Abstract
Patients with sickle cell anemia (SCA) have abnormal hemoglobin (sickle hemoglobin S) leading to the crystallization of hemoglobin chains in red blood cells (RBCs), which assume sickle shape and display reduced flexibility. Sickle RBCs (sRBCs) adhere to vessel walls and block blood flow, thus preventing oxygen delivery to the tissues leading to vaso-occlusive crises (VOC), acute pain and organ damage. SCA patients often have chronic pain that can be attributed to inflammation, vasculopathy, neuropathy, ischemia-reperfusion injury and organ damage. Blood oxygenation level-dependent (BOLD) based functional magnetic resonance imaging (fMRI) technique that is commonly used for noninvasively mapping spontaneous or evoked brain activation in human or animal models has been applied in this study to assess abnormal oxygenation in the brains of mice with SCA. We found that hyperalgesic HbSS-BERK sickle mice with chronic pain display reduced BOLD response to hypoxia manipulation compared to their control HbAA-BERK mice. Hypoxia/reoxygenation (H/R) treated sickle mice under acute pain episode exhibit even smaller BOLD signal changes than sickle mice without H/R, suggestive of correlations between cerebral BOLD signal changes and nociception.
1. Introduction
Previous functional magnetic resonance imaging (fMRI) studies in transgenic mice with sickle cell anemia (SCA) showed positive and larger Blood oxygenation level-dependent (BOLD) responses in transgenic S+SAntilles and NYKO1 mice compared to C57BL/6 control mice under hyperoxia conditions [1]. The hematocrit and P50 of S+SAntilles mice are similar to control mice but show moderately severe disease. In contrast, NY1KO-γH model with a slightly reduced hematocrit and P50 is the least severe of sickle mice showing varying levels of pathology depending upon the expression of hemoglobin F [1,2]. Notably, the S+SAntilles mice express approximately 42% of human βS and 36% of βS-Antilles and show vascular pathology and inflammation, which are further exacerbated by hypoxia, but do not show the severity of pain observed in SCA [3,4]. In the present study, HbSS-BERK transgenic sickle mice and control HbAA-BERK mice were utilized since HbSS-BERK mice express exclusively human α and βS globin chains with ~99% human HbS, but no murine α or β globins [5], exhibiting the characteristic features of sickle pain including chronic hyperalgesia and acute pain evoked by hypoxia/reoxygenation (H/R) [4,6].
The purpose of this preliminary fMRI investigation is to explore the correlation between cerebral BOLD signal changes and nociception in HbSS-BERK sickle mice that shows the hematologic, pathologic and nociceptive features observed in sickle patients.
2. Materials and methods
2.1 Measurement of fMRI BOLD and arterial oxygenation saturation
The fMRI BOLD measurements were conducted on 9.4T horizontal animal scanner and HbAA-, and HbSS-BERK mice under normoxia and following H/R were scanned at different conditions by varying the fraction of inspired oxygen (FiO2) from 40% (baseline) to as low as 8% and as high as 80%. Their arterial oxygen saturation levels at each condition were also measured using mouse pulse oximetry.
2.2 Pain measurement and H/R performance
Hyperalgesia assessed with paw withdrawal frequency (PWF) as well as H/R treatment were described previously [7,8]. PWF was measured before the fMRI scan and 1 & 18 h after two treatments of H/R.
The experimental details can be found in the supplement material.
3. Results
3.1 Sickle mice with chronic and acute pain episodes display reduced change of BOLD signal under hypoxia
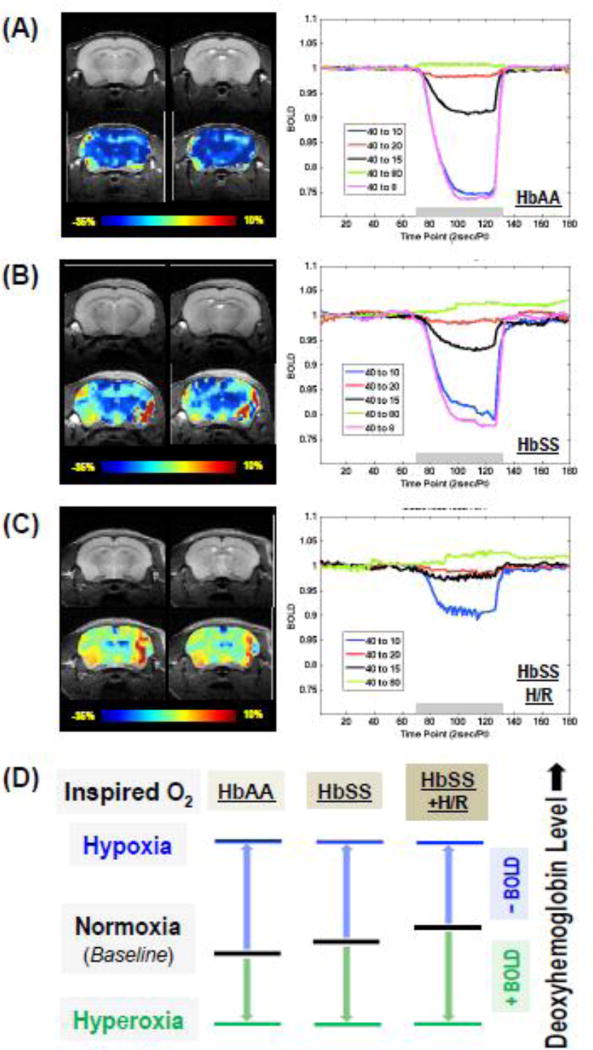
Coronal anatomical image and fMRI BOLD map of mouse brain were obtained in control HbAA-BERK and sickle HbSS-BERK mice before and under H/R (HbSS + H/R). HbAA-BERK mice had BOLD response in time- and oxygen level-dependent manner (Fig.1A), which showed a relatively smooth change from the beginning to the end during a 2 min FiO2 manipulation. The amplitude of the negative BOLD response increased with the reduction of inspired oxygen fraction. Comparatively, sickle mice display weakened BOLD response (Fig.1B), especially when the FiO2 was lower than room air. We also observed a slightly increased positive BOLD response in HbSS-BERK mice under hyperoxia (i.e., FiO2 = 80%) compared to HbAA-BERK (Fig.1A–1B). This finding is consistent with previous observations in sickle mice under hyperoxia (FiO2 = 100%) [1]. Furthermore, the sickle mice pretreated with H/R exhibit even smaller BOLD signal changes at the same condition than that in the HbSS-BERK without H/R (Fig.1B–1C). The correlation between BOLD response change and deoxyhemoglobin level is shown in Fig.1D. The results of fMRI measurements from all animals are summarized in Table 1, which also includes the levels of arterial oxygen saturation obtained in these mice.
Figure 1. Sickle mice show decreased BOLD response to hypoxia.
Representative anatomical and BOLD fMRI images with FiO2 changed from 40% to 10% for 2 minutes (left panels in A–C, color bar indicates the BOLD response in percentage unit), as well as the BOLD time-course within a selected ROI in response to the hypoxia/hyperoxia manipulations (right panels in A–C, FiO2 at 40% as baseline and at 80/20/15/10/8% for 2 minutes during the hypoxia and hyperoxia as indicated by the gray bars) are shown for HbAA (A, n=6), HbSS (B, n=6) and HbSS pretreated with H/R (C, n=2) mice. Note: for ROI selection, the dark red colored areas in the fMRI maps were due to the imaging artifacts, and are excluded from the BOLD quantification. (D) Schematic illustration of the comprehensive correlations between the FiO2 and the change of venous deoxyhemoglobin content (it implies the BOLD percent change) in response to a severe hypoxia challenge or a high degree of hyperoxia; and the distinctions among HbAA control versus HbSS sickle mice with or without H/R. Abbreviations: BOLD, blood oxygen level-dependent; HbAA, HbAA-BERK control mice; HbSS, HbSS-BERK sickle mice; H/R, hypoxia/reoxygenation; Pt, point; sec, second; FiO2, fraction of inspired oxygen; ROI, region of interest.
Table 1.
BOLD responses of the sickle and control mice brains to the hypoxia or hyperoxia manipulation and their corresponding oxygen saturation levels in the arterial blood.
| Fraction of Inspired Oxygen (FiO2) |
BOLD Response (%) | Oxygen Saturation (%) | ||
|---|---|---|---|---|
| HbAA | HbSSa | HbAA | HbSSa | |
| 40% (Baseline) | - | - | 98.9 ± 0.4 | 98.3 ± 0.9 |
| 8–10% | −24.7 ± 5.8 | −15.0 ± 3.9** | 84.1 ± 1.8 | 84.3 ± 6.6 |
| 15–20% | −5.7 ± 2.5 | −3.3 ± 1.4* | 98.1 ± 0.6 | 96.8 ± 2.0 |
| 80% | 0.3 ± 1.3 | 0.6 ± 1.3 | - | 99.0 |
HbSS mice with and without H/R treatment; Statistic Significance (2-tailed t-test):
p < 0.05;
p < 0.005.
3.2 Hyperalgesia assessment in sickle mice may be related to BOLD response
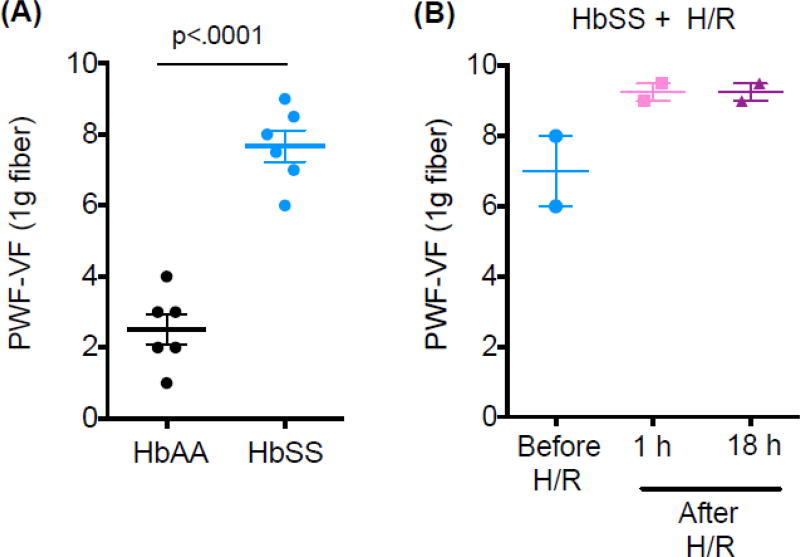
In line with our previous studies [4], we found HbSS-BERK showing significant hyperalgesia as compared to control mice (Fig.2A), which was further worsened after two H/R treatments (Fig.2B). SCA patients also show decreased activity in default-mode-network (DMN) during resting state as compared to healthy subjects [9], which may correlate with SCA-associated chronic pain. Herein, we found that brain regions in sickle mice with chronic hyperalgesia showing minimum BOLD signal change during hypoxia were located in thalamus, specifically in the regions of cerebral crus and zona incerta (Fig.1B, left panel), which are known for their involvement in controlling pain. This finding suggests a low tissue oxygenation level in these brain regions even at normoxia condition. Notably, the basal tissue oxygenation could be further reduced in H/R pretreated sickle mice undergoing acutely evoked hyperalgesia since less BOLD signal changes are observed in these areas during similar hypoxia manipulation (Fig.1C, left panel).
Figure 2. Pain assessments in HbAA control mice and HbSS mice with hyperalgesia.
Baseline of paw withdrawal frequency was measured before fMRI scan in all subjects (HbAA, HbSS; n=6 per group) as well as at 1 &18 h post two treatments of hypoxia/reoxygenation (H/R) in HbSS + H/R mice (n=2). Data are presented as mean ± SEM. Significance is determined by t-test (Unpaired, Two-tailed). Abbreviations: HbAA, HbAA-BERK control mice; HbSS, HbSS-BERK sickle mice; H/R, hypoxia/reoxygenation; PWF, paw withdrawal frequency; VF, von Frey.
4. Discussion
Brain is extremely sensitive to changes in oxygenation and involved in the perception of pain [10]. The detected BOLD contrast (presented as percentage changes of the T2- or T2*-weighted MR signals, T2: transverse relaxation time, T2*: apparent T2) in brain region of interest (ROI) is dependent on the relative changes of the deoxyhemoglobin concentration (or the blood oxygen level in veins and capillaries), which is determined by hemodynamic (cerebral blood flow and volume) and metabolic (cerebral oxygen consumption rate) response associated with the brain activation or other alteration [10,11]. The BOLD contrast, therefore, should be more sensitive to the sickle hemoglobin or sickle red blood cells (sRBCs) in the sickle mice brain because significant presence of defective hemoglobin would lead to lower oxygenation level or higher deoxyhemoglobin content in HbSS mice, while the control mice without sRBCs have better oxygenation or less deoxyhemoglobin at the same basal (e.g., normoxia) condition. When introducing hypoxia to these two animal groups, their BOLD responses are expected to be significantly different assuming a comparable stable cerebral oxygen consumption rate. This hypothesis was confirmed by the experimental results obtained in this study.
It has been shown that the HbSS-BERK mice presented about 29.1% sRBCs and control HbAA-BERK does not show any sRBCs [8]. Moreover, H/R induces approximately 10-fold increase in sRBCs in transgenic sickle mice [12]. We have previously found that HbSS-BERK mice exhibit markedly increased hyperalgesia after two consecutive H/R treatments [4]. These data suggest a positive relationship between sickle hemoglobin level and hyperalgesia. Interestingly, we observed temporal fluctuation of the BOLD time course in HbSS-BERK mice, particularly in sickle mice under H/R with aggravating pain, but not in the control mice. Such characteristic might be an indication of the underlying dynamics of the sRBCs in changing the blood oxygenation level during a severe hypoxia challenge, and deserves further investigation. Table 1 summarizes the results of BOLD fMRI measurements of all animals scanned in this study, as well as their arterial oxygen saturation levels. Statistically significant reduction in the BOLD response to the hypoxia manipulation was observed in HbSS-BERK compared to their counterparts without SCA. Such different BOLD responses between the sickle and control mice were unlikely due to variable oxygen content in their feeding arteries since the arterial oxygen saturation levels were similar in these two groups (see Table 1). Instead, it indicated that less deoxyhemoglobin were generated in HbSS mice under the same hypoxia condition.
It is well known that BOLD signal is a relative measure reflecting the change of deoxyhemoglobin concentration of venous blood in the brain tissue under investigation. As schematically illustrated in Fig.1D, a positive BOLD response correlates with decreased deoxyhemoglobin content, while a negative BOLD response corresponds to a deoxyhemoglobin increase in the ROI. Therefore, the baseline deoxyhemoglobin levels in the sickle mice is expected to be higher than that in control mice, and they are even higher in the animal pretreated with H/R, likely due to the decreased cerebral perfusion in transgenic mouse model of sickle disease assuming the oxygen consumption were not seriously compromised [1]. The lower baseline oxygenation level in the sickle mice limits their ability to further deoxidize hemoglobin, thus, reducing the amplitude of negative BOLD in response to a severe hypoxia challenge.
Our results provide evidence for possible links between cerebral BOLD response in sickle mice under hypoxia manipulation and nociceptive signal perception in the brain. This is in agreement with studies suggesting the role of thalamus in modulating nociceptive inputs [13]. Moreover, it implicates the close correlation of pain intensity and BOLD response under normoxia and hypoxia.
We noticed the variability of BOLD change in brains from different mice in the group of HbSS-BERK sickle mice. The individual variation of sickle mice was also seen with acupuncture treatment [7], which might be attributed to the heterogeneity observed between patients with SCA. Although most fMRI studies rely on BOLD contrast to map the activated brain regions, it is noteworthy that the BOLD signal itself is a relatively scaled quantity reflecting the percent change between two pathophysiological conditions under investigation. Simultaneous imaging of BOLD contrast and the absolute cerebral blood flow (CBF) or its change (ΔCBF) could be considered for future studies to quantitatively determine the cerebral hemodynamic and pathological changes in sickle mice under chronic and acute pain [14]. This study provides a proof of principle of successfully using the fragile HbSS-BERK mice for fMRI to examine the nociceptive mechanisms involving brain function and the significance of examining BOLD response correlative to pain in SCA.
Acknowledgments
This study was supported partly by NIH U01 HL117664 grant to KG, R01 NS070839 to XHZ and R01 MH111413 to WC; Institute for Engineering in Medicine group grant at the University of Minnesota to KG, WC and XHZ; P41 EB015894, P30 NS5076408 and the W.M. Keck Foundation for CMRR facility and resource. We thank Yi Zhang for helping with animal care and fMRI experiment assistance and Ritu Jha and Susan Thompson for breeding sickle and control mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
KG is a Consultant for Ferra Therapeutics LLC.
References
- 1.Kennan RP, Suzuka SM, Nagel RL, Fabry ME. Decreased cerebral perfusion correlates with increased BOLD hyperoxia response in transgenic mouse models of sickle cell disease. Magnetic resonance in medicine. 2004;51(3):525–32. doi: 10.1002/mrm.20014. [DOI] [PubMed] [Google Scholar]
- 2.Fabry ME, Suzuka SM, Weinberg RS, et al. Second generation knockout sickle mice: the effect of HbF. Blood. 2001;97(2):410–8. doi: 10.1182/blood.v97.2.410. [DOI] [PubMed] [Google Scholar]
- 3.Fabry ME, Sengupta A, Suzuka SM, et al. A second generation transgenic mouse model expressing both hemoglobin S (HbS) and HbS-Antilles results in increased phenotypic severity. Blood. 1995;86(6):2419–28. [PubMed] [Google Scholar]
- 4.Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness. British journal of haematology. 2012;156(4):535–44. doi: 10.1111/j.1365-2141.2011.08977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paszty C, Brion CM, Manci E, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–8. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 6.Kohli DR, Li Y, Khasabov SG, et al. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116(3):456–65. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Lei J, Gupta M, et al. Electroacupuncture in conscious free-moving mice reduces pain by ameliorating peripheral and central nociceptive mechanisms. Scientific reports. 2016;6:34493. doi: 10.1038/srep34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent L, Vang D, Nguyen J, et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122(11):1853–62. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang CHMJ, Case M, Datta Y, Nelson S, Gupta K, He B. Resting State Neural Network Properties in Sickle Cell Disease Patients. Blood. 2014;124 [Google Scholar]
- 10.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(24):9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howseman AM, Bowtell RW. Functional magnetic resonance imaging: imaging techniques and contrast mechanisms. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1999;354(1387):1179–94. doi: 10.1098/rstb.1999.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osarogiagbon UR, Choong S, Belcher JD, et al. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96(1):314–20. [PubMed] [Google Scholar]
- 13.Ab Aziz CB, Ahmad AH. The role of the thalamus in modulating pain. The Malaysian journal of medical sciences : MJMS. 2006;13(2):11–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Zhu XH, Zhang Y, Chen W. Simultaneous Imaging of CBF Change and BOLD with Saturation-Recovery-T1 Method. PloS one. 2015;10(4):e0122563. doi: 10.1371/journal.pone.0122563. [DOI] [PMC free article] [PubMed] [Google Scholar]