Abstract
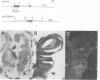
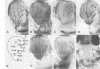
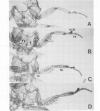
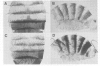
The Drosophila adult cuticle displays a stereotyped pattern of sensory organs (SOs). Its deployment requires the expression of the achaete (ac) and scute (sc) genes. Their products confer to cells of epidermal primordia (imaginal discs and histoblasts) the ability to become SO precursors (SOPs). In imaginal discs, ac and sc expression is spatially restricted to cell clusters within which one or a few cells become SOP(s). With the help of ubiquitous sc expression provided at different developmental times by a heat shock-sc (HSSC) chimeric gene, we have analyzed the response of epidermal primordia to the proneural action of the sc product, and have tested whether the patterned distribution of ac/sc products is necessary to position SOs correctly within the epidermis. Each primordium responds to HSSC expression by developing SOs only during a characteristic developmental period. In the absence of the endogenous ac and sc genes, most SOs induced by HSSC are of the correct type and are located in wild type positions. These results indicate that the capacity of primordia to respond to sc is temporally and spatially regulated, that specification of the type of SO does not depend on ac/sc, and that SO positioning utilizes topological information independent of the spatially restricted distribution of ac/sc products.
Full text
PDF
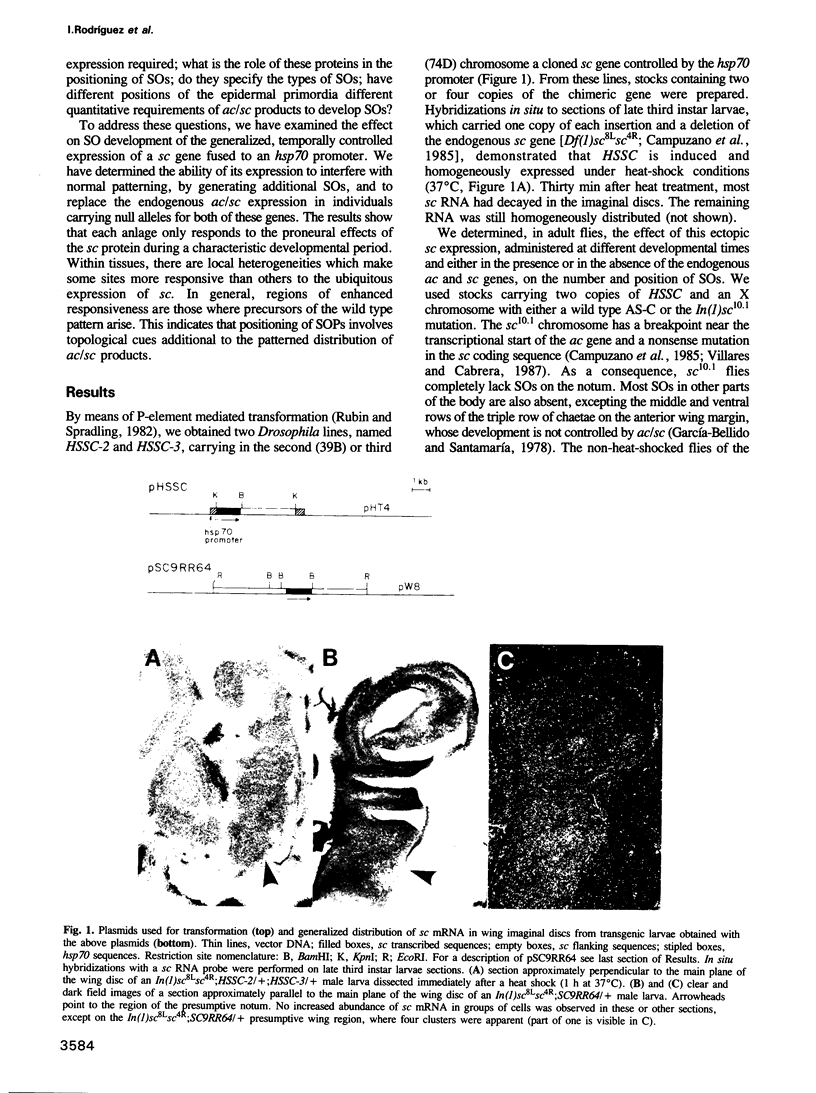
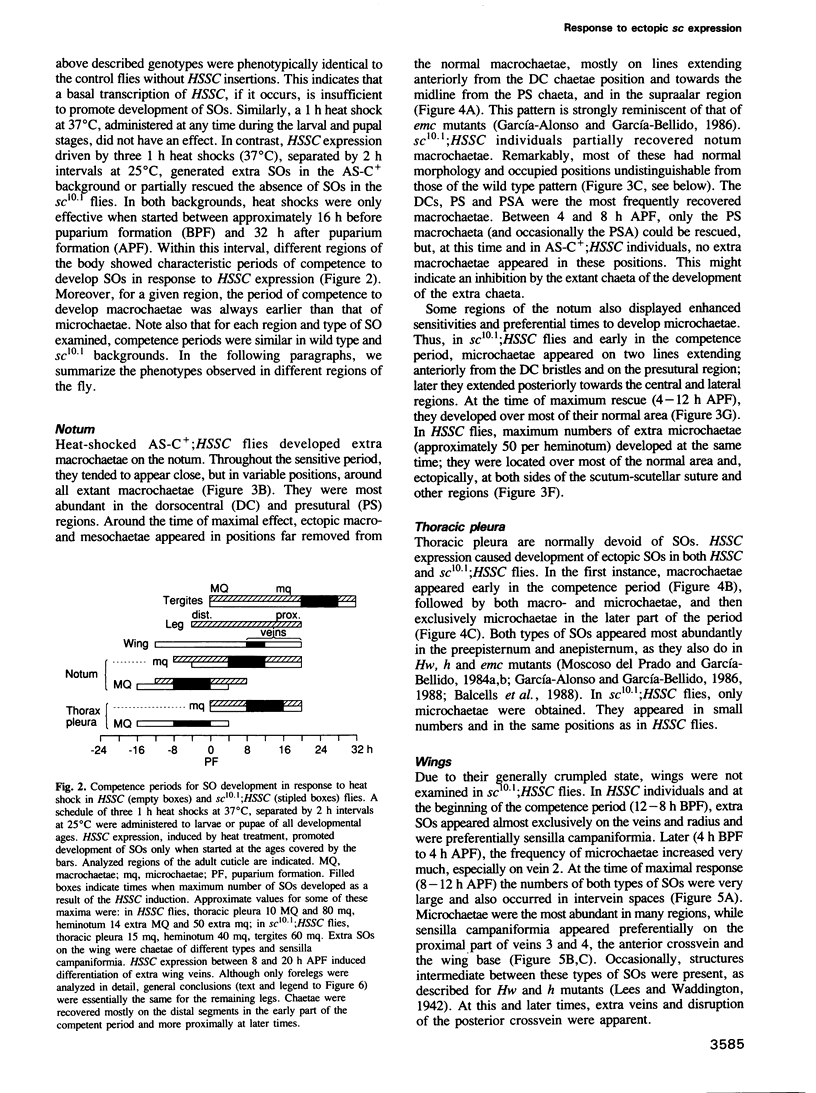
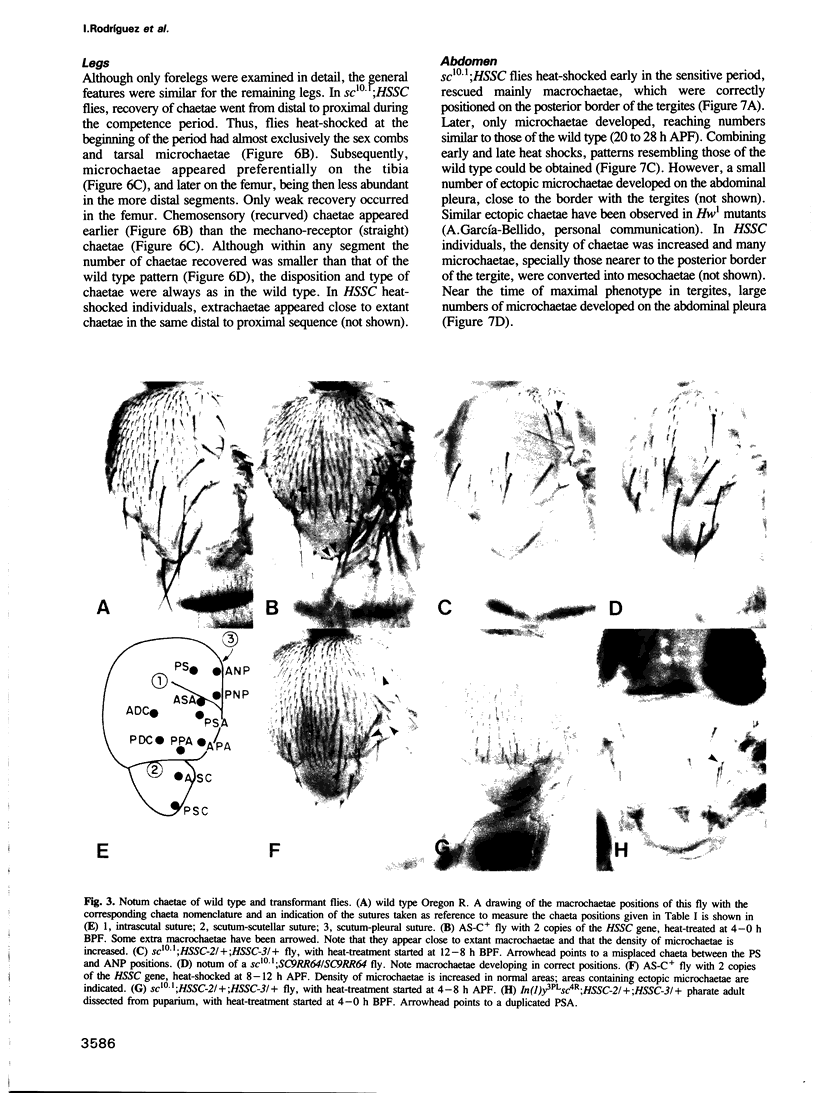

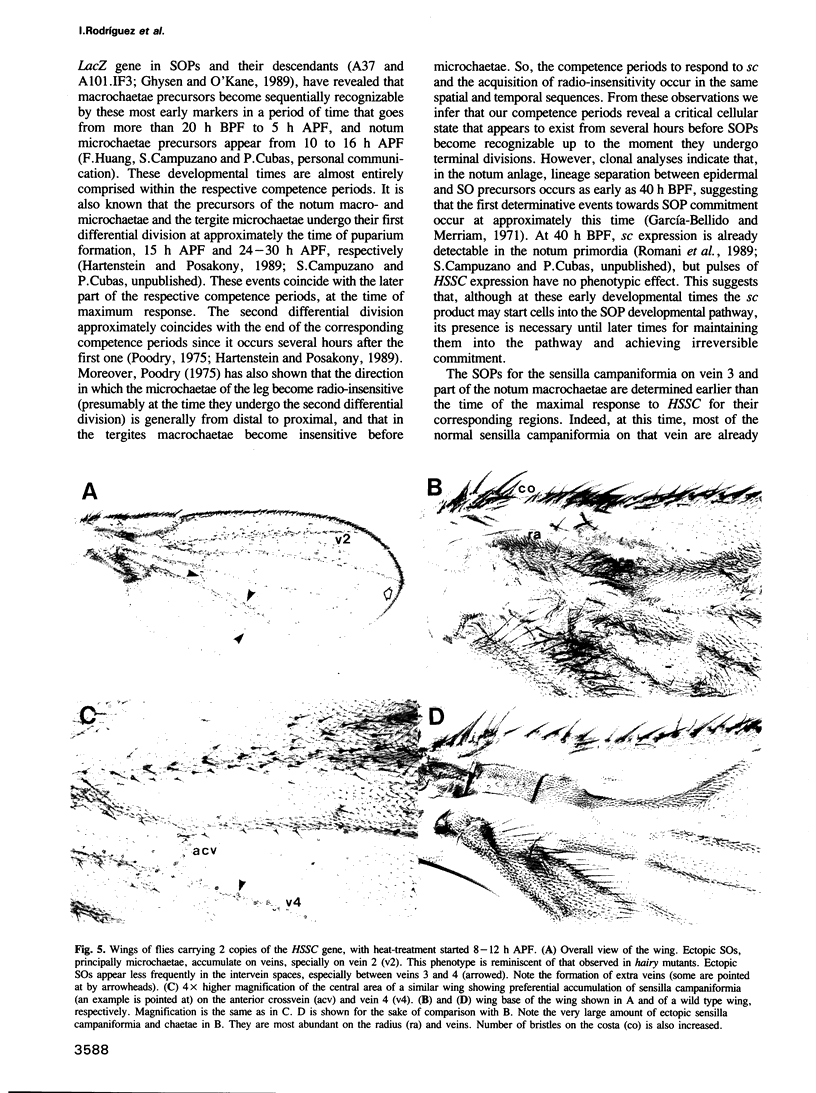

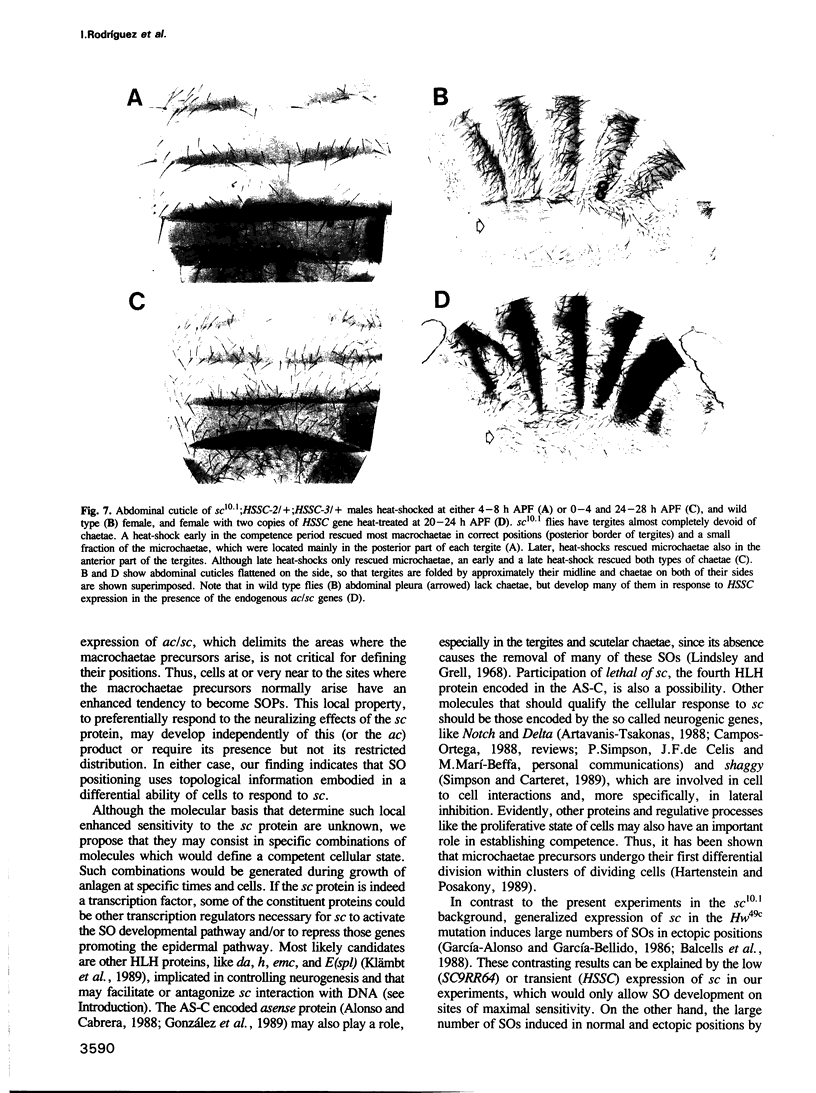
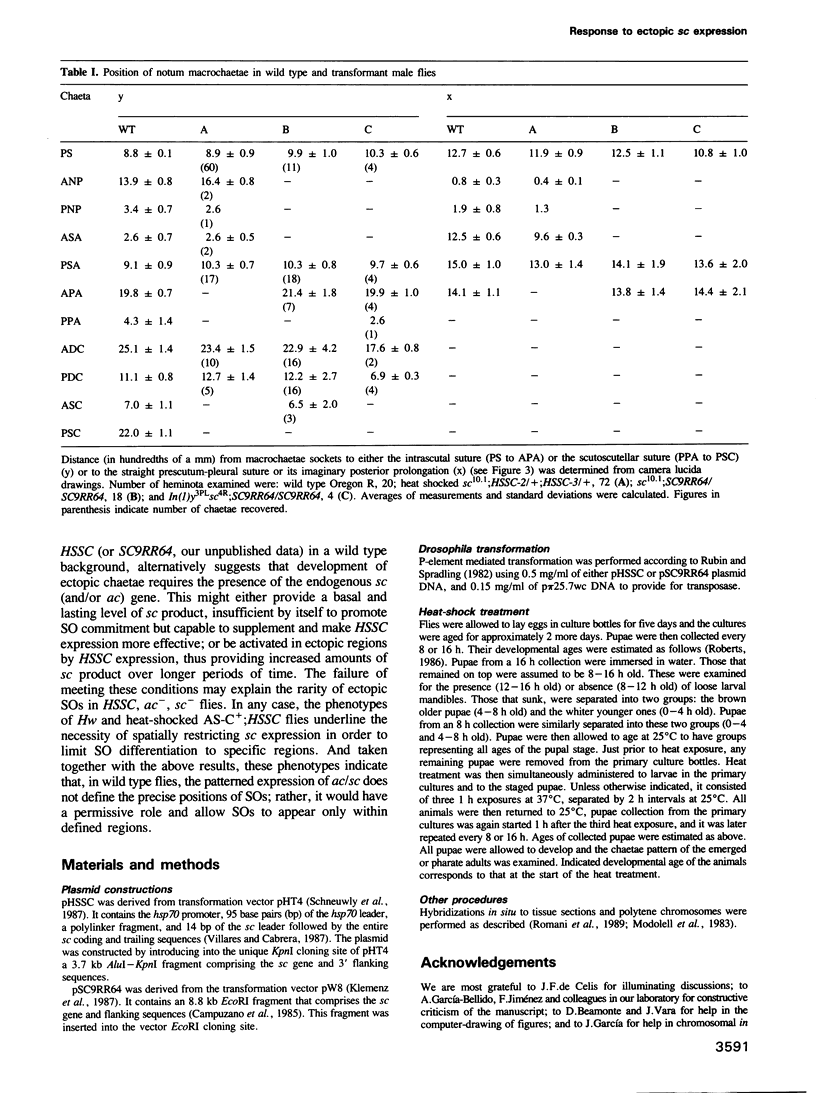

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. C., Cabrera C. V. The achaete-scute gene complex of Drosophila melanogaster comprises four homologous genes. EMBO J. 1988 Aug;7(8):2585–2591. doi: 10.1002/j.1460-2075.1988.tb03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S. The molecular biology of the Notch locus and the fine tuning of differentiation in Drosophila. Trends Genet. 1988 Apr;4(4):95–100. doi: 10.1016/0168-9525(88)90096-0. [DOI] [PubMed] [Google Scholar]
- Balcells L., Modolell J., Ruiz-Gómez M. A unitary basis for different Hairy-wing mutations of Drosophila melanogaster. EMBO J. 1988 Dec 1;7(12):3899–3906. doi: 10.1002/j.1460-2075.1988.tb03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega J. A. Cellular interactions during early neurogenesis of Drosophila melanogaster. Trends Neurosci. 1988 Sep;11(9):400–405. doi: 10.1016/0166-2236(88)90077-x. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Balcells L., Villares R., Carramolino L., García-Alonso L., Modolell J. Excess function hairy-wing mutations caused by gypsy and copia insertions within structural genes of the achaete-scute locus of Drosophila. Cell. 1986 Jan 31;44(2):303–312. doi: 10.1016/0092-8674(86)90764-6. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Carramolino L., Cabrera C. V., Ruíz-Gómez M., Villares R., Boronat A., Modolell J. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell. 1985 Feb;40(2):327–338. doi: 10.1016/0092-8674(85)90147-3. [DOI] [PubMed] [Google Scholar]
- Caudy M., Grell E. H., Dambly-Chaudière C., Ghysen A., Jan L. Y., Jan Y. N. The maternal sex determination gene daughterless has zygotic activity necessary for the formation of peripheral neurons in Drosophila. Genes Dev. 1988 Jul;2(7):843–852. doi: 10.1101/gad.2.7.843. [DOI] [PubMed] [Google Scholar]
- Caudy M., Vässin H., Brand M., Tuma R., Jan L. Y., Jan Y. N. daughterless, a Drosophila gene essential for both neurogenesis and sex determination, has sequence similarities to myc and the achaete-scute complex. Cell. 1988 Dec 23;55(6):1061–1067. doi: 10.1016/0092-8674(88)90250-4. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Cheng P. F., Lassar A. B., Weintraub H. The MyoD DNA binding domain contains a recognition code for muscle-specific gene activation. Cell. 1990 Mar 9;60(5):733–746. doi: 10.1016/0092-8674(90)90088-v. [DOI] [PubMed] [Google Scholar]
- Ellis H. M., Spann D. R., Posakony J. W. extramacrochaetae, a negative regulator of sensory organ development in Drosophila, defines a new class of helix-loop-helix proteins. Cell. 1990 Apr 6;61(1):27–38. doi: 10.1016/0092-8674(90)90212-w. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Merriam J. R. Parameters of the wing imaginal disc development of Drosophila melanogaster. Dev Biol. 1971 Jan;24(1):61–87. doi: 10.1016/0012-1606(71)90047-9. [DOI] [PubMed] [Google Scholar]
- García-Bellido A. Genetic Analysis of the Achaete-Scute System of DROSOPHILA MELANOGASTER. Genetics. 1979 Mar;91(3):491–520. doi: 10.1093/genetics/91.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bellido A., Santamaria P. Developmental Analysis of the Achaete-Scute System of DROSOPHILA MELANOGASTER. Genetics. 1978 Mar;88(3):469–486. doi: 10.1093/genetics/88.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrell J., Modolell J. The Drosophila extramacrochaetae locus, an antagonist of proneural genes that, like these genes, encodes a helix-loop-helix protein. Cell. 1990 Apr 6;61(1):39–48. doi: 10.1016/0092-8674(90)90213-x. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Dambly-Chaudiere C. Genesis of the Drosophila peripheral nervous system. Trends Genet. 1989 Aug;5(8):251–255. doi: 10.1016/0168-9525(89)90097-8. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Dambly-Chaudière C. From DNA to form: the achaete-scute complex. Genes Dev. 1988 May;2(5):495–501. doi: 10.1101/gad.2.5.495. [DOI] [PubMed] [Google Scholar]
- Ghysen A., O'Kane C. Neural enhancer-like elements as specific cell markers in Drosophila. Development. 1989 Jan;105(1):35–52. doi: 10.1242/dev.105.1.35. [DOI] [PubMed] [Google Scholar]
- González F., Romani S., Cubas P., Modolell J., Campuzano S. Molecular analysis of the asense gene, a member of the achaete-scute complex of Drosophila melanogaster, and its novel role in optic lobe development. EMBO J. 1989 Dec 1;8(12):3553–3562. doi: 10.1002/j.1460-2075.1989.tb08527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V., Posakony J. W. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development. 1989 Oct;107(2):389–405. doi: 10.1242/dev.107.2.389. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Weber U., Gehring W. J. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 1987 May 26;15(10):3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klämbt C., Knust E., Tietze K., Campos-Ortega J. A. Closely related transcripts encoded by the neurogenic gene complex enhancer of split of Drosophila melanogaster. EMBO J. 1989 Jan;8(1):203–210. doi: 10.1002/j.1460-2075.1989.tb03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Modolell J., Bender W., Meselson M. Drosophila melanogaster mutations suppressible by the suppressor of Hairy-wing are insertions of a 7.3-kilobase mobile element. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1678–1682. doi: 10.1073/pnas.80.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. A., Schubiger M., Palka J. Neuron differentiation and axon growth in the developing wing of Drosophila melanogaster. Dev Biol. 1984 Aug;104(2):259–273. doi: 10.1016/0012-1606(84)90082-4. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Poodry C. A., Hall L., Suzuki D. T. Developmental properties of Shibire: a pleiotropic mutation affecting larval and adult locomotion and development. Dev Biol. 1973 Jun;32(2):373–386. doi: 10.1016/0012-1606(73)90248-0. [DOI] [PubMed] [Google Scholar]
- Romani S., Campuzano S., Macagno E. R., Modolell J. Expression of achaete and scute genes in Drosophila imaginal discs and their function in sensory organ development. Genes Dev. 1989 Jul;3(7):997–1007. doi: 10.1101/gad.3.7.997. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gómez M., Modolell J. Deletion analysis of the achaete-scute locus of Drosophila melanogaster. Genes Dev. 1987 Dec;1(10):1238–1246. doi: 10.1101/gad.1.10.1238. [DOI] [PubMed] [Google Scholar]
- Rushlow C. A., Hogan A., Pinchin S. M., Howe K. M., Lardelli M., Ish-Horowicz D. The Drosophila hairy protein acts in both segmentation and bristle patterning and shows homology to N-myc. EMBO J. 1989 Oct;8(10):3095–3103. doi: 10.1002/j.1460-2075.1989.tb08461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Klemenz R., Gehring W. J. Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. 1987 Feb 26-Mar 4Nature. 325(6107):816–818. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- Simpson P., Carteret C. A study of shaggy reveals spatial domains of expression of achaete-scute alleles on the thorax of Drosophila. Development. 1989 May;106(1):57–66. doi: 10.1242/dev.106.1.57. [DOI] [PubMed] [Google Scholar]
- Villares R., Cabrera C. V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987 Jul 31;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]