Abstract
Purpose of Review
Arsenic, a known carcinogen and developmental toxicant, is a major threat to global health. While the contribution of arsenic exposure to chronic diseases and adverse pregnancy and birth outcomes is recognized, its ability to impair critical functions of humoral and cell-mediated immunity—including the specific mechanisms in humans—is not well understood. Arsenic has been shown to increase risk of infectious diseases that have significant health implications during pregnancy and early life. Here, we review the latest research on the mechanisms of arsenic-related immune response alterations that could underlie arsenic-associated increased risk of infection during the vulnerable periods of pregnancy and early life.
Recent Findings
The latest evidence points to alteration of antibody production and transplacental transfer as well as failure of T helper cells to produce IL-2 and proliferate.
Summary
Critical areas for future research include the effects of arsenic exposure during pregnancy and early life on immune responses to natural infection and the immunogenicity and efficacy of vaccines.
Keywords: Arsenic exposure, Immune response, Infection, Immunotoxicity, Pregnant women, Newborns, Children
Introduction
Arsenic is a widely distributed, naturally occurring metalloid in the Earth’s crust [1]. Arsenic is also readily mobilized from bedrock and soil into groundwater, making it a ubiquitous drinking water contaminant [1]. Currently, the World Health Organization’s (WHO) drinking water standard for arsenic is 10 μg/L, a concentration that is exceeded in the drinking water of an estimated 200 million people worldwide [2]. Arsenic is a known human carcinogen, causing cancer at many body sites [3–5]. Arsenic also causes adverse effects in many organ systems, including the immune system. In addition to arsenic’s carcinogenic and immunotoxic potential, it is also a known developmental toxicant [] that readily crosses the placenta [6]. This is important because two of the most susceptible life stages that can be critically affected by arsenic exposure are pregnancy and fetal development [7]. Pregnancy induces considerable maternal strain and causes many changes in the body, particularly with respect to the maternal immune system and metabolism [8, 9]. The developing fetus is particularly vulnerable to toxic insult because of its rapid rate of in utero development. A growing body of evidence suggests that arsenic’s immunotoxic potential can occur through impairment of both cell-mediated [10–17, 18•] and humoral [19–24] immunity. There is mounting evidence of an increased risk of infections that have significant health consequences during pregnancy and early life [25–27], including respiratory infections [28, 29••], gastrointestinal infections [26, 28, 30, 31••], and tuberculosis [32]. Despite the knowledge advancements from human studies of arsenic exposure and self-reported infections, specific mechanisms of arsenic’s adverse immunological effects during pregnancy and fetal and newborn development remain poorly understood. In this systematic review, we will discuss what is known about the effects of arsenic exposure on both humoral immunity and cell-mediated immunity in mother and fetus. We will then discuss potential approaches for future research to close these knowledge gaps.
Methodology
In this systematic review, we employed the following search strategy. Our research question encompassed three main concepts: (1) immunotoxicity, (2) arsenic, and (3) in utero exposure. We limited our searches to Pubmed and English language publications. All concepts were linked with “AND” statements. For each concept, we identified a number of Medical Subject Heading (MeSH) and keyword terms and searched on all terms at once, linking the terms with “OR” statements. We limited the search to papers published after January 1, 1990. Completion of this search strategy on November 16, 2016 returned 106 papers. Additionally, we excluded all papers related to therapeutic use of arsenic trioxide for treatment of acute myeloid leukemia. As part of the final review of papers that resulted from our search strategy, we also identified and included papers that were missed based on substantive expertise and knowledge of the arsenic and immune response literature.
Arsenic Exposure and Immune Responses
Human Studies of Arsenic Exposure and Humoral Immune Response
Studies demonstrate that arsenic can affect humoral immunity in many important ways (Fig. 1). Humoral immunity is defined as immunity imparted by antibodies and other circulating macromolecules such as complement [33]. Several studies considered the effects of arsenic exposure on antibody production and transplacental transport, specifically in the context of pregnant women and their children (Table 1). In one such study in Bangladesh, maternal arsenic exposure was directly associated with increased total immunoglobulin G (IgG) levels in maternal serum, but not total IgG levels in cord blood [21]. This raises the possibility that arsenic could impair maternal transplacental transport of IgG to the neonate. One possible explanation for this involves increased competition for a limited number of neonatal Fc receptors (FcRn) on syncytiotrophoblast cells in the placenta [34, 35]. These are the receptors responsible for transplacental transport of maternal serum IgG antibodies to the developing fetus. Several past studies have observed that an increase in maternal total IgG can lead to lower transplacental transfer ratios for IgG antibodies specific to anti-tetanus [36], anti-measles [37, 38], and anti-lipopolysaccharide from E. coli [39]. Consistency of arsenic’s effect on total immunoglobulin (Ig) levels has also been observed among non-pregnant adults, who demonstrate measurably elevated serum total IgG as well as total IgA and IgE [20]. Serum complement component 4 (C4) was also found to be elevated in arsenic patients in a 2012 paper by Islam et al.; however, complement-mediated bactericidal ability of these serum samples was lower than that of controls, indicating impairment of the various complement pathways [40].
Fig. 1.
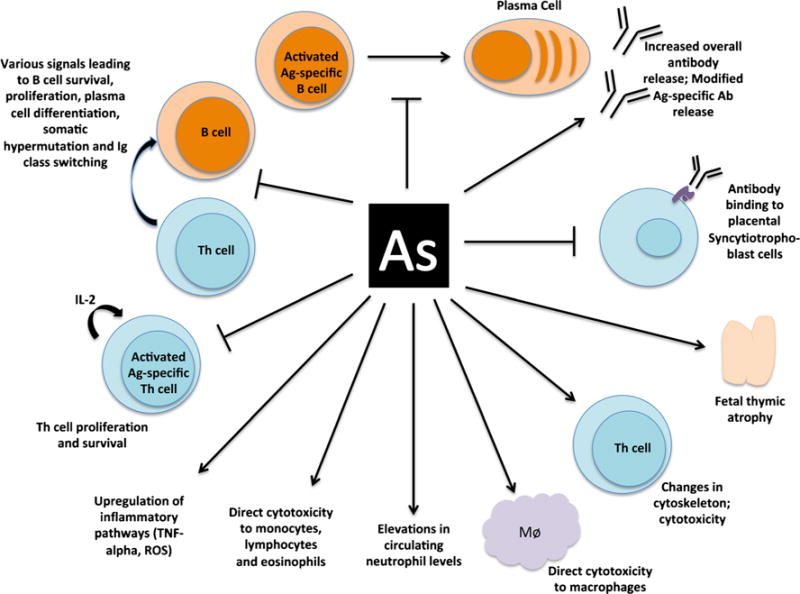
Potential mechanisms of arsenic exposure and alteration of immune responses, including specific functions of humoral and cellular immunity. A line with an arrow at the end indicates “contributes to/leads to/causes.” A line with a perpendicular bar at the end indicates “blocks/inhibits”
Table 1.
Summary of human epidemiologic and in vitro studies of arsenic exposure and altered immune response during pregnancy and/or early life
| Author | Year | Country | Population | Study design | Immune focus | As level | Primary outcomes | Main results |
|---|---|---|---|---|---|---|---|---|
| Heaney et al. | 2015 | Bangladesh | Pregnant women | Nested case-control study | Humoral immunity | Median 61.6 and 65.6 ppb Σurinary arsenic in the non-seroconverting and seroconverting groups, respectively during the first trimester | Authors assessed the urinary arsenic species, HEV-specific serum IgG, and serum cytokines in enrolled women in the first and third trimester as well as 3 months postpartum. | The authors found that the odds ratio of maternal HEV seroconversion was 2.17 per IQR increase in urinary inorganic arsenic plus methylated species (ppb). Serum IL-2 concentration was also found to be positively correlated with increasing maternal urinary arsenic concentration in both seroconverters and non-seroconverters. |
| Ser et al. | 2014 | Bangladesh | Pregnant women | Prospective cohort study in pregnant women | Humoral immunity | 3.1–1356 ppb range in urine | The authors measured total maternal serum IgG as well as cord blood total IgG along with maternal urinary arsenic species. | Increasing arsenic exposure results in a greater concentration of maternal serum IgG, but not cord blood IgG. |
| Kile et al. | 2014 | Bangladesh | Pregnant women | Prospective cohort study in pregnant women | Humoral and cell-mediated immunity | <1–1400 ppb range in drinking water | Authors assessed drinking water arsenic at enrollment along with self-reported symptoms at four visits during pregnancy and after birth. | When analyzing all symptoms together, women in the highest quartile of arsenic exposure were more likely to experience any symptoms than women in the lowest quartile. Notably, women in the second quartile (0.9–2 ppb arsenic) were protected against symptoms (95% CI 0.44–0.88). When looking at individual symptoms alone, women in the highest arsenic exposure quartile were significantly more likely to experience nausea/vomiting and abdominal cramping than women in the lowest quartile. Risk of cold/flu/respiratory infection was the same across quartiles. |
| Saha et al. | 2013 | Bangladesh | Children | Prospective cohort in children 2–5 years of age | Humoral and cell-mediated immunity | Low (mean 6.6 ppb) and high (mean 291.8 ppb) arsenic in urine | The authors measured urinary arsenic species and serum C3 and C4 concentrations. Cholera-specific, diphtheria-specific, tetanus-specific, and measles-specific antibodies were also measured, and the cholera antibody vibriocidal activity was assessed. | Diphtheria and tetanus-specific IgG were higher in the high arsenic-exposed group when compared to the low arsenic-exposed children as well as serum concentrations of C3 and C4. No differences were noted in the vibriocidal activity of the postimmunization antibodies of high-arsenic vs. low-arsenic-exposed children nor a difference between measles-specific igG levels. |
| Farzan et al. | 2013 | USA | Pregnant women and infants | Prospective cohort study in pregnant women in New Hampshire | Humoral and cell-mediated immunity | 0.01–67.5 ppb range in drinking water; 0.45-58.3 ppb range in urine | Relative risk of respiratory or other infection in infants was calculated in relation to a onefold increase in maternal urinary arsenic on a natural logarithmic scale. | Risk of lower respiratory tract infections was significantly positively associated with increasing maternal arsenic exposure. |
| Rahman et al. | 2011 | Bangladesh | Pregnant women and infants | Prospective cohort study in pregnant women and their newborns | Humoral and cell-mediated immunity | 1–1211 ppb range in urine | The authors assessed urinary arsenic levels in pregnant women at GW8 and GW30 along with infant morbidity and mortality by 7-day recall during monthly postnatal follow-up visits. | Risk of lower respiratory tract infection, severe lower respiratory tract infection, and diarrheal illness in the first year of life increased by 69, 54, and 20% when comparing infants in the highest quintile of maternal urinary arsenic with those in the first quintile. Risks for lower respiratory tract infections were more pronounced when based on late gestation maternal urinary arsenic, while risk of diarrheal illness was more pronounced when based on early gestational urinary arsenic. |
| Raquib et al. | 2009 | Bangladesh | Pregnant women and infants | Prospective cohort study in pregnant women and their newborns | Humoral and cell-mediated immunity | 1–2020 ppb range in urine | The authors assessed urinary arsenic levels in pregnant women at GW8 and GW30 along with infant thymic index and lactoferrin and IL-7 in breastmilk at 12 months postpartum. Additionally, the authors looked at maternal diarrheal disease and fever as well as infant acute respiratory infection. | The authors found that increasing maternal urinary arsenic concentration was inversely correlated with infant thymic index and lactoferrin and IL-7 in breastmilk, as well as positively associated with maternal morbidities and acute respiratory illnesses in male infants only. |
| Kile et al. | 2014 | Bangladesh | Infant cord blood | Prospective cohort study in pregnant women | Cell-mediated immunity | <1–510 ppb range in drinking water | Whole cord blood DNA methylation was measured along with drinking water arsenic. | Maternal arsenic exposure was associated with differential cord blood DNA methylation at various CpG sites. Increased methylation was found at CpG sites within genes such at the TNFR superfamily member 10b and CD151. Additionally, increased maternal arsenic was associated with a decreased percentage of CD4+ and an increase in percentage of CD8+ in the cord blood. |
| Nadeau et al. | 2014 | USA | Pregnant women and infants | Prospective cohort study in pregnant women in New Hampshire | Cell-mediated immunity | 5.7 ± 10.8 ppb (mean ± SD) in drinking water | Authors measured maternal urinary arsenic concentration along with drinking water arsenic concentration. They further measured cord blood leukocyte populations by flow cytometry, cord blood T cell proliferation, and cord blood mononuclear cell gene expression. | Authors found that increasing maternal arsenic exposure was positively correlated with placental IL-1β expression (a potent pro-inflammatory cytokine), cord blood T cell proliferative capacity, and changes in cord blood naïve T cell subsets. |
| Bailey et al. | 2014 | Mexico | Pregnant women and infants | Cross-sectional study of pregnant women and their newborns | Cell-mediated immunity | <0.456–236 ppb range in drinking water | Maternal drinking water and urinary arsenic species were determined within 4 weeks of delivery. Fetal cord blood was assessed for proteomic composition. | Proteomic analysis revealed 111 developmental and immune response proteins that were significantly altered (either in an activated or repressed state) with relation to maternal urinary arsenic species. Activator status in male newborns was associated with lower average urinary arsenic, DMA in urine, and neonatal head circumference than repressor males. Pathway analysis revealed TNF as a potential regulator of this urinary arsenic-cord blood proteome relationship. |
| Ahmed et al. | 2014 | Bangladesh | Children | Prospective cohort study of children | Cell-mediated immunity | 12–1228 μg/L urine range, adjusted by specific gravity | Prenatal and early life arsenic exposure was determined utilizing urinary arsenic species from pregnant mothers and children at 4.5 years of age. | The highest quartile of childhood urinary arsenic concentration was associated with a significantly lower plasma IL-2 concentration when compared with all lower quartiles. |
| Burchiel et al. | 2014 | USA | Healthy adult donors | In vitro | Cell-mediated immunity | 0, 0.1, 1, 10, 100 nM As3+ or MMA3+ | Proliferative ability of PBMCs from healthy individuals was assessed after exposure to As3+ or MMA3+. | Authors uncovered differential susceptibility of PBMCs to low-dose arsenic exposure revealed by reduced proliferative capacity when stimulated with anti-CD3 antibody in vitro. Additionally, arsenic-exposed PBMCs from “susceptible” individuals exhibited altered CD4 subset populations. |
| Ahmed et al. | 2012 | Bangladesh | Pregnant women and infants | Prospective cohort study in pregnant women and their newborns | Cell-mediated immunity | 17-481 ppb (5-95 percentile) range at gestational week 8 or 14 in urine | Authors measured maternal urinary, placental, and cord blood arsenic as well as assessing cord blood oxidative stress markers and cord blood PBMC gene expression and sjTREC concentration. | Arsenic concentrations (from all sources) were found to be positively correlated with cord blood oxidative stress and inversely related to expression of several antioxidant genes in cord blood mononuclear cells and sjTREC concentration. |
| Smith et al. | 2011 | Chile | Adults | Retrospective cohort study of adults | Cell-mediated immunity | ∼10–870 ppb range depending on the time period | Authors utilized historical data on arsenic concentrations in municipal water supplies in Antofagasta, Chile, since the 1950s as well as death certificates over this period of time. | The authors found a significant association between periods of high arsenic exposure through municipal drinking water and tuberculosis mortality, using a 5-year latency period. |
| Luna et al. | 2010 | Mexico | Children | Cross-sectional study of children | Cell-mediated immunity | 12.3–1411 μg/g of creatinine in urine | Arsenic exposure was determined through analysis of urine. Nitric oxide and superoxide anion production from PBMCs and isolated peripheral blood monocytes was determined before and after ex vivo stimulation. | Authors identified significant positive associations between inorganic arsenic species in urine and basal levels of nitric oxide in PBMCs and monocytes as well as an association between urinary DMA and basal superoxide anion levels in PBMCs. Authors also noted that superoxide anion production in stimulated monocytes was positively correlated with all species of urinary arsenic. |
| Vega et al. | 1999 | Mexico | Healthy adult donors | In vitro | Cell-mediated immunity | 0, 0.01, 0.1, 1 μM | Authors assessed proliferative ability and IL-2 secretion of primary human PBMCs via stimulation with phytohemagglutinin at various arsenic concentrations. They also looked at IL-2 mRNA concentration, intracellular IL-2 concentration, and electron microscopy of arsenic-exposed cells. | The authors found that increasing arsenic concentrations were inversely correlated with proliferative ability of PBMCs as well as their ability to secrete IL-2, up to 1 μM, at which dose, a few individuals’ PBMCs produced more IL-2. Additionally, the authors found that IL-2 mRNA was unaffected by increasing arsenic dose, while intracellular IL-2 increased slightly, perhaps because of a lack of secretion. Electron microscopy revealed severe morphological changes in PBLs treated with 0.01 μM sodium arsenite. |
| Vega et al. | 2004 | Mexico | Healthy adult donors | In vitro | Cell-mediated immunity | 0, 0.01, 0.1, 1 μM | Authors assessed proliferative ability and IL-2 secretion of primary human PBMCs via stimulation with phytohemagglutinin at various arsenic concentrations. They also performed flow cytometry on stimulated cells. | The authors confirmed earlier findings that increasing doses of arsenic suppress PBMC proliferation, particularly in cells from female donors (except for a small increase in proliferation of cells over control from female donors at the lowest dose, 1.3 ppb). Flow cytometry of stimulated cells revealed that CD4+ T cell populations were severely decreased in cells from female donors at 130 ppb, while CD8+ T cells remained unaffected. This assay also revealed that activated T cells are more susceptible to arsenic toxicity than resting T cells. |
| Smith et al. | 2013 | Bangladesh | Children | Retrospective cohort study of children | Asthma and spirometry | 0–1512 ppb | Arsenic in drinking water sources throughout pregnancy and early life were assessed through a combination of recall questionnaires and historical arsenic data collected from all local tube wells. Children’s lifetime morbidity information was assessed via questionnaire. | Authors found that children in the highest in utero arsenic exposure category had significantly elevated OR of asthma, wheezing while not having a cold, and shortness of breath when walking fast or on flat ground. |
In addition to questions about transplacental transfer of pathogen-specific IgG from mother to fetus, there remain questions about arsenic’s role in impairment of maternal pathogen-specific IgG response. A 2015 study of maternal arsenic exposure and susceptibility to hepatitis E virus (HEV) IgG seroconversion during pregnancy and postpartum in Bangladesh found that the odds ratio (OR) of maternal HEV seroconversion was 2.17 (95% confidence interval 1.07, 4.39) per IQR increase in urinary inorganic arsenic plus methylated species (μg/L) [31••]. A few studies have also assessed the effect of high arsenic exposure on antibody response to vaccination among pregnant women and young children. In a 2013 study by Saha et al., antibody response to inactivated vaccines (oral cholera, intradermal tetanus, diphtheria) in Bangladeshi children was unaltered by high versus low arsenic exposure [24]. However, a reduced antibody response to live attenuated measles vaccination was observed (although not statistically significant) among children with high versus low arsenic exposure. These results suggest that arsenic may affect antibody response differently, depending on the pathogen-specific vaccine target [24]. In a US study utilizing data from NHANES, increased urinary arsenic concentration (μg/L) was related to an increased odds of IgG seronegativity (loss of antibody) for varicella zoster virus, supporting the idea that antibody protection may be lost over time, allowing VZV to reactivate with an increasing arsenic dose. However, arsenic speciation in this study is limited by high levels of undetectable concentrations for arsenite and arsenate and for mononmethylarsonate (MMA) [41]. A separate study involving NHANES data of hepatitis Avirus (HAV) total antibody (IgM and IgG) seroprevalence showed that increasing urinary total arsenic concentration (μg/L) was associated with an increased odds of total HAVantibody seroprevalence [42]. The association showed consistency across strata of individuals’ recall of their HAV vaccination history, suggesting that arsenic exposure may be associated with a lower protective immunity provided by the HAV immunization, which may subsequently result in HAV infection yielding a stronger total antibody response [42].
Despite the advancements made by these pioneering studies, there are still many knowledge gaps to fill in this field. For example, it is unknown what the serum Ig profile of a highly arsenic-exposed newborn is compared to newborns of mothers with low exposure to arsenic. It is also unknown what role various pathogenic exposure factors play in determining the maternal and newborn antibody response to natural infection and/or vaccination in areas of high arsenic exposure and what other co-exposures and covariates, such as micronutrient availability (including those critical for one-carbon metabolism—B vitamins, folate, etc.), may play in mediating this response. There is a need for more epidemiologic research in order to elucidate the mechanisms and pathways through which arsenic exposure can alter humoral immune response in pregnant women and their newborns.
Animal Studies of Arsenic Exposure and Humoral Immune Response
Our literature search returned no toxicological studies of humoral immune response alterations due to arsenic exposure in animal models of pregnant females and neonates (Table 2). In non-pregnant adult rats and goats, however, arsenic exposure has been shown to impair humoral immunity, specifically IgG production. In male rats, arsenic can suppress secondary antibody-mediated response to T cell-dependent antigen stimulation [22]. In a 2013 study by Patra et al., the authors found that female goats exposed daily to 2 mg/kg bodyweight of arsenic for 84 days had reduced antibody levels and reduced total circulating erythrocytes and leukocytes [23]. Additionally, bone marrow cells from these goats were also found to have higher caspase-3 activity and have more nicks in their DNA, both signs of apoptosis. More animal studies, particularly in models of pregnancy, are needed to continue elucidating the drivers and pathways of altered humoral immune responses to vaccination and susceptibility to viral and bacterial challenge.
Table 2.
Summary of animal model studies of arsenic exposure and altered immune response during pregnancy and/or early life
| Author | Year | Model animal | Sex | Immune focus | As level | Main results |
|---|---|---|---|---|---|---|
| Petrick et al. | 2009 | Sprague-Dawley rat | Both | Lung physiology | 0, 500 ppb | In utero exposure to As results in decreased weight of fetal rat lung. Genes and proteins associated with cell motility, cytoskeleton, and adhesion were also altered in the fetal rat lung. |
| Patra et al. | 2013 | Black Bengal Goat(s) | Female | Humoral immunity | 0, 2 mg/kg BW | Serum IgG, hemoglobin, total erythrocyte count, and total leukocyte counts were all significantly reduced in arsenic-exposed animals. Bone marrow cells of arsenic-exposed goats were found to have higher caspase-3 activity and increased DNA nicking. |
| Nain et al. | 2012 | Wistar rat | Male | Humoral immunity | 0, 400, 4,000, 40,000 ppb | Secondary antibody (IgG) production against keyhole limpet hemocyanin antigen was decreased with increasing arsenic exposure, while neutrophil oxidative burst potential was unaffected. |
| Ramsey et al. | 2013a | C57BL/6 mice | Both | Humoral and cell-mediated immunity | 0, 10, 100 ppb | Prenatal exposure of C57BL/6 mice to 100 ppb of arsenic resulted in increased susceptibility of offspring to influenza A infection at 1 week of life. |
| Ramsey et al. | 2013b | C57BL/6 mice | Both | Humoral and cell-mediated immunity | 0, 100 ppb | Prenatal exposure of C57BL/6 mice to 100 ppb of arsenic was correlated with smaller lung size and reduced pulmonary function in pups. Genes involved in mucociliary clearance and innate immunity were also upregulated in pups born to arsenic-exposed dams. |
| Kozul et al. | 2009 | C57BL/6 mice | Male | Humoral and cell-mediated immunity | 0, 100 ppb | Treatment of adult male C57BL/6J mice with 100 ppb As resulted in reduced host immunity against influenza A and increased viremia. |
| Ezeh et al. | 2016 | C57BL/6 mice; 2E8 cell line | Male | Cell-mediated immunity | 0, 5, 50, 500 nM As3+ or MMA3+ | Authors found that the STAT5 signaling pathway was suppressed after exposure of C57BL/6 primary bone marrow cells to low-dose As3+ or MMA3+ and that STAT5 signaling was suppressed in 2E8 cells only with low doses of MMA3+, revealing that this arsenic metabolite is likely responsible for early lymphocyte dysregulation and toxicity, since 2E8 cells lack the AS3MT enzyme needed to metabolize As3+. |
| Xu et al. | 2016a | C57BL/6 mice; D1 cell line | Male | Cell-mediated immunity | 0, 5, 50, 500 nM As3+ or MMA3+ | The authors cultured primary mouse thymocytes and D1 cells at various concentrations of As3+ and MMA3+ and assessed the effects on the STAT5 signaling pathway, an important pathway in the development of pro-B cell and double-negative T cells. They found that both As3+ and MMA3+ suppress Jak1 and Jak3 phosphorylation as well as STAT5 signaling (a downstream phosphorylation target of Jak kinases) and that MMA3+ exposure results in decreased IL-7R (an important downstream developmental receptor of STAT5 signaling) expression on D1 cells. |
| Xu et al. | 2016b | C57BL/6 mice | Male | Cell-mediated immunity | 0, 100, 500 ppb As3+ | The authors exposed mice to varying concentrations of trivalent inorganic arsenic for 30 days, then assessed arsenic speciation and DNA damage in the thymus, bone marrow, and spleen. They found dose-dependent increases in MMA3+ in both the spleen and thymus, but no correlation between arsenic dose and MMA3+ concentration in the spleen. Additionally, dose-dependent increases in DNA damage were observed in the thymus and bone marrow. |
| Cho et al. | 2012 | C57B/6 mice | Female | Cell-mediated immunity | 0, 0.03, 0.06, 0.13, 0.25, 0.5, 1.0, 2.0 μM | Splenocytes were isolated from young adult (2 months) or aged (24–26 months) female mice and co-cultured with varying concentrations of sodium arsenite and a mitogen or anti-CD3 antibody. In young mice, increasing doses of arsenic were associated with decreasing IFN-γ production and decreased IL-10 production in aged mice when co-culture with Con A. |
| Soto-Peña and Vega | 2008 | CD57BL6N mice | Male | Cell-mediated immunity | 0, 10, 100, 1000 ppb | Young adult male mice were gavaged daily with varying doses of dissolved sodium arsenite for 30 days. Spleens were then collected. The investigators found that increasing concentrations of arsenic were associated with reduced splenic Th populations along with a reduced CD4+/CD8+ ratio and a resulting increase in the proportion of splenic CD11b+ cells. Increasing arsenic dose was also related to decreases in lymphocyte proliferation in response to co-culture with a mitogen, which may be related to altered lck kinase phosphorylation kinetics. |
| Conde et al. | 2007 | C57BL/6 mice | Female | Cell-mediated immunity | 0, 1, 10 μM | Mononuclear splenocytes were isolated from young adult female mice and cultured with varying sodium arsenite concentrations. Proliferation, ERK phosphorylation, IL-2 mRNA expression, and secretion were measured. Increasing arsenic concentrations were associated with reduced IL-2 expression, secretion, and mononuclear cell proliferation in response to PHA. The highest concentration of arsenic exposure was found to inhibit ERK1/2 phosphorylation, while both concentrations of arsenic studied reduced the total number of splenic CD8+ cells, while leaving CD4+ cells unaffected. |
| Patterson et al. | 2004 | Balb/c mice | Female | Cell-mediated immunity | 0, 50,000 ppb | Treatment of female mice with 50,000 ppb arsenic via drinking water for 4 weeks resulted in reduced delayed-type contact hypersensitivity, circulating neutrophils, and immune cell proliferation as measured by the local lymph node assay as well as an in vitro lymphoproliferation assay. This study also found a reduction in the recruitment of macrophages to the peritoneal cavity, while no effect was seen in the baseline number of macrophages present in the BALF of arsenic-exposed mice when compared with controls. |
| Goytia-Acevedo et al. | 2003 | C57B/6 mice | Female | Cell-mediated immunity | 1, 10, 100, 1000nM | T lymphocytes were isolated from young adult female mice and co-cultured with mitogens PHA or Con A. Authors found that at the highest doses of sodium arsenite, T lymphocytes underwent less mitogen-stimulated proliferation when pretreated (PHA and Con A) or simultaneously cultured (PHA only) with sodium arsenite. |
Human Studies of Arsenic Exposure and Cell-Mediated Immune Response
In Vitro Studies
Studies probing the effects of arsenic exposure on cell-mediated immunity in pregnant women, developing fetuses and young children, are also scarce (Table 1). Cell-mediated immunity is defined as an immune response wherein antigen-specific T cells and B cells along with cells of the innate immune system play a primary role in immune protection [33]. Another characteristic of this form of immunity is that is cannot be transferred passively via serum. [33]. There are laboratory-based experimental in vitro studies that have begun to assess effects of arsenic exposure on cell-mediated immunity (Table 1); however, their results require some level of extrapolation to arsenic-exposed human populations. Laboratory studies have shown, for example, that arsenic immunotoxicity affects critical aspects of functional cellular immunity. For example, high arsenic exposure has been shown to reduce interleukin-2 (IL-2) production—an important cytokine in cell-mediated immune function—which is associated with a decrease in T cell proliferation [43–45] and activation (Fig. 1) [46]. Briefly, such studies involve isolating peripheral blood mononuclear cells (PBMCs) from healthy human donors, culturing them in the presence of varying levels of arsenic, stimulating them with particular antigens or mitogens, then analyzing their IL-2 secretion via ELISA. Studies utilizing similar in vitro approaches have found that high arsenic concentrations are capable of altering Tcell structure, including the cytoskeleton [47]. Changes in cytoskeletal arrangement could affect secretion of proteins, which may help to explain the decrease in production of certain proteins by these cells, such as IL-2 [47]. In cultures of primary human lymphocytes from healthy volunteers, arsenic was found to be more toxic to helper T (Th) cell subsets (CD4+) compared to cytotoxic T lymphocytes (CTL or CD8+), an effect that was more pronounced in women than men. This result suggests that women may be more susceptible to arsenic immunotoxicity than men [48], although the current evidence is too limited to reach firm conclusions. Generation of T cell-dependent antibody responses requires orchestration of antigen processing and presentation, CD4+ T cell responses, and B cell responses [49]. Although arsenic can induce apoptosis in B cell leukemic cell lines in vitro, little is known about its alteration of B lymphocyte and plasma cell function in humans [50].
A recent paper by Burchiel et al. [51] adds yet another level of complexity to this. In their study, they found that PBMCs from healthy donors cultured in vitro displayed differential levels of susceptibility to low-dose arsenite, as assessed by a T cell proliferation assay [51]. Using intracellular staining of activated, arsenite-treated PBMCs, the authors showed that populations of TH1 (Tbet+) cells and double-positive TH17 (RoRγt+) and TReg (Foxp3+) were diminished among arsenite-susceptible individuals compared with activated, arsenite-treated PBMCs from non-susceptible individuals. This finding confirms the importance of taking interindividual variability into account in studies of arsenic immunotoxicity.
Epidemiologic Studies
While there are an abundance of in vitro studies utilizing human primary PBMCs and human cell lines, there are fewer studies that assessed cell-mediated immune effects of arsenic in exposed human populations (Table 1). A study by Ahmed et al. [18•] of preschool-aged children in rural Bangladesh showed that arsenic alters cell-mediated immune response to Bacillus Calmette-Guerin (BCG) vaccination as indicated by induration at the site of purified protein derivate (PPD) challenge. Briefly, the BCG vaccine was provided early in life to protect against severe tuberculosis morbidity and mortality, while PPD challenges were administered later in life as assessments of residual T cell immunologic memory in the child. In this study, increasing arsenic exposure was associated with increased odds of non-response to PPD challenge. The significance of this non-response is a failure of the BCG-specific CD4+ T cells to respond to challenge with an antigen that they have “seen” before and indicates that the BCG vaccine may no longer have been as effective in children as their arsenic exposure increased. The authors observed effect modification by child underweight status and reports of a recent infection and noted a negative but potentially non-monotonic exposure-response association between urinary arsenic levels and serum IL-2 [18•]. It should be noted that Ahmed et al. reported a combined summary test statistic that was interpreted as a negative monotonic trend of IL-2 across increasing urinary arsenic quartiles. However, their quartile data appear to suggest increasing IL-2 within the lower three quartiles of urinary arsenic exposure (Q1 IL-2 1.6 [0.24, 12.15] pg/mL; Q2 IL-2 2.3 [0.24, 9.78] pg/mL; Q3 IL-2 2.0 [0.24, 7.37] pg/mL) followed by an IL-2 decline at the highest quartile of urinary arsenic exposure (Q4 0.24 [0.24, 6.61] pg/mL) [18•]. This arsenic-IL-2 relation at low to moderate arsenic exposure levels is consistent with a study of HEV IgG seroconversion during pregnancy and pospartum conducted in a region of Bangladesh with low to moderate arsenic exposure. Among pregnant women who seroconverted to HEV, there was an increase in IL-2 levels as arsenic exposure increased [31••]. Yet, these low to moderate arsenic-IL-2 results run counter to what has been observed in vitro, requiring further investigation.
In the Saha et al. [24] study of children living in high versus low arsenic exposure areas of Bangladesh, total white blood cells (WBC) and lymphocytes, monocytes, and eosinophils were significantly reduced among children with the highest arsenic exposure. Results from studies nested within the MINIMat study of arsenic-exposed pregnant women in Matlab, Bangladesh, revealed that arsenic exposure, as measured in maternal blood and urine, was associated with a decrease in thymic size and function in the neonate as well as an increase in infant morbidity [30, 52] and upregulation of cord blood pro-apoptotic and oxidative stress genes [52]. A cohort study by Nadeau et al. [53] of pregnant women from a region of variable exposure in New Hampshire found that increased maternal urinary arsenic concentration (μg/L) was associated with an increase in placental IL-1β expression (a pro-inflammatory cytokine), an increase in cord blood T cell proliferation, and alterations in cord blood T cell subsets. Specifically, the authors noted a decrease in CD45RA+ CD69+ (naïve activated) T cells, while CD45RA+ CD69− CD294+ (naïve resting TH2 cells) increased in cord blood. Additionally, they observed that cord blood-derived T cells exhibited greater proliferative ability in response to in vitro T cell receptor stimulation. Another study by Kile et al. [54] among pregnant Bangladeshi women found that cord blood leukocyte DNA methylation levels as well as CD4+/CD8+ ratio were altered. In particular, for each log10 increase in drinking water arsenic exposure, the proportion of CD8+ cells increased by 7.4%, while the proportion of CD4+ cells decreased by 9.2% [54]. The authors also noted that for each log10 increase in arsenic exposure, B cell proportion decreased by 1.4%, following a non-significant trend (p value = 0.056). Among the genomic loci found to have increased arsenic-associated methylation were genes related to immune function, such as the tumor necrosis factor (TNF) receptor super-family member 10b and CD151, a protein that complexes with integrin to aid in cell trafficking and motility [55]. A proteomic study of the cord blood of arsenic-exposed Mexican infants by Bailey et al. observed that the proteome was significantly altered in a manner dependent upon maternal urinary arsenic and drinking water arsenic concentrations, while pathway analysis revealed that TNF-α expression may have been at the center of this proteomic shift [56]. Finally, a case-control study in California showed that increasing ambient arsenic exposure during pregnancy and early life was associated with an increased odds ratio of the infant developing acute lymphoblastic leukemia [57]. Taken together, these studies suggest mechanisms of arsenic immunotoxicity that may hinge on immune cell subset alterations, changes in the cytokine milieu, as well as reductions in cell trafficking capacity (Fig. 1).
Animal Studies of Arsenic Exposure and Cell-Mediated Immune Response
In Vivo Studies
In addition to the in vitro and epidemiologic literature, several animal studies have investigated cell-mediated mechanisms of arsenic immunotoxicity (Table 2). Xu et al. [58] exposed male C57BL/6 mice via drinking water to 0, 100, or 500 ppb of As3+ for 30 days, then assessed arsenic speciation in the bone marrow, spleen, and thymus of each animal along with levels of DNA damage in each of these tissues. The authors observed a dose-dependent increase in MMA3+ levels in the bone marrow and thymus, which correlated with increased levels of DNA damage, demonstrating the importance of toxicokinetics as well as toxicodynamics in studies of arsenic immunotoxicity. A 2004 study by Patterson et al. attempted a suite of immunotoxicological tests of young adult female mice to evaluate the competence of their cell-mediated immune responses after arsenic exposure. In this study, Balb/c mice were treated with 50,000 ppb arsenic via drinking water for 4 weeks, resulting in reduced delayed-type contact hypersensitivity, circulating neutrophils, and immune cell proliferation, as measured by a local lymph node assay and an in vitro lymphoproliferation assay [59]. This study also observed a reduction in the recruitment of macrophages to the peritoneal cavity, however, the baseline number of peritoneal macrophages was not assessed prior to sensitization while no effect was seen in the baseline number of macrophages present in the BALF of arsenic-exposed mice when compared with controls. Importantly, these results suggest potential dysfunction in immune cell trafficking and ability to perform antigen presentation. A 2008 study of young adult male mice showed that increasing arsenic concentration (administered via gavage) was associated with reduced splenic TH cell populations along with a reduced CD4+/CD8+ ratio and a resulting increase in the proportion of splenic CD11b+ (monocytes) cells [14]. CD11b+ is a cell surface protein expressed on monocytes and macrophages (considered by some to be a pan-macrophage screening tool) but is also expressed on several other cell types. Additionally, the authors showed that increasing arsenic dose was related to decreases in lymphocyte proliferation, an effect that they posited may have been related to increases in the phosphorylation level of specific kinases involved in the T cell receptor activation pathway.
Host resilience against viral challenge following arsenic exposure has been assessed in at least one study by Kozul et al. [60]. This study showed that male C57BL/6J mice exposed to 100 ppb of sodium arsenite for 5 weeks (and throughout infection) suffered worse influenza morbidity than mice not exposed to arsenic, as assessed by pulmonary viral load along with albumin, total cell infiltrate, macrophage, and neutrophil cell counts in the bronchioalveolar lavage fluid (BALF) at day 7 postinoculation. Interestingly, compared to influenza-infected mice, those mice also exposed to arsenic had higher numbers of CD8+ T cells in their BALF. Additionally, the authors observed that dendritic cells and B cells from arsenic-exposed mice were less able to traffic to the mediastinal lymph nodes (the draining lymph nodes of the lungs) at 3 days postinoculation, while primary bone marrow dendritic cells were found to be less capable of migration via a transwell assay. These assays demonstrate that arsenic may reduce the ability of antigen-presenting and antibody-forming immune cells (necessary for ramping up the adaptive immune response) to traffic to nearby lymph nodes in order to orchestrate an organized immune response to the influenza infection (Fig. 1).
Only one study, to our knowledge, addressed questions of host immune competence in neonatal mice after prenatal and early life exposure to arsenic. In their 2013 paper, Ramsey et al. challenged 1-week-old C57BL/6 mice with influenza A virus [29••]. The investigators observed that mice exposed prenatally and perinatally to arsenic carried higher influenza A viral load in their lungs at day 7 postinoculation and higher macrophages as well as total cells in their BALF at day 3 postinoculation compared to arsenic unexposed, influenza-challenged controls. Interestingly, there was no significant difference in total lymphocytes in the BALF, despite the higher influenza A viral load in the arsenic-exposed, influenza A virus-challenged mice, suggesting that while lymphocytes may be trafficking to the site of infection, their cytotoxic capacity may have somehow been impaired by the elevated arsenic exposure.
In Vitro Studies
A few murine in vitro studies have also been conducted to investigate the effects of arsenic exposure on cell-mediated immunity (Table 2). In one study by Goytia-Acevedo et al. [61], T lymphocytes were isolated from young adult female mice and co-cultured with various doses of arsenic and mitogens PHA or Con A. The authors found that at the highest doses of sodium arsenite, T lymphocytes underwent less mitogen-stimulated proliferation when pretreated (in the case of mitogens PHA and Con A) or simultaneously cultured (in the case of mitogen PHA only) with sodium arsenite, similar to results noted by Soto-Peña et al. [14] and Kozul et al. [60]. Another study by Cho et al. [62] found that C57BL/6 splenocytes from young adult female mice exhibited reduced proliferation and IFN-γ production, as well as a non-significant decreasing trend in IL-2 production, when cultured with Con A, and increasing concentrations of sodium arsenite. The same downward trend in IL-2 production with increasing arsenic concentration was also noted for splenocytes cultured with anti-CD3 antibody, and the decrease was found to be significant at the highest dose of arsenic (2 μM). A similar in vitro study by Conde et al. [44] found that IL-2 expression (as measured by mRNA concentration) and secretion were reduced in a dose-dependent manner in mononuclear splenic cells cultured with increasing doses of arsenic. The highest concentration of arsenic exposure was found to inhibit ERK1/2 phosphorylation, while both concentrations of arsenic studied reduced the total number of splenic CD8+ cells but not CD4+ cells. This last result runs contrary to what has been observed in other studies that have demonstrated reduced CD4+ numbers and decreases in CD4+/CD8+ cell ratios in many settings, once again highlighting the need for further research in this area.
Two recent studies undertook a more in-depth investigation of the molecular mechanisms of arsenic immunotoxicity in T and B cells. Xu et al. [63•] found that suppressed Jak1, Jak3, and STAT5 signaling may be at the root of arsenic toxicity in double-negative thymocytes and potentially B cells, which rely on the same signaling pathways to develop. In a related study, Ezeh et al. [64] studied the effects of As3+ and MMA3+ in both primary C57BL/6 male mouse bone marrow cells and the 2E8 murine pre-B cell line. They found that very low doses of MMA3+ were capable of suppressing STAT5 signaling and thus IL-7 signaling, a necessary component of early lymphoid development in both B and Tcells. Additionally, the lack of effect noted in 2E8 cells by As3+ treatment indicates that this mechanism of toxicity is a result of exposure to MMA3+, since 2E8 cells lack the enzyme arsenic-3 methyl-transferase (AS3MT) needed to convert As3+ to MMA3+.
Future Directions
While the immunotoxic effects of arsenic are widely known, the specific mechanisms of these immune function alterations and how they manifest in exposed populations during critical periods of vulnerability—pregnancy, in utero, newborn, and early childhood—remain poorly understood. Considering the potential for significant arsenic-attributable infectious disease burden during these critical periods of growth and development among pregnant women and their children, this is an area that is ripe for future research and discovery.
One important area for future research relates to the impact of arsenic exposure on barrier tissue maintenance, such as in the lungs. While this is not direct immunotoxicity, loss of barrier integrity can be a strong driver of susceptibility to numerous infectious diseases, particularly respiratory infections. A recent study by Ramsey et al. [65] showed that prenatal exposure of C57BL/6 mice to arsenic resulted in neonatal lungs that were smaller, less elastic, and that exhibited differential expression of many genes involved in mucociliary function. The pulmonary effects of arsenic exposure have important human health implications. In a 2013 retrospective cohort study by Smith et al., the authors found that Bangladeshi children in the highest in utero arsenic exposure category had a significantly elevated OR of asthma, wheezing while not having a cold, and shortness of breath when walking fast or on flat ground. A 2007 study by Islam et al. assessed the relationship between arsenic exposure, respiratory symptoms, and serum Ig profiles in arsenicosis patients compared to unexposed controls [20]. The authors found that high arsenic exposure was associated with a high prevalence of respiratory conditions such as asthma, bronchitis, cough, and chest sounds. Others have documented arsenic-related exacerbation of non-malignant lung disease and bronchiectasis severity [67]. All of these studies point to mechanisms of pulmonary toxicity by arsenic, with the potential to lead to immune-mediated disease and infection susceptibility later in life.
Another area for future research would be determining the most critical exposure windows during the prenatal, perinatal, and postnatal periods. Throughout the life of the mother and father as well as during gestation and early life development of the child, there exist critical windows of organ system development as well as changes in DNA methylation status [7, 68–71]. The immune effects that arsenic exposure can elicit in the host, similar to effects associated with other toxic chemicals [72–75], may differ dramatically depending on the developmental period during which exposure occurs. However, little is known about this topic.
Another area for future research is the potential role of arsenic exposure in altering maternal immune response to vaccination and maternal antibody production and transplacental transfer efficiency. Although arsenic [18•, 24] and other environmental toxicants [72–75] appear to impair immune responses to vaccinations in childhood, little is known about whether arsenic can impair maternal antibody responses to vaccination and the transfer of maternal antibody to the newborn [76]. There are several critical prenatal, perinatal, and postnatal and early childhood development periods that would benefit from focused future longitudinal studies. Assessment of maternal and child immune responses to one or more vaccinations that have known health benefits and are recommended for pregnant women and their newborns, such as influenza (recommended at any point during pregnancy [77]) and hepatitis B (recommended at birth by CDC [78]), could improve understanding of arsenic-related mechanisms and pathways of immunotoxicity. Knowledge of specific mechanisms could lead to prioritization of interventions (changes in vaccine dosage; administration of booster doses) in areas where persistent arsenic exposure cannot readily be remediated due to the complexity of exposure sources (e.g., food, water, occupation) and resource limitations. Additionally, by prospectively collecting samples such as peripheral blood serum, drinking water, and urine, researchers will help to build important knowledge of the temporal effects of exposure, including when and how external exposure and arsenic metabolism affect maternal, fetal, and neonatal immunity and how early life exposure may influence infectious disease outcomes into childhood and adulthood. While some studies have demonstrated that arsenic exposure increases total antibody titers in the serum [20, 31••, 79], the effects of arsenic on the production of antigen-specific antibodies seem to differ depending on the antigen [24, 41]. It would be useful to know if this is a result of loss of antigen-specific TH cells, a loss of the ability of these TH cells to home to local lymph nodes, or an inability of these TH cells to properly activate B cells to differentiate into plasma and memory B cells and to initiate affinity maturation (Fig. 1). The precise mechanism or suite of mechanisms involved in this progression remains a fundamental knowledge gap, one that could be closed by future in vivo and epidemiologic studies.
Moreover, factors that affect arsenic metabolism and toxicity outcomes, specifically micronutrients critical for one-carbon metabolism (e.g., folate and vitamins B2, B6, and B12), [80–82], have not been evaluated in studies of arsenic immunotoxicity, correlates of vaccine-induced protection, placental transfer of maternal antibodies, or morbidity in mothers and newborns. In order to fill these knowledge gaps, future studies should measure micronutrient intake as well as external sources of arsenic exposure (e.g., drinking water, food) as well as arsenic metabolism (e.g., inorganic arsenic, dimethylarsinic acid (DMA), and monomethylarsonic acid (MMA) in urine).
Respiratory infections during the prenatal, perinatal, and postnatal period are of great concern for maternal and child health. Animal studies have shown arsenic-related exacerbation of severe influenza infection following in utero and early life arsenic exposure [29••, 60, 83]. Similarly, human studies also indicate arsenic-related exacerbation of self-reported respiratory morbidity symptoms during pregnancy and early life [20, 25, 26, 28, 30, 66], but no human arsenic exposure studies to our knowledge have employed objective measures of respiratory morbidity (e.g., laboratory-confirmed infection via tests of seroconversion and/or pathogen isolation) during pregnancy and early life.
Continuance of these gaps limits understanding of critical immunologic mechanisms, processes, and pathways. This represents an important problem because until filled, optimal points for intervention to prevent arsenic-related immunotoxicity and morbidity during pregnancy and early life will not be known. Future in vitro and epidemiological studies should focus on building upon knowledge of arsenic immunotoxicity mechanisms by integrating enumerative and functional measures of antibody and immune cell (CD4+, CD8+ T cells) responses in regions with varying arsenic exposure to determine how arsenic alters humoral and cell-mediated immunity and susceptibility to infection among pregnant women and their neonates and children during early life.
Acknowledgments
Funding Sources The authors would like to acknowledge their funding sources: NIH grants 1R01ES026973-01A1, R01ES021367, and R01ES025216.
Footnotes
This article is part of the Topical Collection on Mechanisms of Toxicity
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Vahter M. Effects of arsenic on maternal and fetal health. Annu Rev Nutr. 2009;29:381–99. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- 2.George CM, et al. Arsenic exposure in drinking water: an unrecognized health threat in Peru. Bull World Health Organ. 2014;92(8):565–72. doi: 10.2471/BLT.13.128496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdul KS, et al. Arsenic and human health effects: a review. Environ Toxicol Pharmacol. 2015;40(3):828–46. doi: 10.1016/j.etap.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Dangleben NL, Skibola CF, Smith MT. Arsenic immunotoxicity: a review. Environ Health. 2013;12:73. doi: 10.1186/1476-069X-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IARC. Arsenic and Arsenic Compounds. 2012 [Google Scholar]
- 6.Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicol Sci. 1998;44:185–90. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- 7.Dietert RR. Developmental immunotoxicity, perinatal programming, and noncommunicable diseases: focus on human studies. Adv Med. 2014;2014:867805. doi: 10.1155/2014/867805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond MP, Gabriele R. Effects of pregnancy on metabolism Comprehensive physiology. Hoboken: John Wiley & Sons, Inc.; 2011. [Google Scholar]
- 9.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(6):425–33. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lantz RC, et al. Effect of arsenic exposure on alveolar macrophage function. I. Effect of soluble As(III) and As(V) Environ Res. 1994;67(2):183–95. doi: 10.1006/enrs.1994.1073. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee N, et al. Chronic arsenic exposure impairs macrophage functions in the exposed individuals. J Clin Immunol. 2009;29(5):582–94. doi: 10.1007/s10875-009-9304-x. [DOI] [PubMed] [Google Scholar]
- 12.Biswas R, et al. Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Hum Exp Toxicol. 2008;27(5):381–6. doi: 10.1177/0960327108094607. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Castro B, et al. Effect of arsenic on regulatory T cells. J Clin Immunol. 2009;29(4):461–9. doi: 10.1007/s10875-009-9280-1. [DOI] [PubMed] [Google Scholar]
- 14.Soto-Pena GA, Vega L. Arsenic interferes with the signaling transduction pathway of T cell receptor activation by increasing basal and induced phosphorylation of Lck and Fyn in spleen cells. Toxicol Appl Pharmacol. 2008;230(2):216–26. doi: 10.1016/j.taap.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 15.Sikorski EE, et al. Immunotoxicity of the semiconductor gallium arsenide in female B6C3F1 mice. Fundam Appl Toxicol. 1989;13(4):843–58. doi: 10.1016/0272-0590(89)90338-2. [DOI] [PubMed] [Google Scholar]
- 16.Lemarie A, et al. Human macrophages constitute targets for immunotoxic inorganic arsenic. J Immunol. 2006;177(5):3019–27. doi: 10.4049/jimmunol.177.5.3019. [DOI] [PubMed] [Google Scholar]
- 17.Soto-Pena GA, et al. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. FASEB J. 2006;20(6):779–81. doi: 10.1096/fj.05-4860fje. [DOI] [PubMed] [Google Scholar]
- 18•.Ahmed S, et al. Arsenic exposure and cell-mediated immunity in pre-school children in rural Bangladesh. Toxicol Sci. 2014;141(1):166–75. doi: 10.1093/toxsci/kfu113. In this MINIMat substudy, authors use data collected prospectively from pregnant mothers and their children to associate recent and prenatal arsenic exposure with T cell-mediated immunologic memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blakley BR, Sisodia CS, Mukkur TK. The effect of methylmercury, tetraethyl lead, and sodium arsenite on the humoral immune response in mice. Toxicol Appl Pharmacol. 1980;52(2):245–54. doi: 10.1016/0041-008x(80)90111-8. [DOI] [PubMed] [Google Scholar]
- 20.Islam LN, et al. Association of respiratory complications and elevated serum immunoglobulins with drinking water arsenic toxicity in human. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;42(12):1807–14. doi: 10.1080/10934520701566777. [DOI] [PubMed] [Google Scholar]
- 21.Ser PH, et al. Arsenic exposure increases maternal but not cord serum IgG in Bangladesh. Pediatr Int. 2014;57(1):119–125. doi: 10.1111/ped.12396. [DOI] [PubMed] [Google Scholar]
- 22.Nain S, Smits JE. Pathological, immunological and biochemical markers of subchronic arsenic toxicity in rats. Environ Toxicol. 2012;27(4):244–54. doi: 10.1002/tox.20635. [DOI] [PubMed] [Google Scholar]
- 23.Patra PH, et al. Immunotoxic and genotoxic potential of arsenic and its chemical species in goats. Toxicol Int. 2013;20(1):6–10. doi: 10.4103/0971-6580.111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha A, et al. Vaccine specific immune response to an inactivated oral cholera vaccine and EPI vaccines in a high and low arsenic area in Bangladeshi children. Vaccine. 2013;31(4):647–52. doi: 10.1016/j.vaccine.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 25.Farzan SF, et al. In utero arsenic exposure and infant infection in a United States cohort: a prospective study. Environ Res. 2013;126:24–30. doi: 10.1016/j.envres.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kile ML, et al. A prospective cohort study of the association between drinking water arsenic exposure and self-reported maternal health symptoms during pregnancy in Bangladesh. Environ Health. 2014;13(1):29. doi: 10.1186/1476-069X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dangleben NL, Skibola CF, Smith MT. Arsenic immunotoxicity: a review. Environ Health. 2013;12(1):73. doi: 10.1186/1476-069X-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman A, et al. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environ Health Perspect. 2011;119(5):719–24. doi: 10.1289/ehp.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Ramsey KA, et al. Early life arsenic exposure and acute and long-term responses to influenza A infection in mice. Environ Health Perspect. 2013;121(10):1187–93. doi: 10.1289/ehp.1306748. This study outlines an in vivo model of prenatal arsenic exposure and neonatal immune challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raqib R, et al. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicol Lett. 2009;185(3):197–202. doi: 10.1016/j.toxlet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 31••.Heaney CD, et al. Arsenic exposure and hepatitis E virus infection during pregnancy. Environ Res. 2015;142:273–80. doi: 10.1016/j.envres.2015.07.004. This study provides an example of a prospective cohort study assessing the immunotoxic effects of arsenic exposure during the pregnancy and postpartum periods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith AH, et al. Evidence from Chile that arsenic in drinking water may increase mortality from pulmonary tuberculosis. Am J Epidemiol. 2011;173(4):414–20. doi: 10.1093/aje/kwq383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy KTP, Walport M. Immunobiology. Seventh. New York: Garland Science, Taylor & Francis Group, LLC; 2008. [Google Scholar]
- 34.Brambell FW. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966;2(7473):1087–93. doi: 10.1016/s0140-6736(66)92190-8. [DOI] [PubMed] [Google Scholar]
- 35.Palmeira P, et al. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gendrel D, et al. Placental transfer of tetanus antibodies and protection of the newborn. J Trop Pediatr. 1990;36(6):279–82. doi: 10.1093/tropej/36.6.279. [DOI] [PubMed] [Google Scholar]
- 37.Hartter HK, et al. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J. 2000;19(7):635–41. doi: 10.1097/00006454-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Goncalves G, et al. Transplacental transfer of measles and total IgG. Epidemiol Infect. 1999;122(2):273–9. doi: 10.1017/s0950268899002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silveira Lessa AL, et al. Preterm and term neonates transplacentally acquire IgG antibodies specific to LPS from Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2011;62(2):236–43. doi: 10.1111/j.1574-695X.2011.00807.x. [DOI] [PubMed] [Google Scholar]
- 40.Islam LN, et al. Function of serum complement in drinking water arsenic toxicity. J Toxicol. 2012;2012:302817. doi: 10.1155/2012/302817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardenas A, et al. Arsenic exposure and prevalence of the varicella zoster virus in the United States: NHANES (2003–2004 and 2009–2010) Environ Health Perspect. 2015;123(6):590–6. doi: 10.1289/ehp.1408731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardenas A, et al. Arsenic exposure and the seroprevalence of total hepatitis A antibodies in the US population: NHANES, 2003–2012. Epidemiol Infect. 2016;144(8):1641–51. doi: 10.1017/S0950268815003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin-Chouly C, et al. Inorganic arsenic alters expression of immune and stress response genes in activated primary human T lymphocytes. Mol Immunol. 2011;48(6–7):956–65. doi: 10.1016/j.molimm.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Conde P, et al. Sodium arsenite-induced inhibition of cell proliferation is related to inhibition of IL-2 mRNA expression in mouse activated T cells. Arch Toxicol. 2007;81(4):251–9. doi: 10.1007/s00204-006-0152-7. [DOI] [PubMed] [Google Scholar]
- 45.Galicia G, et al. Sodium arsenite retards proliferation of PHA-activated T cells by delaying the production and secretion of IL-2. Int Immunopharmacol. 2003;3(5):671–82. doi: 10.1016/S1567-5769(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 46.Tenorio EP, Saavedra R. Differential effect of sodium arsenite during the activation of human CD4+ and CD8+ T lymphocytes. Int Immunopharmacol. 2005;5(13–14):1853–69. doi: 10.1016/j.intimp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Vega L, et al. Sodium arsenite reduces proliferation of human activated T-cells by inhibition of the secretion of interleukin-2. Immunopharmacol Immunotoxicol. 1999;21(2):203–20. doi: 10.3109/08923979909052758. [DOI] [PubMed] [Google Scholar]
- 48.Vega L, et al. Helper T cell subpopulations from women are more susceptible to the toxic effect of sodium arsenite in vitro. Toxicology. 2004;199(2–3):121–8. doi: 10.1016/j.tox.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Mozdzanowska K, et al. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J Virol. 2005;79(10):5943–51. doi: 10.1128/JVI.79.10.5943-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akao Y, et al. Arsenic induces apoptosis in B-cell leukaemic cell lines in vitro: activation of caspases and down-regulation of Bcl-2 protein. Br J Haematol. 1998;102(4):1055–60. doi: 10.1046/j.1365-2141.1998.00869.x. [DOI] [PubMed] [Google Scholar]
- 51.Burchiel SW, et al. Differential susceptibility of human peripheral blood Tcells to suppression by environmental levels of sodium arsenite and monomethylarsonous acid. PLoS One. 2014;9(10):e109192. doi: 10.1371/journal.pone.0109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmed S, et al. In utero arsenic exposure is associated with impaired thymic function in newborns possibly via oxidative stress and apoptosis. Toxicol Sci. 2012;129(2):305–14. doi: 10.1093/toxsci/kfs202. [DOI] [PubMed] [Google Scholar]
- 53.Nadeau KC, et al. In utero arsenic exposure and fetal immune repertoire in a US pregnancy cohort. Clin Immunol. 2014;155(2):188–197. doi: 10.1016/j.clim.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kile ML, et al. Effect of prenatal arsenic exposure on DNA methylation and leukocyte subpopulations in cord blood. Epigenetics. 2014;9(5):774–82. doi: 10.4161/epi.28153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.NCBI, CD151 CD151 molecule (Raph Blood Group) Homo sapiens (humans) 2016 [Google Scholar]
- 56.Bailey KA, et al. Prenatal arsenic exposure and shifts in the newborn proteome: interindividual differences in tumor necrosis factor (TNF)-responsive signaling. Toxicol Sci. 2014;139(2):328–37. doi: 10.1093/toxsci/kfu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heck JE, et al. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int J Hyg Environ Health. 2014;217(6):662–8. doi: 10.1016/j.ijheh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu HM, Medina S, Lauer FT, Douillet C, Liu KJ, Hudson LG, Styblo M, Burchiel SW. Differential sensitivities of bone marrow, spleen and thymus to genotoxicity induced by environmentally relevant concentrations of arsenite. Toxicol Lett. 2016;262:55–61. doi: 10.1016/j.toxlet.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patterson R, et al. Arsenic-induced alterations in the contact hypersensitivity response in Balb/c mice. Toxicol Appl Pharmacol. 2004;198(3):434–43. doi: 10.1016/j.taap.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Kozul CD, et al. Low-dose arsenic compromises the immune response to influenza a infection in vivo. Environ Health Perspect. 2009;117(9):1441–7. doi: 10.1289/ehp.0900911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goytia-Acevedo RC, Cebrian ME, Calderon-Aranda ES. Differential effects of arsenic on intracellular free calcium levels and the proliferative response of murine mitogen-stimulated lymphocytes. Toxicology. 2003;189(3):235–44. doi: 10.1016/s0300-483x(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 62.Cho Y, et al. Age-related effects of sodium arsenite on splenocyte proliferation and Th1/Th2 cytokine production. Arch Pharm Res. 2012;35(2):375–82. doi: 10.1007/s12272-012-0219-3. [DOI] [PubMed] [Google Scholar]
- 63•.Xu H, et al. Environmentally relevant concentrations of arsenite and monomethylarsonous acid inhibit IL-7/STAT5 cytokine signaling pathways in mouse CD3+CD4-CD8- double negative thymus cells. Toxicol Lett. 2016;247:62–8. doi: 10.1016/j.toxlet.2016.02.014. This recent article provides novel insight into the potential immune signaling cascades responsible for arsenic-induced immunotoxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ezeh PC, et al. Monomethylarsonous acid (MMA+3) inhibits IL-7 signaling in mouse pre-B cells. Toxicol Sci. 2016;149(2):289–99. doi: 10.1093/toxsci/kfv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramsey KA, et al. In utero exposure to arsenic alters lung development and genes related to immune and mucociliary function in mice. Environ Health Perspect. 2013;121(2):244–50. doi: 10.1289/ehp.1205590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith AH, et al. Chronic respiratory symptoms in children following in utero and early life exposure to arsenic in drinking water in Bangladesh. Int J Epidemiol. 2013;42(4):1077–86. doi: 10.1093/ije/dyt120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazumder DNG, et al. Bronchiectasis in persons with skin lesions resulting from arsenic in drinking water. Epidemiology. 2005;16(6):760–5. doi: 10.1097/01.ede.0000181637.10978.e6. [DOI] [PubMed] [Google Scholar]
- 68.Burggren WW, Mueller CA. Developmental critical windows and sensitive periods as three-dimensional constructs in time and space. Physiol Biochem Zool. 2015;88(2):91–102. doi: 10.1086/679906. [DOI] [PubMed] [Google Scholar]
- 69.De Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Australian and New Zealand Journal of Obstetrics and Gynaecolog. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 70.Okae H, et al. Genome-wide analysis of DNA methylation dynamics during early human development. PLoS Genet. 2014;10(12):e1004868. doi: 10.1371/journal.pgen.1004868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skogen JC, Overland S. The fetal origins of adult disease: a narrative review of the epidemiological literature. JRSM Short Rep. 2012;3(8):59. doi: 10.1258/shorts.2012.012048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307(4):391–7. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heilmann C, et al. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006;3(8):e311. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heilmann C, Budtz-Jørgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ Health Perspect. 2010;118(10):1434–8. doi: 10.1289/ehp.1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jusko TA, et al. A birth cohort study of maternal and infant serum PCB-153 and DDE concentrations and responses to infant tuberculosis vaccination. Environ Health Perspect. 2016;124(6):813–21. doi: 10.1289/ehp.1510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraus TA, et al. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol. 2012;32(2):300–11. doi: 10.1007/s10875-011-9627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.CDC. Flu Vaccine Safety and Pregnancy. 2016 [Google Scholar]
- 78.CDC. Immunization Schedule: Child and Adolescent Schedule. 2016 [Google Scholar]
- 79.Ser PH, et al. Arsenic exposure increases maternal but not cord serum IgG in Bangladesh. Pediatr Int. 2015;57(1):119–25. doi: 10.1111/ped.12396. [DOI] [PubMed] [Google Scholar]
- 80.Howe CG, et al. Folate and cobalamin modify associations between S-adenosylmethionine and methylated arsenic metabolites in arsenic-exposed Bangladeshi adults. J Nutr. 2014;144(5):690–7. doi: 10.3945/jn.113.188789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hall MN, et al. Folate, cobalamin, cysteine, homocysteine, and arsenic metabolism among children in Bangladesh. Environ Health Perspect. 2009;117(5):825–31. doi: 10.1289/ehp.0800164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hall M, et al. Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect. 2007;115(10):1503–9. doi: 10.1289/ehp.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petrick JS, et al. Inorganic arsenic as a developmental toxicant: in utero exposure and alterations in the developing rat lungs. Mol Nutr Food Res. 2009;53(5):583–91. doi: 10.1002/mnfr.200800019. [DOI] [PMC free article] [PubMed] [Google Scholar]


