SUMMARY
Objective
Serum sex steroid concentrations may alter body composition and glucose homeostasis in men in a dose-response manner. We evaluated these endpoints in healthy men rendered medically castrate through use of a gonadotropin-releasing hormone antagonist (acyline) with incremental doses of exogenous testosterone (T) gel.
Design
Subjects (n=6–9 per group) were randomly assigned to injections of acyline every 2 weeks plus transdermal T gel (1.25 g, 2.5 g, 5.0 g, 10 g or 15 g) daily or double placebo (injections and gel) for 12 weeks.
Patients
Healthy men, ages 25–55 years, with normal serum total T concentrations.
Measurements
Serum T, dihydrotestosterone (DHT) and estradiol (E2) were measured at baseline and every 2 weeks. Body composition was analyzed by dual-energy x-ray absorptiometry at baseline and week 12. Fasting serum adiponectin, leptin, glucose and insulin concentrations were measured at baseline and week 10.
Results
Forty-eight men completed the study. A significant treatment effect was observed for change in lean mass (ANOVA p=0.01) but not fat mass (p=0.14). Lean mass increased in the 15g T group relative to all lower dose groups, except the 10g T group. When all subjects were analyzed together, changes in lean mass correlated directly and changes in fat mass correlated inversely with serum T, E2 and DHT. No changes were noted in serum glucose, insulin, or adipokine levels.
Conclusions
In healthy men higher serum concentrations of T, DHT and E2 were associated with greater increases in lean mass and decreases in fat mass but not with changes in serum glucose, insulin, or adipokines.
Keywords: Testosterone, estradiol, adipokines, insulin resistance
INTRODUCTION
Untreated hypogonadism adversely impacts body composition, bone mass and sexual function in men [1] and has been associated with an increased risk of diabetes, cardiovascular disease, and even early mortality [2–4]. Testosterone replacement therapy (TRT) has the potential to reduce these risks of hypogonadism; however, the beneficial effects of TRT on cardiometabolic outcomes have not been clearly established, nor have optimal treatment targets for on-therapy concentrations of serum testosterone (T). Recent findings have shown dose-dependent changes produced by exogenous T on body composition and threshold sex steroid concentrations below which tissue-specific symptoms of hypogonadism might develop [5]. Similarly, dose-dependent effects of incremental doses of TRT have been shown in certain tissues [6]. However, whether these effects are direct or indirect, and whether they depend upon the dose of exogenous T given or the concentrations of its downstream metabolites, dihydrotestosterone (DHT) and/or estradiol (E2) achieved, has not been explored. This is particularly important in men receiving transdermal TRT, given variability in sex steroid concentrations achieved by this route [7].
T is metabolized by 5α-reductase to produce DHT and by aromatase to E2. Though the roles DHT and E2 play in developing boys are well characterized [8] [9], their importance in adult men is less clear. Recent work has highlighted the importance of E2 in limiting adipose accumulation and maintaining sexual health in older men [5] while studies utilizing inhibitors of 5α-reductase have demonstrated DHT is not required for androgenic effects on bone, muscle or adipose tissue in the presence of normal levels of circulating T [10].
To understand how the administration of exogenous, transdermal T affects body composition, glucose homeostasis and other cardiometabolic parameters, we performed a randomized, placebo-controlled intervention study in healthy men who were medically castrated with a GnRH antagonist (acyline) and then received variable doses of topical T (ranging from subphysiological to supraphysiological). We hypothesized that lean mass would show dose-dependent increases with supraphysiological T doses while fat mass would show dose-dependent decreases, and that the reverse would be observed with subphysiological T doses (dose-dependency analysis). We further hypothesized that we would demonstrate a dose-dependent improvement in cardiometabolic profiles – with improvement in insulin resistance and more favorable adipokine and lipid profiles. As part of our secondary analysis, we also assessed the relationship between these outcomes and serum sex steroid concentrations (T, DHT and E2) as continuous variables, regardless of dose group (concentration-dependency analysis).
MATERIALS AND METHODS
Presented results are pre-specified secondary outcome measures from a previously reported trial designed to explore the effects of varying T doses on intra-prostatic androgen concentrations and other prostate outcomes in healthy men. Those results have been published [11] with full details of study procedures, safety laboratory assessments, inclusion/exclusion criteria, statistical methods and adverse events, and are summarized briefly below.
Subjects
We recruited healthy male volunteers ages 25–55 years via advertisement. Written informed consent was obtained prior to screening. All study procedures were conducted at the University of Washington Medical Center, Seattle, WA and were approved by the Institutional Review Board. Notable inclusion criteria included general good health, normal serum total T level (>10.4 nmol/L), normal reproductive history and normal physical examination while exclusion criteria included use of any drugs known to affect steroid hormone synthesis, metabolism or effect in the past 3 months, history of illicit drug or alcohol abuse, weight >145 kg or BMI >40. Ninety-eight subjects were screened; 67 were randomized to receive treatment but only 62 chose to initiate treatment and subsequently 9 subjects dropped out during the study; 53 completed the week 12 end-of-treatment visit, but 3 declined the final on-treatment body composition analyses and were excluded from data analysis. Another 2 subjects were excluded from the analyses due to drug non-compliance (n=48 for analysis).
Study design and randomization
Subjects were randomly assigned (using random block allocation with block size of 4) to either a double placebo group (daily placebo transdermal gel + placebo injection every 2 weeks) or 1 of 5 treatment groups. Subjects in the 5 active treatment groups all received the GnRH antagonist acyline (300μg/kg every 2 weeks, provided by NICHD, PolyPeptide Laboratories, San Diego, CA) [12] plus one of the following doses of 1% transdermal T gel packets (Besins, Bangkok, Thailand): 1.25 g, 2.5 g, 5 g, 10 g or 15 g, applied daily and at the same time after bathing. Subjects received 12 weeks of the assigned treatment and had a recovery visit at 18 weeks.
Primary outcomes
Body composition (total lean body mass, total fat mass) was assessed by dual-energy x-ray absorptiometry (DXA) scan using a GE Lunar Prodigy scanner at baseline, end-of-treatment and recovery. The DXA scanner is regularly calibrated to ensure accuracy and reproducibility of results, and a single technician performed all of the study measurements. Based on the respective precision errors of 0.25 and 0.15 kg for fat mass and total body mass, the least significant change for each metric was 0.68 and 0.42 kg, respectively. Blood was collected every 2 weeks during treatment, and sex steroids were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described previously [13]. This assay is validated for T quantification by the CDC using certified standards. The lower limit of quantification for T and DHT was ≤0.035 nmol/L and 7.34 pmol/L for E2. The intra-assay coefficients of variation were <5% for all three sex steroids. Normal reference ranges for serum T and DHT using these methods were established from 118 morning samples from healthy men ages 18–55.
Secondary outcomes
Fasting serum lipids and adiponectin, leptin, glucose and insulin concentrations were measured at baseline, week 10 and recovery. These were not measured at end-of-treatment (week 12) as subjects were not fasting at that visit while multiple other study procedures took place. The homeostatic model assessment for insulin resistance (HOMA-IR) was calculated from the fasting glucose and insulin concentrations [14]. Serum glucose concentrations were measured enzymatically using Roche reagents on a Roche Module P Chemistry autoanalyzer (Roche Diagnostics Inc.) with intra- and inter-assay coefficients of variation of ≤ 1.1% and ≤ 1.7%, respectively. Serum insulin concentrations were measured by a two-site immune-enzymo-metric assay on a TOSOH 2000 autoanalyzer (TOSOH Bioscience Inc.) with an inter- and intra-assay coefficients of variation ≤ 2.8%. Adiponectin and leptin were measured by radioimmunoassay (Millipore, Inc., Billerica, MA) with intra-assay co-efficients of variation of 6.2 and 3.7%, respectively. Fasting serum lipids (LDL-C, total cholesterol, HDL-C, triglycerides) were measured by the Northwest Lipid Research Center (Seattle, WA) using standard assays [15].
Statistical analyses
The original study was powered to identify changes in intra-prostatic androgen concentrations as previously described [11]. Serum sex steroid concentrations achieved in the treatment groups were not normally distributed, so non-parametric statistical analyses were performed, and the results are presented as medians with interquartile ranges (IQR). For comparisons among groups at a given time point we used a Kruskal-Wallis ANOVA (p<0.05 considered significant) with a Wilcoxon rank-sum post-hoc test for pairwise comparisons with an adjusted Bonferroni p-value of less than 0.008 being considered significant. To assess changes within a group during the study, a Wilcoxon signed rank test with an unadjusted p<0.05 was considered significant. Spearman’s correlation was used to evaluate the associations between serum hormone concentrations and various study end points. We calculated average on-treatment serum T, DHT and E2 concentrations in all study groups using concentrations measured every 2 weeks from weeks 2–12, to account for intra-individual variability in T concentrations observed with transdermal T gel [7]. The changes in serum T, DHT and E2 concentrations for each subject were calculated by subtracting the baseline (Day 0) value from the average on-treatment value (weeks 2–12). All analyses were performed using GraphPad Prism 6.0 (La Jolla, CA). Intention to treat analysis was not performed and only subjects that completed the study were included in the analysis.
RESULTS
Subjects
Forty-eight subjects were included in the data analysis. There were no significant differences at baseline among subjects in the 6 treatment groups except in serum adiponectin concentrations (Table 1).
Table 1.
Baseline characteristics of study subjects by treatment group (median (25th, 75th percentiles))
| Variable | Placebo | 1.25 g T | 2.5 g T | 5 g T | 10 g T | 15 g T | p-value |
|---|---|---|---|---|---|---|---|
| Number of subjects | 8 | 9 | 6 | 9 | 8 | 8 | |
| Age (years) | 45 (36,48) |
45 (41,46) |
43 (39,48) |
34 (30,44) |
42 (34,45) |
51 (48,53) |
0.15 |
| BMI (kg/m2) | 28 (26,31) |
28 (25,33) |
27 (24,28) |
25 (25,27) |
25 (23,28) |
27 (25,28) |
0.50 |
| Serum Hormones | |||||||
| Testosterone (nmol/L) | 15.6 (11.2,17.8) |
15.6 (12,17.7) |
16.9 (15.6,20.4) |
14.5 (13.4,17.5) |
16.7 (15.3,19.9) |
15 (13.1,18.6) |
0.71 |
| Dihydrotestosterone (nmol/L) | 1.2 (1,1.6) |
1.3 (0.8,1.5) |
1.4 (1.3,1.5) |
1.3 (0.6,1.7) |
1.7 (1.5,1.8) |
1.4 (1,2) |
0.35 |
| Estradiol (pmol/L) | 77.1 (60.5,104.8) |
82.9 (73.2,95.1) |
47.5 (46.4,76.6) |
84.8 (55.1,122.4) |
62.9 (54.5,71.3) |
79 (54,95) |
0.29 |
| Sex-hormone binding globulin (nmol/L) | 22.1 (16.4,34.5) |
20.1 (18.6,25.1) |
25 (22.4,42.3) |
20.6 (15.7,27.2) |
31.9 (30.1,33.6) |
30.5 (23.9,37.9) |
0.14 |
| Serum Lipids | |||||||
| Cholesterol (mmol/L) | 5.5 (5.2,6) |
4.9 (4.5,5.5) |
4.6 (4.2,5.7) |
5.1 (4.5,5.7) |
4.5 (4.2,5.1) |
4.7 (4.4,6.2) |
0.68 |
| Triglycerides (mmol/L) | 1.7 (1.4,2.3) |
1.5 (0.9,1.6) |
1.1 (0.9,1.2) |
1.0 (0.9,1.3) |
1.4 (1.2,1.9) |
1.6 (0.9,2.1) |
0.24 |
| LDL (mmol/L) | 3.4 (2.9,3.8) |
3.0 (2.5,3.4) |
2.6 (2.2,3) |
2.9 (2.4,3.6) |
2.6 (2.4,2.9) |
3.0 (2.7,3.6) |
0.50 |
| HDL (mmol/L) | 1.3 (1.1,1.4) |
1.3 (1.1,1.7) |
1.6 (1.5,1.7) |
1.6 (1.2,1.8) |
1.3 (1.1,1.6) |
1.2 (1,1.2) |
0.31 |
| Metabolic parameters | |||||||
| Glucose (mmol/L) | 5.2 (5.2,5.3) |
5.4 (4.9,5.6) |
5.7 (5.5,6) |
5.5 (5,5.6) |
5.2 (5.1,5.3) |
5.4 (5.1,5.6) |
0.25 |
| Insulin (pmol/L) | 51 (44,104) |
47 (31,71) |
57 (49,81) |
63 (47,81) |
49 (41,69) |
70 (56,87) |
0.73 |
| HOMA-IR | 11.8 (10,25.3) |
11.4 (6.5,17.8) |
14.7 (12.2,22.4) |
14 (12,20.1) |
10.8 (9.1,16.7) |
16.1 (12.5,21.4) |
0.71 |
| Adiponectin (mcg/mL) | 10.1 (5.7,17.2) |
8.9 (8,12) |
10.8 (7.9,14.1) |
7 (5.1,8.1) |
15.7 (11.6,19.8) |
7.3 (5.1,8.8) |
0.01* |
| Leptin (ng/mL) | 6.5 (4.5,13.7) |
9.5 (5.5,15) |
5.7 (3.3,10.6) |
5.7 (4.3,14.1) |
3.9 (2.5,6) |
4.7 (2.1,9.3) |
0.51 |
= p-value <0.05 on Kruskal-Wallis ANOVA
kg/m2 = kilograms per meter(squared). nmol/L = nanomoles per liter. pmol/L = picomoles per liter. mIU/mL = milli-international units per milliliter. mmol/L = millimoles per liter. mcg/mL = micrograms per milliliter. ng/mL = nanograms per milliliter.
Serum hormones
On-treatment serum T (Figure 1A), DHT (Figure 1B) and E2 (Figure 1C) concentrations significantly differed with escalating doses of exogenous T (ANOVA p<0.001 for all). Serum T and E2 concentrations ranged from below normal to the higher end of the normal range, while serum DHT concentrations were above the upper limit of the normal range in all but the placebo and lowest dose T groups (1.25 g). The ratio of serum DHT/T was higher in all the treatment groups compared to the placebo group (Figure 1D), however, there was no dose-dependent difference in this ratio between the treatment groups.
Figure 1. Scatter plots of serum T (A), DHT (B) and E2 (C) concentrations and DHT/T ratio (D) during treatment (weeks 2–12).

Significant pair-wise comparisons (p<0.008) are indicated. * = compared to 15 g T group; # = compared to 10 g T group; $ = compared to 5 g T group; Ω = compared to Placebo. Dotted lines represent normal reference ranges.
Body Composition
Body weight remained stable in all groups throughout the study (Supplementary Figure 1). Absolute change in body fat (kg) was not significantly different across the treatment groups (ANOVA p=0.14, Figure 2A). Although there was no significant inter-group treatment effect on fat mass, subjects in the 1.25 g T group uniformly exhibited increases in fat mass (Wilcoxon matched-pairs signed rank test p=0.004) with a median increase of 1.9 kg (IQR 1.1–2.3 kg), as did 4 out of 6 subjects in the 2.5 g T group, though this did not achieve statistical significance in the group as a whole. In contrast, change in lean mass differed significantly across treatment groups (ANOVA p=0.011, Figure 2B). In pairwise comparisons, lean mass increased in men in the 15 g T group relative to all other groups except the 10 g T group with a median increase of 2.9 kg compared to baseline (IQR of 1.3–3.1 kg) (Wilcoxon matched-pairs signed rank test p=0.02). In contrast, men in the 1.25 g T group had a median decrease in lean mass of 1.3 kg (IQR 0.8–2.1 kg) (Wilcoxon matched-pairs signed rank test p=0.004), and 5 out of 6 men in the 2.5 g T group also showed decreases in lean mass, though this did not achieve statistical significance (Wilcoxon matched-pairs signed rank test p=0.09) compared to baseline. Despite normalization of serum T concentrations by week 18 in all subjects (data not shown), these changes in body composition persisted with the exception of the loss of lean mass in the 1.25 g T group which had returned to baseline (p=0.91 versus baseline). However, due to the shorter duration of the recovery period (6 weeks) compared to the treatment period (12 weeks) we were unable to assess whether changes in body composition were maintained with sufficient, sustained recovery of the gonadal axis.
Figure 2. Scatter plot of changes in body fat mass (kg) (A) and lean mass (kg) (B) during treatment.
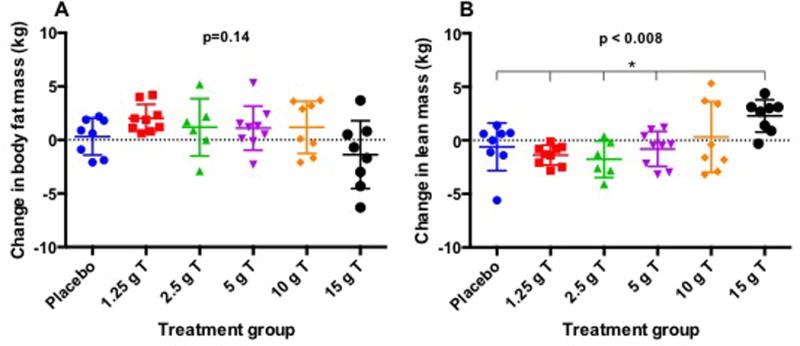
* = Significant compared to 15 g T group.
In our concentration-dependency analysis, change in lean mass exhibited strong correlations with average on-treatment serum T and E2 concentrations (Figure 3) and showed similar, positive correlations with changes in serum T and E2 concentrations (Supplementary Figure 2). In contrast, change in fat mass inversely correlated with on-treatment serum sex steroids with a stronger correlation with serum E2 than T concentration (Figure 4). Change in fat mass also inversely correlated with changes in serum concentrations of T and E2 (Supplementary Figure 3). We also performed a sensitivity analysis by excluding the placebo group in these correlations as this group did not receive any GnRH antagonist or T and so had no net change in sex steroid concentrations, but this did not affect the results for serum T and E2. However, when looking at correlations between change in body composition and average on-treatment (Figure 5) and change in serum DHT (Supplementary Figure 4), strong positive correlations with DHT and change in lean mass (Figure 5A) and negative correlations with change in body fat (Figure 5B) were noted when the placebo group was excluded from the analysis. More modest, but still significant, correlations were seen when the placebo subjects were included in the analysis of DHT and body composition (Spearman’s r for correlation of average serum DHT with change in lean mass 0.38, p=0.007 and with change in fat mass −0.37, p=0.009).
Figure 3. Spearman’s correlations of change in lean mass (kg) with average serum T (A) and E2 (B).
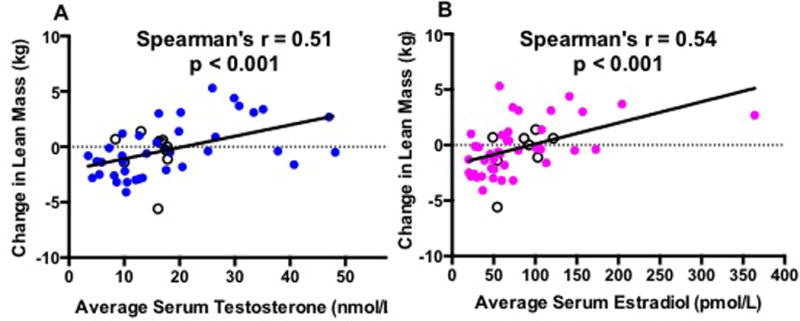
Black hollow circles are subjects from the placebo group.
Figure 4. Spearman’s correlations of change in body fat mass (kg) with average serum T (A) and E2 (B).
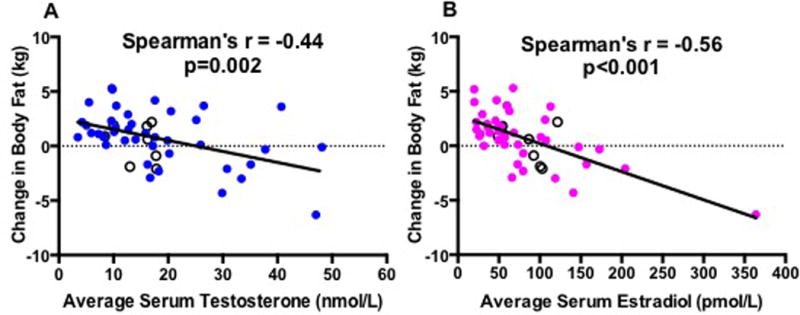
Black hollow circles are subjects from the placebo group.
Figure 5. Spearman’s correlations of average serum DHT with change in lean mass (kg) (A) and change in fat mass (kg)(B).

Note: Placebo subjects (n=8) have been excluded from this analysis.
Lipids
The changes in serum cholesterol (p=0.82), LDL-C (p=0.72), HDL-C (p=0.09), VLDL (p=0.95) and triglyceride (p=0.93) concentrations during treatment were not significantly different across the treatment groups (data not shown). No treatment group had a significant (within group) change in any lipid parameter during the treatment period.
Metabolic parameters
Changes in fasting glucose and insulin concentrations were not significantly different across the treatment groups (p=0.41 and p=0.47 in overall ANOVA, respectively), nor were there significant within-group changes in either of these parameters. Consistent with this absence of treatment effect, change in HOMA-IR also did not differ by group (p=0.47). Adiponectin concentrations showed a trend towards increasing during the treatment period in the lower dose T groups (1.25 g, 2.5 g, 5 g) and towards decreasing in the higher dose T groups (10 g, 15 g), but this finding did not quite achieve statistical significance (p=0.05 in overall ANOVA). However, serum adiponectin concentrations at week 10 (Supplementary Figure 5) were significantly lower in the 15 g T group compared to both the 1.25 and 2.5 g T groups (p=0.006 and p=0.005 respectively, in pairwise comparisons), suggesting a treatment effect. The change in serum leptin concentration was not different across groups (p=0.11 in overall ANOVA).
In the concentration-dependency analysis, changes in serum adiponectin showed moderate, inverse correlations with average on-treatment serum T (Spearman’s r = −0.29, p=0.04), DHT (Spearman’s r = −0.40, p=0.01) and E2 (Spearman’s r = −0.36, p=0.01) concentrations as well as with changes in serum T (Spearman’s r= −0.39, p=0.006), DHT (Spearman’s r = −0.36, p=0.02) and E2 (Spearman’s r = −0.47, p<0.001) concentrations during treatment. Similar inverse correlations were also noted between changes in serum leptin and average on-treatment serum T (Spearman’s r = −0.38, p=0.008), DHT (Spearman’s r = −0.59, p<0.001) and E2 (Spearman’s r = −0.41, p=0.004) concentrations as well as changes in serum T (Spearman’s r= −0.30, p=0.04), DHT (Spearman’s r = −0.51, p<0.001) and E2 (Spearman’s r = −0.35, p=0.01) concentrations. There was a strong positive correlation between change in serum leptin concentration and change in body fat (kg) (Spearman’s r=0.66, p<0.001).
DISCUSSION
Our findings support a concentration-dependent relationship between serum sex steroids and body composition changes in men. We achieved a wide range of serum T, E2, and DHT concentrations in healthy young men by suppressing endogenous sex steroid production and administering incremental doses of exogenous T. Serum T and E2 concentrations ranged from below normal to the high-normal range for healthy young men. We found a significant effect of T dose on change in lean mass. Increased lean mass strongly and positively correlated with serum concentrations of all three sex steroids, since these rise in a co-linear fashion with transdermal T administration. Although the overall effect of treatment group was not statistically significant for change in fat mass, increases in fat mass were seen uniformly among subjects receiving the 1.25 g dose of exogenous T. Moreover, in our concentration-dependency analysis, an inverse correlation between change in fat mass and on-treatment sex steroid concentrations was observed.
Our results are consistent with those of Bhasin et al [16] who utilized a GnRH agonist and escalating doses of injectable T for 20 weeks. These investigators noted increases in lean mass in the men receiving supra-physiologic doses of T replacement and a strong, positive correlation between the changes in lean mass and serum T concentrations. They also found no significant effect of T dose on change in fat mass but did report an inverse correlation between changes in fat mass and serum T levels. Notably, in contrast to Bhasin et al, we used transdermal T as compared to intramuscular T, with a shorter study interval and lower overall exposure to T and E2 as a result of both the pharmacokinetics of transdermal gel versus injections and the lower doses of T administered. In addition, these authors did not explore the relationship between serum DHT and body composition in their analyses.
Our findings also are consistent with prior studies that suppressed endogenous sex steroid production in men and administered transdermal T gel in varying doses [17] [5]. Similar to our study design, two prior studies gave healthy, eugonadal men a GnRH antagonist with varying doses of T gel add-back. In these subject cohorts, on-treatment serum T and E2 concentrations were comparable to those achieved in our study. As in the study by Finkelstein et al, our findings demonstrate reductions in lean mass at the 1.25 g dose of exogenous T and increases in lean mass at the highest administered T dose. Our results indicate that the body composition changes seen at 16 weeks in the Finkelstein study already are evident by 12 weeks of treatment. Further, our results suggest that lean mass continues to increase with a T dose increment of 10 g to 15 g daily, though this inter-group comparison did not reach statistical significance. Finally, findings from both studies suggest that T doses of 5 g daily or more are needed to prevent increases in fat mass in medically castrated healthy men. Importantly, in both previously published studies, observed changes in fat mass appeared more strongly attributable to changes in serum E2 levels than changes in serum T levels. Our study was not designed to make this distinction, but the results of our correlation analyses are consistent with a role for E2 in the regulation of adiposity in men. Additionally, we have expanded on these findings by assessing the relationship between serum DHT concentrations and the changes noted in body composition. The inclusion of a double-placebo group, where subjects underwent no changes to their gonadal status, is a strength of this analyses, allowing us to control for body composition and metabolic changes that were purely a function of time.
Although T is considered the dominant circulating androgen in men, serum DHT concentrations may play an important role in some androgen-mediated end-organ effects. Although serum DHT concentrations are 10–100 fold lower than T, DHT is a more potent androgen, binding the androgen receptor with greater affinity than T [18]. Two observational studies by Shores and colleagues [19, 20] as well as a recent systematic review and meta-analysis [21] suggest that serum DHT concentrations correlate with ischemic stroke and CVD risk more strongly than serum T. The role of DHT in body composition regulation has not been clearly determined. An absence of DHT, in the setting of TRT, does not impact TRT-related changes in body composition [22] [10]. While DHT may not be required for androgen-mediated changes in body composition, exogenous DHT (that suppresses endogenous T) supports favorable, androgen related body composition changes. One study using 3 months of DHT gel in healthy older men showed that supra-physiologic serum DHT concentrations lowered their fat mass significantly (~2 kg) while not changing lean mass [23]. When used as androgen replacement, transdermal DHT gel decreased fat mass and increased lean mass in hypogonadal middle-aged men [24]. Serum DHT concentrations rise to much higher levels in men receiving TRT by transdermal than by an injectable route [21], likely due to robust 5α-reductase activity in the skin [25]. Consistent with this, we noted that serum DHT concentrations rose to supra-physiologic concentrations even with sub-physiologic doses of exogenous T gel. Interestingly, we observed a strong correlation between serum DHT and changes in body composition, similar to that observed for E2 suggesting that perhaps this potent androgen plays a role in body composition regulation despite the low serum concentrations of DHT. Whether higher concentrations of DHT in the setting of mid-range T levels synergize to support greater body composition changes has not been addressed in clinical studies but may be of clinical relevance in choosing a TRT replacement strategy for men. In future studies, direct comparison of the metabolic effects of these different routes of T administration could add insight into the relative importance of circulating DHT for the regulation of body composition and other cardiometabolic outcome measures.
Exogenous T administration within the normal physiologic range in healthy young men has not been shown to markedly affect plasma lipid levels [26], in contrast to anabolic steroid abuse in athletes [27]. Consistent with these findings, we did not find a significant treatment effect in this study on plasma lipid concentrations, nor were significant changes observed within any treatment group during the intervention period. Despite positive changes in body composition, no changes in fasting insulin, fasting glucose or HOMA-IR were observed in this study. Of note, however, our healthy volunteers were not insulin resistant, markedly obese or hypogonadal at baseline. Consistent with our results, testosterone-mediated changes in insulin sensitivity/resistance are not consistently observed in healthy, young men receiving exogenous T [26]. In contrast, older, hypogonadal men receiving TRT often demonstrate modest improvements in insulin resistance [28]. The difference in the metabolic responses of healthy men compared to hypogonadal men when exposed to exogenous androgens may stem from changes that result from either prolonged hypogonadism or baseline metabolic dysfunction in hypogonadal individuals. Alternatively, the lack of change in insulin resistance we observed in the study presented here may result from insufficient power for these endpoints.
Reductions in serum T concentrations have been shown to increase serum adiponectin concentrations [29, 30] while supraphysiologic T administration in healthy men [29], as well as TRT in hypogonadal men [31] decreases serum adiponectin concentrations. Consistent with these findings, our study showed lower adiponectin concentrations in the highest dose T group when compared with the two lowest dose T groups. It is possible, but unproven, that decreases in adiponectin resulting from exogenous T partially mitigate otherwise positive metabolic effects of T treatment. In contrast to adiponectin, changes in serum leptin concentrations correlated more strongly with changes in fat mass than with changes in serum sex steroid concentrations. These results are consistent with observations in other studies [32].
The primary limitation of our study is its small sample size, which represents the most likely reason a significant treatment group effect was not seen for change in fat mass. However, our study has notable strengths and adds to the existing literature. We demonstrated a concentration-dependent relationship between serum sex steroids and body composition changes and adipokines. When androgens are administered intramuscularly or orally, dose-response and concentration-response are likely to be collinear. However, this might not be the case for transdermal TRT, given the marked variability in absorption. Therefore, differentiating between the changes in various androgen-dependent outcomes based on androgen dose administered versus sex steroid concentrations achieved is an important and necessary analysis. Further, we show evidence that body composition changes in healthy men become evident within a short time frame of sex steroid manipulation. Interestingly, our findings suggest that in this time frame, favorable changes in body composition do not translate into the expected corresponding changes in cardio-metabolic factors such as insulin resistance and lipid profiles. Finally, our results underscore the importance of considering the respective metabolic effects of E2 and DHT in men in contrast to the historical focus on T. Ultimately, this line of investigation has the potential to better define optimal TRT regimens on an individual basis and thereby enable delivery of a therapy that confers the desired androgenic effects while minimizing cardio-metabolic risk.
Supplementary Material
Acknowledgments
AT had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We are grateful to NICHD who provided acyline at no cost to the study (IND # 53,539). We would like to thank our research study co-ordinator, Ms. Kathryn Torrez Duncan and study nurse, Marilyn Buscher (RN) as well as the research subjects without whom this work would not be possible
Grant Support:
This study was funded by the National Institute of Aging, NIH through RO1AG037603 grant to STP. Additional support was received as follows: 5T32DK007247-37 grant to AT; Robert B. McMillen Professorship and the Eunice Kennedy Shriver National Institute of Child Health and Development (HD042454) (STP); University of Washington Nutrition Obesity Research Center Pilot and Feasibility Award P30 DK035816 and the American Heart Association Clinical Research Program (KBR); VA Advanced Fellowship in Geriatrics (LAC) and resource and facilities use at VA Puget Sound Health Care System in Seattle, WA (BTM and AMM). JKA is supported, in part, by K24HD082231, from the Eunice Kennedy Shriver NICHD, a division of the NIH.
Disclosure statement:
Transdermal T gel and placebo gel were provided at no cost to the study by Besins Healthcare (Bangkok, Thailand) as an investigator-initiated grant. Besins Healthcare had no input into the design, conduct, data analyses, or manuscript preparation and provided no other financial support for the study.
John Amory: Grant support from Clarus
Alvin Matsumoto: Grant support from Abbott Laboratories and GlaxoSmithKline; Consultant, Abbott Laboratories, Lilly USA, LLC, Endo Pharmaceuticals, Clarus
Footnotes
Clinical Trials Registration Number:
This study is registered at ClinicalTrials.gov, trial identifier: NCT 01327495
References
- 1.Matsumoto AM. Fundamental aspects of hypogonadism in the aging male. Rev Urol. 2003;5(Suppl 1):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 2.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. The Journal of clinical endocrinology and metabolism. 2008;93(1):68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vikan T, Schirmer H, Njølstad I, Svartberg J. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromsø Study. Eur J Endocrinol. 2009;161(3):435–442. doi: 10.1530/EJE-09-0284. [DOI] [PubMed] [Google Scholar]
- 4.Vikan T, Schirmer H, Njølstad I, Svartberg J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. Eur J Endocrinol. 2010;162(4):747–754. doi: 10.1530/EJE-09-0943. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein JS, Yu EW, Burnett-Bowie SA. Gonadal steroids and body composition, strength, and sexual function in men. The New England journal of medicine. 2013;369(25):2457. doi: 10.1056/NEJMc1313169. [DOI] [PubMed] [Google Scholar]
- 6.Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281(6):E1172–1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 7.Swerdloff RS, Pak Y, Wang C, et al. Serum Testosterone (T) Level Variability in T Gel-Treated Older Hypogonadal Men: Treatment Monitoring Implications. The Journal of clinical endocrinology and metabolism. 2015;100(9):3280–3287. doi: 10.1210/JC.2015-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imperato-Mcginley J, Peterson RE, Gautier T, Sturla E. Androgens and the evolution of male-gender identity among male pseudohermaphrodites with 5alpha-reductase deficiency. The New England journal of medicine. 1979;300(22):1233–1237. doi: 10.1056/NEJM197905313002201. [DOI] [PubMed] [Google Scholar]
- 9.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. The Journal of clinical endocrinology and metabolism. 1995;80(12):3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 10.Bhasin S, Travison TG, Storer TW, et al. Effect of testosterone supplementation with and without a dual 5alpha-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. Jama. 2012;307(9):931–939. doi: 10.1001/jama.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thirumalai A, Cooper LA, Rubinow KB, et al. Stable Intraprostatic Dihydrotestosterone in Healthy Medically Castrate Men Treated with Exogenous Testosterone. The Journal of clinical endocrinology and metabolism. 2016:jc20161483. doi: 10.1210/jc.2016-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbst KL, Coviello AD, Page S, Amory JK, Anawalt BD, Bremner WJ. A single dose of the potent gonadotropin-releasing hormone antagonist acyline suppresses gonadotropins and testosterone for 2 weeks in healthy young men. The Journal of clinical endocrinology and metabolism. 2004;89(12):5959–5965. doi: 10.1210/jc.2003-032123. [DOI] [PubMed] [Google Scholar]
- 13.Roth MY, Page ST, Lin K, et al. Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. J Clin Endocrinol Metab. 2010;95(8):3806–3813. doi: 10.1210/jc.2010-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Guy J, Ogden L, Wadwa RP, et al. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for Diabetes in Youth case-control study. Diabetes Care. 2009;32(3):416–420. doi: 10.2337/dc08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. The Journal of clinical endocrinology and metabolism. 2005;90(2):678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 17.Herbst KL, Anawalt BD, Amory JK, Matsumoto AM, Bremner WJ. The male contraceptive regimen of testosterone and levonorgestrel significantly increases lean mass in healthy young men in 4 weeks, but attenuates a decrease in fat mass induced by testosterone alone. The Journal of clinical endocrinology and metabolism. 2003;88(3):1167–1173. doi: 10.1210/jc.2002-020918. [DOI] [PubMed] [Google Scholar]
- 18.Deslypere JP, Young M, Wilson JD, Mcphaul MJ. Testosterone and 5 alpha-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992;88(1–3):15–22. doi: 10.1016/0303-7207(92)90004-p. [DOI] [PubMed] [Google Scholar]
- 19.Shores MM, Arnold AM, Biggs ML, et al. Testosterone and dihydrotestosterone and incident ischaemic stroke in men in the Cardiovascular Health Study. Clinical endocrinology. 2014;81(5):746–753. doi: 10.1111/cen.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shores MM, Biggs ML, Arnold AM, et al. Testosterone, dihydrotestosterone, and incident cardiovascular disease and mortality in the cardiovascular health study. The Journal of clinical endocrinology and metabolism. 2014;99(6):2061–2068. doi: 10.1210/jc.2013-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borst SE, Shuster JJ, Zou B, et al. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC medicine. 2014;12:211. doi: 10.1186/s12916-014-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. The Journal of clinical endocrinology and metabolism. 2005;90(3):1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 23.Ly LP, Jimenez M, Zhuang TN, Celermajer DS, Conway AJ, Handelsman DJ. A double-blind, placebo-controlled, randomized clinical trial of transdermal dihydrotestosterone gel on muscular strength, mobility, and quality of life in older men with partial androgen deficiency. The Journal of clinical endocrinology and metabolism. 2001;86(9):4078–4088. doi: 10.1210/jcem.86.9.7821. [DOI] [PubMed] [Google Scholar]
- 24.Idan A, Griffiths KA, Harwood DT, et al. Long-term effects of dihydrotestosterone treatment on prostate growth in healthy, middle-aged men without prostate disease: a randomized, placebo-controlled trial. Annals of internal medicine. 2010;153(10):621–632. doi: 10.7326/0003-4819-153-10-201011160-00004. [DOI] [PubMed] [Google Scholar]
- 25.Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22(3):168–171. doi: 10.1111/exd.12024. [DOI] [PubMed] [Google Scholar]
- 26.Singh AB, Hsia S, Alaupovic P, et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. The Journal of clinical endocrinology and metabolism. 2002;87(1):136–143. doi: 10.1210/jcem.87.1.8172. [DOI] [PubMed] [Google Scholar]
- 27.Basaria S. Androgen abuse in athletes: detection and consequences. The Journal of clinical endocrinology and metabolism. 2010;95(4):1533–1543. doi: 10.1210/jc.2009-1579. [DOI] [PubMed] [Google Scholar]
- 28.Corona G, Giagulli VA, Maseroli E, et al. THERAPY OF ENDOCRINE DISEASE: Testosterone supplementation and body composition: results from a meta-analysis study. European journal of endocrinology/European Federation of Endocrine Societies. 2016;174(3):R99–116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 29.Page ST, Herbst KL, Amory JK, et al. Testosterone administration suppresses adiponectin levels in men. Journal of andrology. 2005;26(1):85–92. [PubMed] [Google Scholar]
- 30.Host C, Gormsen LC, Hougaard DM, Christiansen JS, Pedersen SB, Gravholt CH. Acute and short-term chronic testosterone fluctuation effects on glucose homeostasis, insulin sensitivity, and adiponectin: a randomized, double-blind, placebo-controlled, crossover study. The Journal of clinical endocrinology and metabolism. 2014;99(6):E1088–1096. doi: 10.1210/jc.2013-2807. [DOI] [PubMed] [Google Scholar]
- 31.Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clinical endocrinology. 2004;60(4):500–507. doi: 10.1111/j.1365-2265.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 32.Gale SM, Castracane VD, Mantzoros CS. Energy homeostasis, obesity and eating disorders: recent advances in endocrinology. J Nutr. 2004;134(2):295–298. doi: 10.1093/jn/134.2.295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


