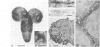Abstract
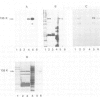
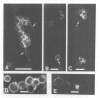
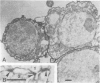
Neurotactin is a 135 kd membrane glycoprotein which consists of a core protein, with an apparent molecular weight of 120 kd, and of N-linked oligosaccharides. In vivo, the protein can be phosphorylated in presence of radioactive orthophosphate. Neurotactin expression in the larval CNS and in primary embryonic cell cultures suggests that it behaves as a contact molecule between neurons or epithelial cells. Electron microscopy studies reveal that neurotactin is uniformly expressed along the areas of contacts between cells, without, however, being restricted to a particular type of junction. It putative adhesive properties have been tested by transfecting non adhesive Drosophila S2 cells with neurotactin cDNA. Heat shocked transfected cells do not aggregate, suggesting that neurotactin does not mediate homophilic cell adhesion. However, these transfected cells bind to a subpopulation of embryonic cells which probably possess a related ligand. The location at cellular junctions between specific neurons or epithelial cells, the heterophilic binding to a putative ligand and the ability to be phosphorylated are consistent with the suggestion that neurotactin functions as an adhesion molecule.
Full text
PDF

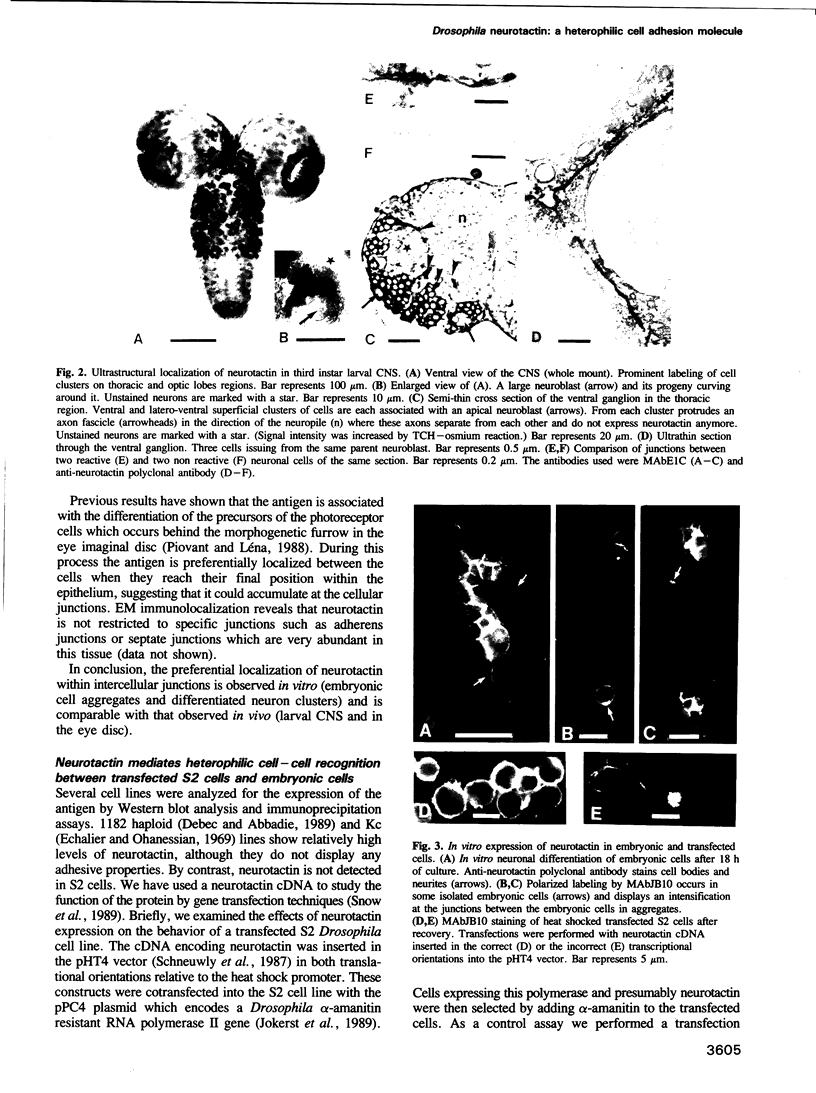
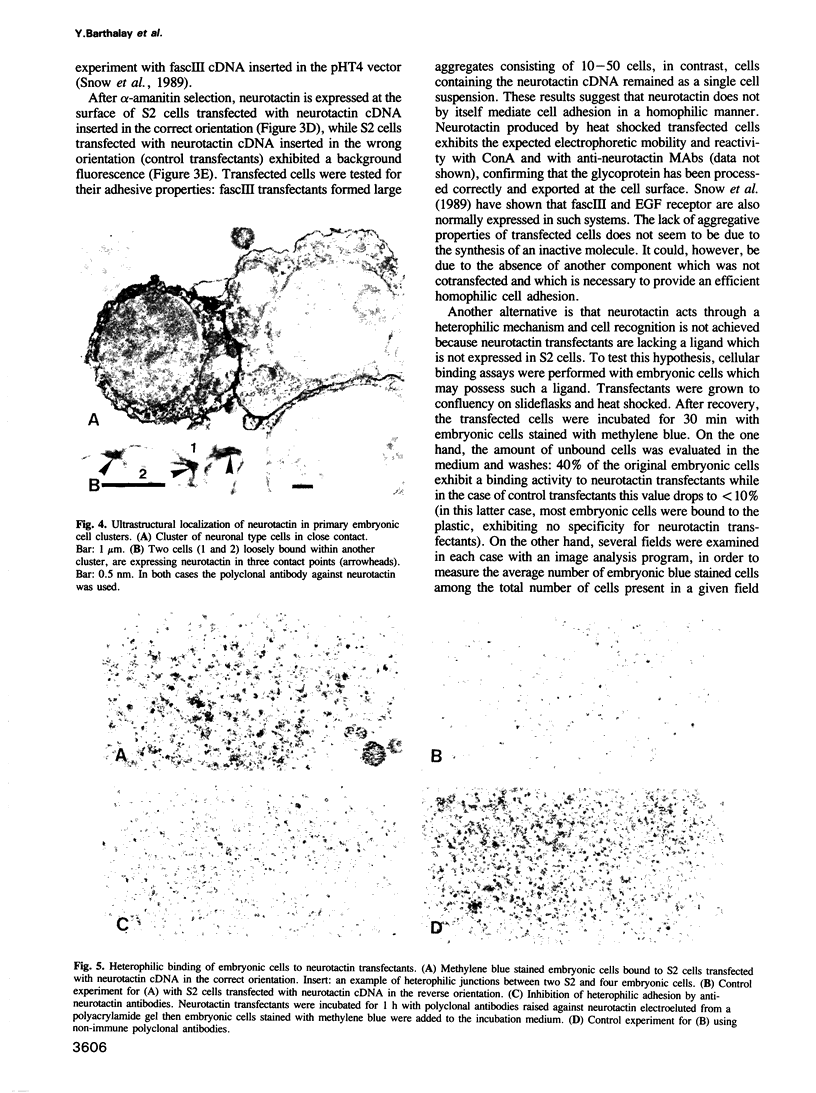



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson A., Rutishauser U. Neural cell adhesion molecule regulates cell contact-mediated changes in choline acetyltransferase activity of embryonic chick sympathetic neurons. J Cell Biol. 1988 Feb;106(2):479–486. doi: 10.1083/jcb.106.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas J. A., Chaix J. C., Steinmetz M., Goridis C. Differential splicing and alternative polyadenylation generates distinct NCAM transcripts and proteins in the mouse. EMBO J. 1988 Mar;7(3):625–632. doi: 10.1002/j.1460-2075.1988.tb02856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Debec A., Abbadie C. The acentriolar state of the Drosophila cell lines 1182. Biol Cell. 1989;67(3):307–311. [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. Isolement, en cultures in vitro, de lignées cellulaires diploïdes de Drosophila melanogaster. C R Acad Sci Hebd Seances Acad Sci D. 1969 Mar 31;268(13):1771–1773. [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Fehon R. G., Kooh P. J., Rebay I., Regan C. L., Xu T., Muskavitch M. A., Artavanis-Tsakonas S. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990 May 4;61(3):523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Deb C., Wasserkrug H. L., Seligman A. M. Staining tissue for light and electron microscopy by bridging metals with multidentate ligands. Science. 1966 Jun 17;152(3729):1631–1634. doi: 10.1126/science.152.3729.1631. [DOI] [PubMed] [Google Scholar]
- Hogervorst F., Kuikman I., von dem Borne A. E., Sonnenberg A. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J. 1990 Mar;9(3):765–770. doi: 10.1002/j.1460-2075.1990.tb08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen K. M., Fehon R. G., Artavanis-Tsakonas S. The notch gene product is a glycoprotein expressed on the cell surface of both epidermal and neuronal precursor cells during Drosophila development. J Cell Biol. 1989 Nov;109(5):2427–2440. doi: 10.1083/jcb.109.5.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokerst R. S., Weeks J. R., Zehring W. A., Greenleaf A. L. Analysis of the gene encoding the largest subunit of RNA polymerase II in Drosophila. Mol Gen Genet. 1989 Jan;215(2):266–275. doi: 10.1007/BF00339727. [DOI] [PubMed] [Google Scholar]
- Kidd S., Baylies M. K., Gasic G. P., Young M. W. Structure and distribution of the Notch protein in developing Drosophila. Genes Dev. 1989 Aug;3(8):1113–1129. doi: 10.1101/gad.3.8.1113. [DOI] [PubMed] [Google Scholar]
- Lander A. D. Understanding the molecules of neural cell contacts: emerging patterns of structure and function. Trends Neurosci. 1989 May;12(5):189–195. doi: 10.1016/0166-2236(89)90070-2. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988 Dec 1;7(12):3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989 Nov;1(1):37–44. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson P. F., Fessler L. I., Nelson R. E., Sterne R. E., Campbell A. G., Fessler J. H. Glutactin, a novel Drosophila basement membrane-related glycoprotein with sequence similarity to serine esterases. EMBO J. 1990 Apr;9(4):1219–1227. doi: 10.1002/j.1460-2075.1990.tb08229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovant M., Léna P. Membrane glycoproteins immunologically related to the human insulin receptor are associated with presumptive neuronal territories and developing neurones in Drosophila melanogaster. Development. 1988 May;103(1):145–156. doi: 10.1242/dev.103.1.145. [DOI] [PubMed] [Google Scholar]
- Salvaterra P. M., Bournias-Vardiabasis N., Nair T., Hou G., Lieu C. In vitro neuronal differentiation of Drosophila embryo cells. J Neurosci. 1987 Jan;7(1):10–22. doi: 10.1523/JNEUROSCI.07-01-00010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Schneuwly S., Klemenz R., Gehring W. J. Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. 1987 Feb 26-Mar 4Nature. 325(6107):816–818. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- Schuch U., Lohse M. J., Schachner M. Neural cell adhesion molecules influence second messenger systems. Neuron. 1989 Jul;3(1):13–20. doi: 10.1016/0896-6273(89)90111-6. [DOI] [PubMed] [Google Scholar]
- Seeger M. A., Haffley L., Kaufman T. C. Characterization of amalgam: a member of the immunoglobulin superfamily from Drosophila. Cell. 1988 Nov 18;55(4):589–600. doi: 10.1016/0092-8674(88)90217-6. [DOI] [PubMed] [Google Scholar]
- Snow P. M., Bieber A. J., Goodman C. S. Fasciclin III: a novel homophilic adhesion molecule in Drosophila. Cell. 1989 Oct 20;59(2):313–323. doi: 10.1016/0092-8674(89)90293-6. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Springer T. A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989 May 4;339(6219):61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Naitoh Y. Amino acid sequence of a novel integrin beta 4 subunit and primary expression of the mRNA in epithelial cells. EMBO J. 1990 Mar;9(3):757–763. doi: 10.1002/j.1460-2075.1990.tb08170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillens S., Ludgate M., Mercken L., Dumont J. E., Vassart G. Analysis of sequence and structure homologies between thyroglobulin and acetylcholinesterase: possible functional and clinical significance. Biochem Biophys Res Commun. 1986 May 29;137(1):142–148. doi: 10.1016/0006-291x(86)91187-3. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Ready D. F. Neuronal differentiation in Drosophila ommatidium. Dev Biol. 1987 Apr;120(2):366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Tougard C., Picart R. Use of pre-embedding ultrastructural immunocytochemistry in the localization of a secretory product and membrane proteins in cultured prolactin cells. Am J Anat. 1986 Feb-Mar;175(2-3):161–177. doi: 10.1002/aja.1001750206. [DOI] [PubMed] [Google Scholar]
- Vila-Porcile E., Picart R., Tixier-Vidal A., Tougard C. Cellular and subcellular distribution of laminin in adult rat anterior pituitary. J Histochem Cytochem. 1987 Mar;35(3):287–299. doi: 10.1177/35.3.3029213. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Escalera S., Bockamp E. O., Moya F., Piovant M., Jiménez F. Characterization and gene cloning of neurotactin, a Drosophila transmembrane protein related to cholinesterases. EMBO J. 1990 Nov;9(11):3593–3601. doi: 10.1002/j.1460-2075.1990.tb07570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]