missing data were considered missing at random. One participant had an inconsistent response style, and another failed to complete 42% of the total survey; data from these two individuals were excluded from analyses based on these reasons. To prevent score deflation on the FSFI, a measure known to overestimate dysfunction among individuals reporting sexual inactivity (Rosen et al., 2000), imputation of missing values was initially considered. Mean item score imputation for the FSFI, however, generated total scores that correlated with raw scores at .98 or higher, indicating that the use of imputed values did not meaningfully change results. The analyses presented herein thus used raw scores, rather than scores with imputed values.
Frequency and descriptive statistics were performed. Demographic, diagnostic and sexual morbidity data were compared between the married/partnered and single/unpartnered subsamples, using independent samples t-tests, Pearson’s χ2, and Fisher’s Exact tests, based on previous research showing that sexual distress scores, among other potential factors, differ by marital status (Panjari et al., 2011).
To explore associations among the independent and dependent variables, Pearson’s correlations, independent samples t-tests and one-way ANOVA analyses were performed. Correlation matrices included the four sexual morbidity outcomes (sexual function [FSFI], sexual distress [FSDS-R], body change stress [BITS] and body satisfaction [BASS]), and current age, time since diagnosis, depression (CES-D), quality of life at diagnosis (SF-12 total, mental and physical) and marital satisfaction. Independent samples t-tests compared mean scores on each outcome variable based on the following dichotomous variables: mastectomy, chemotherapy, treatment-induced (i.e. premature) menopause and treatment-induced weight gain. One-way ANOVA analyses evaluated the AJCC cancer stage at diagnosis as a predictor of sexual morbidity outcomes.
Finally, multiple linear regressions were performed to compare the influence of a priori psychosocial and medical factors on sexual morbidity outcomes. Factors entered into regression equations included the following: (1) age, (2) marital status, (3) mastectomy, (4) premature menopause, (5) post-treatment weight gain (yes/no), (6) current depression score (CES-D), (7) physical quality of life score at diagnosis (SF-12 PCS) and (8) mental quality of life score at diagnosis (SF-12 MCS). Each sexual morbidity outcome was regressed separately on these factors. To test the significance of changes in marital satisfaction since diagnosis, separate models were performed on the married/ partnered subsample, in which marital satisfaction was also entered. Assumption testing yielded non-significant results for heteroscedasticity, non-linearity and multicolinearity.
Results
Demographic and cancer characteristics
Table 1 displays demographic and clinical information for the full sample and subsamples by marital status. Mean participant (N = 83) age was 49.34 years (SD = 8.47) at diagnosis and 56.21 years (SD = 8.78) at follow-up. Median time from diagnosis was seven years (SD = 1.68) and ranged from 3 to 12 years. Participants were primarily Caucasian (83%), married or in stable romantic relationships (i.e. married/partnered; 70%) and employed full-time (51%). Forty percent of participants held graduate or professional degrees and 46% reported household incomes above $100,000 per year.
Table 1.
Demographic and clinical characteristics.
| Variable | Total sample (N = 83) |
Married/partnered (n = 58) |
Single/unpartnered (n = 25) |
pa | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age: mean (SD) | 56.21 | (8.78) | 56.60 | (8.89) | 55.25 | (8.61) | .529 |
| Years since diagnosis: median (SD) | 7.00 | (1.68) | 7.00 | (1.70) | 7.00 | (1.61) | .215 |
| Education | .035 | ||||||
| High school degree | 10 | 12.2 | 8 | 13.8 | 2 | 8.3 | |
| Some undergraduate school | 12 | 14.6 | 5 | 8.6 | 7 | 29.2 | |
| Undergraduate degree | 18 | 22.0 | 11 | 19.0 | 7 | 29.2 | |
| Some graduate/prof school | 9 | 11.0 | 9 | 15.5 | 0 | 0.0 | |
| Graduate/prof degree | 33 | 40.2 | 25 | 43.1 | 8 | 33.3 | |
| Employment status | .026 | ||||||
| Employed FT | 42 | 50.6 | 23 | 39.7 | 19 | 76.0 | |
| Employed PT | 10 | 12.0 | 9 | 15.5 | 1 | 4.0 | |
| Unemployed/student | 11 | 13.3 | 10 | 17.2 | 1 | 4.0 | |
| Retired/disabled | 20 | 24.1 | 16 | 27.6 | 4 | 16.0 | |
| Race/ethnicity | .286 | ||||||
| Caucasian | 67 | 83.8 | 49 | 87.5 | 18 | 75.0 | |
| Black/African-American | 5 | 6.0 | 2 | 3.6 | 3 | 12.5 | |
| Hispanic/Latino | 2 | 2.5 | 2 | 3.6 | 0 | 0.0 | |
| American Indian/Alaskan | 2 | 2.5 | 1 | 1.8 | 1 | 4.2 | |
| Other | 4 | 5.0 | 2 | 3.6 | 2 | 8.3 | |
| AJCC cancer stageb | .823 | ||||||
| Stage 0 | 7 | 8.4 | 6 | 10.5 | 1 | 4.0 | |
| Stage I | 37 | 44.6 | 26 | 45.6 | 10 | 40.0 | |
| Stage IIA, IIB | 27 | 32.5 | 18 | 31.6 | 9 | 36.0 | |
| Stage IIIA, IIIB | 11 | 13.6 | 6 | 10.5 | 5 | 20.0 | |
| Stage IV | 1 | 1.2 | 1 | 1.8 | 0 | 0.0 | |
| Tumor laterality | .699 | ||||||
| Right only | 42 | 50.6 | 30 | 52.6 | 12 | 48.0 | |
| Left only | 40 | 48.8 | 27 | 47.4 | 13 | 52.0 | |
| Positive lymph nodes at dxb | 49 | 59.0 | 37 | 63.8 | 12 | 48.0 | .179 |
| Breast reconstructionb | 33 | 41.2 | 20 | 36.4 | 13 | 54.2 | .140 |
| Treatment | |||||||
| Surgery | 83 | 100 | 58 | 100 | 25 | 100 | – |
| Chemotherapy | 48 | 57.8 | 31 | 53.5 | 17 | 68.0 | .218 |
| Radiation | 62 | 74.7 | 43 | 74.1 | 19 | 76.0 | .858 |
| Hormone therapy | 54 | 65.1 | 38 | 65.5 | 16 | 64.0 | .894 |
| Surgery typeb | .184 | ||||||
| Lumpectomy | 43 | 53.8 | 33 | 60.0 | 10 | 40.0 | |
| Unilateral mastectomy | 22 | 27.5 | 14 | 25.5 | 8 | 32.0 | |
| Bilateral mastectomy | 15 | 18.8 | 8 | 14.5 | 7 | 28.0 | |
| Current hormone therapy | 23 | 28.0 | 18 | 31.0 | 5 | 20.0 | .303 |
| Other cancer history | 2 | 2.4 | 2 | 3.4 | 0 | 0.0 | .574 |
| Premature menopause after tx | 32 | 38.6 | 22 | 38.6 | 10 | 40.0 | .859 |
| Weight control harder after tx | 46 | 55.4 | 27 | 46.6 | 19 | 76.0 | .013 |
| BC recurrence | 5 | 6.0 | 4 | 6.9 | 1 | 4.0 | .681 |
Comparison of partnered subsample (n = 58) to unpartnered subsample (n = 25); two-sided Pearson’s χ2 and Fisher’s exact tests.
Based on diagnostic data.
Participants reported mostly AJCC Stage I (45%) and IIA/B (33%) breast cancer diagnoses. All underwent surgical resection, with just under half (46%) receiving unilateral/bilateral mastectomy (vs. lumpectomy). Forty-two percent of participants reported breast reconstructive surgery following resection. In addition, 75% received radiation treatment, 65% received hormone therapy and 58% received chemotherapy. Twenty-eight percent reported current hormone therapy at the time of survey completion. Over one-third (39%) of survivors experienced premature menopausal symptoms from cancer treatment and 6% reported a breast cancer recurrence. Most participants (55%) reported that weight control was more challenging after treatment and, of those, 73% cited gains of 4.5 kg (10 lbs) or more.
Psychosocial and sexual morbidity characteristics
Quality of life scores at diagnosis were comparable to normative values of age-appropriate (50–64 years) females (Office of Public Health Assessment, 2004) on the physical domain of the SF-12 (PCS, M = 49 vs. M = 47, respectively), while mental quality of life was well below (MCS, M = 46 vs. M = 52, respectively). Current average depression on the CES-D was low to moderate (M = 10.94, SD = 10.83), with 25% of survivors meeting criteria for clinically significant depression (i.e. CES-D ≥ 16). Over half (54%) of the sample rated their current health (SF-12 item 1) as very good or excellent, 30% good and 16% fair or poor. Mean marital satisfaction scores among married/partnered women were high both at diagnosis (M = 3.43 out of 4, SD = .60) and at present (M = 3.27, SD = .68).
Sexual morbidity data at follow-up are displayed in Table 2. Sixty-five percent of participants reported partnered sexual activity in the previous six months, while approximately half (48%) reported sexual activity in the previous four weeks. Twenty-eight percent of single/unpartnered women reported four-week sexual activity compared with 57% of married/partnered women. Over three-quarters of all participants (77%) qualified for sexual dysfunction based on FSFI score. Approximately half (51%) of the full sample met criteria for female sexual dysfunction based on a cut-off of 11 on the FSDS-R, including 28% of single/unpartnered women and 60% of married/partnered women. Using a higher FSDS-R cut-off of 15, 37% of participants met criteria for female sexual dysfunction.
Table 2.
Sexual morbidity characteristics.
| Variable | Total sample (N = 83) Mean (SD) |
Married/partnered (n = 58) Mean (SD) |
Single/unpartnered (n = 25) Mean (SD) |
p* |
|---|---|---|---|---|
| Sexual activity in the past six months | ||||
| Any (w/or w/o partner; %) | 60 (72.3%) | 43 (74.1%) | 17 (68.0%) | .567 |
| With partner (%) | 54 (65.1%) | 41 (70.7%) | 13 (52.0%) | .101 |
| Sexual activity freq (w/partner) in past four weeks | .014 | |||
| Every other day | 1 (1.2%) | 0 (0.0%) | 1 (4.0%) | |
| Half the days (%) | 4 (4.8%) | 2 (3.4%) | 2 (8.0%) | |
| Once per week (%) | 15 (18.1%) | 13 (22.4%) | 2 (8.0%) | |
| Once every other week (%) | 20 (24.1%) | 18 (31.0%) | 2 (8.0%) | |
| Not at all (%) | 43 (51.8%) | 25 (43.1)% | 18 (72.0%) | |
| Meets criteria for sexual dysfunction: | ||||
| FSFI ≤ 26.5 (%) | 62 (76.5%) | 44 (77.2%) | 18 (75.0%) | .832 |
| FSDS-R ≥ 11 (%) | 42 (50.6%) | 35 (60.3%) | 7 (28.0%) | .006 |
| FSDS-R ≥ 15 (%) | 31 (37.3%) | 26 (44.8%) | 5 (20.0%) | .027 |
| Female sexual function index (FSFI) | 16.89 (10.92) | 17.66 (10.55) | 15.07 (11.78) | .333 |
| Desire | 2.72 (1.26) | 2.60 (1.20) | 3.00 (1.37) | .199 |
| Arousal | 2.74 (2.01) | 2.86 (1.93) | 2.45 (2.21) | .407 |
| Lubrication | 2.76 (2.20) | 2.86 (2.09) | 2.59 (2.48) | .672 |
| Orgasm | 3.03 (2.41) | 3.21 (2.37) | 2.62 (2.50) | .319 |
| Satisfaction | 3.12 (1.95) | 3.35 (1.88) | 2.57 (2.03) | .096 |
| Pain | 2.74 (2.56) | 3.11 (2.52) | 1.85 (2.48) | .042 |
| Female sexual distress scale-revised (FSDS-R) | 13.81 (12.75) | 15.10 (12.54) | 10.80 (12.97) | .160 |
| Bothered by low sexual desire (%) | 20 (24.4%) | 17 (29.3%) | 3 (12.5%) | .059 |
| Breast impact of treatment Scale (BITS) | 20.63 (14.92) | 19.88 (13.74) | 22.36 (17.55) | .490 |
| Body areas satisfaction Scale (BASS) | 2.60 (1.13) | 2.67 (1.11) | 2.44 (1.17) | .394 |
Comparison of partnered subsample (n = 58) to unpartnered subsample (n = 25); two-sided Pearson’s χ2 and Fisher’s exact tests.
Mean sexual function (FSFI, M = 16.89, SD = 10.92) and sexual distress (FSDS-R, M = 13.81, SD = 12.75) were subnormal based on a FSFI clinical cut-off of 26.5 and a FSDS-R cut-off of 11 (but not 15). The married/partnered subsample, however, met the higher FSDS-R threshold (FSDS-R, M = 15.10, SD = 12.54). Twenty-four percent of participants reported either ‘always’ or ‘frequently’ feeling ‘bothered by low sexual desire’ on item 13 of the FSDS-R. Body change stress (BITS, M = 20.63, SD = 14.92) fell between scores from published breast cancer samples surveyed after breast-conserving surgery (M = 16.09, SD = 12.65) or radical mastectomy (M = 29.18, SD = 13.14; Frierson et al., 2006). Average body satisfaction (BASS, M = 2.60, SD = 1.13) was lower than published normative data (M = 3.23, SD = .74, p < .001; Cash & Henry, 1995).
Compared with single/unpartnered women, married/partnered individuals reported significantly better physical quality of life at diagnosis (p = .014). At follow-up, a greater proportion of married/partnered survivors reported past-month sexual activity (p = .014). However, married/partnered women were also more likely to qualify for sexual dysfunction based on FSDS-R sexual distress criteria, using both lower (p = .006) and higher (p = .027) clinical cut-offs, and experienced greater sex-related pain on the FSFI (p = .042). Single/unpartnered survivors were more likely to rate post-treatment weight control as difficult (p = .013). No other significant differences between subgroups emerged.
To explore the influence of sexual activity status on sexual function and distress scores, post hoc analyses were conducted among women reporting some amount of sexual activity in the previous four weeks (n = 40). Regardless of relationship status, 60% of sexually active women met criteria for sexual dysfunction based on the FSFI, while 50 and 33% met criteria based on FSDS-R cut-offs of 11 and 15, respectively, indicating little change in sexual distress by restricting analyses based on sexual activity. Results showed substantially better FSFI scores (M = 25.26, SD = 5.89) compared with the full participant sample (i.e. sexually active and inactive), though the mean remained below the FSFI clinical cut-off.
Bivariate associations
Table 3 displays Spearman’s correlations between sexual morbidity scores and continuous psychosocial data gathered at diagnosis for the parent study and for the current study. Significant intercorrelations across sexual morbidity outcomes were observed (data not shown), with body satisfaction (BASS) and body change stress (BITS) generating the most notable bivariate (and negative) association (r = −.53, p < .001). Sexual function (FSFI) was not significantly associated with scores on the BASS or BITS. In addition, mastectomy surgery (vs. lumpectomy) was significantly associated with worse body change stress (t[78] = −5.94, p < .001) and sexual distress (t[78] = −3.83, p < .001), and lower (i.e. worse) scores on the FSFI pain (t[75] = 2.00, p = .049) and satisfaction (t[76] = 3.13, p = .003) subscales. Premature menopause was significantly associated with worse body satisfaction (t[81] = 2.30, p = .024), body change stress (t[81] = −2.54, p = .013) and sexual distress (t[81] = −3.37, p = .001). Participants reporting post-treatment weight control problems also reported significantly worse body image (t[81] = 4.53, p < .001) and body change stress (t[78] = −3.36, p = .001). Chemotherapy treatment was associated with less body satisfaction (t[81] = 2.07, p = .043) and greater body change stress (t[81] = −2.21, p = .03). AJCC cancer stage at diagnosis was positively associated with body change stress; survivors diagnosed with more advanced breast cancer (e.g. stages 3a, 3b) reported worse body change stress than survivors diagnosed with early disease (e.g. stages 0, 1; F[6, 76] = 2.29, p = .044). No other significant associations emerged.
Table 3.
Correlations between sexual morbidity outcomes and psychosocial variables (N = 83).a
| Variable | Sexual function (FSFI) | Sexual distress (FSDS-R) | Body change Stress (BITS) | Body satisfaction (BASS) |
|---|---|---|---|---|
| Diagnosis | ||||
| Marital satisfactionb | .16 | −.08 | −.02 | −.07 |
| Physical QOL (SF-12 PCS) | .24* | −.11 | −.20 | .39*** |
| Mental QOL (SF-12 MCS) | .06 | −.19 | −.33** | .04 |
| Overall QOL (SF-12 Total) | .22* | −.17 | −.31** | .26* |
| Present | ||||
| Age | −.03 | −.33** | −.41*** | .27* |
| Time since diagnosis (yrs) | .05 | .12 | .03 | −.02 |
| Marital satisfactionb | .35** | −.33** | −.25* | .16 |
| Marital satisfaction changeb | .29* | −.33** | −.24 | .17 |
| Depressive symptoms (CES-D) | −.19 | .24* | .31** | −.30** |
Spearman’s correlations (non-parametric).
Married/partnered group only (n = 58).
p < .05.
p ≤ .01.
p ≤ .001.
Multiple linear regression models
Table 4 displays results of multiple linear regression analyses. No significant predictors of sexual function on the FSFI emerged. Controlling for age, premature menopause, post-treatment weight gain, and physical and mental quality of life at diagnosis, mastectomy (B = 8.57, CI [3.33–13.81], p = .002), being married/partnered (B = 6.73, CI [1.41–12.10], p = .014) and concurrent depressive symptoms (B = .31, CI [.02–.61], p = .039) significantly predicted greater sexual distress. Body change stress was significantly and positively associated with mastectomy (B = 12.41, CI [6.88–17.95], p < .001) and post-treatment weight gain (B = 5.56, CI [.21–10.91], p = .042). Finally, post-treatment weight gain predicted worse body satisfaction (B = −.79, CI [−1.26 to −.32], p = .001), and better physical quality of life scores at diagnosis predicted better body satisfaction (B = .03, CI [.01 to .05], p = .01). The variance accounted for by the full regression models was 19% for sexual function (F[8, 68] = 2.02, p = .057), 42% for sexual distress (F[8, 70] = 6.22, p < .001), 52% for body change stress (F[8, 70] = 9.64, p < .001) and 32% for body satisfaction (F[8, 70] = 4.17, p < .001).
Table 4.
Multiple linear regression models (N = 83).
| R2 | B (95% CI) | |
|---|---|---|
| Female sexual function index (FSFI) | .19 | |
| 1. Age | – | −.05 (−.38 to .27) |
| 2. Married/partnered (y/n) | – | 3.87 (−1.49 to 9.22) |
| 3. Mastectomy (y/n) | – | −3.71 (−8.94 to 1.53) |
| 4. Premature menopause (y/n) | – | 3.20 (−2.35 to 8.75) |
| 5. Post-tx weight gain (y/n) | – | 4.63 (−.49 to 9.76) |
| 6. Current depressive symptoms (CES-D) | – | −.22 (−.51 to .08) |
| 7. Physical quality of life at diagnosis (SF-12 PCS) | – | .16 (−.06 to .38) |
| 8. Mental quality of life at diagnosis (SF-12 MCS) | – | −.02 (−.31 to .27) |
| Female sexual distress scale-revised (FSDS-R) | .42*** | |
| 1. Age | – | −.10 (−.43 to .23) |
| 2. Married/partnered (y/n) | – | 6.73 (1.41 to 12.10)* |
| 3. Mastectomy (y/n) | – | 8.57 (3.33 to 13.81)** |
| 4. Premature menopause (y/n) | – | 4.65 (−.91 to 10.20) |
| 5. Post-tx weight gain (y/n) | – | .89 (−4.18 to 6.00) |
| 6. Current depressive symptoms (CES-D) | – | .31 (.02 to .61)* |
| 7. Physical quality of life at diagnosis (SF-12 PCS) | – | −.03 (−.26 to .19) |
| 8. Mental quality of life at diagnosis (SF-12 MCS) | – | −.09 (−.39 to .20) |
| Breast impact of treatment scale (BITS) | .52*** | |
| 1. Age | – | −.33 (−.68 to .01) |
| 2. Married/partnered (y/n) | – | 2.16 (−3.46 to 7.78) |
| 3. Mastectomy (y/n) | – | 12.41 (6.88 to 17.95)*** |
| 4. Premature menopause (y/n) | – | −.49 (−6.36 to 5.37) |
| 5. Post-tx weight gain (y/n) | – | 5.56 (.21 to 10.91)* |
| 6. Current depressive symptoms (CES-D) | – | .19 (−.12 to .51) |
| 7. Physical quality of life at diagnosis (SF-12 PCS) | – | −.10 (−.33 to .14) |
| 8. Mental quality of life at diagnosis (SF-12 MCS) | – | −.30 (−.61 to .01) |
| Body areas satisfaction scale (BASS) | .32*** | |
| 1. Age | – | .01 (−.02 to .05) |
| 2. Married/partnered (y/n) | – | −.11 (−.60 to .39) |
| 3. Mastectomy (y/n) | – | −.09 (−.57 to .40) |
| 4. Premature menopause (y/n) | – | −.18 (−.69 to .34) |
| 5. Post-tx weight gain (y/n) | – | −.79 (−1.26 to −.32)*** |
| 6. Current depressive symptoms (CES-D) | – | −.01 (−.04 to .01) |
| 7. Physical quality of life at diagnosis (SF-12 PCS) | – | .03 (.01 to .05)** |
| 8. Mental quality of life at diagnosis (SF-12 MCS) | – | −.01 (−.04 to .01) |
Note: R2 = % variance accounted for by regression equation.
p ≤ .05.
p ≤ .01.
p ≤ .001.
Post-hoc analyses showed that marital satisfaction was non-significant for sexual morbidity in the married/partnered subgroup (n = 58). This was true for mean marital satisfaction scores at diagnosis and at present, in addition to the change in score since diagnosis. Among participants who reported partnered sexual activity within the previous four weeks (n = 40), only marital status predicted sexual function scores (FSFI, B = −7.42, CI [−12.55 to −2.29], p = .006), reflecting that having a partner was the best predictor of better sexual function.
Discussion
Sexual morbidity prevalence
The present study identified substantial self-reported sexual impairment in a sample of middle-aged, long-term breast cancer survivors a median of seven years (range, 3–12 years) post diagnosis. Reported absence of four-week sexual activity was high (52% in full sample, 43% in married/partnered group), with an additional quarter of participants reporting partnered sexual activity occurring no more than twice per month. These rates are comparable to (or somewhat lower than) previous studies of middle-aged breast and ovarian cancer survivors (Taylor, Basen-Engquist, Shinn, & Bodurka, 2004; Fobair et al., 2006). Still, reported sexual activity in this sample and in female cancer populations in general is consistently lower than healthy post-menopausal women (Carmack Taylor et al., 2004; Thirlaway, Fallowfield, & Cuzick, 1996). This suggests a specific, negative and persistent impact of cancer on sexual behaviour. Furthermore, participants’ reported sexual function was inferior to non-clinical and sexual dysfunction samples (Wiegel et al., 2006), in addition to published data from breast cancer survivors of comparable age (Speer et al., 2005). While sexual inactivity may explain some of these differences, analyses among survivors reporting past-month sexual activity revealed that FSFI scores remained below the clinical cut-off for dysfunction and substantially worse than normative scores (Wiegel et al., 2006). Additionally, a greater proportion of the total participant sample (77%) and a similar proportion of the sexually active subsample (60%) qualified for sexual dysfunction based on FSFI score compared with a published sample of middle-aged gynecologic cancer survivors (64%; Carpenter et al., 2009). This finding is surprising given the extensive sexual effects imposed by gynecological cancers.
Prior research has depicted sexual distress as a truer representation of dysfunction than sexual activity status or physical sexual function alone (Bancroft, Loftus, & Long, 2003; Basson et al., 2000; DeRogatis, et al., 2002). While comparative distress data were scant, sexual distress scores fell between known samples with and without female sexual dysfunction (DeRogatis et al., 2011). Single/unpartnered women were substantially less distressed about their sex lives than married/partnered women; this finding is supported by limited previous research on sexual distress in breast cancer patients (Panjari et al., 2011). In addition, fewer women met criteria for female sexual dysfunction based on FSDS-R score (51% using cut-off of 11, 37% using cut-off of 15) compared with FSFI-based diagnoses. Agreement between FSFI and FSDS-R dysfunction diagnoses was low (36% using the higher FSDS-R cut-off) in the full participant sample; 33 (41%) participants received a FSFI diagnosis but not a FSDS-R diagnosis and only 2 (3%) received a FSDS-R diagnosis but not a FSFI diagnosis. Identical analyses in the sexually active subsample reflected similar discrepancies. While the limitations of both the FSFI and FSDS-R are recognised, these results indicate evidence of diagnostic inflation in assessments of physiological function. Reported sexual distress, in contrast, may render fewer ‘false positives’ while retaining adequate diagnostic sensitivity.
Participants demonstrated mid-range body change stress, or traumatic stress symptoms related to perceived body changes since treatment. They reported less body change stress than a female sample that received radical mastectomy, but higher stress than those who received breast-conserving surgery (Frierson et al., 2006). These results align with the approximately even division of lumpectomy vs. mastectomy procedures reported by survivors in the current study (54% vs. 46%, respectively). Body satisfaction scores, however, revealed relatively poor body image among participants in relation to a US normative sample (Cash & Henry, 1995). Body satisfaction also approximated a sample of adult (M = 40 years), obese treatment-seeking females (Foster, Wadden & Vogt, 1997). Body mass index data were not collected in the current study for comparison; however, participants’ generally positive health status suggests good physical fitness and low obesity prevalence, and therefore extreme overweight would not likely account for observed body disturbances. Prior research has also shown remarkable stability in body satisfaction (outside of external influences, such as disease) across the lifespan, which negates older age as a confounding factor (Siegel, 2010; Tiggemann, 2004). Once again, these results point to the breast cancer experience as uniquely and continuously disruptive to survivors’ long-term self-esteem and well-being.
Sexual morbidity correlates
A second aim was to evaluate potential medical and psychosocial predictors of sexual morbidity. Mastectomy emerged as a significant predictor of both distress measures (i.e. sexual distress and body change stress) after controlling for age, marital status, premature menopause, post-treatment weight gain, depression and quality of life (i.e. mental and physical SF-12 scores) at diagnosis. The associations did not appear to be meaningfully confounded by disease stage at diagnosis. While these findings are generally supported in the extant literature (Panjari et al., 2011; Piot-Ziegler, Sassi, Raffoul, & Delaloye, 2010; Rowland et al., 2000; Sheppard & Ely, 2008), the persistent negative effects of invasive surgery are striking. Conversely, analyses revealed depression and quality of life at diagnosis as surprisingly weak correlates of sexual morbidity, depression being significant only for sexual distress, and physical quality of life predicting only body satisfaction. This may reflect differences in diagnostic instruments, as previous research has frequently used measures designed for the medical setting (e.g. Patient Health Questionnaire, Hospital Anxiety and Depression Scale, and FACT scales; Falk Dahl, Reinertsen, Nesvold, Fossa, & Dahl, 2010; Hodgkinson et al., 2007). It may also be that general well-being is not an accurate marker of patient risk for sexual problems after breast cancer. Clinicians should directly address the possibility of sexual effects with all patients, regardless of psychological state.
Intractable weight gain, a common by-product of cancer treatment in this participant pool, predicted worse body change stress and lesser body satisfaction in regression analyses. While these associations are not surprising, it is important that patients are informed of the potential for weight gain and related complications. Adoption of weight management and physical activity regimens has successfully improved body image and reduced distress both during and after cancer treatment (Pinto, Clark, Maruyama, & Feder, 2003; Rooney & Wald, 2007). The high prevalence of weight control issues may provide some explanation for the non-significance of premature menopause after controlling for other predictors; fluctuations in weight and body composition may partially mediate the effects of menopause (Freedman et al., 2004). In addition, ‘natural’ menopause may have obscured the impact of premature menopause since most participants were of menopausal age.
Strengths and weaknesses
To the knowledge of the authors, this study represents the most comprehensive examination of sexual morbidity in a group of long-term breast cancer survivors to date. The representation of multiple facets of sexual morbidity is an important strength of the study. Using four validated instruments, the present study reported on sexual distress, body satisfaction, body change stress and sexual function; in contrast, the majority of research has focused on either body image or sexual function. As previously discussed, a disruption in sexual function alone is not sufficient for a clinical diagnosis of sexual dysfunction in the absence of marked distress. Therefore, distress data add necessary dimension. An additional strength is the scope of medical and psychosocial data presented. While most research has focused on depression/quality of life or relationship sequelae in breast cancer, the present study also collected diagnostic, treatment and post-treatment data. The results provide a thorough examination of the relevant factors in sexual morbidity and an analysis of their relative contributions.
The study findings are primarily limited by the small sample size, restricted general-isability, limitations of sexual survey measures and reliance on self-report. A larger sample may uncover associations among the observed variables that were not apparent due to inadequate statistical power. In addition, participants were largely well educated, Caucasian and of post-menopausal age; while this profile is generally consistent with the prototypical breast cancer survivor described in the literature (ACS, 2011; Ganz et al., 2004), future research should focus on minorities and survivors of lower socioeconomic status to investigate potential differences across demographic groups. The diagnostic limitations of the FSFI, specifically sensitivity to participant sexual activity and relationship quality (Baser, Li, & Carter, 2012), must be considered when interpreting the present study data. However, FSFI scores among the subsample of women who were sexually active indicated that sexual problems remained more severe in this survivor group compared with the general population. Furthermore, the FSFI has demonstrated sound psychometric properties and ability to identify sexual dysfunction among female cancer survivors (Baser et al., 2012). Additionally, self-report is likely to be less accurate than direct physiological measures of sexual function. Recall bias may affect the validity of reported post-treatment weight control and, to a lesser extent, past-month sexual activity and function. Participants were also not directly questioned about sexual changes since their breast cancer diagnoses, outside of body change stress. Future longitudinal studies should track changes in sexual life for multiple years into the survivorship period. Similarly, the primarily cross-sectional data disallow conclusions of causation. This is especially important for sexual distress, which may precede or follow changes in sexual activity and/or sexual arousal processes. Finally, the absence of sexual partner information (e.g. physical, mental and sexual health) prohibits a comprehensive assessment of dyadic function. Marital satisfaction likely accounts for some, though not all, of these missing pieces. Future research should survey both survivors and sexual partners.
Conclusions
Despite any shortcomings, the present study provides a rich account of the clinical and sexual experience of breast cancer survivors in the years following cancer treatment. While most participants reported very good overall physical health and minimal cancer recurrence, they also experienced higher rates of sexual problems than healthy women of comparable age. These findings largely mirror published data but add valuable information regarding sex- and body-related distress (Bancroft et al., 2003; Ganz et al., 2002, 2004). The importance of sexual distress may also support a psychosocial model of assessment in lieu of the more traditional medical approach. Providers should inform patients of their risk for long-term sexual problems prior to treatment, especially those undergoing mastectomy. Sexual counselling and targeted weight control efforts may also help ameliorate sexual and body image issues in the post-treatment period.
Acknowledgments
The authors would like to recognise the valuable contributions of the survivors who participated in this study.
Funding
The parent study was supported by the National Institutes of Health [R01MH063172].
Footnotes
Copyright of Psychology & Health is the property of Routledge and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder’s express written permission. However, users may print, download, or email articles for individual use.
References
- Alicikus Z, Gorken I, Sen R, Kentli S, Kinay M, Alanyali H, Harmancioglu O. Psychosexual and body image aspects of quality of life in Turkish breast cancer patients: A comparison of breast conserving treatment and mastectomy. Tumori. 2009;95:212–218. doi: 10.1177/030089160909500213. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Breast cancer facts & figures 2009–2010 [Brochure] Atlanta, GA: Author; 2009. [Google Scholar]
- American Cancer Society. Breast cancer [Brochure] Atlanta, GA: Author; 2011. [Google Scholar]
- American Cancer Society. Breast cancer. 2013 Retrieved from http://www.cancer.org/cancer/breastcancer/detailedguide/index.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Bancroft J, Loftus J, Long S. Distress about sex: A national survey of women in heterosexual relationships. Archives of Sexual Behavior. 2003;32:193–208. doi: 10.1023/a:1023420431760. [DOI] [PubMed] [Google Scholar]
- Baser RE, Li Y, Carter J. Psychometric validation of the female sexual function index (FSFI) in cancer survivors. Cancer. 2012;118:4606–4618. doi: 10.1002/cncr.26739. [DOI] [PubMed] [Google Scholar]
- Basson R, Berman J, Burnette A, Derogatis L, Ferguson D, Fourcroy J, Whipple B. Report of the international consensus development conference on female sexual dysfunction: Definitions and classifications. Journal of Urology. 2000;163:888–893. [PubMed] [Google Scholar]
- Befort C, Austin H, Klemp J. Weight control needs and experiences among rural breast cancer survivors. Psycho-Oncology. 2011;20:1069–1075. doi: 10.1002/pon.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berscheid E, Walster E, Bohrnstedt G. The happy American body: A survey report. Psychology Today. 1973;7:119–131. [Google Scholar]
- Camoriano JK, Loprinzi CL, Ingle JN, Therneau TM, Krook JE, Veeder MH. Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. Journal of Clinical Oncology. 1990;8:1327–1334. doi: 10.1200/JCO.1990.8.8.1327. [DOI] [PubMed] [Google Scholar]
- Carmack Taylor C. Spousal intimacy after cancer. Gynecologic Oncology. 2005;99:S217–S218. doi: 10.1016/j.ygyno.2005.07.089. [DOI] [PubMed] [Google Scholar]
- Carpenter K, Andersen B, Fowler J, Maxwell G. Sexual self-schema as a moderator of sexual and psychological outcomes for gynecologic cancer survivors. Archives of Sexual Behavior. 2009;38:828–841. doi: 10.1007/s10508-008-9349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash TE, Henry PE. Women’s body images: The results of a national survey in the U.S.A. Sex Roles. 1995;13:19–28. [Google Scholar]
- Demark-Wahnefried W, Rimer BK, Winer EP. Weight gain in women diagnosed with breast cancer. Journal of the American Dietetic Association. 1997;97:519–526. doi: 10.1016/s0002-8223(97)00133-8. [DOI] [PubMed] [Google Scholar]
- DeRogatis L, Clayton A, Lewis-D’Agostino D, Wunderlich G, Fu Y. Validation of the female sexual distress scale-revised for assessing distress in women with hypoactive sexual desire disorder. Journal of Sexual Medicine. 2008;5:357–364. doi: 10.1111/j.1743-6109.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- DeRogatis LR, Clayton AH, Goldstein A, Lewis-D’Agostino D, Wunderlich G, Cotton D. Diary and female sexual distress scale© in evaluating distress in hypoactive sexual desire disorder (HSDD) Journal of Sex Research. 2011;48:565–572. doi: 10.1080/00224499.2010.524321. [DOI] [PubMed] [Google Scholar]
- DeRogatis L, Rosen R, Leiblum S, Burnett A, Heiman J. The female sexual distress scale (FSDS): Initial validation of a standardized scale for assessment of sexually related personal distress in women. Journal of Sex and Marital Therapy. 2002;28:317–330. doi: 10.1080/00926230290001448. [DOI] [PubMed] [Google Scholar]
- Falk Dahl C, Reinertsen K, Nesvold I, Fossa S, Dahl A. A study of body image in long-term breast cancer survivors. Cancer. 2010;116:3549–3557. doi: 10.1002/cncr.25251. [DOI] [PubMed] [Google Scholar]
- Fobair P, Stewart S, Chang S, D’Onofrio C, Banks P, Bloom J. Body image and sexual problems in young women with breast cancer. Psycho-oncology. 2006;15:579–594. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- Foster G, Wadden T, Vogt R. Body image in obese women before, during, and after weight loss treatment. Health Psychology. 1997;16:226–229. doi: 10.1037//0278-6133.16.3.226. [DOI] [PubMed] [Google Scholar]
- Freedman RJ, Aziz N, Albanes D, Hartman T, Danforth D, Hill S, Yanovski JA. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. The Journal of Clinical Endocrinology & Metabolism. 2004;89:2248–2253. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- Frierson G, Fiel D, Andersen B. Body change stress for women with breast cancer: The breast-impact of treatment scale. Annals of Behavioral Medicine. 2006;32:77–81. doi: 10.1207/s15324796abm3201_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz P, Desmond K, Belin T, Meyerowitz B, Rowland J. Predictors of sexual health in women after a breast cancer diagnosis. Journal of Clinical Oncology. 1999;17:2371–2380. doi: 10.1200/JCO.1999.17.8.2371. [DOI] [PubMed] [Google Scholar]
- Ganz P, Desmond K, Leedham B, Rowland J, Meyerowitz B, Belin T. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. Journal of the National Cancer Institute. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- Ganz P, Kwan L, Stanton A, Krupnick J, Rowland J, Meyerowitz B, Belin T. Quality of life at the end of primary treatment of breast cancer: First results from the moving beyond cancer randomized trial. Journal of the National Cancer Institute. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Petersen L, Zibecchi L, Ganz PA. Factors related to sexual function in postmenopausal women with a history of breast cancer. Menopause: The Journal of The North American Menopause Society. 2001;8(2):111–119. doi: 10.1097/00042192-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Hendrickx L, Gijs L, Enzlin P. Distress, Sexual Dysfunctions, and DSM: Dialogue at Cross Purposes? The Journal of Sexual Medicine. 2013;10:630–641. doi: 10.1111/j.1743-6109.2012.02971.x. [DOI] [PubMed] [Google Scholar]
- Hodgkinson K, Butow P, Fuchs A, Hunt GE, Stenlake A, Hobbs KM, Wain GE. Long-term survival from gynecologic cancer: Psychosocial outcomes, supportive care needs and positive outcomes. Gynecologic oncology. 2007;104:381–389. doi: 10.1016/j.ygyno.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Kinsinger S, Laurenceau JP, Carver C, Antoni M. Perceived partner support and psychosexual adjustment to breast cancer. Psychology & Health. 2011;26:1571–1588. doi: 10.1080/08870446.2010.533771. [DOI] [PubMed] [Google Scholar]
- Kuo A, Wiggins D, Dizon D. Sexual dysfunction after breast cancer treatment: Common problems, diagnosis, and management. Breast Cancer SRM. 2008;6:17–20. [Google Scholar]
- Lemieux J, Bordeleau LJ, Goodwin PJ. Medical, psychosocial, and health-related quality of life issues in breast cancer survivors. In: Ganz Patricia A., editor. Cancer survivorship. New York, NY: Springer; 2007. pp. 122–144. [Google Scholar]
- Levin A, Carpenter K, Fowler J, Brothers B, Andersen B, Maxwell L. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. International Journal of Gynecological Cancer. 2010;20:461–470. doi: 10.1111/IGC.0b013e3181d24ce0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz B, Desmond K, Rowland J, Wyatt G, Ganz P. Sexuality following breast cancer. Journal of Sex and Marital Therapy. 1999;25:237–250. doi: 10.1080/00926239908403998. [DOI] [PubMed] [Google Scholar]
- Montazeri A, Vahdaninia M, Harirchi I, Ebrahimi M, Khaleghi F, Jarvandi S. Quality of life in patients with breast cancer before and after diagnosis: An eighteen months follow-up study. BMC Cancer. 2008;8:330. doi: 10.1186/1471-2407-8-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Aging. Midlife in the United States II (MIDUS II) self-administered questionnaire 2. 2002. Items 1a.-1l. in the protocol. [Google Scholar]
- Ochsenkuhn R, Hermelink K, Clayton A, von Schonfeltd V, Gallwas J, Ditsch N, Kahlert S. Menopausal status in breast cancer patients with past chemotherapy determines long-term hypoactive sexual desire disorder. Journal of Sexual Medicine. 2011;8:1486–1494. doi: 10.1111/j.1743-6109.2011.02220.x. [DOI] [PubMed] [Google Scholar]
- Office of Public Health Assessment. Health status in Utah: The medical outcomes study SF-12 (2001 Utah Health Status Survey Report) Salt Lake City, UT: Utah Department of Health; 2004. [Google Scholar]
- Palmer SC, Taggi A, DeMichele A, Coyne JC. Is screening effective in detecting untreated psychiatric disorders among newly diagnosed breast cancer patients? Cancer. 2012;118:2735–2743. doi: 10.1002/cncr.26603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjari M, Bell R, Davis S. Sexual function after breast cancer. Journal of Sexual Medicine. 2011;8:294–302. doi: 10.1111/j.1743-6109.2010.02034.x. [DOI] [PubMed] [Google Scholar]
- Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psycho-oncology. 2003;12:118–126. doi: 10.1002/pon.618. [DOI] [PubMed] [Google Scholar]
- Piot-Ziegler C, Sassi M, Raffoul W, Delaloye J. Mastectomy, body deconstruction, and impact on identity: A qualitative study. British Journal of Health Psychology. 2010;15:479–510. doi: 10.1348/135910709X472174. [DOI] [PubMed] [Google Scholar]
- Qualtrics Labs Inc. Provo, UT, USA. 2009 Retrieved from http://www.qualtrics.com.
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reese J, Shelby R, Keefe F, Porter L, Abernethy A. Sexual concerns in cancer patients: A comparison of GI and breast cancer patients. Support Care Center. 2010;18:1179–1189. doi: 10.1007/s00520-009-0738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M, Kristjanson L. The impact on sexual functioning of chemotherapy-induced menopause in women with breast cancer. Cancer Nursing. 2001;25:57–65. doi: 10.1097/00002820-200202000-00011. [DOI] [PubMed] [Google Scholar]
- Rooney M, Wald A. Interventions for the management of weight and body composition changes in women with breast cancer. Clinical Journal of Oncology Nursing. 2007;11:41–52. doi: 10.1188/07.CJON.41-52. [DOI] [PubMed] [Google Scholar]
- Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, D’Agostino R. The female sexual function index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. Journal of Sex and Marital Therapy. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- Rowland JH, Desmond KA, Meyerowitz BE, Belin TR, Wyatt GE, Ganz PA. Role of breast reconstructive surgery in physical and emotional outcomes among breast cancer survivors. Journal of the National Cancer Institute. 2000;92:1422–9. doi: 10.1093/jnci/92.17.1422. [DOI] [PubMed] [Google Scholar]
- Sheppard LA, Ely S. Breast cancer and sexuality. The Breast Journal. 2008;14:176–181. doi: 10.1111/j.1524-4741.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Siegel I. Does body weight dissatisfaction change with age? A cross-sectional analysis of American women. The New School Psychology Bulletin. 2010;7:42–50. [Google Scholar]
- Speer J, Hillenberg B, Sugrue D, Blacker C, Kresge C, Decker V, Decker D. Study of sexual functioning determinants in breast cancer survivors. The Breast Journal. 2005;11:440–447. doi: 10.1111/j.1075-122X.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- Taylor CLC, Basen-Engquist K, Shinn EH, Bodurka DC. Predictors of sexual functioning in ovarian cancer patients. Journal of Clinical Oncology. 2004;22:881–889. doi: 10.1200/JCO.2004.08.150. [DOI] [PubMed] [Google Scholar]
- Thirlaway K, Fallowfield L, Cuzick J. The sexual activity questionnaire: A measure of women’s sexual functioning. Quality of Life Research. 1996;5:81–90. doi: 10.1007/BF00435972. [DOI] [PubMed] [Google Scholar]
- Tiggemann M. Body image across the adult life span: Stability and change. Body Image. 2004;1:29–41. doi: 10.1016/S1740-1445(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Ware J, Kosinski M, Keller S. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): Cross-validation and development of clinical cutoff scores. Journal of Sex and Marital Therapy. 2006;31(1):1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- Zimmerman T, Scott J, Heinrichs N. Individual and dyadic predictors of body image in women with breast cancer. Psycho-oncology. 2010;19:1061–1068. doi: 10.1002/pon.1660. [DOI] [PubMed] [Google Scholar]


