Summary
Background
Recently emerged H7N9 avian influenza viruses are characterized by enhanced virulence and presence of mammalian adaptation markers, suggesting their pandemic potential. Specific influenza vaccines remain the key defense against a possible H7N9 pandemic. We report here the safety and immunogenicity results from a phase 1 clinical trial of H7N9 live attenuated influenza vaccine (LAIV) candidate in healthy adult volunteers.
Methods
This study was a phase 1, double-blind, individually randomised, placebo-controlled trial of H7N9 LAIV conducted in Saint Petersburg, Russia. Eligible participants were healthy adults aged 18 to 49 years who provided informed consent and met eligibility criteria. The participants were randomised 3:1 to receive live vaccine or placebo using a computerized randomisation scheme generator. Two doses of vaccine or placebo were administered intranasally 28 days apart. After each administration, subjects remained as inpatients for seven days, to allow close observation of subject safety. To assess immune responses to H7N9 LAIV, nasal swab, saliva and serum specimens were collected prior to vaccination and at day 28 after each vaccine dose. This trial is registered with ClinicalTrials.gov, number NCT02480101, and is closed to new participants.
Findings
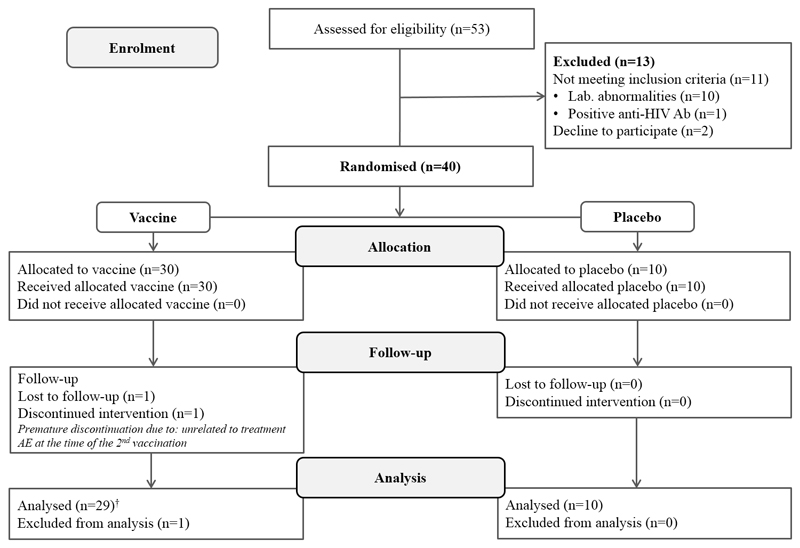
Between October 21, 2014, and October 31, 2014, we randomly assigned forty healthy adults to our study groups. Thirty-nine (97.5%) of the 40 subjects were included in the per-protocol analysis (29 – vaccine, 10 – placebo). No differences in the frequency of adverse events between vaccine and placebo groups were registered. Proportions of seroconversions measured by microneutralization assay were 14/29 (48.3%, 95% CI 31.4–65.6) after the first vaccine dose and 21/29 (72.4%, 95% CI 54.3–85.3) after the second vaccine dose. Cumulative analysis of the immune responses, which included hemagglutination inhibition and microneutralization assays, detection of serum IgA and IgG and mucosal IgA antibodies, as well as measurement of virus–specific T cells, showed that 27 of 29 recipients (93.1%, 95% CI 77.2–99.2) responded to the vaccine.
Interpretation
The H7N9 LAIV was well tolerated and safe. The immune responses to H7N9 LAIV detected in our study present the best results for immunogenicity among all pandemic LAIVs tested in humans so far.
Funding
World Health Organization
Keywords: live attenuated influenza vaccine, H7N9 influenza, influenza pandemic, immunogenicity, safety, vaccine virus shedding, transmissibility, genetic stability
Introduction
New H7N9 avian influenza viruses emerged in the human population in China in early 2013, and by April 2015 at least 630 laboratory-confirmed human infections had been documented, with a fatality rate of over 30%.1 A recent study found evidence of an increased transmission potential of H7N9 viruses during the second outbreak wave.2 H7N9 viruses were shown to bind both avian-type (α2,3-linked sialic acid) and, to a lesser extent, human-type (α2,6-linked sialic acid) receptors.3, 4 Despite the lack of effective respiratory droplet transmission between ferrets, this dual-receptor specificity could be a critical feature for sustained human-to-human transmission, should more adaptive changes occur in the receptor-binding site.5, 6 The enhanced virulence and presence of multiple mammalian adaptation markers, as well as the persistence of the virus in the avian reservoir, suggest that H7N9 viruses have pandemic potential. Given that the H7N9 viruses have been shown to easily acquire resistance to neuraminidase inhibitors in experimental animal models7, 8, specific influenza vaccines remain the key defense against a possible H7N9 pandemic.
Inactivated influenza vaccines (IIVs) administered intramuscularly usually provide short-term and strain-specific humoral immunity. Live attenuated influenza vaccines (LAIVs) are believed to be immunologically superior, because they induce diverse types of adaptive immune responses, including serum antibodies, mucosal immunity and cytotoxic T lymphocytes targeted to conserved virus epitopes.9–12 Other advantages of LAIV over traditional IIV are a much cheaper and quicker manufacturing process and a non-invasive route of administration (by intranasal spray). These features could be critical in ensuring adequate vaccination coverage during the emergency response to the first wave of a pandemic.
We report here the safety and immunogenicity results from a phase 1 clinical trial of an H7N9 LAIV candidate in healthy adult volunteers. The vaccine was found to be safe, infectious, genetically stable and immunogenic after a single dose, indicating that it has the potential to protect populations during the first months of a pandemic.
Methods
Study design and participants
This study was a phase 1, double-blind, individually randomised, placebo-controlled trial conducted at single site in Saint Petersburg, Russia. The study population was forty healthy adults both sexes, aged 18–49 years, meeting all eligibility criteria (appendix). All study participants provided written informed consent prior to study initiation. The study subjects were randomised 3:1 to receive live vaccine or placebo. Two doses of the study vaccine and placebo were administered intranasally 28 days apart, and subjects remained admitted to an inpatient isolation unit during 7 days after administration of each dose. For feasibility reasons and in order for a Safety Monitoring Committee (SMC) to review safety data in a portion of subjects initially, the total cohort of 40 subjects was enrolled in two sub-cohorts of 20 subjects each. Subjects from each sub-cohort were vaccinated in two stages two weeks apart. SMC reviewed individual and cumulative participant safety data (all AEs, including clinical laboratory evaluations and shedding data) on or soon after Days 7 and 35 for each sub-cohort and made recommendations regarding the safe continuation of the study. The protocol for the study and its amendments were reviewed and approved by the study’s Independent Ethics Committee (IEC), Institutional Review Board (IEC/IRB) and the WHO Ethics Review Committee. The trial is registered with ClinicalTrials.gov under the identifier NCT02480101.
Randomisation and masking
A computarised randomisation scheme generator was used to allocate subjects to vaccine or placebo. An allocation code was randomly assigned to each subject using a method that maintains the 3:1 ratio of study vaccine to placebo in each study sub-cohort. Study vaccine and placebo had similar formulations. However, because study vaccine and placebo were clearly labeled as such, an unblinded study clinician was assigned to prepare all study vaccine and placebo preparations. This study clinician did not administer the preparations and did not reveal to either other study staff or to the subject which preparation was given. All the other study-site personnel, investigators and participants remained blinded throughout the trial.
Procedures
The H7N9 LAIV tested in this study was formulated from a vaccine reassortant virus A/17/Anhui/2013/61 (H7N9) at a dose of 7.5 log EID50 per 0.5 ml. This reassortant virus is comprised of haemagglutinin (HA) and neuraminidase (NA) genes of human isolate A/Anhui/1/2013 (H7N9) and the remaining six genes of cold-adapted master donor virus A/Leningrad/134/17/57 (H2N2).13
All subjects received two doses of vaccine or placebo, one on Day 0 and one on Day 28. Vaccinations took place in the inpatient isolation unit (2 to 4 volunteers per room), without special safety precautions, so the placebo vaccinated subjects were in close contact with volunteers from the vaccine group. After each administration, subjects remained as inpatients for seven days (Day 0 through Day 6, and Day 28 through Day 34), to allow close observation of subject safety and collection of nasal swabs to assess viral shedding, nasal wicks and saliva specimens to assess mucosal immunoglobulin class A (IgA), and blood samples to evaluate serum antibodies and cellular immune response. In addition, blood and urine specimens were collected for clinical laboratory evaluations.
Nasal swab specimens collected from all study participants were tested for evidence of influenza virus using either real-time reverse transcription polymerase chain reaction (rRT-PCR) or virus culture in eggs. All procedures related to the specimen collection and processing have been described elsewhere.14 All the live virus isolates were tested for the presence of attenuating mutations within their internal protein gene segments using the partial sequencing strategy described previously.15 In addition, full-length sequencing of HA and NA genes of isolated vaccine virus was performed.
Outcomes
The primary outcome was the safety profile of two intranasal doses of H7N9 LAIV in healthy adults 18 through 49 years of age. Safety was closely assessed daily in all study subjects in the isolation unit, for one week after administration of each dose; in addition, subjects kept a diary card for the following three weeks to record any abnormalities. They exited the study on day 56. Safety results were expressed as the proportion of subjects experiencing adverse effects, related or not related, in the following four categories:
immediate reactions occurring within two hours of administration of either dose;
adverse events commonly associated with intranasal vaccination (solicited local and systemic reactions) occurring more than two hours after administration of either dose up to 6 days following administration,;
all other adverse events (including unsolicited events) occurring during the 6 days following either dose; this included abnormal laboratory findings from blood specimens and urine collected on Days 3, 6, 31 and 34;
all serious adverse events (SAEs) occurring within 4 weeks of any dose; this included abnormal laboratory findings from blood specimens collected on Days 28 (before administration of the second dose) and 56.
The secondary outcome measures were the immune responses to the vaccine detected by several immunological assays on specimens collected prior to the first dose (Day 0), and on Days 28 and 56 of the study. Blood specimens were used for assessment of serum hemagglutination inhibition (HAI) antibodies,16 neutralising antibodies by microneutralisation (MN) assay,17 serum IgA or immunoglobulin class G (IgG) antibodies by enzyme-linked immunosorbent assay (ELISA), 14 and Virus-specific CD4+ and CD8+ T cells in peripheral blood mononuclear cells (PBMCs).15 Nasal wick and saliva specimens were used for evaluation of secretory IgA antibodies by ELISA (appendix). For immunological Virus shedding was parameterised as the proportion of subjects shedding virus (detected by rRT-PCR in nasal swabs) at any time-point, as well as the duration of virus shedding (appendix). Molecular characterization of any shed virus isolated in chicken embryos was performed with special attention to the conservation of attenuation mutations.
Statistical analyses
Immunogenicity analyses were done in the per-protocol population of participants who received both doses of vaccine or placebo, whereas safety analysis included all subjects who received at least one dose of intervention. A sample size of 30 vaccine recipients allows recognition of serious adverse events occurring at a frequency of 12 percent or higher. In these conditions, the probability of observing at least one vaccine-related serious adverse event among 30 subjects is very high (97.8%).
Given that there is a low probability to detect anti-H7 HAI antibodies in the naïve subjects (serum samples are titrated starting from 1:5 dilution, the lowest GMT is 2.5, SD=1.25), and the defined four-fold increase in antibody titer as an effective measure of the immune response (GMT=10, SD=5), the sample size of 30 vaccinated subjects and 10 controls allows conducting nonparametric analyses with statistical power 93.6%.18
HAI, MN and ELISA titers were summarised as geometric means and 95% confidence intervals (Cis) using t test on log-transformed data. Levels of virus-specific T cell subsets were presented as medians. Mann-Whitney U test was used to compare these values between the two treatment groups. Wilcoxon matched-pairs test was utilised to assess significance of their increases within each group at days 28 and 56 compared with the baseline levels.
All safety endpoints and seroconversions were calculated as percentages along with 95% CIs using Clopper-Pearson method, and compared with Fisher’s exact test (two-tailed). The differences were considered significant at p<0.05. No multiplicity adjustment to the error rate were made because there were no multiple statistical hypotheses and all analyses were descriptive.
The data were analysed with Statistica 6 and GraphPad Prizm 5 software. All the protocol-specified analysis, including the immunogenicity assays, was conducted blind. Unblinding of the results took place only after all safety and immunogenicity data had been collected.
Role of the funding source
The sponsor of the study (WHO) was involved in trial design, monitoring of study implementation, data collection, analysis and interpretation of the study results. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Demographic and other baseline characteristics of study participants
Of 53 screened volunteers, 40 (75.5%) were included in the study. A schematic summary of subject disposition is given in Figure 1. Of the 40 enrolled subjects, 30 were randomly assigned to receive the study vaccine and 10 to receive placebo. All 40 (100%) subjects received their assigned treatment on day 0; 39 (97.5%) subjects received their assigned treatment on day 28 and completed the study as per protocol. One study subject who received a first dose of the study vaccine discontinued prematurely and did not receive the second vaccine dose. This subject presented with increased temperature (37.2 C) and pharynx hyperaemia on day 28. These events were considered to be not related to treatment.
Figure 1.
Trial profile
†Safety analyses included all vaccinated subjects who received at least one dose of H7N9 LAIV.
The key demographic and other baseline characteristics of the study subjects are summarised in Table 1. Physical examination on entry to the study found all subjects to be normal. No subjects had a history of allergic reactions.
Table 1.
Baseline demographic characteristics of study subjects
| Vaccine (N=30) | Placebo (N=10) | ||
|---|---|---|---|
| Sex | |||
| Male, n (%) | 15 (50.0) | 5 (50.0) | |
| Female, n (%) | 15 (50.0) | 5 (50.0) | |
| Race | |||
| White, n (%) | 30 (100) | 10 (100) | |
| Other, n(%) | 0 (0.0) | 0 (0.0) | |
| Age (years) | |||
| Mean±SD | 27.6±8.2 | 27.2±8.8 | |
| Median (Q1, Q3) | 25.5 (22.0, 30.0) | 25.5 (21.0, 29.0) | |
| Min. – Max. | 19.0–49.0 | 19.0–46.0 | |
| Weight (kg) | |||
| Mean±SD | 65.6±11.6 | 67.6±14.5 | |
| Median (Q1, Q3) | 62.0 (58.8, 71.0) | 64.5 (57.0, 76.0) | |
| Min. – Max. | 43.0–89.0 | 50.0–101.0 | |
| Height (cm) | |||
| Mean±SD | 172.7±11.2 | 173.1±9.2 | |
| Median (Q1, Q3) | 172.0 (164.0, 185.0) | 172.0 (167.0, 178.0) | |
| Min. – Max. | 148.0–190.0 | 162.0–191.0 | |
SD=standard deviation; Q1=upper quartile; Q3=lower quartile
Safety of H7N9 LAIV
The dataset for safety analysis includes all subjects who received either 2 doses orat least one dose of vaccine or placebo. The adverse reactions and adverse events that occurred during the seven days after administration of either dose 1 or dose 2 are summarised in Table 2. No reactions were reported within the first 2 hours after administration of either dose. In the seven days after administration of dose 1, 19 (63.3%) subjects given the study vaccine and 9 (90.0%) given placebo reported solicited local and systemic adverse events (AEs). No differences in the frequency of AEs between vaccine and placebo groups were registered. Local AEs were reported by 12 (40.0%) vaccine recipients and 7 (70.0%) subjects given placebo. The most frequent local symptoms were catarrhal nasopharynx and nasal congestion. Systemic AEs, mostly fever, were reported by 6 (20.0%) vaccine recipients and 1 (10.0%) subject in the placebo group (Table 2).
Table 2.
Adverse reactions and adverse events in the seven days following administration of dose 1 and dose 2
| Worst grade | After dose 1 |
After dose 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccine group (N=30) | Placebo group (N=10) | Vaccine group (N=29) | Placebo group (N=10) | ||||||
| n (%**) | 95% CI | n (%**) | 95% CI | n (%**) | 95% CI | n (%**) | 95% CI | ||
| Reactions within 2 hours of dose 1 | 0 (0.0) | 0.0-11.6 | 0 (0.0) | 0.0-30.8 | 0 (0.0) | 0.0-11.9 | 0 (0.0) | 0.0-30.8 | |
| Any solicited local or systemic reaction* | 19 (63.3) | 43.9-80.1 | 9 (90.0) | 55.5-99.7 | 9 (31.0) | 15.3-50.8 | 4 (40.0) | 12.2-73.8 | |
| Local reactions* | 12 (40.0) | 22.7-59.4 | 7 (70.0) | 34.8-93.3 | 4 (13.8) | 3.9-31.7 | 0 (0.0) | 0.0-30.8 | |
| Catarrhal nasopharynx | mild | 3 (10.0) | 2.1-26.5 | 2 (20.0) | 2.5-55.6 | 1 ( 3.4 ) | 0.1-17.8 | 0 (0.0) | 0.0-30.8 |
| Dryness of the nose | mild | 1 (3.3) | 0.1-17.2 | 1 (10.0) | 0.3-44.5 | 0 (0.0) | 0.0-11.9 | 0 (0.0) | 0.0-30.8 |
| Nasal congestion | mild | 4 (13.3) | 3.8-30.7 | 1 (10.0) | 0.3-44.5 | 2 (6.9) | 0.8-22.8 | 0 (0.0) | 0.0-30.8 |
| Ticklish throat | mild | 0 (0.0) | 0.0-11.6 | 1 (10.0) | 0.3-44.5 | 0 (0.0) | 0.0-11.9 | 0 (0.0) | 0.0-30.8 |
| Nose bleed | mild | 1 (3.3) | 0.1-17.2 | 1 (10.0) | 0.3-44.5 | 1 (3.4) | 0.1-17.8 | 0 (0.0) | 0.0-30.8 |
| Serous nasal discharge | mild | 2 (6.7) | 0.8-22.1 | 1 (10.0) | 0.3-44.5 | 0 (0.0) | 0.0-11.9 | 0 (0.0) | 0.0-30.8 |
| Runny nose | mild | 1 (3.3) | 0.1-17.2 | 0 (0.0) | 0.0-30.8 | 0 (0.0) | 0.0-11.9 | 0 (0.0) | 0.0-30.8 |
| Systemic reactions* | 7 (23.3) | 9.9-42.3 | 2 (20.0) | 2.5-55.6 | 5 (17.2) | 5.8-35.8 | 4 (40.0) | 12.2-73.8 | |
| Sore throat | mild | 1 (3.3) | 0.1-17.2 | 0 (0.0) | 0.0-30.8 | 1 ( 3.4 ) | 0.1-17.8 | 1 (10.0) | 0.3-44.5 |
| Fever | mild | 6 (20.0) | 7.7-38.6 | 1 (10.0) | 0.3-44.5 | 3 (10.3) | 2.2-27.4 | 1 (10.0) | 0.3-44.5 |
| Dizziness | mild | 0 (0.0) | 0.0-11.6 | 1 (10.0) | 0.3-44.5 | 0 (0.0) | 0.0-11.9 | 0 (0.0) | 0.0-30.8 |
| Headache | mild | 0 (0.0) | 0.0-11.6 | 0 (0.0) | 0.0-30.8 | 1 ( 3.4 ) | 0.1-17.8 | 1 (10.0) | 0.3-44.5 |
| Nonproductive cough | mild | 0 (0.0) | 0.0-11.6 | 0 (0.0) | 0.0-30.8 | 0 (0.0) | 0.0-11.9 | 1 (10.0) | 0.3-44.5 |
| Any serious adverse event | 0 | 0 | 0 | 0 | |||||
CI = confidence interval (%)
A subject with more than one finding in a specific category was counted only once.
Percentages based on total number of subjects in each treatment group.
In the seven days after administration of dose 2, four local AEs (13.8%) were observed in the vaccine group and none in the placebo group. Five systemic AEs (17.2%) were recorded in the vaccine group and four (40%) in the placebo group (Table 2). All registered AEs were evaluated as mild and self-limiting.
Unsolicited adverse events following dose 1 and dose 2 were associated with minor changes in urine and blood test results and were graded as mild in accordance with the FDA Toxicity Grading Scale (Table 3). Clinical laboratory evaluations showed no significant abnormalities and were deemed to be of no clinical significance by the study physicians. No serious adverse events occurred during the study and no significant changes in vital signs or physical findings were observed. These results show that LAIV H7N9 vaccine administered in two intranasal doses was well tolerated and safe.
Table 3.
Laboratory abnormalities observed in the seven days following administration of dose 1 and dose 2
| Worst grade | After dose 1 | After dose 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccine group (N=30) | Placebo group (N=10) | Vaccine group (N=29) | Placebo group (N=10) | ||||||
| n (%**) | 95% CI | n (%**) | 95% CI | n (%**) | 95% CI | n (%**) | 95% CI | ||
| Any laboratory abnormalities* | 4 (13.3) | 3.8–30.7 | 1 (10.0) | 0.3–44.5 | 1 (3.4) | 0.1–17.8 | 1 (10.0) | 0.3–44.5 | |
| Blood chemistry | |||||||||
| Blood bilirubin increased | mild | 1 (3.3) | 0.1–17.2 | 0 (0.0) | 0.0–30.8 | 0 (0.0) | 0.0–11.9 | 0 (0.0) | 0.0–30.8 |
| Haematological blood tests | |||||||||
| Monocyte count increased | mild | 2 (6.6) | 0.8–22.1 | 0 (0.0) | 0.0–30.8 | 0 (0.0) | 0.0–11.9 | 0 (0.0) | 0.0–30.8 |
| Eosinophil count increased | mild | 1 (3.3) | 0.1–17.2 | 0 (0.0) | 0.0–30.8 | 0 (0.0) | 0.0–11.9 | 0 (0.0) | 0.0–30.8 |
| Lymphocyte count increased | mild | 1 (3.3) | 0.1–17.2 | 2 (20.0) | 2.5–55.6 | 0 (0.0) | 0.0–11.9 | 0 (0.0) | 0.0–30.8 |
| Neutrophil count decreased | mild | 2 (6.6) | 0.8–22.1 | 0 (0.0) | 0.0–30.8 | 0 (0.0) | 0.0–11.9 | 0 (0.0) | 0.0–30.8 |
| Erythrocyte sedimentation rate | mild | 2 (6.6) | 0.8–22.1 | 0 (0.0) | 0.0–30.8 | 0 (0.0) | 0.0–11.9 | 0 (0.0) | 0.0–30.8 |
| Urinalysis | |||||||||
| Traces of protein | mild | 0 (0.0) | 0.0–30.8 | 1 (3.4) | 0.1–17.8 | 1 (10.0) | 0.3–44.5 | ||
| Any serious adverse event | 0 | 0 | 0 | 0 | |||||
CI = confidence interval (%)
A subject with more than one finding in a specific category was counted only once.
Percentages based on total number of subjects in each treatment group.
Vaccine virus shedding, genetic stability and transmissibility
The H7N9 LAIV was infectious for humans: after the first dose, virus was detected in over 93.3% of the vaccinated subjects by PCR, and viable virus was isolated by culture from 60% of the vaccinees (Table S1). The mean duration of shedding was two days, with several subjects shedding the virus as long as four days, indicating that the vaccine virus had replicated in the upper respiratory tract (Table S2). A lower proportion of vaccinated subjects shed the virus after the second dose: fewer than 60% were positive by PCR, and only seven subjects (24%) were positive by virus culture. After the second dose, viable virus was isolated only the following day (Day 29), however the viral RNA continued to be detected up to Day 4 after revaccination (Day 32, Table S2).
Altogether, 45 vaccine virus specimens were isolated from vaccinated subjects on different days after vaccine administration. All these isolates were genotyped using partial sequencing strategy and were confirmed to retain their 6:2 reassortant genotype. Sequencing of the gene regions adjacent to the specific attenuating mutations described for the master donor virus Len/17 demonstrated that all these mutations were conserved across all the virus isolates (data not shown). Full-length sequencing of the HA and NA genes revealed one amino acid change (Leu-68-Phe) in the HA2 subunit of three vaccine virus isolates recovered from two immunized subjects (on day 2 in one subject and on days 2 and 3 in the other). No changes were detected in the NA genes of all tested isolates. Importantly, no vaccine virus was detected in any of the placebo recipients (Table S1).
Immunogenicity of H7N9 LAIV
Immune response to the vaccine was primarily assessed by HAI and MN tests. Although the differences in HAI geometric mean titre (GMT) and in the proportion of seroconversions did not differ significantly between the two study groups after the first vaccine dose, the HAI titres within the H7N9 LAIV group increased significantly (p=0.0002), whereas no difference was detected in the placebo group (Table 4). The MN assay also revealed a significant increase in neutralising antibody titres on day 28 of the study: almost half the subjects seroconverted, and the GMT was 3.4 times higher than the baseline level (Table 4). It is worth noting that, among the responders, the HAI and MN antibody titres on day 28 had increased by as much as 4 and 8 times, respectively.
Table 4.
Serum antibody immune responses in subjects immunised with H7N9 LAIV or placebo
| Assay | Haemagglutination inhibition |
Microneutralisation |
||
|---|---|---|---|---|
| Study group | Placebo | Vaccine | Placebo | Vaccine |
| No. of participants | 10 | 29 | 10 | 29 |
| Baseline | ||||
| Reciprocal geometric mean titre (95% CI) | 3.3 (2.6–4.2) | 3.0 (2.6–3.3) | 5.4 (4.6–6.2) | 5.2 (4.4–6.3) |
| Subjects with titre ≥1:40, % (95% CI) | 0 | 0 | 0 | 0 |
| Day 28 | ||||
| Reciprocal geometric mean titre (95% CI) | 3.8 (3.0–4.8) | 5.19 (4.3–6.1) | 6.6 (5.2–8.4) | 17.73,11 (12.0–26.2) |
| Subjects with seroconversion, % (95% CI) | 0.0 (0.0–30.8) | 10.3 (2.2–27.4) | 0.0 (0.0–30.8) | 48.34 (29.4–67.5) |
| Reciprocal geometric mean titre in subjects with seroconversion (95% CI) | - | 10.0† | - | 44.2 (31.1–62.2) |
| Subjects with titre ≥1:40, % (95% CI) | 0.0 (0.0-30.8) | 0.0 (0.0–11.9) | 0.0 (0.0–30.8) | 37.95 (20.7–57.7) |
| Day 56 (28 days after dose 2) | ||||
| Reciprocal geometric mean titre (95% CI) | 3.5 (2.8-4.5) | 10.21,10(7.4–14.3) | 5.4 (4.6–6.2) | 27.36,12(18.8–39.8) |
| Subjects with seroconversion, % (95% CI) | 0.0 (0.0–30.8) | 65.52(45.7–82.1) | 0.0 (0.0–30.8) | 72.47(52.8–87.3) |
| Reciprocal geometric mean titre in subjects with seroconversion (95% CI) | - | 17.3 (13.3–22.5) | - | 45.6 (35.3–59.0) |
| Subjects with titre ≥1:40, % (95% CI) | 0.0 (0.0–30.8) | 13.8 (3.9–31.7) | 0.0 (0.0–30.8) | 58.68(38.9–76.5) |
Significant differences in proportions of subjects (Fisher exact test, two-tailed) and GMT (Mann-Whitney U-test) between the two treatment groups are shown in bold: 1 p=0.001; 2 p=0.0004; 3 p=0.005; 4 p=0.007; 5 p=0.037; 6 p=0.0001; 7 p=0.0001; 8 p=0.002.
Significantly higher antibody titres at days 28 and 56 compared with baseline levels (Wilcoxon matched-pairs test, exact two-sided p values) are underlined: 9 p=0.0001; 10p=0.00003; 11 p=0.00005; 12 p=0.00002;
All three subjects had an HAI antibody titre of 1:10. CI, confidence interval.
The second dose of the H7N9 LAIV further enhanced the immune responses, bringing the total proportion of responders up to 65.5% and 72.4%, as measured by HAI and MN assays, respectively. A high proportion of subjects given vaccine (38%) were found to have neutralising antibody titres of ≥1:40 after the first vaccine dose; this proportion was almost 60% after the second dose of vaccine. No subjects from the placebo group had measurable HAI or MN antibody responses (Table 4).
We also measured serum IgG and IgA, as well as mucosal IgA antibodies by ELISA against whole-virus H7N9 LAIV antigen. Figure S1 shows individual antibody titres at baseline, on day 28 and on day 56. Serum IgG and IgA antibodies, as well as IgA antibody in saliva specimens, significantly increased in the vaccine group after the first dose (p=0.002, p=0.0013 and p=0.0028, respectively). The antibody levels further increased after the second dose and the mean titres of all the tested antibodies, both serum and mucosal, were significantly higher than baseline levels. As expected, no significant increases in serum or mucosal antibodies were detected in the placebo group (Figure S1).
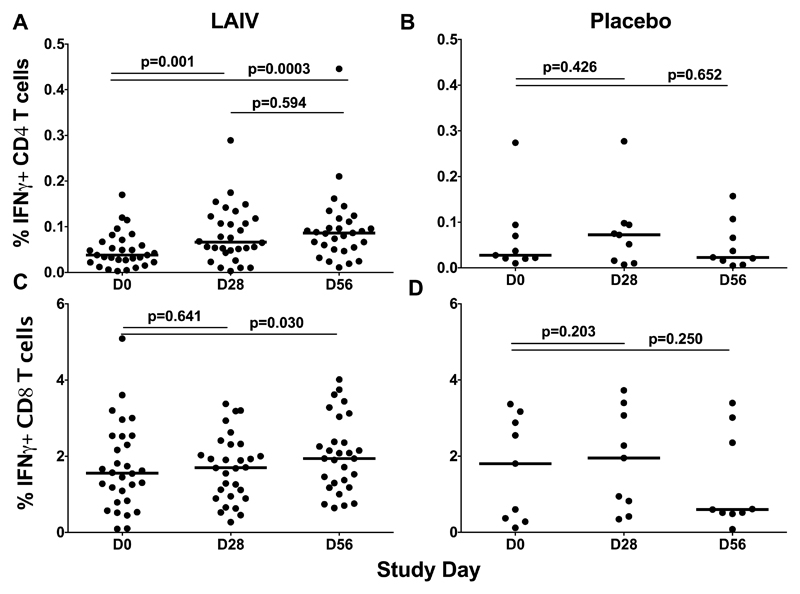
Cell-mediated immunity (CMI) was evaluated in terms of the increases in the levels of virus-specific CD4+ and CD8+ T-cells following administration of the study vaccine or placebo, compared with baseline levels. Interestingly, the majority of the participants had some pre-existing cross-reactive virus-specific CD4+ and CD8+ T-cells, with a median of 0.037% and 1.57%, respectively (Figure 2, day 0). The CD4+ T-cells increased 1.8 times by day 28 after the first vaccine dose. The second LAIV dose further increased these cells and by day 56 their level was 2.3 times higher than baseline (Figure 2A); however, the difference between the virus-specific CD4+ cells in the LAIV group at day 28 and day 56 was not statistically significant. In contrast to the CD4+ T-cells, two doses of the H7N9 LAIV were required to significantly increase virus-specific CD8+ T-cells (Figure 2C). No statistically significant increases in the levels of CD4+ or CD8+ virus-specific T-cells were detected in the placebo group at any time point (Figure 2B and 2D).
Figure 2.
Cell-mediated immune responses in subjects before and after administration of H7N9 LAIV or placebo (given on Day 0 and Day 28). The percentage of virus-specific IFNɣ-positive CD4+ and CD8+ T cells was estimated by ICS after subtracting background. T cell levels at different time-points were compared using the Wilcoxon matched-pairs test. The exact two-sided p values, medians (bars) and individual data (dots) are shown.
In addition, the induction of CD4+ and CD8+ central and effector memory T-cell subsets was evaluated. The gating strategy and individual levels of these subsets are presented in supplementary material. Interestingly, only the levels of CD4+ (but not CD8+) memory T-cells increased after vaccination, and were significantly higher after the first and the second dose, compared with the baseline level (Figure S3). No significant increases were detected in the placebo group (Figure S4). Subjects with cell-mediated immune responses were detected as described earlier15, and visual representation is given in Figures S5 and S6.
Overall, 25 of 29 (86.7%) vaccinated subjects responded to the first dose of H7N9 LAIV in at least one of the immunological assays employed in this study, and this proportion increased up to 93.1% (27 of 29) when the second dose of the vaccine was applied (Table S3). Importantly, the majority of these subjects had an immune response as measured by several assays, and 31% of the vaccinees had all three arms of adaptive immune response: systemic humoral, local (mucosal) and CMI (Figure S7), indicating that H7N9 LAIV has high immunogenic potential.
Discussion
All novel influenza viruses that are capable to infect humans, including H7N9 avian influenza viruses, pose a threat to human health. Our study was designed to evaluate the safety and immunogenicity of H7N9 LAIV candidate in healthy adults aged 18–49 years. As with other A/Leningrad/134/17/57-based pandemic LAIV candidates, the H7N9 LAIV was well tolerated and did not cause any serious adverse events.19
Since H7N9 influenza viruses possess multiple mammalian adaptation markers6, the H7N9 LAIV was expected to replicate better in the upper respiratory tract of naïve volunteers than the other H7 subtype LAIV candidates. Indeed, 60% and 24% of H7N9 LAIV-immunized subjects shed vaccine virus detectable by virus culture after the first and the second dose, respectively, with a mean duration of 2 days. This was in contrast to results with other H7 subtype LAIV clinical trials, where viable virus was isolated by culture only after the first dose, at frequencies ranging from 13% to 24%.15, 20, 21 Importantly, in spite of a good replication pattern, the H7N9 LAIV was not transmitted from vaccinated subjects to their close contacts, which reinforces the safety profile of the tested vaccine.
One of the main purposes of this trial was to evaluate human immune responses to two doses of the H7N9 LAIV candidate. Unlike other pandemic LAIVs, significant increases in H7N9-specific serum (measured by HAI, MN, IgA and IgG ELISA assays) and mucosal (measured by IgA ELISA in saliva) antibody titres were detected after a single dose of the H7N9 LAIV. In addition, a relatively high proportion of subjects achieved neutralising titre of 1:40 or higher at the same time. The second dose of the H7N9 LAIV further enhanced humoral and cell-mediated immunity, bringing the total proportion of responders up to 93%. More importantly, the induced responses were of high quality, since the majority of subjects achieved HAI and/or MN antibody titres of 1:40 or higher, and many of them also showed significant increases in the proportions of virus-specific CD4+ T-cells, a known correlate of protection against wild-type influenza.22 Altogether, these data present the best results for immunogenicity among all pandemic LAIVs tested in humans so far.19, 23
Previous studies found that an H7N3 LAIV candidate based on A/Leningrad/134/17/57 backbone induced antibody immune responses that cross-reacted with new H7N9 viruses, both in animals and humans.24, 25 However, the H7N3 LAIV was restricted in replication and the HAI and MN antibody titres in the vaccinated subjects were fairly low, with no subject achieving a seroprotective level of 1:40, although the seroconversion rate was over 75% after two doses of the vaccine.15 Immunity established after primary immunization with the H7N3 LAIV might be sufficient to induce memory immune responses resulting in prompt and robust antibody production to a single suboptimal dose of IIV given several months or years later.21, 26, 27 In the case of H7N9 LAIV, the induced primary immune response is itself capable of protecting vaccinees from wild-type infection at the beginning of a pandemic, since high neutralizing titres and virus-specific CD4+ T-cells were detected after the first vaccine dose.
Given that immune responses induced by LAIVs are usually cross-reactive, the H7N9 LAIV seems an attractive vaccine candidate to be used in the first wave of a pandemic, even one caused by an antigenically different variant. One significant advantage of LAIV over IIV is the induction of herd immunity when a high percentage of the population is immunised, which is usually the case at the beginning of a pandemic. This could be due to the ability of LAIV to induce mucosal immunity in the respiratory tract which may contribute to limiting virus replication and spread.28 A previous study demonstrated that, when more than 50% of schoolchildren were immunized with seasonal LAIV, the morbidity among their unvaccinated contacts (teachers and classmates) was reduced by two-thirds.29 Therefore, the protection provided by pandemic LAIV might be beneficial not only for the vaccinees, but also for those who cannot be safely immunized for health reasons. In addition, the induction of memory immune responses after primary immunization with LAIV will result in a prompt and robust immune response to a low dose of inactivated vaccine given several months later, allowing significant vaccine dose-sparing and as a result, higher vaccination coverage.
H7N9 vaccine candidates based on several other vaccine platforms have been evaluated previously in clinical trials. Interestingly, regardless of the vaccine platform used, all the unadjuvanted H7N9 vaccines were poorly immunogenic in adults. However, the addition of either ISCOMATRIX or MF59 adjuvant significantly improved the immunogenicity of the vaccines with a potential of dose-sparing, although the adjuvants increased the frequency of local reactions to the vaccines.30–32 It should be noted that the H7N9 LAIV was produced by classical reassortment, and the vaccine reassortant is characterized by high yield in eggs, which is the result of positive selection by the host system. These high-yield and immunogenic properties of the H7N9 LAIV are also encouraging, since countries such as India, China and Thailand have already established their own LAIV production capacities.19, 33 Should a pandemic begin, a large number of doses could quickly become available to protect their high-density populations. Thus, in 2009 LAIVs were among the first vaccines released for public use.23, 33 A prompt vaccination campaign with H7N9 LAIV combined with high vaccination coverage might be an effective preventive measure to combat an H7N9 pandemic.
Supplementary Material
Acknowledgements
We are thankful to Oleg Kiselev for the help in organizing this clinical trial; to Svetlana Donina and Galina Petukhova for the help in conducting serological assays; to Vera Krivitskaya and Maria Pisareva for conducting microneutralization and qRT-PCR assays. We are also thankful to Alexandra Nikiforova for the help in preparation study protocol and getting approval from study’s Independent Ethics Committee and Institutional Review Board; and to Microgen Company for the production of the vaccine lot.
Funding
The study was funded by World Health Organization. The results of the study will be used for the production of the vaccine in developing countries, according to the agreement with WHO. The sponsor of the study was involved in trial design, monitoring of study implementation, data collection, analysis and interpretation of the study results.
Footnotes
Contributors
LR was the principal investigator for the study, supervised the development of vaccine strain, oversaw the study design, study implementation, data analysis and interpretation, and writing of the manuscript. IIS developed the vaccine strain, demonstrated safety and efficacy of the vaccine strain in pre-clinical trials and prepared the dossier for Ethic Committee, was involved in trial design and data analysis, and wrote the first draft of the manuscript. AN performed serological assays, supervised data collection, analysed and interpreted the data. IK performed virological assays and analised the virus shedding data. MS was responsible for trial monitoring and compliance with Good Clinical Practice guidelines, analysed and interpreted the data. ME was involved in trial design and monitoring of the study implementation at the study site, supervised data collection and interpreted the safety data. DK assessed cell-mediated immune responses, analysed and interpreted the data. VM performed sequencing analysis of the vaccine virus isolates and interpreted the data. ES was involved in trial design and monitoring, and finalised the manuscript. MPK was the senior advisor for trial design and implementation.
Declaration of interests
We declare no competing interests.
References
- 1.Su S, Bi Y, Wong G, Gray GC, Gao GF, Li S. Epidemiology, Evolution, and Recent Outbreaks of Avian Influenza Virus in China. Journal of virology. 2015;89(17):8671–6. doi: 10.1128/JVI.01034-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kucharski AJ, Mills HL, Donnelly CA, Riley S. Transmission Potential of Influenza A(H7N9) Virus, China, 2013-2014. Emerging infectious diseases. 2015;21(5):852–5. doi: 10.3201/eid2105.141137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Y, Zhang W, Wang F, et al. Structures and receptor binding of hemagglutinins from human-infecting H7N9 influenza viruses. Science. 2013;342(6155):243–7. doi: 10.1126/science.1242917. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Wang D, Gao R, et al. Biological features of novel avian influenza A (H7N9) virus. Nature. 2013;499(7459):500–3. doi: 10.1038/nature12379. [DOI] [PubMed] [Google Scholar]
- 5.Belser JA, Gustin KM, Pearce MB, et al. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature. 2013;501(7468):556–9. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herfst S, Imai M, Kawaoka Y, Fouchier RA. Avian influenza virus transmission to mammals. Current topics in microbiology and immunology. 2014;385:137–55. doi: 10.1007/82_2014_387. [DOI] [PubMed] [Google Scholar]
- 7.Gillman A, Nykvist M, Muradrasoli S, et al. Influenza A(H7N9) Acquires Resistance Related NA-I222T Substitution when Infected Mallards are Exposed to Low Levels of Oseltamivir in Water. Antimicrobial agents and chemotherapy. 2015 doi: 10.1128/AAC.00886-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh Y, Shichinohe S, Nakayama M, et al. Emergence of H7N9 Influenza A Virus Resistant to Neuraminidase Inhibitors in Nonhuman Primates. Antimicrobial agents and chemotherapy. 2015;59(8):4962–73. doi: 10.1128/AAC.00793-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden FG, Howard WA, Palkonyay L, Kieny MP. Report of the 5th meeting on the evaluation of pandemic influenza prototype vaccines in clinical trials: World Health Organization, Geneva, Switzerland, 12-13 February 2009. Vaccine. 2009;27(31):4079–89. doi: 10.1016/j.vaccine.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 10.Bardiya N, Bae JH. Influenza vaccines: recent advances in production technologies. Applied microbiology and biotechnology. 2005;67(3):299–305. doi: 10.1007/s00253-004-1874-1. [DOI] [PubMed] [Google Scholar]
- 11.Jin H, Subbarao K. Live Attenuated Influenza Vaccine. In: Oldstone MBA, Compans RW, editors. Influenza Pathogenesis and Control - Volume II. Springer International Publishing; 2015. pp. 181–204. [Google Scholar]
- 12.Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Japanese journal of infectious diseases. 2005;58(4):195–207. [PubMed] [Google Scholar]
- 13.Isakova-Sivak I, Chen LM, Matsuoka Y, et al. Genetic bases of the temperature-sensitive phenotype of a master donor virus used in live attenuated influenza vaccines: A/Leningrad/134/17/57 (H2N2) Virology. 2011;412(2):297–305. doi: 10.1016/j.virol.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Isakova-Sivak I, Stukova M, Erofeeva M, et al. H2N2 live attenuated influenza vaccine is safe and immunogenic for healthy adult volunteers. Human vaccines & immunotherapeutics. 2015;11(4):970–82. doi: 10.1080/21645515.2015.1010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudenko L, Kiseleva I, Naykhin AN, et al. Assessment of human immune responses to H7 avian influenza virus of pandemic potential: results from a placebo-controlled, randomized double-blind phase I study of live attenuated H7N3 influenza vaccine. PloS one. 2014;9(2):e87962. doi: 10.1371/journal.pone.0087962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. WHO Manual on animal influenza diagnosis and surveillance. [accessed 12 August 2013];2002 Available from: http://www.bvsde.paho.org/bvsacd/cd52/animal.pdf.
- 17.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. Journal of clinical microbiology. 1999;37(4):937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior research methods. 2009;41(4):1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 19.Rudenko L, Isakova-Sivak I. Pandemic preparedness with live attenuated influenza vaccines based on A/Leningrad/134/17/57 master donor virus. Expert review of vaccines. 2015;14(3):395–412. doi: 10.1586/14760584.2015.979159. [DOI] [PubMed] [Google Scholar]
- 20.Talaat KR, Karron RA, Callahan KA, et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a Phase I trial in healthy adults. Vaccine. 2009;27(28):3744–53. doi: 10.1016/j.vaccine.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babu TM, Levine M, Fitzgerald T, et al. Live attenuated H7N7 influenza vaccine primes for a vigorous antibody response to inactivated H7N7 influenza vaccine. Vaccine. 2014;32(50):6798–804. doi: 10.1016/j.vaccine.2014.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zens KD, Farber DL. Memory CD4 T cells in influenza. Current topics in microbiology and immunology. 2015;386:399–421. doi: 10.1007/82_2014_401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coelingh KL, Luke CJ, Jin H, Talaat KR. Development of live attenuated influenza vaccines against pandemic influenza strains. Expert review of vaccines. 2014;13(7):855–71. doi: 10.1586/14760584.2014.922417. [DOI] [PubMed] [Google Scholar]
- 24.Rudenko L, Isakova-Sivak I, Donina S. H7N3 live attenuated influenza vaccine has a potential to protect against new H7N9 avian influenza virus. Vaccine. 2013;31(42):4702–5. doi: 10.1016/j.vaccine.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Carter DM, Bloom CE, Kirchenbaum GA, et al. Cross-protection against H7N9 influenza strains using a live-attenuated H7N3 virus vaccine. Vaccine. 2015;33(1):108–16. doi: 10.1016/j.vaccine.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Rudenko L, Naykhin A, Donina S, et al. Assessment of immune responses to H5N1 inactivated influenza vaccine among individuals previously primed with H5N2 live attenuated influenza vaccine. Human vaccines & immunotherapeutics. 2015 doi: 10.1080/21645515.2015.1069931. Accepted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talaat KR, Luke CJ, Khurana S, et al. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. The Journal of infectious diseases. 2014;209(12):1860–9. doi: 10.1093/infdis/jiu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barria MI, Garrido JL, Stein C, et al. Localized mucosal response to intranasal live attenuated influenza vaccine in adults. The Journal of infectious diseases. 2013;207(1):115–24. doi: 10.1093/infdis/jis641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudenko LG, Slepushkin AN, Monto AS, et al. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. The Journal of infectious diseases. 1993;168(4):881–7. doi: 10.1093/infdis/168.4.881. [DOI] [PubMed] [Google Scholar]
- 30.Fries LF, Smith GE, Glenn GM. A recombinant viruslike particle influenza A (H7N9) vaccine. The New England journal of medicine. 2013;369(26):2564–6. doi: 10.1056/NEJMc1313186. [DOI] [PubMed] [Google Scholar]
- 31.Mulligan MJ, Bernstein DI, Winokur P, et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2014;312(14):1409–19. doi: 10.1001/jama.2014.12854. [DOI] [PubMed] [Google Scholar]
- 32.Bart SA, Hohenboken M, Della Cioppa G, Narasimhan V, Dormitzer PR, Kanesa-Thasan N. A cell culture-derived MF59-adjuvanted pandemic A/H7N9 vaccine is immunogenic in adults. Science translational medicine. 2014;6(234):234ra55. doi: 10.1126/scitranslmed.3008761. [DOI] [PubMed] [Google Scholar]
- 33.Rudenko L, van den Bosch H, Kiseleva I, et al. Live attenuated pandemic influenza vaccine: clinical studies on A/17/California/2009/38 (H1N1) and licensing of the Russian-developed technology to WHO for pandemic influenza preparedness in developing countries. Vaccine. 2011;29(Suppl 1):A40–4. doi: 10.1016/j.vaccine.2011.04.122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.