Abstract
Background
Chronic alcohol consumption leads to increased fracture risk and an elevated risk of osteoporosis by decreasing bone accrual through increasing osteoclast activity and decreasing osteoblast activity. We have shown that this mechanism involves the generation of reactive oxygen species (ROS) produced by NADPH oxidases (NOX). It was hypothesized that different dietary antioxidants, N-acetyl cysteine (NAC, 1.2mg/kg/d) and α-tocopherol (VitE, 60 mg/kg/d)) would be able to attenuate the NOX-mediated ROS effects on bone due to chronic alcohol intake.
Methods
To study the effects of these antioxidants, female mice received a Lieber DeCarli liquid diet containing ethanol (EtOH) with or without additional antioxidant for 8 weeks.
Results
Tibias displayed decreased cortical bone mineral density in both the EtOH and EtOH+antioxidant groups compared to pair-fed (PF) and PF+antioxidant groups (P<0.05). However, there was significant protection from trabecular bone loss in mice fed either antioxidant (P<0.05). MicroCT analysis demonstrated a significant decrease in bone volume (BV/TV) and trabecular number (Tb.N) (P<0.05), along with a significant increase in trabecular spacing (Tb.Sp) in the EtOH compared to PF (P<0.05). In contrast, the EtOH+NAC and EtOH+α-tocopherol did not statistically differ from their respective PF controls. Ex vivo histological sections of tibias were stained for nitrotyrosine, an indicator of intracellular damage by ROS, and tibias from mice fed EtOH exhibited significantly more staining than PF controls. EtOH treatment significantly increased the number of marrow adipocytes per mm as well as mRNA expression of aP2, an adipocyte marker in bone. Only NAC was able to reduce the number of marrow adipocytes to PF levels. EtOH fed mice exhibited reduced bone length (P<0.05) and had a reduced number of proliferating chondrocytes within the growth plate. NAC and Vitamin E prevented this (P<0.05).
Conclusions
These data show that alcohol’s pathological effects on bone extend beyond decreasing bone mass and suggest a partial protective effect of the dietary antioxidants NAC and α-tocopherol at these doses with regard to alcohol effects on bone turnover and bone morphology.
INTRODUCTION
Chronic alcohol consumption is a well-known risk factor for osteoporosis and low bone mass (Sampson, 2002; Chakkalakal, 2005; Berg et al., 2008). Bone remodeling is controlled by a delicate equilibrium between osteoclast activity, the removal of old bone, and osteoblast activity, the formation of new bone (Zaidi, 2007;Sims and Vrahnas, 2014). Multiple investigators have shown that alcohol affect both aspects of this balance; inhibiting osteoblastogenesis (Turner et al., 2010; Chen et al., 2010) and stimulating osteoclastogenesis (Dai et al., 2000; Wezeman et al., 2000; Mercer et al., 2014). Alcohol also affects the lineage commitment of bone marrow mesenchymal stromal cells (MSCs), altering their progression toward osteoblasts and redirecting them to become adipocytes (Chen et al., 2010). Chronic alcohol consumption impacts the bone in other ways as well. Alcohol reduces the number of proliferating chondrocytes in the tibial growth plate of rats fed ethanol via total enteral nutrition (Shankar et al., 2006). Chondrocytes secrete the cartilaginous matrix essential for the process of endochondral ossification. The ordered progression of chondrocyte differentiation and columnar arrangement of the chondrocytes proceeds from the articular ends to the shaft of long bones. The continued proliferation of less mature chondrocytes at the extremities, followed by their differentiation into hypertrophic chondrocytes, and finally their replacement by trabecular bone near the center results in bone longitudinal growth (St Jacques et al., 1999). Additionally, ethanol has been shown to increase bone cell senescence (Chen et al., 2009) and apoptosis (Mercer et al., 2012). . Extensive oxidative stress causes damage to DNA, proteins, and lipids (Droge, 2002; Balaban et al., 2007) and may play a critical role in alcohol-induced osteopenia (Ronis et al., 2011; Mercer et al., 2014).
Oxidative stress plays a key role in several pathologies such as cancer (Filaire et al., 2013; Paschos et al., 2013; Hardbower et al., 2013), cardiovascular disease (Donato et al., 2015), and ageing (Kong et al., 2014). Dietary antioxidant supplementation has garnered attention over the last decade as means of disease prevention. Dietary supplements with antioxidant properties have been shown to exhibit profound effects on bone. Soy Protein Isolate (Chen et al., 2013), blueberries (Zhang et al., 2013), and genistein (Yang et al., 2014) have all been reported to have bone anabolic effects in vivo. Other dietary antioxidants have been tested in attempts to identify substances that are protective against ROS-related bone loss. For example, N-acetyl cysteine (NAC) is a small molecule amino acid derivative and glutathione precursor. It also has antioxidant properties and is currently in clinical use as an antidote for acetaminophen overdose. Alone, dietary NAC supplementation was shown to increase bone volume and bone mineral density as well as decrease osteoclast differentiation in mice (Cao and Picklo, 2014). In a study to determine the effectiveness of NAC as a post-fracture osteogenic supplement molecule, a collagenous sponge enhanced with NAC was compared to the sponge itself in the ability to help repair a bone defect. The NAC supplemented sponge supported bone healing significantly better than the sponge alone (Yamada et al., 2013). In addition, NAC has previously been shown to block osteopenia in rats fed EtOH intragastrically via total enteral nutrition and to prevent alcohol-related deficient fracture healing in mice (Chen et al. 2012; Roper et al. 2016).
Vitamin E, α-tocopherol (Vit.E) is a polyisoprenoid derivative with antioxidant activities which is a true free radical scavenger. However, the true effect of α-tocopherol on bone is uncertain. A review of studies on Vitamin E in fracture repair suggested that α-tocopherol may impact bone remodeling and healing due to its antioxidant properties but cited the need for more data to be collected (Borhanuddin et al., 2012). In a study using mice genetically unable to import α-tocopherol, mice displayed decreased bone mass due to increased osteoclast fusion. Upon feeding of α-tocopherol to these mice, a normal bone mass phenotype was restored and osteoclast fusion remained elevated (Fujita et al., 2012). Despite these findings, in a study using ovariectomy-induced bone loss, α-tocopherol at 60mg/kg per day and pure-tocotrienol at the same dose were completely protective (Muhammad et al., 2012).
Given that alcohol-related bone loss involves ROS-induced signaling pathways, in this study two different dietary antioxidants with different mechanisms of action were tested as protective agents against alcohol-induced pathology in the skeletons of female mice.
Materials and Methods
Animals and Experimental Design
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. Mice were housed in an Association Assessment and Accreditation of Laboratory Animal Care approved animal facility. Eighty, 6-week-old WT female mice (Jackson Laboratories, Bar Harbor, ME) were randomly assigned to 6 weight-matched groups: a 28% EtOH liquid diet (n = 10); a corresponding pair-fed (PF) control (n = 10); a 28% EtOH liquid diet plus NAC [1.2mg/kg/d] (n=10); a corresponding PF control plus NAC (PF+NAC) (n = 10); a 28% EtOH liquid diet plus (Vit.E) [60mg/kg/d] (n=10); a corresponding PF control plus Vit.E (PF+Vit.E) (n = 10). All groups had access to water ad libitum. EtOH was added to the Lieber–DeCarli liquid diet (35% of energy from fat, 18% from protein, 47% from carbohydrates) by slowly substituting carbohydrate calories for EtOH calories (#710260; Dyets, Inc., Bethlehem, PA) in a stepwise manner until 28% total calories were reached, which constitutes final EtOH concentration of 4.9 % (v/v), respectively, and maintained until sacrifice (40 days; Mercer et al., 2012). Mice being fed a Lieber–DeCarli control diet (Dyets#710027) were isocalorically matched to their corresponding EtOH group based on the diet consumptions of the previous day (PF). We have previously published data showing that under these conditions, mice reach blood ethanol concentrations of 150-200 mg/dL, a value close to twice the current legal limit of 80 mg/dL for driving while impaired and easily attainable in chronic alcoholics (Mercer et al. 2012). The dose of NAC chosen was that we have previously reported to be protective against alcoholic osteopenia in rats (Chen et al. 2012). The dose of Vit E chosen was based on the dose reported by Muhammad et al. (2012) to protect against ovariectomy-induced bone loss in female rats. At sacrifice, trunk blood was collected and femurs were frozen at −80°C for mRNA extraction. Right tibial bones were fixed in formalin for peripheral quantitative computerized tomography (pQCT) and micro-computed tomography (μCT) analyses, and left tibial bones were fixed in EtOH for immunohistochemistry.
pQCT Analyses
Ex vivo cortical BMD, cortical bone area, and trabecular BMD were measured in the tibias collected from all PF and EtOH-treated mouse groups using a STRATEC XCT Research SA+ pQCT (Orthometrix, White Plains, NY) in a blinded fashion as previously described (Shankar et al., 2008). Proximal tibias were analyzed using the manufacturer's software version 5.40. Three contiguous sections, 1 mm apart, distal to the proximal end were measured for cortical and trabecular BMD and cortical area with a spatial resolution of 100 μm. Thresholds of 207 mg/cm3 and 285 mg/cm3 were used to distinguish trabecular and cortical bone respectively. Average values for all slices were calculated for statistical analysis.
MicroCT Analyses
All MicroCT analyses were consistent with current guidelines for the assessment of bone microstructure in rodents (Bouxsein et al., 2010). Formalin-fixed tibias were imaged using a MicroCT 40 (Scanco Medical AG, Bassersdorf, Switzerland) using a 12 μm isotropic voxel size in all dimensions. The region of interest selected for analysis comprised 240 transverse CT slices representing the entire medullary volume extending 1.24 mm distal to the end of the primary spongiosa with a border lying 100 μm from the cortex. Bone was segmented from soft tissue using the same threshold, 247 mg HA/cm3 or trabecular bone. Fractional bone volume (bone volume/tissue volume; BV/TV) and architectural properties of trabecular bone (trabecular thickness [Tb.Th, mm], trabecular number [Tb.N, per mm], and connectivity density [Conn. D, mm3]) were calculated using previously published methods (Suva et al., 2008).
Real Time Reverse Transcriptase Polymerase Chain Reaction Analyses
Total RNA was isolated by crushing whole femur shaft in RLT buffer (Qiagen) and using the RNeasy RNA isolation kit (Qiagen) as per manufacturer’s instructions. All RNA was reverse transcribed using IScript cDNA synthesis (Bio-Rad Laboratories, Hercules, CA) according to manufacturer’s instructions, and subsequent real-time PCR analysis was performed using SYBR green and an ABI 7500 sequence detection system (Applied Biosystems, Foster City, CA). Gene expression from the entire femoral shaft was quantified using the deltaCT method relative to 18s then the appropriate control. Comparisons of raw CT values did not differ between groups, indicating that 18s was an appropriate normalizer. Gene-specific primers were: 18s – Fwd GAG GCC CTTG TAA TTG GAA TGA G, Rev CGC TAT TGG AGG TGG AAT TAC C; aP2 – Fwd CAA AAT GTG TGA TGC CTT TGT G, Rev CTC TTC CTT TGG CTC ATG CC; p21 – Fwd TGG GAT GCA CTG GGT GTT CT, Rev CCT TCC TCA CCT GTG TCG TCT T.
Estimation of fat volume/TV
To estimate the relative volume occupied by fat cells in the tibia, decalcified paraffin embedded histologic sections (5 μm) were stained with H&E. Fat area measurements were performed in the secondary spongiosa ~1 mm below the lowest portion of the growth plate using OsteoMeasure software (OsteoMetrics, Decatur, GA, USA). Measurements were performed throughout the entire width of the bone (until the cortical surface), covering area ~4–5 mm below the growth plate.
Number of proliferating chondrocytes in the tibial growth plate and assessment of bone length
The number of proliferating chondrocytes in the growth plate of control, EtOH-fed, and antioxidant-fed animals was enumerated from hematoxylin and eosin-stained histologic sections. Average numbers of cells in a ×400 magnification were counted in three separate fields from three mice per group using an EVOS-FL microscope (Advanced Microscopy Group, Bothell, WA) and imaging software (Olympus, Melville, NY). Proliferating chondrocytes were identified by their semi-flattened appearance, deep nuclear staining and location within the growth plate. The distance from the proximal tibial head to the tibia-fibula junction was assessed using digital calipers (Thermo Scientific, Rockford, IL, USA) and measurements were made on all formalin fixed tibias from all feeding groups.
Nitrotyrosine Staining
For immunohistochemical analysis, antigen retrieval was performed by heating sections in 10 mM sodium citrate buffer (pH 6.0) for 20 min. Endogenous peroxidase was quenched by incubating the sections with Peroxidase Suppressor (Thermo Scientific, Rockford, IL, USA) for 15 min at RT. The slides were blocked with Non-Serum Protein Block (Dako, Carpinteria, CA, USA) for 20 min at RT. Primary antibodies were prepared in antibody diluent solution (0.5% non-fat dry milk and 1% BSA in TBS) and incubated overnight at 4 °C. The concentration of primary antibody and dilution for Anti-Nitrotyrosine was 1:6000 (Millipore, Temecula, CA, USA). The specificity of the nitrotyrosine antibody binding was confirmed by blocking the antibody with 3-nitrotyrosine (10 mM). Immunoreactivity was detected by Dako Envision+ System-HRP (Dako, Carpinteria, CA, USA).
Statistical Analysis
Data were expressed as means ± S.E.M. Two-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc analysis was used to compare groups. Values were considered statistically significant at p < 0.05 and are reported as such.
RESULTS
Study Observations
There was no observed effect of liquid diet feeding with respect to weight gain, the final weights from each group were: control PF 22.7 g (S.E.M. 0.6), EtOH 22.3 g (S.E.M. 0.8), NAC 22.5 g (S.E.M. 0.5), NAC/EtOH 22.8 g (S.E.M. 0.3), Vit.E 23.3 g (S.E.M. 0.4), and Vit.E/EtOH 21.9 g (S.E.M. 0.6). Feeding of EtOH resulted in a blood ethanol concentration of 189 mg/dL (S.E.M. 28). In groups fed NAC with EtOH, blood ethanol concentration was 182 mg/dL (S.E.M. 28), and in the group fed Vit.E with EtOH, blood ethanol was 164.mg/dL (S.E.M. 36), There were no significant differences between blood EtOH levels in mice fed an antioxidant with EtOH and mice fed EtOH alone.
Dietary antioxidants protect against alcohol-induced trabecular bone loss but not cortical bone loss in female mice
pQCT analysis of fixed tibias showed that chronic alcohol consumption (EtOH) significantly reduced trabecular bone density and cortical bone density compared to pair-fed (PF) controls (Table 1). Neither antioxidant, N-acetyl cysteine (NAC) nor α-tocopherol (Vit.E) was able to protect against alcohol induced reduction of cortical bone density. However, Vit.E was partially protective against decreased cortical area due to alcohol (Table 1). Mice in the PF+NAC group exhibited significantly lower trabecular bone density compared to mice receiving the PF diet alone. However, pQCT showed that there were no significant differences between trabecular bone densities in either of the EtOH+antioxidant groups compared to their respective PF+antioxidant controls.
Table 1.
pQCT analysis of tibial bone from PF-control mice, EtOH-fed mice, NAC-fed, NAC+EtOH fed, Vit.E-fed, and Vit.E+EtOH-fed mice. Data are expressed as the mean with S.E.M. in parentheses. Statistical significance was determined by two-way ANOVA followed by Student Newman-Keuls post hoc analysis. Values with different letter subscripts are statistically different from each other (p<0.05).
| PF | EtOH | PF/NAC | EtOH/NAC | PF/Vit.E | EtOH/Vit.E | |
|---|---|---|---|---|---|---|
| pQCT Trabecular Bone | ||||||
| Density mg/cm3 | 222.17 (5.842)a | 186.43 (4.161)b | 193.73 (12.57)a | 196.34 (10.28)a | 210.06 (6.713)a | 205.89 (7.773)a |
| Area mm2 | 0.541 (0.035)a | 0.552 (0.052)a | 0.449 (0.540)b | 0.498 (0.026)b | 0.488 (0.029)b | 0.513 (0.044)b |
| pQCT Cortical Bone | ||||||
| Density mg/cm3 | 476.89 (3.807)a | 439.78 (4.075)b | 483.17(5.787)a | 443.75 (5.852)b | 477.70 (4.023)a | 433.63 (2.921)b |
| Area mm2 | 1.906 (0.032)a | 1.550 (0.040)b | 1.853 (0.041)a | 1.647 (0.026)b | 1.830 (0.041)a | 1.704 (0.064)a,b |
α-tocopherol and N-acetyl cysteine feeding resulted in different levels of protection against alcohol-induced trabecular bone loss
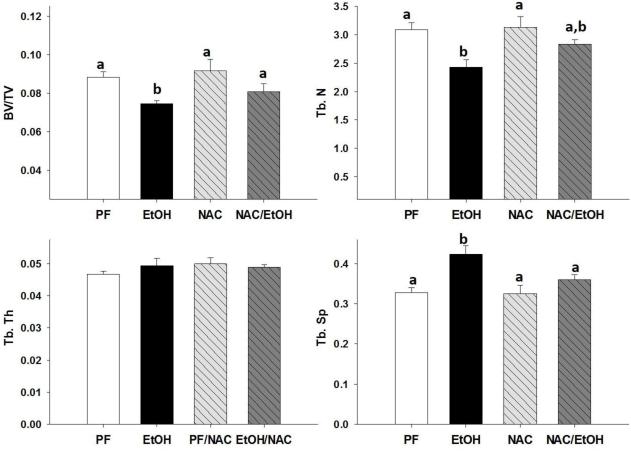
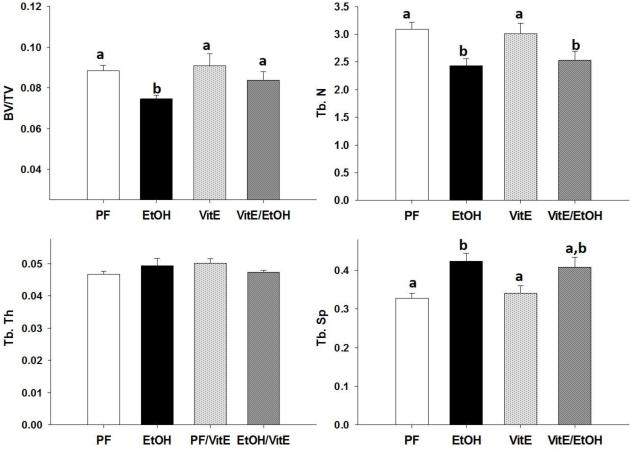
MicroCT analysis of trabecular bone performed on fixed tibias revealed that EtOH significantly reduced bone volume (BV/TV) and trabecular number (Tb.N) and increased trabecular separation (Tb.Sp.) compared to tibias from PF controls. There was no effect on trabecular thickness observed regardless of antioxidant supplementation, with or without alcohol (Figures 1 and 2 A, B, D). NAC completely protected against the EtOH induced decrease in BV/TV% and increase in Tb.Sp. NAC was partially protective against reduced Tb.N due to EtOH feeding (Figure 1 A, B, D). Vit.E completely protected against the decrease in BV/TV due to chronic EtOH and partially protected against the EtOH associated increase in Tb.Sp. However, Vit.E was unable to protect against the EtOH induced decrease in Tb.N (Figure 2 A, B, D).
Figure 1.
MicroCT analysis of tibial trabecular bone (A) BV/TV%, (B) Tb. N, (C) Tb. Th., (D) Tb. Sp., in EtOH-fed mice, PF-control mice, NAC-fed mice, and NAC+EtOH-fed mice. Data are expressed as the mean ± S.E.M. Statistical significance was determined by two-way ANOVA followed by Student Newman-Keuls post hoc analysis. Values with different letter subscripts are statistically different from each other (p<0.05).
Figure 2.
MicroCT analysis of tibial trabecular bone (A) BV/TV%, (B) Tb. N, (C) Tb. Th., (D) Tb. Sp., in EtOH-fed mice, PF-control mice, Vit.E-fed mice, and Vit.E+EtOH-fed mice. Data are expressed as the mean ± S.E.M. Statistical significance was determined by two-way ANOVA followed by Student Newman-Keuls post hoc analysis. Values with different letter subscripts are statistically different from each other (p<0.05).
N-acetyl cysteine completely blocks the increase of reactive oxygen species in bone due to chronic alcohol consumption
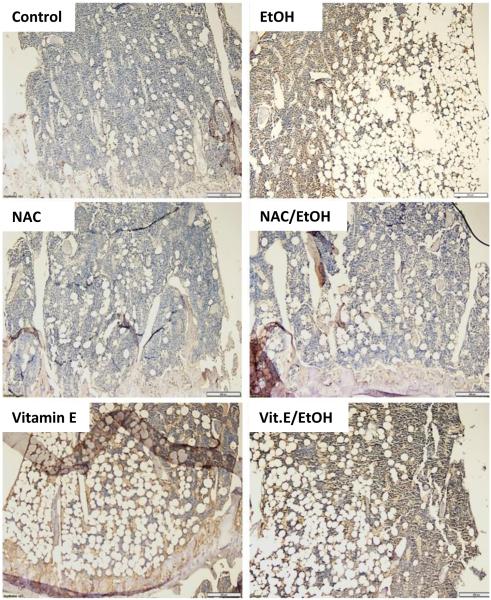
Previous studies from our lab have shown that the effects of chronic EtOH on bone can be attributed to increased oxidative stress (Chen et al., 2008;Chen et al., 2010;Chen et al., 2011;Mercer et al., 2014). It was shown in vitro, through DCF-fluorescence, that the addition of EtOH to osteoblast cultures caused an increase in oxidative stress and this could be blocked by NAC or estradiol (Chen et al., 2008). In this study we go further and demonstrate using nitrotyrosine staining, that chronic EtOH feeding increases ROS-associated protein adducts, and therefore that EtOH does indeed cause excess production of ROS in bone in vivo.
Tibias from EtOH fed mice show a dramatic increase in nitrotyrosine staining compared to PF controls (Figure 3). No staining was observed in tibia sections from mice fed NAC. Similarly, in sections from mice fed EtOH+NAC, there was no visible nitrotyrosine staining indicating complete protection Interestingly, in sections from mice fed Vit.E alone and sections from EtOH+Vit.E fed mice, nitrotyrosine staining was visible (Figure 3).
Figure 3.
Oxidative stress in the bone was measured by immunohistochemistry for protein-nitrotyrosine adducts (brown staining). Representative images (200x original magnification) showing the area directly under the tibial growth plate from bones of PF-control mice, EtOH-fed mice, NAC-fed, NAC+EtOH fed, Vit.E-fed, and Vit.E+EtOH-fed mice.
N-acetyl cysteine protects against the alcohol-induced increase in adipocyte protein 2 expression and the increase in number of bone marrow adipocytes
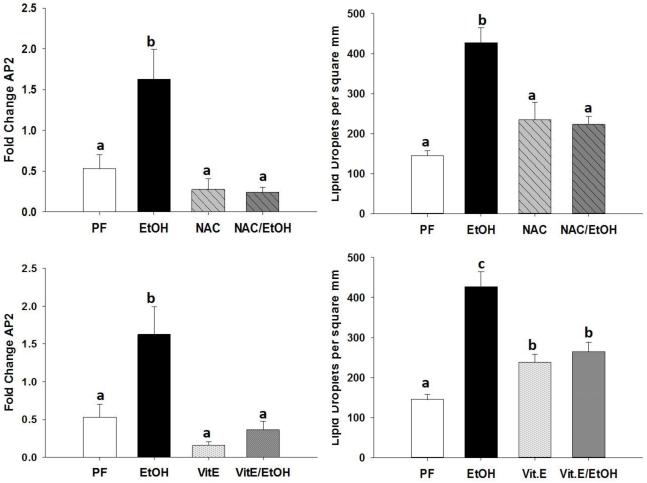
Adipocyte protein 2 (aP2) is a commonly used marker for fully differentiated adipocytes, aP2 has been used previously to measure marrow adiposity associated with alcohol consumption(Chen et al., 2008). Indeed, EtOH feeding significantly increased the expression of aP2 compared to PF control mice. Feeding NAC completely blocked the increase in aP2 associated with EtOH (Figure 4 A). Vit.E was also protective against the increase in aP2 expression due to EtOH (Figure 4 C). Decalcified sections from tibias were stained with H&E to assess marrow adiposity. Tibias from mice chronically fed EtOH exhibited a significantly higher number of adipocytes per square millimeter compared to PF control mice. Mice fed EtOH+NAC were protected against the increase in adipocyte number (Figure 4 B). Vit.E supplementation significantly increased the number of marrow adipocytes compared to controls, but there was no difference in adipocyte number between tibias from EtOH+Vit.E and Vit.E alone (Figure 4 D).
Figure 4.
Assessment of bone marrow adiposity by measuring (A and C) aP2 mRNA expression from femurs of PF-control mice, EtOH-fed mice, NAC-fed, NAC+EtOH fed, Vit.E-fed, and Vit.E+EtOH-fed mice. From H&E stained sections of tibias, number of marrow adipocytes per square millimeter were counted (B and D). Data are expressed as the mean ± S.E.M. Statistical significance was determined by two-way ANOVA followed by Student Newman-Keuls post hoc analysis. Values with different letter subscripts are statistically different from each other (p<0.05).
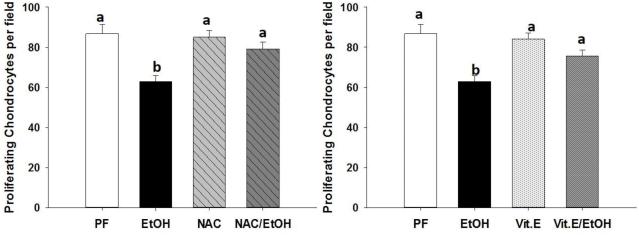
Both N-acetyl cysteine and α-tocopherol feeding prevent alcohol-induced reduction of proliferating chondrocytes
In tibia sections stained with H&E, proliferating chondrocytes in the growth plate were identified and counted. Previously we have observed that rats fed EtOH via total enteral nutrition have significantly reduced numbers of proliferating chondrocytes in the growth plate compared to controls (Shankar et al., 2006). We observed a similar phenomenon in mice fed EtOH using a Lieber-DeCarli liquid diet. NAC and Vit.E feeding were both able to block the alcohol-induced decrease in number of proliferating chondrocytes within the growth plate (Figure 5). Chronic alcohol feeding resulted in a significant, 1mm, reduction in the distance from the proximal tibia head to the tibia-fibula junction. Mice fed NAC exhibited a significantly reduced distance compared to control PF mice, but not to the degree of EtOH fed controls. There was no difference in tibia distances between mice fed EtOH+NAC and NAC alone (Table 2). Mice fed Vit.E also displayed a significantly lesser distance from the proximal tibia head to the tibia-fibula junction, but again not to the extent of EtOH fed control mice. Tibia distances were not statistically different between mice fed EtOH+Vit.E and Vit.E alone (Table2).
Figure 5.
Numbers of proliferating chondrocytes within the growth plate were counted (A and B) from H&E stained sections of tibias. Data are expressed as the mean ± S.E.M. Statistical significance was determined by two-way ANOVA followed by Student Newman-Keuls post hoc analysis. Values with different letter subscripts are statistically different from each other (p<0.05).
Table 2.
Bone length measurements of fixed tibias from PF-control mice, EtOH-fed mice, NAC-fed, NAC+EtOH fed, Vit.E-fed, and Vit.E+EtOH-fed mice. Data are expressed as the mean followed by the S.E.M. Statistical significance was determined by two-way ANOVA followed by Student Newman-Keuls post hoc analysis. Values with different letter subscripts are statistically different from each other (p<0.05).
| Diet | Distance from Tibial Head to Tibia-Fibula Junction (mm) |
S.E.M. |
|---|---|---|
| Pair Fed | 11.29a | 0.204 |
| EtOH | 10.21c | 0.061 |
| N-acetylcysteine | 10.53b | 0.114 |
| N-acetylcysteine + EtOH | 10.77b | 0.236 |
| α-tocopherol | 10.63b | 0.048 |
| α-tocopherol + EtOH | 10.71b | 0.127 |
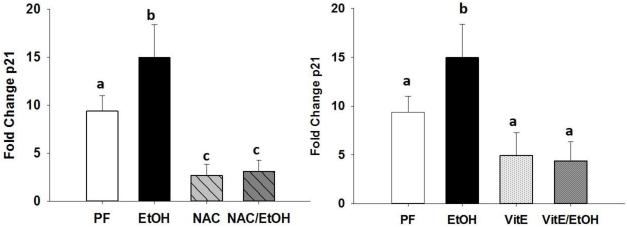
N-acetyl cysteine and α-tocopherol protect against alcohol associated increase in p21 expression
Aside from affecting osteoblastogenesis and osteoclastogenesis, it has been shown previously that alcohol stimulates bone cell senescence (Chen et al., 2009). mRNA expression of the senescence marker p21 was measured and there was a significant increase in femurs from mice fed EtOH. Both NAC and Vit.E feeding protected against the increase in p21 expression due to chronic alcohol consumption (Figure 6).
Figure 6.
Assessment of bone cell senescence by measuring (A and B) p21 mRNA expression from femurs of PF-control mice, EtOH-fed mice, NAC-fed, NAC+EtOH fed, Vit.E-fed, and Vit.E+EtOH-fed mice. Data are expressed as the mean ± S.E.M.
DISCUSSION
It is well established that EtOH-induced skeletal pathology is driven by ROS signaling pathways that reduce bone accrual by altering bone remodeling homeostasis. Dietary antioxidants have proven useful in preventing disease by reducing ROS signaling. In the present study, two dietary antioxidants, NAC or Vit E were shown to partially prevent EtOH-induced osteopenia by reducing ROS and its subsequent signaling effects. Female mice received EtOH liquid diets with or without antioxidant supplementation for 8 wks. As expected, chronic EtOH treatment induced a clear increase in ROS as measured by nitrotyrosine staining and resulted in decreased cortical and trabecular BMD (Table1). Both NAC and Vit.E were able to protect against trabecular bone loss due to EtOH but not against cortical bone loss. NAC has been shown to function by multiple mechanisms of action, with free radical scavenging capabilities as well as being able to increase intracellular glutathione, and it also is able to act as a reducing agent through a thiol-sulfide exchange activity (Zafarullah et al., 2003; Kim et al., 2001). Alpha-tocopherol was shown to have the highest antioxidant efficiency of all of the tocopherols and its primary function is to scavenge peroxyl radicals in order to protect poly unsaturated fatty acids and lipid membranes (Traber and Atkinson, 2007). These data suggest that the protective effects of NAC on EtOH-induced osteopenia are not unique or related solely to effects on GSH levels, but are more generally related to its antioxidant and free radical scavenging properties.
The broad effects of EtOH on bone turnover have been well documented (Turner, 2000; Chakkalakal, 2005; Ronis et al., 2011). EtOH also has several indirect effects on bone, including inhibition of growth hormone secretion (Badger et al., 1993), reductions in fibroblast growth factors (Zhao et al., 2015), and suppression of the calcium-Vitamin D axis (Shankar et al. 2008a; Mercer et al., 2012). In addition, EtOH has been shown to have direct apoptotic effects on bone cells and activate cellular senescence pathways (Mercer et al., 2012; Chen et al., 2009). In a rat model of total enteral nutrition (TEN), the feeding of chronic EtOH resulted in decreased bone mineral density, trabecular bone volume, and osteoblast number while increasing trabecular separation, eroded surface, and osteoclast number (Chen et al., 2011). These effects were completely ameliorated when rats were fed either the antioxidant NAC or the pan-NADPH oxidase inhibitor diphenyleneiodonium (DPI), which suggested a role for NADPH oxidases in the pathology of alcohol in bone.
Previous studies using a rat TEN model have demonstrated that oxidative stress caused by ethanol impacts the lineage commitment of mesenchymal stromal cells. Specifically, ethanol was shown to inhibit β-catenin translocation to the nucleus thereby blocking Wnt/ β-catenin signaling reducing osteoblastogenesis and promoting adipogenesis. These EtOH effects were blocked by treatment with NAC (Shankar et al. 2008b; Chen et al. 2010; 2011). Marrow adipocytes and bone marrow adiposity are becoming increasingly recognized as important regulators of bone remodeling (Kawai et al., 2012;Scheller et al., 2014). There is now compelling evidence that bone marrow adipose tissue acts as an endocrine organ, specifically the inhibition of marrow adipose tissue accumulation elevates levels of adiponectin (Cawthorn et al., 2014). In this study, both NAC and Vit.E were able to block the EtOH-induced increase in aP2 mRNA expression in femur and that NAC protected against the increase in number of marrow adipocytes per square millimeter due to chronic ethanol consumption. Vit.E feeding alone increased the number of marrow adipocytes compared to PF controls and no difference was observed between Vit.E and EtOH+Vit.E groups. It is possible that at the dose used in these studies, Vit.E is having pro-oxidant effects by itself. EtOH induced ROS clearly impacts mesechymal stromal cell differentiation and thus the bone marrow cellular milleu. It is likely that chronic EtOH consumption is also impacting the endocrine activity of bone marrow adipose tissue. Further studies are needed to determine the impact of EtOH on marrow adipose tissue’s endocrine control of adiponectin.
Chondrocytes are another bone cell phenotype derived from mesenchymal stromal cells (Johnstone et al., 1998) which are essential for normal endochondral bone formation and long bone growth. Both chondrogenesis and chondrocyte hypertrophy are activated by canonical Wnt/β-catenin signaling specifically through Sox9 (Yano et al., 2005). Among the many negative effects of chronic EtOH consumption on bone, EtOH has been shown to both reduce the number of proliferating chondrocytes in the growth plate of rats (Shankar et al., 2006) and block Wnt/β-catenin signaling in mesenchymal stromal cells by inhibiting the translocation of β-catenin to the nucleus (Chen et al., 2010). Previously, ROS has been shown to impact chondrocyte differentiation in vitro through the stimulation of MAPK/ERK-activating kinase (MEK/ERK) pathway and via p38 (Morita et al., 2007). We have previously demonstrated that EtOH causes sustained ERK expression in osteoblasts which drives RANKL induction (Chen et al., 2006). In this study, we show for the first time that both antioxidants, NAC and Vit.E, were able to block the inhibitory effect of EtOH on chondrogenesis, suggesting that EtOH-induced ROS inhibits chondrogenic signaling in mice in vivo.
Chondrocyte proliferation and differentiation is critical for bone lengthening (Kronenberg, 2003). As expected, EtOH-fed mice that had less proliferating chondrocytes had significantly shorter bones. The measurement of bone length in this study, from the proximal tibia head to the tibia-fibula junction, was chosen to eliminate any potential error in the total tibia length which could occur during dissection of the distal tibia. Measuring from the proximal tibia head to the tibia-fibula junction provided a distance between two unbiased fixed points. While NAC and Vit.E were able to protect against the alcohol-induced decrease in proliferating chondrocytes, mice fed the antioxidants with EtOH had longer bones compared to EtOH-fed groups but that they were still significantly shorter than bones from PF-control mice. Elevated ROS in growth plates of mice has previously been shown to disrupt the columnar proliferation of chondrocytes and their hypertrophic differentiation (Morita et al., 2007). Our data suggests that in addition to ROS-mediated effects on chondrogenesis, indirect, non-ROS mediated effects of chronic alcohol consumption on growth hormone/IGF-1 signaling (Badger et al., 1993; Srivastava et al. 2002; Wang et al. 2004); the calcium/Vitamin D axis (Shankar et al. 2008a, Mercer et al., 2012) or effects on regulation of local growth factors in the growth plate such as Indian hedgehog, FGFs, or BMPs (Wei et al. 2016) may also significantly impact bone lengthening.
EtOH has been implicated in stimulating bone cell senescence by increasing estrogen receptor alpha and beta mRNA expression and by activating p53 and p21(Chen et al., 2009). We observed that NAC and Vit.E completely protected against the increase in p21 mRNA expression associated with EtOH in vivo. There are previous reports of ROS being important for regulating tumor suppressor genes and senescence(Colavitti and Finkel, 2005;Rufini et al., 2013). We have previously reported that estradiol treatment is able to suppress EtOH-induced p21 expression and β-galactosidase activity and suggested that ROS is involved in the negative cross-talk of estradiol on EtOH signaling in bone cells in vitro (Chen et al., 2009). These findings confirm that alcohol-induced ROS is involved in the stimulation of bone cell senescence.
Mice that received EtOH+NAC or EtOH+Vit.E in this study were protected against trabecular bone loss but not cortical bone loss due to EtOH. This suggests compartment-specific effects of EtOH on bone in the mouse. It is possible that previously reported endocrine disruption produced by chronic EtOH consumption such as reductions in plasma estradiol or calcium and vitamin D3 (Shankar et al. 2006; Shankar et al.2008a) are responsible for ROS-independent cortical bone loss. Alternatively, previously reported apoptotic effects of EtOH on osteocytes may impact the cortical bone more than the trabecular bone in this species (Mercer et al. 2012). The findings of this manuscript also contribute evidence to support the idea that the trabecular and cortical compartments of bone are regulated differently. Recent reports suggest that the cortical compartment is regulated through estrogen receptor signaling, specifically estrogen receptor alpha activating factor 2 (Borjesson et al., 2011; Khosla et al., 2011; Borjesson et al., 2013). Moreover, a novel member of the Wnt family Wnt16 has recently been shown to have compartment-specific effects on bone mass (Moverare-Skrtic et al. 2015). We and others have previously reported that chronic EtOH treatment results in extensive disruption of the Wnt-β-catenin system which is critical for osteoblastogenesis, including reduced expression of a number of Wnts including Wnt3a (Chen et al., 2010, Callaci et al. 2010; Lauing et al. 2012). However, effects of EtOH on expression of Wnt16 in bone are currently unknown.
In conclusion, the findings presented here demonstrate that chronic EtOH is causing elevated ROS production in the bone which contributes to its negative effects. EtOH-induced ROS not only impacts bone formation and resorption, but as we demonstrate here also impacts bone cell senescence and, report for the first time in the mouse, also impacts chondrocyte proliferation and subsequent long bone length. It is clear from these findings that ROS signaling is involved in many different processes in bone, including regulation of trabecular bone turnover, and the disruption of those signals by EtOH is highly detrimental to the skeleton. Collectively, these data demonstrate that concurrent feeding of both water- and lipid-soluble dietary antioxidants NAC and Vit.E are able to block specific effects but not all of the effects of alcohol consumption on the skeleton of growing female mice.
Acknowledgments
Footnotes
Funded in part by NIH National Institute on Alcohol Abuse and Alcoholism [R37 AA018282] (M.J.J.R), National Institute of General Medical Sciences [T32GM106999-01] "Systems Pharmacology and Toxicology” Training Program (University of Arkansas for Medical Sciences), University of Arkansas for Medical Sciences and University of Arkansas for Medical Sciences CUMG Funds.
Footnotes
Authorship Contributions
Participated in research design: Ronis, Mercer, Alund, Chen, Badger
Conducted Experiments: Alund, Pulliam, Suva
Performed data analysis: Alund
Wrote or contributed to the writing of the manuscript: Alund, Ronis, Suva, Mercer
The authors of this manuscript declare no conflicts of interest.
Reference List
- Badger TM, Ronis MJ, Lumpkin CK, Valentine CR, Shahare M, Irby D, Huang J, Mercado C, Thomas P, Ingelman-Sundberg M. Effects of chronic ethanol on growth hormone secretion and hepatic cytochrome P450 isozymes of the rat. J.Pharmacol.Exp.Ther. 1993;264:438–447. [PubMed] [Google Scholar]
- Balaban YH, Korkusuz P, Simsek H, Gokcan H, Gedikoglu G, Pinar A, Hascelik G, Asan E, Hamaloglu E, Tatar G. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann.Hepatol. 2007;6:242–250. [PubMed] [Google Scholar]
- Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA, Jr., Malik R, Arnsten JH. Association between alcohol consumption and both osteoporotic fracture and bone density. Am.J.Med. 2008;121:406–418. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borhanuddin B, Mohd Fozi NF, Naina M. Vitamin e and the healing of bone fracture: the current state of evidence. Evid.Based.Complement Alternat.Med. 2012;2012:684510. doi: 10.1155/2012/684510. I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjesson AE, Farman HH, Engdahl C, Koskela A, Sjogren K, Kindblom JM, Stubelius A, Islander U, Carlsten H, Antal MC, Krust A, Chambon P, Tuukkanen J, Lagerquist MK, Windahl SH, Ohlssen C. The role of activation functions 1 and 2 of estrogen receptor-α for the effects of estradiol and selective estrogen receptor modulators in male mice. J Bone Miner Res. 2013;28(5):1117–26. doi: 10.1002/jbmr.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjesson AE, Windahl SH, Lagerquist MK, Engdahl C, Frenkel B, Moverare-Skrtic S, Sjogren K, Kindblom JM, Stubelius A, Islander U, Antal MC, Krust A, Chambon P, Ohlssen C. Roles of transactivating function 1 and 2 of estrogen receptor-alpha in bone. Proc Natl Acad Sci U S A. 2011;108(15):6288–93. doi: 10.1073/pnas.1100454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaci JJ, Himes R, Lauing K, Roper P. Long-term modulations in the vertebral transcriptome of adolescent-stage rats exposed to binge alcohol. Alcohol Alcohol. 2010;45:332–346. doi: 10.1093/alcalc/agq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JJ, Picklo MJ. N-acetylcysteine supplementation decreases osteoclast differentiation and increases bone mass in mice fed a high-fat diet. J.Nutr. 2014;144:289–296. doi: 10.3945/jn.113.185397. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, Bredella MA, Fazeli PK, Klibanski A, Horowitz MC, Rosen CJ, Macdougald OA. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin.Exp.Res. 2005;29:2077–2090. doi: 10.1097/01.alc.0000192039.21305.55. [DOI] [PubMed] [Google Scholar]
- Chen JR, Haley RL, Hidestrand M, Shankar K, Liu X, Lumpkin CK, Simpson PM, Badger TM, Ronis MJ. Estradiol protects against ethanol-induced bone loss by inhibiting up-regulation of receptor activator of nuclear factor-kappaB ligand in osteoblasts. J.Pharmacol.Exp.Ther. 2006;319:1182–1190. doi: 10.1124/jpet.106.109454. [DOI] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Haley RL, Blackburn ML, Badger TM, Ronis MJ. Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways, whereas estradiol attenuates the effects of ethanol in osteoblasts. J.Bone Miner.Res. 2009;24:221–230. doi: 10.1359/jbmr.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ. A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. J.Bone Miner.Res. 2010;25:1117–1127. doi: 10.1002/jbmr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Lumpkin CK, Badger TM, Ronis MJ. Inhibition of NADPH oxidases prevents chronic ethanol-induced bone loss in female rats. J.Pharmacol.Exp.Ther. 2011;336:734–742. doi: 10.1124/jpet.110.175091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Shankar K, Nagarajan S, Badger TM, Ronis MJ. Protective effects of estradiol on ethanol-induced bone loss involve inhibition of reactive oxygen species generation in osteoblasts and downstream activation of the extracellular signal-regulated kinase/signal transducer and activator of transcription 3/receptor activator of nuclear factor-kappaB ligand signaling cascade. J.Pharmacol.Exp.Ther. 2008;324:50–59. doi: 10.1124/jpet.107.130351. [DOI] [PubMed] [Google Scholar]
- Chen JR, Zhang J, Lazarenko OP, Cao JJ, Blackburn ML, Badger TM, Ronis MJ. Soy protein isolates prevent loss of bone quantity associated with obesity in rats through regulation of insulin signaling in osteoblasts. FASEB J. 2013;27:3514–3523. doi: 10.1096/fj.12-226464. [DOI] [PubMed] [Google Scholar]
- Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB.Life. 2005;57:277–281. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- Dai J, Lin D, Zhang J, Habib P, Smith P, Murtha J, Fu Z, Yao Z, Qi Y, Keller ET. Chronic alcohol ingestion induces osteoclastogenesis and bone loss through IL-6 in mice. J.Clin.Invest. 2000;106:887–895. doi: 10.1172/JCI10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J.Mol.Cell Cardiol. 2015;89:122–135. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Filaire E, Dupuis C, Galvaing G, Aubreton S, Laurent H, Richard R, Filaire M. Lung cancer: what are the links with oxidative stress, physical activity and nutrition. Lung Cancer. 2013;82:383–389. doi: 10.1016/j.lungcan.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Fujita K, Iwasaki M, Ochi H, Fukuda T, Ma C, Miyamoto T, Takitani K, Negishi-Koga T, Sunamura S, Kodama T, Takayanagi H, Tamai H, Kato S, Arai H, Shinomiya K, Itoh H, Okawa A, Takeda S. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat.Med. 2012;18:589–594. doi: 10.1038/nm.2659. [DOI] [PubMed] [Google Scholar]
- Hardbower DM, de Sablet T, Chaturvedi R, Wilson KT. Chronic inflammation and oxidative stress: the smoking gun for Helicobacter pylori-induced gastric cancer? Gut Microbes. 2013;4:475–481. doi: 10.4161/gmic.25583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp.Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- Kawai M, de Paula FJ, Rosen CJ. New insights into osteoporosis: the bone-fat connection. J.Intern.Med. 2012;272:317–329. doi: 10.1111/j.1365-2796.2012.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Melton LJ, 3rd, Riggs BL. The unitary model for estrogen receptor deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res. 2011;26(3):441–51. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Rhim T, Choi I, Kim SS. N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J.Biol.Chem. 2001;276:40591–40598. doi: 10.1074/jbc.M100975200. [DOI] [PubMed] [Google Scholar]
- Kong Y, Trabucco SE, Zhang H. Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Interdiscip.Top.Gerontol. 2014;39:86–107. doi: 10.1159/000358901. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Lauing KL, Roper PM, Nauer RK, Callaci JJ. Acute alcohol exposure impairs fracture healing and deregulates β-catenin signaling in the fracture callus. Alc. Clin. Exp. Res. 2012;36:2095–2103. doi: 10.1111/j.1530-0277.2012.01830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer KE, Sims CR, Yang CS, Wynne RA, Moutos C, Hogue WR, Lumpkin CK, Suva LJ, Chen JR, Badger TM, Ronis MJ. Loss of functional NADPH oxidase 2 protects against alcohol-induced bone resorption in female p47phox−/− mice. Alcohol Clin.Exp.Res. 2014;38:672–682. doi: 10.1111/acer.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer KE, Wynne RA, Lazarenko OP, Lumpkin CK, Hogue WR, Suva LJ, Chen JR, Mason AZ, Badger TM, Ronis MJ. Vitamin D supplementation protects against bone loss associated with chronic alcohol administration in female mice. J.Pharmacol.Exp.Ther. 2012;343:401–412. doi: 10.1124/jpet.112.197038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Miyamoto T, Fujita N, Kubota Y, Ito K, Takubo K, Miyamoto K, Ninomiya K, Suzuki T, Iwasaki R, Yagi M, Takaishi H, Toyama Y, Suda T. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J.Exp.Med. 2007;204:1613–1623. doi: 10.1084/jem.20062525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Wu J, Henning P, Gustafsson KL, Sjogren K, Windahl SH, Koskela A, Tuukkanen J, Borjesson AE, Lagerquist MK, Lerner UH, Zhang F-P, Gustafsson J-A, Poutanen M, Ohlsson C. The bone sparing effects of estrogen and WNT16 are independent of each other. Proc. Natl. Acad. Sci. U.S.A. 2015;112:14972–14977. doi: 10.1073/pnas.1520408112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad N, Luke DA, Shuid AN, Mohamed N, Soelaiman IN. Two different isomers of vitamin e prevent bone loss in postmenopausal osteoporosis rat model. Evid.Based.Complement Alternat.Med. 2012;2012:161527. doi: 10.1155/2012/161527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos A, Pandya R, Duivenvoorden WC, Pinthus JH. Oxidative stress in prostate cancer: changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic.Dis. 2013;16:217–225. doi: 10.1038/pcan.2013.13. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Mercer K, Chen JR. Effects of nutrition and alcohol consumption on bone loss. Curr.Osteoporos.Rep. 2011;9:53–59. doi: 10.1007/s11914-011-0049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper PM, Abbasnia P, Vuchkovska A, Natoli RM, Callaci JJ. Alcohol-related deficient fracture healing is associated with activation of FoxO transcription factors in mice. J. Orthop. Res. 2016 doi: 10.1002/jor.23235. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013;32:5129–5143. doi: 10.1038/onc.2012.640. [DOI] [PubMed] [Google Scholar]
- Sampson HW. Alcohol and other factors affecting osteoporosis risk in women. Alcohol Res.Health. 2002;26:292–298. [PMC free article] [PubMed] [Google Scholar]
- Scheller EL, Troiano N, Vanhoutan JN, Bouxsein MA, Fretz JA, Xi Y, Nelson T, Katz G, Berry R, Church CD, Doucette CR, Rodeheffer MS, Macdougald OA, Rosen CJ, Horowitz MC. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol. 2014;537:123–139. doi: 10.1016/B978-0-12-411619-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar K, Hidestrand M, Haley R, Skinner RA, Hogue W, Jo C-H, Simpson P, Lumpkin CK, Aronson J, Badger TM, Ronis MJJ. Different molecular mechanisms underlie ethanol-induced bone loss in cycling and pregnant rats. Endocrinology. 2006;147:166–178. doi: 10.1210/en.2005-0529. [DOI] [PubMed] [Google Scholar]
- Shankar K, Haley R, Badger TM, Lumpkin CK, Chen J-R, Nagaragian S, Liu X, Ronis MJJ. Disruption of vitamin D3 homeostasis in rats fed ethanol via total enteral nutrition is the result of induction of CYP24A1. Endocrinology. 2008a;149:1748–1756. doi: 10.1210/en.2007-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar K, Hidestrand M, Liu X, Chen JR, Haley R, Perrien DS, Skinner RA, Lumpkin CK, Jr., Badger TM, Ronis MJ. Chronic ethanol consumption inhibits postlactational anabolic bone rebuilding in female rats. J.Bone Miner.Res. 2008b;23:338–349. doi: 10.1359/jbmr.071023. [DOI] [PubMed] [Google Scholar]
- Sims NA, Vrahnas C. Regulation of cortical and trabecular bone mass by communication between osteoblasts, osteocytes and osteoclasts. Arch.Biochem.Biophys. 2014;561C:22–28. doi: 10.1016/j.abb.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Dearth RK, Hiney JK, Chandrashekar V, Mattison JA, Bartke A, Dees WL. Alcohol suppresses insulin-like growth factor-1 gene expression in prepubertal transgenic female mice overexpressing the bovine growth hormone gene. Alc. Clin. Exp. Res. 2002;26:1697–1702. doi: 10.1097/01.ALC.0000036922.18456.EF. [DOI] [PubMed] [Google Scholar]
- Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic.Biol.Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT. Skeletal response to alcohol. Alcohol Clin.Exp.Res. 2000;24:1693–1701. [PubMed] [Google Scholar]
- Turner RT, Rosen CJ, Iwaniec UT. Effects of alcohol on skeletal response to growth hormone in hypophysectomized rats. Bone. 2010;46:806–812. doi: 10.1016/j.bone.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA. Evidence supporting dual, IGF-1 independent and IGF-1-dependent, role for growth hormone in promoting longitudinal bone growth. J. Endocrinol. 2004;180:247–255. doi: 10.1677/joe.0.1800247. [DOI] [PubMed] [Google Scholar]
- Wei X, Hu M, Mishina Y, Liu F. Developmental regulation of the growth plate and cranial synchondrosis. J. Dent. Res. 2016 doi: 10.1177/0022034516651823. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezeman FH, Emanuele MA, Moskal SF, Steiner J, Lapaglia N. Alendronate administration and skeletal response during chronic alcohol intake in the adolescent male rat. J.Bone Miner.Res. 2000;15:2033–2041. doi: 10.1359/jbmr.2000.15.10.2033. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tsukimura N, Ikeda T, Sugita Y, Att W, Kojima N, Kubo K, Ueno T, Sakurai K, Ogawa T. N-acetyl cysteine as an osteogenesis-enhancing molecule for bone regeneration. Biomaterials. 2013;34:6147–6156. doi: 10.1016/j.biomaterials.2013.04.064. [DOI] [PubMed] [Google Scholar]
- Yang CS, Mercer KE, Alund AW, Suva LJ, Badger TM, Ronis MJ. Genistein supplementation increases bone turnover but does not prevent alcohol-induced bone loss in male mice. Exp.Biol.Med.(Maywood.) 2014;239:1380–1389. doi: 10.1177/1535370214532759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano F, Kugimiya F, Ohba S, Ikeda T, Chikuda H, Ogasawara T, Ogata N, Takato T, Nakamura K, Kawaguchi H, Chung UI. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem.Biophys.Res.Commun. 2005;333:1300–1308. doi: 10.1016/j.bbrc.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol.Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi M. Skeletal remodeling in health and disease. Nat.Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lazarenko OP, Kang J, Blackburn ML, Ronis MJ, Badger TM, Chen JR. Feeding blueberry diets to young rats dose-dependently inhibits bone resorption through suppression of RANKL in stromal cells. PLoS.One. 2013;8:e70438. doi: 10.1371/journal.pone.0070438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Liu Y, Xiao J, Liu L, Chen S, Mohammadi M, McClain CJ, Li X, Feng W. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J.Lipid Res. 2015;56:1481–1491. doi: 10.1194/jlr.M058610. [DOI] [PMC free article] [PubMed] [Google Scholar]