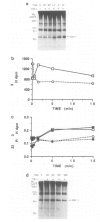
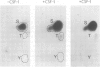
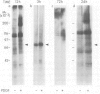
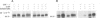Abstract
The serine/threonine kinase RAF-1 is phosphorylated in intact macrophages in response to CSF-1 at 37 degrees C The augmented phosphorylation of RAF-1 and a concomitant increase in RAF-1 associated serine/threonine kinase activity are kinetically later events than CSF-1 induced protein tyrosine phosphorylation. Furthermore, phosphoamino acid analysis of RAF-1 reveals the presence of phosphoserine, trace amounts of phosphothreonine but no phosphotyrosine and the phosphorylated RAF-1 does not react with anti-phosphotyrosine antibodies. In contrast to CSF-1 induced protein tyrosine phosphorylation, RAF-1 phosphorylation and activation are temperature dependent and do not occur at 4 degrees C. Furthermore, coprecipitation experiments failed to reveal any noncovalent association of RAF-1 with the CSF-1 receptor. Thus, while RAF-1 is not a direct substrate for the CSF-1 receptor tyrosine kinase in vivo, its temperature dependent phosphorylation and activation represent an intriguing aspect of the CSF-1 response.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosio L., Mahowald A. P., Perrimon N. Requirement of the Drosophila raf homologue for torso function. Nature. 1989 Nov 16;342(6247):288–291. doi: 10.1038/342288a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Oppermann H., Seeburg P., Kerby S. B., Gunnell M. A., Young A. C., Rapp U. R. The complete coding sequence of the human raf oncogene and the corresponding structure of the c-raf-1 gene. Nucleic Acids Res. 1986 Jan 24;14(2):1009–1015. doi: 10.1093/nar/14.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Downing J. R., Rettenmier C. W., Sherr C. J. Ligand-induced tyrosine kinase activity of the colony-stimulating factor 1 receptor in a murine macrophage cell line. Mol Cell Biol. 1988 Apr;8(4):1795–1799. doi: 10.1128/mcb.8.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Tremble P. M., Williams L. T. Evidence for the platelet-derived growth factor-stimulated tyrosine phosphorylation of the platelet-derived growth factor receptor in vivo. Immunopurification using a monoclonal antibody to phosphotyrosine. J Biol Chem. 1984 Jun 25;259(12):7909–7915. [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Hart C. E., Seifert R. A., Ross R., Bowen-Pope D. F. Synthesis, phosphorylation, and degradation of multiple forms of the platelet-derived growth factor receptor studied using a monoclonal antibody. J Biol Chem. 1987 Aug 5;262(22):10780–10785. [PubMed] [Google Scholar]
- Huhn R. D., Posner M. R., Rayter S. I., Foulkes J. G., Frackelton A. R., Jr Cell lines and peripheral blood leukocytes derived from individuals with chronic myelogenous leukemia display virtually identical proteins phosphorylated on tyrosine residues. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4408–4412. doi: 10.1073/pnas.84.13.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W., Schultz A. M., Oppermann H., Rapp U. R. Preparation of raf-oncogene-specific antiserum with raf protein produced in E. coli. Biochim Biophys Acta. 1988 Feb 28;949(2):233–239. doi: 10.1016/0167-4781(88)90087-5. [DOI] [PubMed] [Google Scholar]
- Kumjian D. A., Wahl M. I., Rhee S. G., Daniel T. O. Platelet-derived growth factor (PDGF) binding promotes physical association of PDGF receptor with phospholipase C. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8232–8236. doi: 10.1073/pnas.86.21.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Moelling K., Heimann B., Beimling P., Rapp U. R., Sander T. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature. 1984 Dec 6;312(5994):558–561. doi: 10.1038/312558a0. [DOI] [PubMed] [Google Scholar]
- Morgan C., Pollard J. W., Stanley E. R. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC1.2F5. J Cell Physiol. 1987 Mar;130(3):420–427. doi: 10.1002/jcp.1041300316. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Browning P. J., White M. F., Roberts T. M. Tyrosine phosphorylations in vivo associated with v-fms transformation. Mol Cell Biol. 1988 Jan;8(1):176–185. doi: 10.1128/mcb.8.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rapp U., Roberts T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölders H., Defesche J., Müller D., Bonner T. I., Rapp U. R., Müller R. Integration of transfected LTR sequences into the c-raf proto-oncogene: activation by promoter insertion. EMBO J. 1985 Mar;4(3):693–698. doi: 10.1002/j.1460-2075.1985.tb03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Look A. T., Roussel M. F., Sherr C. J. Tandem linkage of human CSF-1 receptor (c-fms) and PDGF receptor genes. Cell. 1988 Nov 18;55(4):655–661. doi: 10.1016/0092-8674(88)90224-3. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Dull T. J., Rettenmier C. W., Ralph P., Ullrich A., Sherr C. J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Copeland T. D., Mark G. E., Rapp U. R., Oroszlan S. Detection of the myristylated gag-raf transforming protein with raf-specific antipeptide sera. Virology. 1985 Oct 15;146(1):78–89. doi: 10.1016/0042-6822(85)90054-6. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Copeland T., Oroszlan S., Rapp U. R. Identification and characterization of c-raf phosphoproteins in transformed murine cells. Oncogene. 1988 Feb;2(2):187–193. [PubMed] [Google Scholar]
- Sengupta A., Liu W. K., Yeung Y. G., Yeung D. C., Frackelton A. R., Jr, Stanley E. R. Identification and subcellular localization of proteins that are rapidly phosphorylated in tyrosine in response to colony-stimulating factor 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8062–8066. doi: 10.1073/pnas.85.21.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung Y. G., Jubinsky P. T., Sengupta A., Yeung D. C., Stanley E. R. Purification of the colony-stimulating factor 1 receptor and demonstration of its tyrosine kinase activity. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1268–1271. doi: 10.1073/pnas.84.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]