Abstract
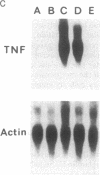
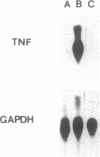
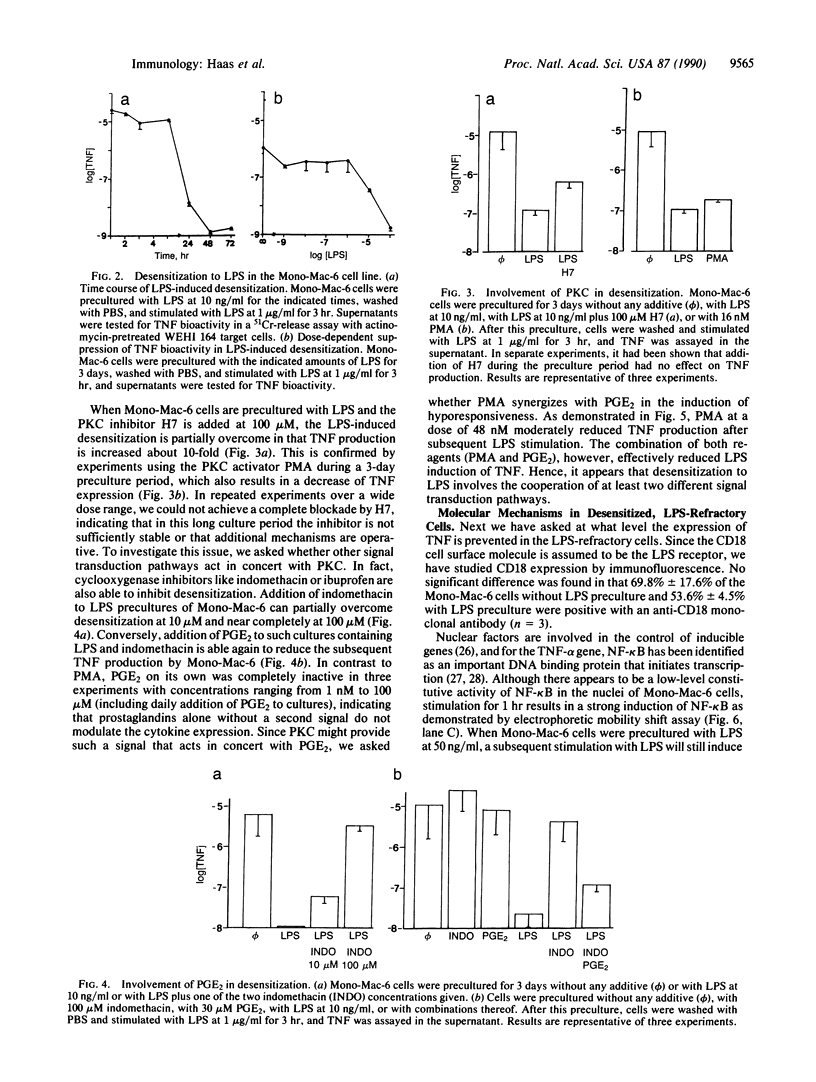
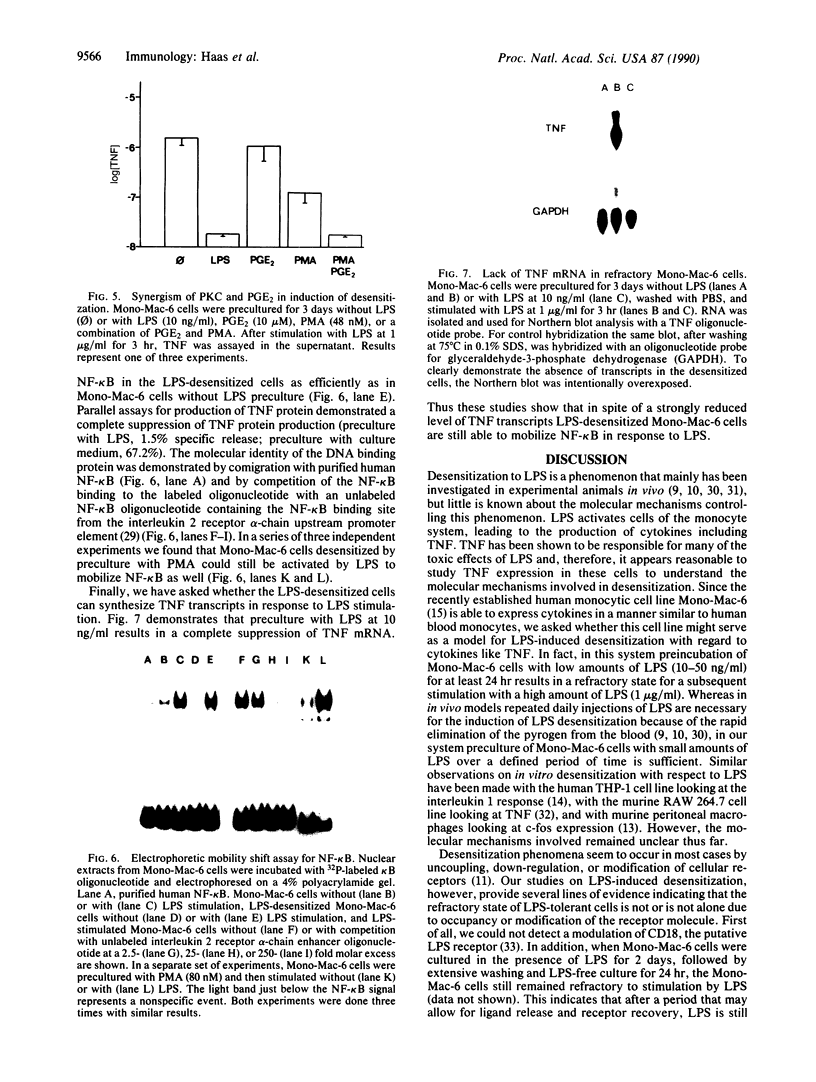
Excessive production of tumor necrosis factor (TNF) after stimulation by lipopolysaccharide (LPS) may result in fever, intravascular coagulation, and lethal shock. An efficient way of preventing the excessive TNF production is desensitization of monocytes/macrophages to LPS. We have analyzed the molecular mechanisms involved in the induction of desensitization and the mechanisms operative in the desensitized, LPS-refractory cells by employing the human monocytic cell line Mono-Mac-6. Similar to human blood monocytes, treatment of Mono-Mac-6 cells with LPS (1 microgram/ml) results in a rapid and transient expression of TNF. When Mono-Mac-6 cells are precultured in medium containing low levels of LPS, they become refractory to subsequent LPS stimulation and show no or little secretion of TNF protein. Desensitization can be blocked by the inhibition of cyclooxygenase and protein kinase C; both prostaglandin E2 (together with a second signal) and phorbol 12-myristate 13-acetate can mimic desensitization. By employing prostaglandin E2 and low concentrations of phorbol 12-myristate 13-acetate, a synergism in the induction of desensitization can be demonstrated. Hence, our studies show that two distinct pathways are involved in the induction of hyporesponsiveness. In both LPS-responsive and LPS-desensitized Mono-Mac-6 cells, LPS was able to induce the transcription factor NF-kappa B in the nucleus. Still, the prevalence of TNF-specific mRNA was dramatically reduced in the desensitized cells. These data indicate that LPS-desensitized Mono-Mac-6 cells are able to activate initial steps of signal transduction up to the level of the NF-kappa B transcription factor. The absence of TNF transcripts, however, indicates that additional nuclear factors may be missing or that silencers may be active such that transcription of the TNF gene is prevented.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Cohen D. S., Wright S. D., Cohn Z. A. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986 Jul 1;164(1):165–179. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Blumenstein M., Schmidt B., Ward R. A., Ziegler-Heitbrock H. W., Gurland H. J. Altered interleukin-1 production in patients undergoing hemodialysis. Nephron. 1988;50(4):277–281. doi: 10.1159/000185187. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Censini S., Bartalini M., Tagliabue A. Regulation of arachidonic acid metabolism in macrophages by immune and nonimmune interferons. J Immunol. 1985 Jul;135(1):502–505. [PubMed] [Google Scholar]
- Böhnlein E., Lowenthal J. W., Siekevitz M., Ballard D. W., Franza B. R., Greene W. C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988 Jun 3;53(5):827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Baeuerle P., Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990 Apr;10(4):1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. L., Hunsaker W. R. Improved hybridization assays employing tailed oligonucleotide probes: a direct comparison with 5'-end-labeled oligonucleotide probes and nick-translated plasmid probes. Anal Biochem. 1985 Dec;151(2):211–224. doi: 10.1016/0003-2697(85)90168-x. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale B. E., Shin H. S. Activation of macrophages for tumor cell cytotoxicity: identification of indomethacin sensitive and insensitive pathways. J Immunol. 1981 Aug;127(2):760–765. [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fenton M. J., Vermeulen M. W., Clark B. D., Webb A. C., Auron P. E. Human pro-IL-1 beta gene expression in monocytic cells is regulated by two distinct pathways. J Immunol. 1988 Apr 1;140(7):2267–2273. [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988 May;56(5):1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., Woodward W. E. Mechanisms of endotoxin tolerance. IV. Specificity of the pyrogenic refractory state during continuous intravenous infusions of endotoxin. J Exp Med. 1966 Nov 1;124(5):983–1000. doi: 10.1084/jem.124.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introna M., Bast R. C., Jr, Johnston P. A., Adams D. O., Hamilton T. A. Homologous and heterologous desensitization of proto-oncogene cfos expression in murine peritoneal macrophages. J Cell Physiol. 1987 Apr;131(1):36–42. doi: 10.1002/jcp.1041310107. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Kovacs E. J., Radzioch D., Young H. A., Varesio L. Differential inhibition of IL-1 and TNF-alpha mRNA expression by agents which block second messenger pathways in murine macrophages. J Immunol. 1988 Nov 1;141(9):3101–3105. [PubMed] [Google Scholar]
- Nedwin G. E., Naylor S. L., Sakaguchi A. Y., Smith D., Jarrett-Nedwin J., Pennica D., Goeddel D. V., Gray P. W. Human lymphotoxin and tumor necrosis factor genes: structure, homology and chromosomal localization. Nucleic Acids Res. 1985 Sep 11;13(17):6361–6373. doi: 10.1093/nar/13.17.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Havell E. A. The antitumor function of tumor necrosis factor (TNF) II. Analysis of the role of endogenous TNF in endotoxin-induced hemorrhagic necrosis and regression of an established sarcoma. J Exp Med. 1988 Mar 1;167(3):1086–1099. doi: 10.1084/jem.167.3.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990 Jan 1;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Taplits M. S., Henkart P. A., Hodes R. J. T helper cell cytoplasmic granules. Exocytosis in response to activation via the T cell receptor. J Immunol. 1988 Jul 1;141(1):1–9. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Virca G. D., Kim S. Y., Glaser K. B., Ulevitch R. J. Lipopolysaccharide induces hyporesponsiveness to its own action in RAW 264.7 cells. J Biol Chem. 1989 Dec 25;264(36):21951–21956. [PubMed] [Google Scholar]
- Wallach D., Holtmann H., Engelmann H., Nophar Y. Sensitization and desensitization to lethal effects of tumor necrosis factor and IL-1. J Immunol. 1988 May 1;140(9):2994–2999. [PubMed] [Google Scholar]
- Weiss E. H., Cheah K. S., Grosveld F. G., Dahl H. H., Solomon E., Flavell R. A. Isolation and characterization of a human collagen alpha 1(I)-like gene from a cosmid library. Nucleic Acids Res. 1982 Mar 25;10(6):1981–1994. doi: 10.1093/nar/10.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Levin S. M., Jong M. T., Chad Z., Kabbash L. G. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989 Jan 1;169(1):175–183. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Möller A., Linke R. P., Haas J. G., Rieber E. P., Riethmüller G. Tumor necrosis factor as effector molecule in monocyte mediated cytotoxicity. Cancer Res. 1986 Nov;46(11):5947–5952. [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W. The biology of the monocyte system. Eur J Cell Biol. 1989 Jun;49(1):1–12. [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Thiel E., Fütterer A., Herzog V., Wirtz A., Riethmüller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988 Mar 15;41(3):456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]