Abstract
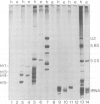
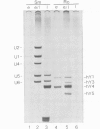
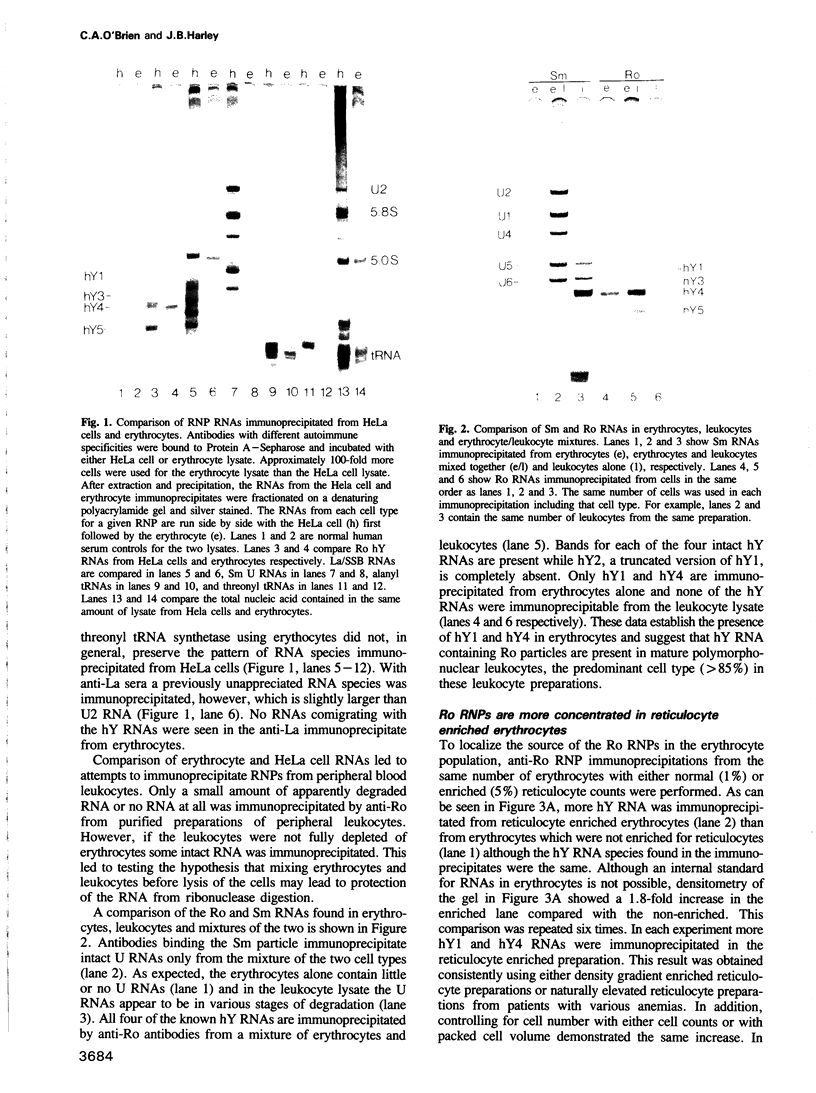
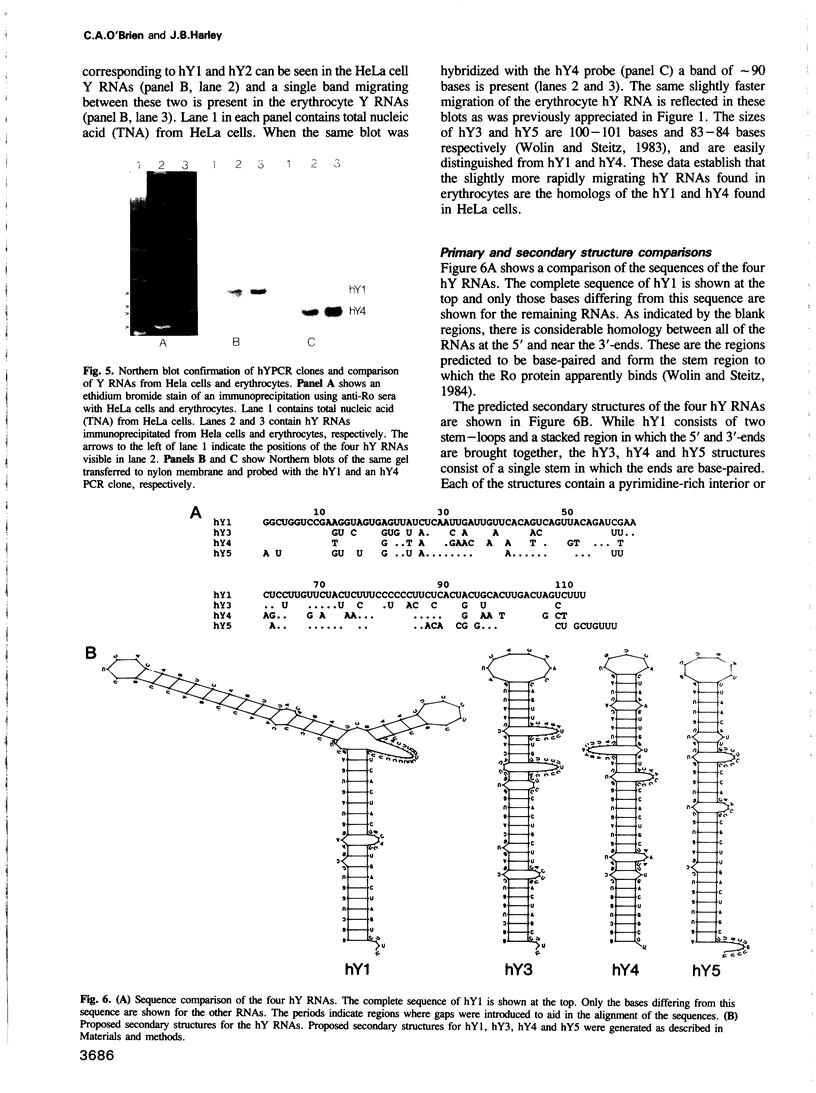
The Ro autoantigen is a mammalian cellular ribonucleoprotein (RNP) of unknown function. We have demonstrated that hY1 and hY4 Ro RNAs are associated with erythrocyte Ro RNPs and represent a subset of the four hY RNAs found in HeLa cell and leukocyte Ro RNPs. We have cloned and sequenced hY4 RNA, the only hY RNA not sequenced previously, from a polymerase chain reaction amplified erythrocyte hY cDNA library. Sequencing of the erythrocyte hY RNAs in conjunction with Northern blot analysis confirms that the erythrocyte hY RNAs contain the same sequences as the respective HeLa cell RNAs of similar mobility. Ribonuclease inhibition activity has been found in erythrocytes and this activity inhibits the degradation of hY3 and hY5 in leukocyte lysates thereby favoring the possibility that the presence of hY1 and hY4 in erythrocytes is the result of differential expression of the hY RNAs in erythrocyte precursors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann M., Pfeifer K., Schröder H. C., Müller W. E. The La antigen shuttles between the nucleus and the cytoplasm in CV-1 cells. Mol Cell Biochem. 1989 Feb 21;85(2):103–114. doi: 10.1007/BF00577106. [DOI] [PubMed] [Google Scholar]
- Ben-Chetrit E., Chan E. K., Sullivan K. F., Tan E. M. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988 May 1;167(5):1560–1571. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G., Reichlin M., Tomasi T. B., Jr Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythmatosus. J Immunol. 1969 Jan;102(1):117–122. [PubMed] [Google Scholar]
- Deutscher S. L., Harley J. B., Keene J. D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9479–9483. doi: 10.1073/pnas.85.24.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forman M. S., Nakamura M., Mimori T., Gelpi C., Hardin J. A. Detection of antibodies to small nuclear ribonucleoproteins and small cytoplasmic ribonucleoproteins using unlabeled cell extracts. Arthritis Rheum. 1985 Dec;28(12):1356–1361. doi: 10.1002/art.1780281207. [DOI] [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither K. K., Fox O. F., Yamagata H., Mamula M. J., Reichlin M., Harley J. B. Implications of anti-Ro/Sjögren's syndrome A antigen autoantibody in normal sera for autoimmunity. J Clin Invest. 1987 Mar;79(3):841–846. doi: 10.1172/JCI112892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S., Mizuno D. Degradation of RNA in rat reticulocytes. II. Relationship between RNase and its corresponding inhibitor in rat reticulocyte. Arch Biochem Biophys. 1971 Jul;145(1):71–77. doi: 10.1016/0003-9861(71)90011-7. [DOI] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. The RNA binding protein La influences both the accuracy and the efficiency of RNA polymerase III transcription in vitro. EMBO J. 1989 Mar;8(3):841–850. doi: 10.1002/j.1460-2075.1989.tb03445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Nucleotide sequence of 4.5S RNA (C8 or hY5) from HeLa cells. Biochem Biophys Res Commun. 1982 Sep 16;108(1):363–370. doi: 10.1016/0006-291x(82)91875-7. [DOI] [PubMed] [Google Scholar]
- Kraft N., Shortman K. A suggested control function for the animal tissue ribonuclease-ribonuclease inhibitor system, based on studies of isolated cells and phytohaemagglutinin-transformed lymphocytes. Biochim Biophys Acta. 1970 Sep 17;217(1):164–175. doi: 10.1016/0005-2787(70)90133-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., O'Brien C. A., Harley J. B., Hardin J. A. The Ro ribonucleoprotein particle: induction of autoantibodies and the detection of Ro RNAs among species. Clin Immunol Immunopathol. 1989 Sep;52(3):435–446. doi: 10.1016/0090-1229(89)90158-x. [DOI] [PubMed] [Google Scholar]
- Rader M. D., O'Brien C., Liu Y. S., Harley J. B., Reichlin M. Heterogeneity of the Ro/SSA antigen. Different molecular forms in lymphocytes and red blood cells. J Clin Invest. 1989 Apr;83(4):1293–1298. doi: 10.1172/JCI114014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Tan E. M., Henning D., Nohga K., Busch H. Detection of a nucleolar 7-2 ribonucleoprotein and a cytoplasmic 8-2 ribonucleoprotein with autoantibodies from patients with scleroderma. J Biol Chem. 1983 Feb 10;258(3):1383–1386. [PubMed] [Google Scholar]
- Reichlin M. Significance of the Ro antigen system. J Clin Immunol. 1986 Sep;6(5):339–348. doi: 10.1007/BF00915372. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A. E. Purification and characterization of adenosine triphosphate: ribonucleic acid adenyltransferase from Escherichia coli. Eur J Biochem. 1973 Aug 1;37(1):31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettore L., De Matteis M. C., Zampini P. A new density gradient system for the separation of human red blood cells. Am J Hematol. 1980;8(3):291–297. doi: 10.1002/ajh.2830080307. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. Genes for two small cytoplasmic Ro RNAs are adjacent and appear to be single-copy in the human genome. Cell. 1983 Mar;32(3):735–744. doi: 10.1016/0092-8674(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Harley J. B., Reichlin M. Molecular properties of the Ro/SSA antigen and enzyme-linked immunosorbent assay for quantitation of antibody. J Clin Invest. 1984 Aug;74(2):625–633. doi: 10.1172/JCI111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]