Abstract
Purpose
Circulating Tumor cells (CTC) are prognostic in metastatic breast cancer (MBC). We tested whether the EpCAM based capture system (CellSearch®) is effective in patients with triple negative (TN) MBC, and whether CTC-apoptosis and clustering enhances the prognostic role of CTC.
Experimental Design
CTC enumeration and apoptosis was determined using the CXC CellSearch® kit at baseline and days 15 and 29 in blood drawn from TN MBC patients who participated in a prospective randomized phase II trial of nanoparticle albumin-bound paclitaxel (nab-PAC) with or without tigatuzumab (TIG). Association between levels of CTC and patient outcomes was assessed using logistic regression, Kaplan Meier curves, and Cox proportional hazards modeling.
Results
Nineteen of 52 (36.5%), 14/52 (26.9%), and 13/49 (26.5%) patients who were evaluable had elevated CTC (≥5CTC/7.5 ml WB) at baseline, days 15 and 29, respectively. Patients with elevated vs. not elevated CTC at each time point had worse progression free survival (PFS) (p=0.005, 0.0003, 0.0002, respectively). The odds of clinical benefit response for those who had elevated vs. low CTC at baseline and days 15 and 29 were 0.25 (95% CI: 0.08–0.84, p=0.024), 0.19 (95% CI: 0.05–0.17, p=0.014), and 0.06 (95% CI: 0.01–0.33, p=0.001), respectively. There was no apparent prognostic effect comparing CTC-apoptosis vs. non-apoptosis. Presence of CTC-cluster at day 15, and day 29 was associated with shorter PFS.
Conclusions
CTC were detected using CellSearch® assay in approximately one-third of TN MBC patients. Elevated CTC at baseline and days 15 and 29 were prognostic, and reductions in CTC levels reflected response.
Keywords: Circulating Tumor Cells (CTC), Breast Cancer, triple negative, M-30, apoptosis
INTRODUCTION
Approximately 20% of all breast cancers fail to express either estrogen or progesterone receptors (ER, PgR) or the human epidermal growth factor receptor 2 (HER2)(1). While chemotherapy is effective for these so-called “triple negative” (TN) breast cancers, no targeted therapies are available for this subtype. Preclinical studies have demonstrated activity of tigatuzumab (TIG), a humanized anti-death receptor agonist monoclonal antibody, which triggers apoptosis within basal-like breast cancer(2). The Translational Breast Cancer Research Consortium (TBCRC) conducted a randomized phase 2 trial comparing nanoparticle albumin-bound paclitaxel (nab-PAC) with or without TIG to determine if the latter has evidence of activity in patients with TN metastatic breast cancer (MBC), reported as a separate companion manuscript (Forero A. et al.).
Circulating tumor cells (CTC) are prognostic at baseline and follow-up in patients with metastatic breast cancer (MBC)(3–5). The CellSearch® system (Janssen Diagnostics, LLC, Raritan, NJ) is based on a capture strategy using ferromagnetic particles coated with an antibody to epithelial cell adhesion molecule (anti-EpCAM)(4, 6). Overall, approximately one-half of patients with MBC have ≥5 CTC/7.5 ml whole blood (WB), when evaluated using CellSearch®, at baseline before starting a new therapy, whether first-line or later in their clinical course(4, 6). Subset analyses of prior studies have failed to consistently identify a clinical or biological subgroup of patients with MBC for whom CTC, as enumerated by CellSearch®, are not prognostic(3, 4). However, there has been concern regarding the performance of the CellSearch® assay in detecting CTC in patients with TN breast cancer, since these cancers tend to fall into the “basal” intrinsic subtype which appears to express lower levels of EpCAM than luminal or HER2 positive breast cancers(7).
In addition to enumeration, CTC genotyping and phenotyping might provide additional clinical and biological information. In this regard, early apoptosis, as might be induced by TIG, can be detected with the monoclonal antibody M-30, which is directed against a neo-epitope of cytokeratin 18 disclosed by caspase cleavage(8). We have previously reported detection and semi-quantification of apoptotic CTC in patients with MBC using the CellSearch® platform(9, 10).
We investigated whether CTC are elevated in patients with TN MBC and hypothesized that CTC-enumeration, CTC-apoptosis, and CTC-clusters might be prognostic, predict response to TIG, or provide a surrogate indication of response to either nab-PAC alone or the combination of nab-PAC and TIG. Therefore, we studied CTC in patients with TN MBC who participated in the TBCRC randomized phase II trial (overall results reported by Forero A. et al., in a companion manuscript).
MATERIAL AND METHODS
Study design and objectives
This study was a correlative study of an open label, randomized 2:1 phase II trial of nab-PAC, with or without TIG in patients with measurable TN MBC (overall results reported separately in the companion manuscript by Forero A. et al.).
The study was approved by the Institutional Review Board (IRB) of each participating center and all the enrolled subjects provided written consent prior to entry. Eligibility was limited to patients with TN MBC who were either chemotherapy naïve or who had progressed on prior chemotherapy. Patients were stratified for randomization by three categories: no prior chemotherapy for MBC, prior taxane therapy in the metastatic setting, or no prior taxane therapy in the metastatic setting. Sixty four patients were accrued into the clinical trial and 60 patients received at least 1 cycle of therapy.
The primary objective of this correlative study was to determine the feasibility of detecting CTC prior to initiating therapy (baseline) and their prognostic role in TN MBC. Secondary objectives were designed to investigate the prognostic, predictive, and monitoring roles of CTC levels, CTC-apoptosis, and CTC-clusters at baseline, day 15, and day 29 after initiation of therapy.
Patients staging and follow-up
Details of eligibility, accrual, and conduct of the clinical trial are reported in the companion manuscript (Forero A. et al). Relevant to this report, prior to being enrolled into the study, all patients had measurable disease at baseline, and the primary endpoint of the trial was response as determined according to the Response Evaluation Criteria in Solid Tumor (RECIST version 1.1). Throughout the manuscript, the term clinical response refers either to overall response rate (ORR) defined as complete response (CR) and partial response (PR) or clinical benefit rate (CBR) defined as stable disease (SD), PR, and CR disease for > 4 cycles. Assessment of tumor response was performed every two cycles (every 8 weeks).
Blood draw, CTC enumeration and characterization
Blood draws for CTC enumeration and characterization were scheduled at baseline, day 15, and first follow-up at day 29. At each time point, approximately 10 ml of whole blood (WB) were collected into a 10 cc vacutainer tube that contained a cellular fixative (CellSave Preservative Tubes, Janssen Diagnostics, LLC, Raritan, NJ). Blood specimens were maintained and shipped at room temperature, and processed within a maximum of 96 hours after blood drawing. Once the samples were received and blinded at the processing laboratory, they were re-suspended into 7.5 ml aliquots. CTC enumeration and M-30 (apoptosis) determination was performed using CellSearch® CXC kits (Janssen Diagnostics, LLC) as previously described(9). Enumeration of CTC was determined after staining with DAPI (double stranded DNA), and fluoresceinated anti-cytokeratin and anti-CD45 antibodies, using criteria previously described for CellSearch. The 4th “empty” channel of CellSearch® was used to measure M-30 expression using monoclonal antibody M-30 (Peviva, Stockholm, Sweden) conjugated to phycoerythrin (PE). CTC-apoptosis was further determined by visual inspection for nucleic condensation and/or fragmentation, as well as granular cytokeratin. As previously reported(9), as a positive control for the apoptosis marker for each run, cultured human breast cancer apoptotic MCF-7 cells, were generated by culturing for seven days in RPMI 1640 media and therefore inducing overgrowth and apoptosis. The supernatant containing floating and loosely adherent cells was then fixed in 0.3% paraformaldehyde, and spiked into 7.5 ml human WB from healthy donors, processed and analyzed with CellSearch®. MCF-7 cell line was purchased through ATCC. CTC-apoptosis was defined as any M-30 staining and/or visual evidence of apoptosis (Supplementary Table 1). A specimen was considered to be positive for CTC-apoptosis if 25% or more of the CTC met these criteria.
CTC-clusters were defined as a group of CTC containing three or more distinct nuclei, and with contiguous cytoplasm membranes, as previously described (Supplementary Figure 1)(11).
CTC-enumeration, CTC-clusters, and CTC-M-30 were determined by two reviewers (CP, MM). CTC-visual apoptosis was determined independently by two reviewers (CP, KA). The results generated by each operator were then compared, and discordant results were reconciled by joint readings.
Healthy donors and sample collection
Blood for CTC-apoptosis positive controls was drawn from healthy volunteers who gave their informed consent approved by the University of Michigan IRB.
Statistical Analysis
As per the convention used by Smerage et al.(4), we designated 3 patient subgroups according to baseline and subsequent CTC level: Group A = patients with CTC<5/7.5 ml WB at baseline; Group B day 15 (B15) and Group B day 29 (B29) = patients with ≥5CTC/7.5 ml WB at baseline which were reduced to <5CTC/7.5 ml WB at day 15 (B15) or day 29 (B29), respectively; Group C day 15 (B15) and Group C day 29 (C29) = patients with ≥5CTC/7.5 ml at baseline which persisted as ≥5CTC/7.5 ml at day 15 (C15) or day 29 (C29), respectively. The analysis primarily consisted of comparing group A to groups B and C. CTC enumeration was tabulated for all patients and by treatment arm at baseline, day 15, and day 29. Comparison of the proportion of those in group A versus groups B and C between treatment arms was assessed using chi-square tests of independence or Fisher’s exact tests. Associations between levels of CTC, CTC-apoptosis, and CTC-clusters at baseline, day 15, and day 29 and ORR and CBR were assessed using chi-square or Fisher’s exact tests. Odds ratios were computed with 95% confidence intervals and corresponding p-values for ORR and CBR. Associations between level of CTC, CTC-apoptosis, and CTC-clusters at baseline, day 15 and day 29 and PFS were assessed with Kaplan Meier curves and log-rank tests. PFS was defined from the time of first treatment to the time of disease recurrence or the last follow up. Patients who had no recurrence were considered as right censored. For associations at day 29, benchmark analysis was used for the patients who had not yet progressed by that time and had CTC information. Hazard ratios, 95% confidence intervals and the corresponding p-values were computed with Cox proportional hazards models. No covariates were entered into any of the models due to the sample size. Multiple comparisons were not explicitly controlled for due to the small sample size and exploratory nature of the analysis.
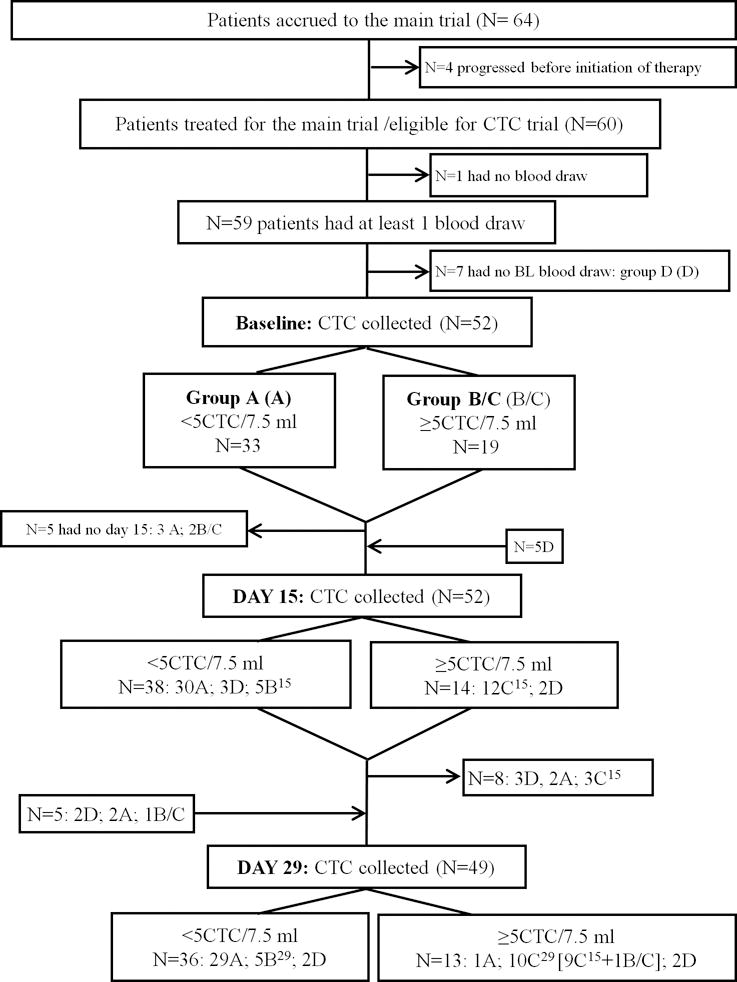
We report this study according to the REMARK guidelines(12) (see Figure 1).
Figure 1.
RESULTS
Patient enrollment
Only 60 of the 64 enrolled patients received any treatment. Of these 60, blood specimens were not drawn in one patient, and thus 59 patients were enrolled in this correlative study and had at least one blood draw within the trial (Figure 1). Overall demographic details are provided in a separate report of the main therapeutic trial results (Forero A. et al). However, demographics are briefly outlined as follows: the median age was 51 (range, 32 to 72), and 51 (range, 34 to 75) years, 33% and 32% had no prior chemotherapy in the metastatic setting, median number of prior therapy regimens was 2 (range, 0–5) and 1(range, 0–4), in the TIG/nab-PAC and in nab-PAC arm, respectively.
CTC-enumeration
Incidence
Other investigators have suggested that basal-like, TN breast cancer cells do not express sufficient EpCAM to be captured and enumerated with anti-EpCAM based systems, such as CellSearch®(7). In this trial that specifically addressed TN MBC, CTC were drawn in 52, 52, and 49 patients at baseline, day 15, and day 29, respectively. Seven patients did not have a blood draw at baseline (Figure 1, Group D). However, they had blood drawn at least at one other time point. At baseline, CTC were ≥5 CTC/7.5 ml WB in 19 (36.5%) patients (Table 1A; Figure 1). Thus, 63.5% of patients did not have elevated CTC by the criteria of the study at baseline (Group A), while 36.5% had ≥5 CTC/7.5 ml (Figure 1; Group B/C).
Table 1.
Incidence of CTC, CTC apoptosis, and CTC clusters.
| A. Incidence of CTC | ||||||
|---|---|---|---|---|---|---|
| CTC/7.5ml WB | ≥5 CTC/7.5 ml WB By Randomized Arm | |||||
| ≥1 | ≥3 | ≥5 | nab-PAC | nab-PAC + TIG | p-value | |
| Baseline | 61.5% (32/52) | 50.0% (26/52) | 36.5% (19/52) | 31.6% (6119) | 39.4% (13/33) | 0.57 |
| Day 15 | 42 .3% (22/52) | 32.7% (17/52) | 26.9% (14/52) | 15.8 (3119) | 33 .3% (11/33) | 0.17 |
| Day 29 | 38.8% (19/49) | 28.6% (14/49) | 26.5% (13/49) | 22.2% (4118) | 290% (9/31) | 0.74 |
| B. Incidence of CTC-apoptosis and CTC-clusters | |||||
|---|---|---|---|---|---|
| CTC-Apoptosisa | CTC-Clustersb | ||||
| Baseline | Day 15 | Day 29 | Baseline | Day 15 | Day 29 |
| 5/19 (26.3%) | 7/14 (50.0%) | 8/13 (61.5%) | 8/32 (25.0%) | 4/22 (18.2%) | 7/19 (36.8%) |
| C. Apoptosis according to single vs. clustered CTC at any time during the trial | |||
|---|---|---|---|
| Single CTC | CTC-cluster | ||
| # of single CTC cells | % single cell positive for apoptosis | # of CTC in the cluster | % CTC cluster with 1 cell positive for apoptosis |
| 8393 | 20% | 943 | 0.4% |
| D. Apoptosis according to single vs. clustered CTC at baseline, day 15, and day 29 | |||
|---|---|---|---|
| Baseline | Day 15 | Day 29 | |
| Single cell | 516/2678 (19%) |
363/1505 (24%) |
795/4210 (19%) |
| Cluster | 1/16 1 (0.6%) |
0/283 (0%) |
3/499 (0.6%) |
Of those ≥ 5CTC/7.5 ml WB;
Of those with ≥ 1 CTC/7.5 1111 WE.
A cross-sectional analysis at each time point was performed. For each separate time point, all patients who had a blood draw were included, regardless of whether they had prior blood draws. At day 15 and day 29, 14/52 (26.9%) and 13/49 (26.5%) patients had elevated (≥5 CTC/7.5 ml WB), respectively. Note that while these patient groups overlap considerably, they are not identical, since 5 patients who had blood draws at baseline did not have them at day 15, while a separate 5 patients who did not have baseline specimens, did have them drawn at day 15. Likewise, 5 patients who had blood drawn at baseline, and 3 patients who did not have blood drawn at baseline did not have specimens drawn at day 29. Further, 3 patients who had blood drawn at day 29 and baseline did not have blood drawn at day 15 and 2 patients who did not have blood drawn at baseline did have blood drawn at day 29.
The patients that had <5 CTC at baseline (Group A) were scheduled to have further blood draws at day 15 and day 29. In particular at baseline, 33 patients had low CTC level (<5 CTC). At day 15, 3 of these patients did not have blood draw. The remaining 30 patients continued to have low CTC level at day 15. Two of the three patients that did not have blood drawn at day 15, had blood drawn at day 29 with low CTC levels (<5 CTC). Only one patient converted CTC levels from low (<5 CTC) at baseline to high (≥5 CTC) at day 29. The rest of the patients who had blood drawn at subsequent time points continued to have low CTC level (<5) throughout (Figure 1).
For serial analyses for “CTC-response,” 19 patients had elevated CTC (Group B/C) at baseline. At day 15, blood specimens were not drawn on two patients. Of the remaining 17 patients, CTC levels declined to <5CTC/7.5 ml in 5 (29%) (B15), while CTC remained elevated in the other 12 (C15) (Supplementary Figure 2). At day 29, blood was not obtained from 4 patients who had elevated CTC at baseline. Of the remaining 15 patients who had elevated CTC at baseline and had a blood specimen at day 29, 10 (67%) continued to have elevated CTC (C29) (Supplementary Figure 2). Thus, approximately one-third of patients experienced a “CTC-response” to therapy.
The incidence of elevated CTC (≥5 CTC/7.5 ml WB) at baseline was similar for patients in the nab-PAC and nab-PAC + TIG arms (31.6% vs. 39.4%, p=0.57). There was no significant difference in CTC enumeration between the arms at day 15 or day 29 (Table 1).
Prognosis based on CTC enumeration
In the overall clinical trial, TIG had no beneficial effect (see Forero A. et al., companion manuscript). However, five patients in the combination arm had long progression free survival (PFS) (11.1, 15.3, 22.4, 26.0, and 34.2 months). All of these patients began the trial with <5 CTC and their CTC levels remained low through day 15 and 29. Addionally, there was one patient with long term PFS in the control arm (1004 days) who also maintained <5 CTC throughout the first 29 days. An exploratory analysis failed to demonstrate any difference in outcomes in the TIG-treated patients versus the control group according to baseline CTC levels (Supplementary Figure 3). Thus, for the remaining analyses, all patients were considered regardless of the arm to which they were assigned.
Numerous reports have demonstrated that CTC, as enumerated by CellSearch®, are prognostic in MBC(3, 4, 6). However, none of these has prospectively addressed the specific prognostic role of CTC in patients with TN MBC. At baseline, patients with elevated CTC (≥5CTC/7.5 ml WB) had a significantly worse PFS than patients with <5CTC/7.5 ml WB. Median PFS for patients with elevated vs. not elevated CTC at baseline = 3.6 vs. 1.9 months (p=0.005) (Figure 2A).
Figure 2.
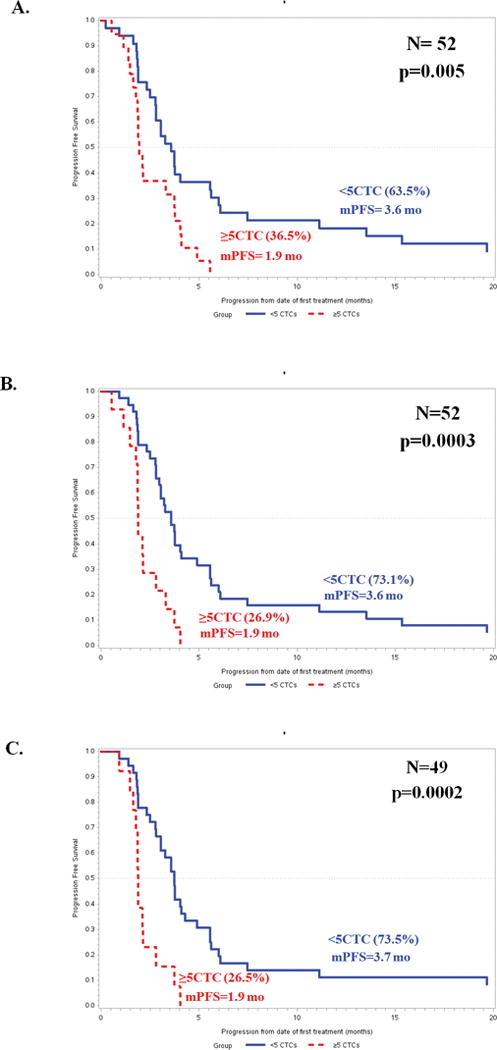
Likewise, failure to clear CTC by day 15 and by day 29 was also associated with worse outcomes: median PFS elevated vs. not elevated = 3.6 vs. 1.9 months at day 15 and 3.7 vs. 1.9 months at day 29 (p = 0.0003 and p= 0.0002, respectively) (Figure 2B and 2C). The hazard ratios (95% confidence intervals) for progression for elevated (≥5 CTC/7.5 ml WB) compared to non-elevated CTC at baseline, day 15, and day 29 were 2.3 (1.3–4.4; p=0.007), 3.2 (1.6–6.4; p=0.0007), and 3.5 (1.7–7.2; p=0.0005), respectively (Figure 2A–C).
Response rate according to CTC enumeration
Overall, the response rate in the clinical trial was 26%, and the CBR was 45%, with no apparent difference in the assigned arms (see Forero A. et al., companion manuscript). We correlated CTC levels with RECIST ORR and CBR (Figure 3). Of the 19 patients with ≥5CTC at baseline, only four (21.1%) had a CR or PR, while 12 of the 33 (36.4%) who did not have elevated CTC experienced a response (p=0.25). Likewise, at days 15 and 29, 3/14 (21.4%), and 1/13(7.7%) patients who had elevated CTC had CR or PR, compared to 14/38 (36.8%) and 14/36 (38.9%) of patients without elevated CTC who had objective responses (p=0.34, 0.043, respectively). Although not statistically different, the odds of overall response for those who had elevated vs. low CTC at baseline and days 15 and 29 were 0.47 (95% CI 0.13–1.73, p=0.25), 0.47 (95% CI 0.11–1.97, p=0.30), and 0.13 (95% CI 0.02–1.12, p=0.064).
Figure 3.
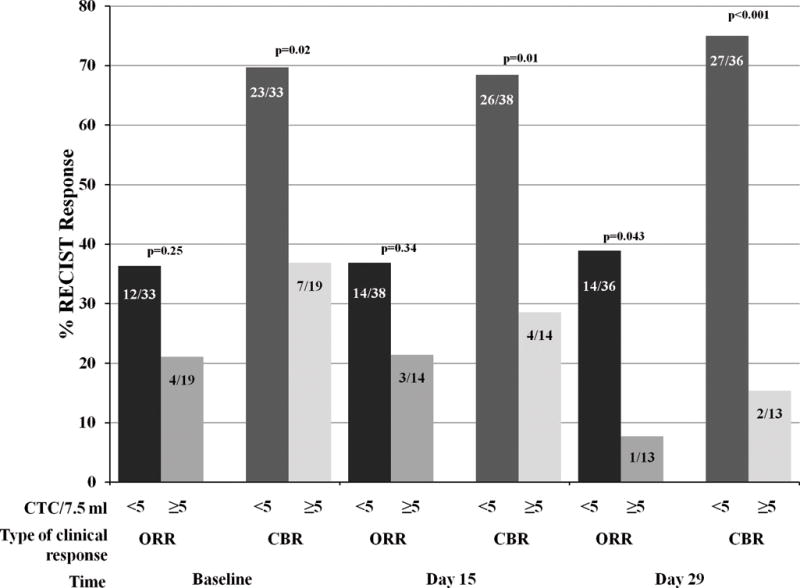
Since most TN MBC are rapidly growing, one would expect stable disease to be a function of therapeutic benefit rather than indolent disease. To increase the power of our exploratory analysis, we correlated CTC-response with CBR (Figure 3). Of the patients with elevated CTC at baseline, only 7/19 (36.8%) had clinical benefit, while 23/33 (69.7%) who did not have elevated CTC experienced clinical benefit. Likewise, at day 15, clinical benefit was observed in 4/14 (28.6%) compared to 26/38 (68.4%) patients without elevated CTC. The results at day 29 were similar (clinical benefit = 2/13 (15.4%) vs. 27/36 (75.0 %) for elevated vs. non-elevated CTC). The odds of clinical benefit response for those who had elevated vs. low CTC at baseline and days 15 and 29 were 0.25 (95% CI: 0.08–0.84, p=0.024), 0.19 (95% CI: 0.05–0.17, p=0.014), and 0.06 (95% CI: 0.01–0.33, p=0.001), respectively. These results were statistically significant.
Correlation of CTC-response with clinical response
For those patients with elevated CTC at baseline (Supplementary Figure 2), we have designated reduction of CTC to <5/7.5 ml WB as a “CTC response.” We performed an exploratory analysis to determine if CTC response correlated with ORR and CBR (Supplementary Figure 4). Of the 19 patients in Groups B/C at baseline, five had a decline to <5CTC/7.5 ml WB (B15), while 12 did not (C15) (blood was not obtained at day 15 and 29 in 1 patient and at day 15 for another patient) (Supplementary Figure 2). The ORR at day 15 in B15 was 40% (2/5 patients) compared to 17% (2/12 patients) in C15 (Figure 4, p=0.54). CBR was 80% (4/5 patients) for Group B, but only 25% (3/12 patients) in Group C (p=0.10). Power was quite limited to effectively determine if this difference in response rates between those in groups B and C was statistically significant or due to play of chance. However, there was a trend for higher response rates for those who cleared their CTC by day 15. Similar results were observed at day 29 (Supplementary Figure 4).
Taken together, these data regarding CTC and ORR and CBR suggest that patients with elevated CTC at baseline or early follow-up are less likely to respond to chemotherapy.
CTC-apoptosis
Incidence of CTC-apoptosis
At baseline, five of the 19 (26.3%) patients who had ≥5 CTC/7.5 ml WB were considered to be positive for CTC-apoptosis (defined as having ≥25% of the CTC positive for apoptosis) (Table 1B). At day 15 and 29, 7/14 (50.0%) and 8/13 (61.5%) patients were considered to have CTC-apoptosis.
Prognostic role of CTC-apoptosis
Median PFS was not significantly different for those with or without CTC-apoptosis at baseline (1.9 vs. 2.1 months, respectively). Although power was limited there was no suggestion that the presence or absence of CTC-apoptosis at any time point predicted PFS (Table 2).
Table 2.
Association of CTC enumeration, apoptosis, and clusters with outcomes.
| Baseline | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTC-Enumerationa | CTC-Apoptosisb | CTC-Clustersc | ||||||||||||||||
| ORR | p | CBR | p | PFS | p | ORR | p | CBR | p | PFS | p | ORR | p | CBR | p | PFS | p | |
| No | 12/33 (36.4) |
0.25 | 23/33 (69.7) |
0.02 1 | 3.6 | 0.005 | 3/14 (21.4) |
1.0 | 6/14 (42.9) |
0.60 | 1.9 | 0.47 | 5/24 (20.8) |
1.0 | 11124 (45.8) |
1.0 | 2.3 | 0.34 |
| Yes | 4/19 (21.1) |
7/19 (36.8) |
1.9 | 1/5 (20.0) |
1/5 (20.0) |
2.1 | 1/8 (12.5) |
3/8 (37.5) |
2.0 | |||||||||
| Day 15 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTC-Enumerationa | CTC-Apoptosisb | CTC-Clustersc | ||||||||||||||||
| ORR | P | CBR | P | PFS | P | ORR | P | CBR | P | PFS | P | ORR | P | CBR | P | PFS | P | |
| No | 14/3 8 (36.8) |
0.34 | 26/38 (684) |
0.010 | 3.6 | 0.0003 | 2/7 (28.6) |
1.0 | 2/7 (28.6) |
1.0 | 1.8 | 0.11 | 5/18 (27 .8) |
0.54 | 7/18 (3 8.9) |
0.26 | 2.5 | 0.028 |
| Yes | 3/14 (21.4) |
4/14 (28.6) |
1.9 | 1/7 (14.3) |
2/7 (28.6) |
2.1 | 0/4 (0) |
0/4 (0) |
1.8 | |||||||||
| Day 29 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTC-Enumerationa | CTC-Apoptosisb | CTC-Clustersc | ||||||||||||||||
| ORR | P | CBR | P | PFS | P | ORR | P | CBR | P | PFS | P | ORR | P | CBR | P | PFS | P | |
| No | 14/36 (38.9) |
0.043 | 27/36 (75.0) |
0.0002 | 3.7 | 0.0002 | 0/5 (0) |
1.0 | 115 (20.0) |
1.0 | 1.8 | 0.73 | 4112 (33.3) |
0.25 | 6112 (50.0) |
0.04 4 | 2.7 | 0.009 |
| Yes. | 1/13 (7.7) |
2/13 (15.4) |
1.9 | 1/8 (12.5) |
1/8 (12.5) |
2.0 | 0/7 (0) |
0/7 (0) |
1.8 | |||||||||
For CTC-enumeration No<5 CTC/7.5 ml, Yes≥5CTC/7.5 ml;
For CTC-Apoptosis No ≥5CTC/7.5 ml with <25% apoptic, Yes≥5CTC/7.5 ml with 2>25% apoptotic;
CTC-cluster No ≥1 CTC/7.5 ml with no cluster; Yes ≥1 CTC with at least 1 cluster; ORR = overall response rate, sum of complete response (CR) plus partial response (PR); CBR=clinical benefit rate stable disease (SD) plus PR, and CR; PFS=Progression free survival; p=p value. (Note values in parentheses are numbers expressed in %; PFS is median time to progression in months.
CTC-apoptosis and response
The ORR and CBR were explored according to CTC-apoptosis. ORR was 20% (1/5 patients), 14%, (1/7 patients), and 13% (1/8 patients) at baseline, day 15, and day 29, respectively for patients who had CTC-apoptosis compared to 21% (3/14 patients), 29% (2/7 patients), and 0% (0/5 patients) for those who had CTC but did not meet the criteria for CTC-apoptosis (Table 2, Supplementary Figure 5).
Likewise, CBR was 20% (1/5), 29% (2/7) and 13% (1/8) for patients with CTC-apoptosis at baseline, day 15, and day 29, respectively, compared to 43% (6/14), 29% (2/7), and 20% (1/5) for patients who had elevated CTC but did not have CTC-apoptosis.
CTC-clusters
Incidence of CTC-clusters
We hypothesized that CTC in a cluster might have prognostic implications that differ from a single cell, even though both are counted only as one CTC by the CellSearch® clinical algorithm. At baseline, eight (25.0%) of the 32 patients with any CTC had one or more clusters (Table 1B). Of the 19 patients who had ≥5 CTC/7.5 ml WB, 7 (36.8%) had CTC-clusters. In contrast of the 13 patients with 1–4 CTC as per CellSearch® criteria, 1 (7.7% %) had CTC-clusters (p=0.10).
Prognostic role of CTC-clusters
At baseline, median PFS was around 2.0 months for those with or without CTC-clusters (Table 2). There was no difference in PFS between those who had presence of CTC clusters at baseline and those who did not, p=0.34. Although there were smaller sample sizes, there was a significant difference in PFS between those with clusters at day 15 and day 29 than those without (p=0.028, 0.009 respectively), such that those with clusters had worse PFS.
CTC-clusters and response
We also performed an exploratory analysis of ORR and CBR according to CTC-clusters. ORR was 13% (1/8), 0% (0/4), and 0% (0/7) for patients who had CTC-clusters compared to 21% (5/24), 28% (5/18), and 33% (4/12) for those who had CTC but did not meet the criteria for CTC-clusters at baseline, day 15 and day 29, respectively (Table 2).
Likewise, CBR was 38% (3/8), 0% (0/4) and 0% (0/7) for patients with CTC-clusters at baseline, day 15, and day 29, respectively, compared to 46% (11/24), 39% (7/18) and 50% (6/12) for patients who had elevated CTC but did not have CTC-clusters.
CTC-clusters and Apoptosis
A total of 194 CTC-clusters, containing 943 CTC, were detected among the 8 patients at any time within the trial. Only 4 of the cells (0.4%) found in clusters were apoptotic, as determined either due to M-30 staining and/or visual evidence of apoptosis (Table C). In contrast, 1674 of 8393 single CTC (20%) that were identified in all the patients at any time within the trial were found to be apoptotic (Table 1C). In particular, CTC-apoptosis was 19% (516/2678), 24%, (363/1505), and 19% (795/4210) at baseline, day 15, and day 29, respectively for single cells compared to 0.6% (1/161), 0% (0/283), and 0.6% (3/499) for cells within clusters (Table 1D).
DISCUSSION
In this correlative study to TBCRC study 019, we have confirmed that the performance of a CTC assay system based on anti-EpCAM immunocapture (CellSearch®) is feasible in patients with TN MBC. Approximately one-third of such patients enrolled in this clinical trial had ≥5 CTC/7.5 ml WB at baseline. Furthermore, baseline elevated CTC were associated with a worse prognosis (PFS), and failure to reduce CTC to <5/7.5 ml WB by first follow-up was highly suggestive of resistance to chemotherapy, in this case nab-PAC.
These data are similar to those previously reported for patients with MBC regardless of biologic, or intrinsic, subtype(3, 4, 6). In this regard, a recently published pooled analysis of CTC results from 54 European centers demonstrated that 44% of 746 patients with TN MBC had ≥5 CTC/7.5 ml WB(3). Our prospective study further confirms that the performance characteristics of the CellSearch® assay are similar in patients with TN MBC to those in patients with hormone receptor or HER2 positive breast cancer. Taken together, although the sensitivity of CellSearch® may be slightly lower in TN vs. Non-TN MBC, these data refute the claim of other authors who have suggested that an EpCAM-based assay might not be applicable to patients with TN or “basal”-like breast cancers due to low expression of this marker(7).
Our results are also consistent with those of a prospective, randomized clinical trial conducted by SWOG (S0500), which demonstrated that patients who have elevated CTC at baseline before starting a new, first-line chemotherapy for MBC, and who fail to reduce them to <5/7.5 ml WB by first follow-up (Group C), appear to be relatively, if not absolutely, resistant to the chemotherapy regimen they received. Further, in S0500, the very short overall survival of patients in Group C (median OS 13 months) suggested that their cancers are more likely to be resistant to most other types of chemotherapy that might be applied subsequently(4). In TBCRC 019, all patients had TN MBC and were stratified to 1st or later line of chemotherapy. Therefore, their prognosis is even worse than the more general population who enrolled in SWOG S0500 or those in the pooled analysis. Indeed, PFS for those with elevated CTC at baseline, or at 2 or 4 weeks after starting therapy, was < 2 months. Overall survival was not a measured endpoint in this trial, and is unavailable.
In addition, the secondary objectives were to determine the prognostic, predictive, and monitoring roles of CTC levels, CTC-apoptosis, and CTC-clusters at baseline, day 15, and day 29 after initiation of therapy. In particular, we investigated whether CTC-apoptosis was predictive of benefit of the addition of the anti-death receptor agent, TIG. Unfortunately, in the parent trial, there was no discernable difference in any endpoint between those who did or did not receive TIG. Although CTC levels were prognostic overall, there was no evidence in an exploratory analysis that patients who either did or did not have elevated CTC received any benefit from TIG.
We did not detect a statistically significant association between CTC enumeration and ORR at baseline or day 15. However, the statistical power to do so was quite poor due to a very low number of true responses. Taken together, these data suggest that patients with elevated CTC at baseline or early follow-up are less likely to respond to chemotherapy and that CTC-response could serve as a surrogate for clinical response in future phase II trials of new agents.
There was an association between CTC at baseline and CBR, but again power was limited to determine if the visual observation of this association at subsequent time points was statistically significant or due to play of chance. Likewise, there was a visual, but not statistically significant association between CTC-response and clinical response (ORR and CBR) at days 15 and 29.
Taken together, these data suggest that patients with elevated CTC at baseline or early follow-up are less likely to respond to chemotherapy and that CTC-response could serve as a surrogate for clinical response in future phase II trials of new agents.
Since TIG appears to function by activation of the death receptor, we had hypothesized that the early appearance of CTC-apoptosis might serve as an early indication of TIG activity. In this study, we failed to show that the presence or absence of CTC-apoptosis at any time point predicted PFS, ORR, or CBR, either in association with TIG or overall.
Published data regarding CTC-apoptosis are conflicting. Hou et al(13) reported that CTC apoptosis (assigned by nuclear morphology) at baseline in patients with metastatic small cell lung cancer (SCLC) was associated with worse PFS an OS compared to their absence, a finding similar to that of Smerage et al (9) in MBC patients. In contrast, Rossi et al (10) have suggested that appearance of CTC-apoptosis in 8 patients with MBC was associated with response to chemotherapy. The issue of CTC-apoptosis and therapy response requires further investigation. The failure to show that the presence or absence of CTC-apoptosis at any time point predicted PFS, ORR, or CBR, either in association with TIG or overall in our trial is not surprising, since TIG was inactive in the overall trial.
CTC-clusters have been previously reported in the blood of patients with lung, renal, prostate cancer and recently breast cancer(11, 13–16). In our study, at baseline, 25% of patients of the 32 patients with ≥1 CTC/7.5 ml had one or more clusters. Although there was no difference in PFS between those who had CTC-clusters at baseline and those who did not, we detected a significant difference in PFS between those with residual clusters at day 15 and day 29 compared to those with CTC but without clusters. Hou et al. have shown that the presence of CTC-clusters was significantly associated with worse prognosis in SCLC (13). Likewise, Aceto et al.(16) have recently reported that the presence of CTC-clusters isolated by a novel microfluidic device in blood from patients with MBC and prostate cancer was associated with shorter PFS(16).
Of interest, Hou et al reported that in their study of CTC in patients with SCLC, no CTC within a cluster exhibited apoptotic morphology. Although the incidence of CTC-clusters in our population of TN MBC was lower than that observed by Hou et al in SCLC, we similarly observed few apoptotic CTC (by M30 expression and visual inspection) within clusters (0.4%), while single CTC were much more likely to appear apoptotic (20%). Further, during treatment with NabPAC, although the relative percent of patients with elevated CTC declined (reflecting a CTC response), approximately one-fifth of the single cell CTC remained apoptotic, while the incidence of observed apoptosis in CTC-clusters remained less than 1%. The numbers of patients in these categories were too small to perform meaningful evaluation of outcomes according to these evaluations, but these data suggest that clustering of CTC may confer relative resistance to cytotoxic drugs.
A strength of this correlative study is that all patients were prospectively enrolled into a phase II clinical trial with controlled eligibility criteria, prescribed treatments, and high quality outcomes assessment. However, a weakness of this study is the small sample size, which limits our CTC-based subgroup analyses, and the lack of activity of the investigational agent, TIG.
In summary, the results of this correlative trial validate that CTC are indeed elevated and are prognostic in TN MBC patients receiving chemotherapy. CTC-apoptosis needs further investigation with a larger sample size. In addition, quantification of CTC-clusters might add additional prognostic information to simple CTC-enumeration.
Supplementary Material
Statement of translational relevance.
Circulating Tumor Cells (CTC) are associated with worse prognosis in metastatic breast cancer (MBC) patients. However, the role of CTC is unclear in triple negative breast cancer due to low expression of epithelial cell adhesion molecule (EpCAM) compared to other subtypes of breast cancers. Using CellSearch®, we have demonstrated that CTC are prognostic in the subgroup of patients with triple negative breast cancer, further substantiating the clinical role of monitoring CTC. Exploratory analyses suggest that evaluation of CTC-clusters may provide further clinical and biological insights into the mechanisms of the metastatic process.
Acknowledgments
We are grateful to all the patients who generously volunteered to participate in the study. We thank the TBCRC investigators, research nurses, & study coordinators for their efforts on the behalf of the patients.
Funding: Supported with funds provided to the TBCRC by its three foundation partners: The AVON Foundation, The Breast Cancer Research Foundation, and Susan G. Komen for the Cure. In addition this work was supported by Veridex/Janssen, LLC, Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale™ (DFH), and by a studentship from the Italian Foundation for Cancer Research (FIRC) - Milan Italy and the Associazione “Sandro Pitigliani”-Prato Italy (CP), Susan G. Komen for the cure and the Triple Negative Breast Cancer Foundation for the funding of the Promise Grant.
Abbreviations
- CBR
clinical benefit rate
- CR
complete response
- CTC
Circulating Tumor Cells
- EpCAM
epithelial cell adhesion molecule
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- IRB
institutional Review Board
- MBC
metastatic breast cancer
- nab-PAC
nanoparticle albumin-bound paclitaxel
- ORR
overall response rate
- PE
phycoerythrin
- PD
progression disease
- PFS
progression free survival
- PgR
progesterone receptor
- PR
partial response
- RECIST
Response Evaluation Criteria In Solid Tumor
- SCLC
small cell lung cancer
- SD
stable disease
- TBCRC
Translational Breast Cancer Research Consortium
- TIG
tigatuzumab
- TN
triple negative
- WB
whole blood
Footnotes
Conflict of interest: D.F.H. received support from Veridex/Janssen, LLC, the commercial vendor of CellSearch®, to support clinical and laboratory research support. Dr. Hayes is the inventor named on a patent regarding CTC-ETI held by the University of Michigan and licensed to Janssen Diagnostics, LLC. The following authors have the following conflict of interest to disclose: T.A.T received research support from Janssen, but not the diagnostic arm. With the past 24 months, M.C.L. has received CTC related research funding from Celgene, ClearBridge, Eisai, and Janssen/Veridex. All funding went to the institution with no personal compensation. She has no other relevant relationships to disclose. A. F-T. does not have any conflict of interest except research support from Daiichi Sankyo paid to his University to support the trial.
The remaining authors have no conflict of interest to disclose.
Presentation / Publications: Data from this work have been partially reported at the San Antonio Breast Cancer Symposium 2013 (poster # P1-04-01) for which Dr. Paoletti was awarded an AACR Scholar-In-Training Award.
References
- 1.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchsbaum DJ, Zhou T, Grizzle WE, Oliver PG, Hammond CJ, Zhang S, et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res. 2003;9:3731–41. [PubMed] [Google Scholar]
- 3.Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–14. doi: 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- 4.Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, et al. Circulating Tumor Cells and Response to Chemotherapy in Metastatic Breast Cancer: SWOG S0500. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.56.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paoletti C, Smerage J, Hayes DF. Circulating tumor cells as a marker of prognosis. Princip Prac Oncol. 2012;26:1–8. [Google Scholar]
- 6.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 7.Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009;101:61–6. doi: 10.1093/jnci/djn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567–72. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Smerage JB, Budd GT, Doyle GV, Brown M, Paoletti C, Muniz M, et al. Monitoring apoptosis and Bcl-2 on circulating tumor cells in patients with metastatic breast cancer. Mol Oncol. 2013;7:680–92. doi: 10.1016/j.molonc.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi E, Basso U, Celadin R, Zilio F, Pucciarelli S, Aieta M, et al. M30 neoepitope expression in epithelial cancer: quantification of apoptosis in circulating tumor cells by CellSearch analysis. Clin Cancer Res. 2010;16:5233–43. doi: 10.1158/1078-0432.CCR-10-1449. [DOI] [PubMed] [Google Scholar]
- 11.Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178:989–96. doi: 10.1016/j.ajpath.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–4. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 13.Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–32. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 14.Kats-Ugurlu G, Roodink I, de Weijert M, Tiemessen D, Maass C, Verrijp K, et al. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J Pathol. 2009;219:287–93. doi: 10.1002/path.2613. [DOI] [PubMed] [Google Scholar]
- 15.Molnar B, Ladanyi A, Tanko L, Sreter L, Tulassay Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res. 2001;7:4080–5. [PubMed] [Google Scholar]
- 16.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–22. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


