Abstract
Objective
To examine the role of comorbidity and pain in the associations of hand osteoarthritis (OA) with self-reported and performance-based physical function in a general population of elderly persons.
Methods
We studied data from 2,942 participants ages 65-85 years in the European Project on OSteoArthritis, a collaborative observational study of 6 European cohorts (from Germany, Italy, The Netherlands, Spain, Sweden and the UK). Outcomes measures included self-reported physical function of the hands measured by the Australian/Canadian Osteoarthritis Hand Index (AUSCAN) for hand OA physical function subscale and performance-based grip strength measured using a strain gauge dynamometer.
Results
Comorbidity was not a confounder in the association of hand OA with self-reported and performance-based functional limitations, while the role of pain as a mediator was confirmed. Anxiety, depression, stroke and osteoporosis were associated with AUSCAN scores reflecting more impairment. Depression and osteoporosis were associated with less grip strength.
Conclusions
Although comorbidity was decidedly and independently associated with hand functional limitation, it had no effect on the relationship of hand OA with physical function. Hand OA was found to be associated with both self-reported and performance-based physical function impairment; the association was found to be partially mediated by pain, which reduces its impact.
Keywords: osteoarthritis, comorbidity, functional limitations, AUSCAN, pain
Osteoarthritis (OA) is a widespread disease that is receiving specific attention from researchers, clinicians and public health professionals because of its increased occurrence given the progressive aging of the population. The disease represents a major burden for both the individual and society because of its impact on quality of life and the economic consequences linked to loss of autonomy (1,2).
To date, most epidemiologic studies have focused on radiographic OA, and relatively few population-based studies have targeted symptomatic disease, especially symptomatic hand OA. Symptomatic hand OA is less frequent than radiographic OA, suggesting that radiographic features are not necessarily symptomatic (3).The prevalence of radiographic hand OA has been reported to range from 27% to >80% (4). Frequently involving the carpometacarpal (CMC), peripheral interphalangeal (PIP) and distal interphalangeal (DIP) joints, it may be associated with pain, stiffness and functional impairment, which have important implications particularly with regard to personal care, preparing meals and eating, household management (5,6). It has been demonstrated that persistent pain and activity limitations and the frustration linked to them may deeply affect the quality of life of patients with hand OA (7).
Old age, comorbidity, and hand OA have all been linked to loss of independence in older individuals (8). Since the impact of both somatic and mental comorbidities on physical functioning is important when assessing the effect of selected conditions, it is essential to evaluate both self-reported and performance-based parameters in order to plan appropriate interventions, including drug treatments, occupational and physical therapy (9). The current analysis, which was carried out on population-based cohorts of elderly persons from 6 European countries, aimed to assess: 1) whether comorbidity is a confounder in the association between hand OA and self-reported and performance-based functional limitations in daily activities and 2) the impact of selected diseases, considering the mediating role of pain.
Patients and Methods
Study design and sample characteristics
We analyzed baseline data of 2,942 subjects (1,416 men and 1,526 women) ages 65-85 years who participated in the European Project on OSteoArthritis (EPOSA), a collaborative observational study of several European cohorts (from Germany, Italy, The Netherlands, Spain, Sweden and the UK). All of the medical ethics committees of the institutions involved in the study granted approval. A detailed description of the study design and data collection procedure of the EPOSA study has been reported elsewhere (10). Written informed consent was obtained from participants. Data were collected from November 2010 to November 2011.
A trained researcher interviewed the participants at their home or in a medical center. At that time, subjects were asked to complete a standardized questionnaire regarding demographics, comorbidities, cognitive and psychological status/functioning, medications being taken, and physical activity limitations. Participants also underwent a short physical performance battery and were subjected to a clinical examination that included an assessment of OA status.
Outcomes Measures
Outcome variables included self-reported physical function of the hands as measured by the Australian/Canadian OA Hand Index (AUSCAN) (11) and a performance-based test (grip strength test). The AUSCAN for hand OA physical function subscale contains 9 items focusing on hand problems during common daily activities (turning, fastening, opening, carrying, grabbing, and squeezing various objects) in the 48 hours before the participant took the test. A 5-point Likert scale was used that assigned numerical values to answers about activities as causing no difficulty (none) to extreme difficulty (none=0, mild=1, moderate=2, severe=3, and extreme=4). The maximum possible score was 36; the final score was normalized to 100 (range 0-100). Higher scores reflect greater difficulty in daily activities.
Grip strength was measured using a strain gauge dynamometer (12). While seated in a chair with arms, subjects were asked to rest their forearm on the arms of the chair with the elbow at right angles and the wrist in a neutral position. The examiner demonstrated how to use the dynamometer, indicating that gripping very tightly registers the best score. The hand was positioned so that the thumb went around one side of the handle and the other 4 fingers went around the other side. The hand had to feel comfortable holding the instrument, so the position was altered if necessary. The dynamometer was supported lightly by the examiner. The subject was encouraged to squeeze as long and as tightly as possible or until the needle stopped rising. The result was recorded to the nearest kilogram. Grip strength was measured alternately, beginning with the right hand. It was measured a total of 2 times on each side. To calculate the total score, the maximum values of the right and the left hands were summed and divided by two. If only one hand could be used, the values of that subject were not considered in the analysis.
Clinical diagnosis of hand OA
Hand OA was diagnosed on the basis of the patient’s medical history and the physical examination he/she underwent, using algorithms based on the clinical criteria developed by the American College of Rheumatology (ACR) (13) and on recommendations of the European League Against Rheumatism (14). For the AUSCAN pain and stiffness sections, the responses are categorized on a 5-point Likert scale ranging from none to extreme difficulty (none=0, mild=1, moderate=2, severe=3, and extreme=4) (pain score for hand OA cutoff ≥3; stiffness score for hand OA cutoff ≥1). In addition, a diagnosis of OA required fulfillment of at least 2 of the following criteria: hard tissue enlargement of ≥2 of the second and third DIP joints, second and third PIP joints, or first CMC joint of at least 1 hand; hard tissue enlargement of ≥2 DIP joints of at least 1 hand; deformity of at least 1 of the second and third DIP joints, second and third PIP joints, first CMC joint of at least 1 hand. Swelling of the metacarpophalangeal joints, which is also included in the ACR classification criteria as a measure to exclude rheumatoid arthritis, was assessed only in the UK and Germany.
Demographic data
We collected participants’ demographic data including age, gender, country of origin and education level. Education level was dichotomized as less than secondary education (“elementary school not completed”, “elementary school completed”) versus the highest level of education (“vocational education/general secondary education”, and “college or university education”).
Comorbidity
During the health assessment height and weight of the participants were measured, and obesity was defined as a body mass index of ≥30 kg/m2. Cognitive impairment was defined as a score of ≤23 on the 22-item Mini-Mental State Examination, which has a maximum possible score of 30 (15).
The Hospital Anxiety Depression Scale, which includes 14 items with a maximum possible score of 21 for each of the two subscales (16), was used to evaluate anxiety and symptoms of depression. Participants with a score of ≥8 on either or both of the subscales were considered anxious and/or depressed.
Direct questions concerning diseases/symptoms lasting at least 3 months or which caused the participant to seek a physician’s attention were used to assess some chronic conditions. Those questions were phresed as follows: “Do you have or have you ever had any of the following diseases: chronic non-specific lung disease (i.e. asthma, chronic bronchitis or pulmonary emphysema, etc.), cardiovascular disease (i.e. cardiac valve disease, coronary heart diseases, arrhythmia, a pacemaker, cardiac arrest, etc.), peripheral artery disease, stroke, diabetes, cancer, or osteoporosis?” Participants were asked to give more details if the answer was affirmative. Participants reporting ≥3 coexisting disorders (third quartile) were classified as having comorbidity.
Pain
The AUSCAN for hand OA pain subscale, which contains 5 specific items concerning pain felt in the hand (at rest and during gripping, lifting, turning, and squeezing objects), was used to assess pain experienced during the previous 48 hours. As outlined above, the responses were categorized on a 5-point Likert scale ranging from no pain (none) to extreme pain, and the scores were summed to obtain a pain subscale score ranging from 0 to 20. The participants’ final scores were normalized to 100 (range 0–100). A higher score reflects more pain.
Statistical Analysis
Descriptive and association analyses
These analyses were performed using a set of weights calculated per sex and per 5-year age category considering the 2010 European Standard Population. The comparisons carried out refer to baseline characteristics including continuous variables such as age, the AUSCAN physical function and pain domain scores, and grip strength, and categorical variables such as sex, country of origin, education level, comorbidity, selected diseases, and clinical hand OA. Continuous variables were expressed as medians and interquartile range (IQR) and categorical variables were expressed as proportions.
Due to partial collection of informations, the median and the interquartile range of the AUSCAN physical function and pain scores for participants from Germany were zero, and their data were not included when we examined associations between the study variables and the 2 outcomes. As a result, only the 2,242 subject with complete records were considered in the analysis. The first outcome, the AUSCAN for hand OA physical function subscale score, was dichotomized at the highest quartile versus the lower quartiles, and the more impaired category was defined as subjects with scores of >11.1. The second outcome, grip strength score, was dichotomized as the lowest tertile versus the higher tertiles of subsample distributions stratified by sex and country of origin. The category of less strength was defined for women as a score <20.0 (kg) in Italy and in The Netherlands, <15.0 (kg) in Spain, <19.0 (kg) in Sweden, and <17.5 (kg) in the UK. For men it was defined as a score <34.0 (kg) in Italy and in The Netherlands, <27.0 (kg) in Spain, <34.5 (kg) in Sweden, and <31.0 (kg) in the UK. Comparisons among and between countries or categories were assessed by Kruskal-Wallis test, Wilcoxon rank-sum test for continuous variables and Chi-squared test for categorical variables.
Multivariable analysis
The sequential modeling approach using multiple logistic regressions was implemented to assess the effect of hand OA on all study outcomes. The first model was adjusted for sex, age, country of origin, and education level (less than secondary education). In the second, comorbidity (≥ 3 diseases) was added to assess whether it was a confounding factor. In the third model, the AUSCAN assessment of hand pain was added to determine whether this variable was a potential mediator of hand OA. A further logistic model on hand OA was run; this model was adjusted for sex, age, country of origin, and education level, as well as for pain considering specific diseases such as obesity, cognitive impairment, anxiety, and depression and self-reported physician diagnosis of chronic lung disease, cardiovascular disease, peripheral artery disease, diabetes mellitus, stroke, cancer, and osteoporosis.
SAS macro, implemented by Valeri and VanderWeele (17), was used to test the pain mediation effect and to obtain estimates and confidence intervals for the direct and indirect effects of hand OA on hand functional limitation. All interaction effects were investigated, particularly those regarding the country of origin, to assess whether a stratified analysis was appropriate; the first interaction with a P values less than 0.10 was added to the model. Two-sided P values less than 0.05 were considered significant. Analyses were performed using SAS software, version 9.4 (SAS Institute).
Results
Sample characteristics
With regard to the baseline characteristics of the total EPOSA study population stratified by country of origin (weighted data), approximately 25% of the participants showed ≥3 comorbidities and 17% were affected by hand OA (Table 1); significant differences were found between the countries of origin. The prevalence of clinical hand OA ranged from 11.3% in The Netherlands cohort to 24.2% in the Italian cohort. Grip strength was highest in the German and Italian cohorts and lowest in the Spanish cohort.
Table 1.
Weighted baseline characteristics of the EPOSA study subjects, stratified by country of origin*
| All subjects (n = 2,942) |
Germany (n = 407) |
Italy (n = 468) |
The Netherlands (n = 574) |
Spain (n = 539) |
Sweden (n = 510) |
UK (n = 444) |
|
|---|---|---|---|---|---|---|---|
| Sociodemographic characteristics | |||||||
| Women | 51.9 | 40.3 | 53.2 | 55.2 | 49.7 | 60.0 | 50.0 |
| Age, median (IQR) years | 74 (70-78) | 73 (70-77) | 73 (69-77) | 75 (70-80) | 75 (70-80) | 71 (68-75) | 75 (73-77) |
| Less than secondary education | 45.0 | 49.9 | 77.6 | 25.5 | 72.4 | 23.3 | 20.9 |
| Health status | |||||||
| Obesity | 26.1 | 24.4 | 26.1 | 25.6 | 33.2 | 16.9 | 31.2 |
| Cognitive impairment | 9.0 | 0.9 | 19.1 | 4.6 | 13.4 | 5.7 | 10.0 |
| Anxiety | 20.7 | 14.2 | 53.0 | 16.5 | 17.6 | 7.3 | 16.4 |
| Depression | 11.9 | 7.7 | 20.9 | 12.7 | 19.5 | 1.3 | 7.4 |
| Chronic lung disease | 14.0 | 16.0 | 6.9 | 13.4 | 21.2 | 12.5 | 13.7 |
| Cardiovascular disease | 25.4 | 21.4 | 23.5 | 28.2 | 30.8 | 25.4 | 19.6 |
| Peripheral artery disease | 11.9 | 13.6 | 12.2 | 21.2 | 11.0 | 8.5 | 1.5 |
| Diabetes mellitus | 13.0 | 11.4 | 12.1 | 15.1 | 17.9 | 7.9 | 13.1 |
| Stroke | 5.6 | 3.9 | 4.6 | 6.8 | 6.0 | 6.2 | 5.6 |
| Cancer | 14.5 | 19.9 | 13.9 | 14.0 | 10.2 | 14.9 | 16.6 |
| Osteoporosis | 17.1 | 10.7 | 20.4 | 19.5 | 22.8 | 9.0 | 19.6 |
| Comorbidity (≥3 diseases) | 25.2 | 17.9 | 34.6 | 27.6 | 35.4 | 12.8 | 20.0 |
| Clinical hand osteoarthritis, (%) | 17.1 | 13.3 | 24.2 | 11.3 | 19.3 | 19.4 | 14.7 |
| AUSCAN subscale scores, median (IQR) | |||||||
| Physical functiona | 0.0 (0.0-11.1) | 0.0 (0.0-0.0) | 0.0 (0.0-11.1) | 0.0 (0.0-13.9) | 0.0 (0.0-13.9) | 0.0 (0.0-13.9) | 0.0 (0.0-11.1) |
| Painb | 0.0 (0.0-5.0) | 0.0 (0.0-0.0) | 0.0 (0.0-10.0) | 0.0 (0.0-0.0) | 0.0 (0.0-10.0) | 0.0 (0.0-10.0) | 0.0 (0.0-5.0) |
| Grip strengthc, median (IQR), kg | 25.5 (19.5-34.5) | 27.5 (20.0-36.5) | 27.0 (22.0-36.0) | 25.5 (20.5-37.0) | 21.5 (16.0-30.0) | 25.5 (20.5-35.0) | 24.5 (18.5-33.0) |
Number of subjects, age, and sex were unweighted data. Except were indicated otherwise, values are percent of subjects. EPOSA = European Project on OSteoArthritis; IQR = interquartile range; AUSCAN = AUStralian/CANadian Osteoarthritis Hand Index.
Possible hand physical function scores ranges from 0 to 100 (standardized x 100/36), with 0 indicating no limitations.
Possible hand pain scores ranges from 0 to 100 (standardized x 100/20), with 0 indicating no pain.
Lower values indicate worse function.
AUSCAN physical function and grip strength
Table 2 shows characteristics of subjects belonging to the category of more impaired (compared with others) according to the AUSCAN for hand OA physical function subscale or belonging to the category of less strength (compared with others) based on grip strength values (weighted data). Subjects belonging to the more impaired category according to the AUSCAN for hand OA physical function subscale (n = 604) were older, mostly women, had a lower level of education, and had significantly different prevalences of nearly all the diseases.
Table 2.
Weighted baseline characteristics of the EPOSA study subjects, stratified by AUSCAN for hand OA physical function subscale score and grip strength*
| AUSCAN for hand OA physical function | Grip strength | |||||
|---|---|---|---|---|---|---|
| More impaireda (n = 604) |
Other (n = 1,638) |
P | Lessb (n=680) |
Other (n = 1,562) |
P | |
| Women | 84.64 | 46.91 | <0.0001 | 58.19 | 57.23 | 0.6778 |
| Age, median (IQR), years | 74.3 (69.5-79.8) | 72.4 (68-77) | <0.0001 | 76.0 (72-81) | 72.0 (68-76) | <0.0001 |
| Country of origin | 0.1783 | 0.4717 | ||||
| Italy | 18.00 | 21.17 | 18.29 | 21.11 | ||
| Netherlands | 25.76 | 23.04 | 24.47 | 23.52 | ||
| Spain | 21.86 | 20.71 | 21.94 | 20.65 | ||
| Sweden | 21.95 | 20.21 | 19.89 | 21.04 | ||
| UK | 12.44 | 14.87 | 15.41 | 13.67 | ||
| Less than secondary education | 49.03 | 42.00 | 0.0030 | 47.76 | 42.39 | 0.0214 |
| Obesity | 29.87 | 24.23 | 0.0069 | 24.46 | 26.38 | 0.3505 |
| Cognitive impairment | 13.49 | 8.06 | 0.0001 | 13.37 | 8.02 | 0.0001 |
| Anxiety | 31.25 | 17.82 | <0.0001 | 24.36 | 20.44 | 0.0428 |
| Depression | 20.28 | 9.39 | <0.0001 | 18.20 | 10.03 | <0.0001 |
| Chronic lung disease | 16.07 | 11.53 | 0.0044 | 14.88 | 11.94 | 0.0608 |
| Cardiovascular disease | 27.56 | 24.59 | 0.1525 | 29.95 | 23.53 | 0.0017 |
| Peripheral artery disease | 11.66 | 11.22 | 0.7711 | 14.25 | 10.13 | 0.0057 |
| Diabetes mellitus | 11.83 | 13.19 | 0.3941 | 14.72 | 12.00 | 0.0838 |
| Stroke | 7.28 | 4.50 | 0.0094 | 6.46 | 4.79 | 0.1119 |
| Cancer | 14.89 | 12.25 | 0.0993 | 12.81 | 13.07 | 0.8740 |
| Osteoporosis | 29.68 | 12.71 | <0.0001 | 23.05 | 15.15 | <0.0001 |
| Comorbidity (≥3 diseases) | 38.42 | 20.41 | <0.0001 | 33.91 | 21.88 | <0.0001 |
| Clinical hand osteoarthritis | 46.90 | 5.68 | <0.0001 | 27.01 | 13.18 | <0.0001 |
| AUSCAN for hand OA pain suscale scorec, median (IQR) | 20 (0-35) | 0 (0-0) | <0.0001 | 0 (0-20) | 0 (0-25) | <0.0001 |
Except were indicated otherwise, values are percent of subjects. EPOSA = European Project on OSteoArthritis; IQR = interquartile range;
A score higher than the third quartile on the AUStralian/CANadian Osteoarthritis Hand Index (AUSCAN) for hand osteoarthritis (OA) physical function subscale.
A score at the first tertile of subsample distribution stratified by sex and country of origin
Possible hand pain scores ranges from 0 to 100 (standardized x 100/20), with 0 indicating no pain.
Subjects with less grip strength (n = 680) were older, less educated, and had more comorbidities, particularly cognitive impairment, anxiety, depression, cardiovascular diseases, peripheral artery diseases and osteoporosis.
Clinical hand OA and AUSCAN physical function
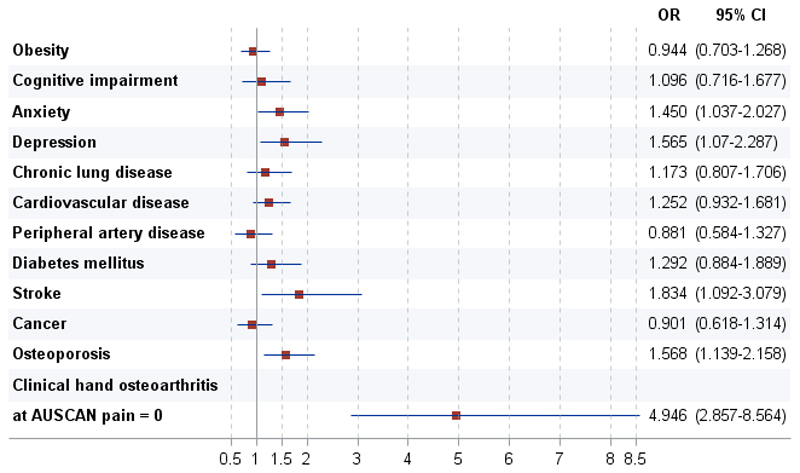
Multivariable logistic regressions analyses to evaluate the effect of hand OA on AUSCAN physical function limitations, controlling for confounding and assessing mediating effects, are shown in Table 3. There were no major differences in the association of clinical hand OA with physical function limitations between the first model (model 1; adjusted for sex, age, country of origin, and education level) and the second model (model 2; also controlled for comorbidity), suggesting that comorbidity is not a confounder in that relationship. When the pain variable was included, there was evidence of both a statistical adjustment (model 3) and a significant interaction (model 4) with respect to the effect of OA. The test to assess mediation confirmed that pain was indeed a mediator, or more specifically, that part of the effect of hand OA on physical function works through pain. When pain and other variables were examined, the odds ratio for physical function limitations was 5.1 for hand OA (versus non-OA) (model 4). Substituting the specific diseases for the number of comorbidities (see Supplementary Table 1), Figure 1 shows that beyond hand OA (for which the association was partially mediated by pain), anxiety, depression, stroke, and osteoporosis were associated to physical function limitation.
Table 3.
Multivariable logistic regression analyses for the association of AUSCAN for hand OA physical function subscale score with clinical hand OA*
| Model | Clinical hand osteoarthritis | βa | Standard Error | P | Δβ%b | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| 1 | Plus sex, age, country of origin, education level (less than secondary) | 2.7806 | 0.1503 | <0.0001 | 16.129 | 12.015-21.652 | |
| 2 | Plus sex, age, country of origin, education level (less than secondary), and comorbidity (≥ 3 diseases) | 2.7642 | 0.1522 | <0.0001 | 0.6 | 15.866 | 11.773-21.382 |
| 3 | Plus sex, age, country of origin, education level (less than secondary), comorbidity (≥ 3 diseases), and AUSCAN for hand OA pain subscale scorec | 0.9977 | 0.2042 | <0.0001 | 177.1 | 2.712 | 1.817-4.047 |
| 4 | Plus sex, age, country of origin, education level (less than secondary), comorbidity (≥ 3 diseases), AUSCAN for hand OA pain subscale scorec, and interaction of clinical hand OA with AUSCAN for hand OA pain subscale score | 1.6275 | 0.2775 | <0.0001 | # |
The outcome being measured was the likelihood of having more impairment (an AUStralian/CANadian Osteoarthritis Hand Index [AUSCAN] for hand osteoarthritis [OA] physical function score in the highest quartile) versus not having more impairment (a score in the lower quartiles).
Estimated coefficients.
Amount of adjustment = 100 x (β previous Model – β Model) / β Model.
Possible AUSCAN for hand OA pain subscale scores range from 0 to 100, with 0 indicating no pain.
Controlled direct effect: P <0.0001, odds ratio (OR) 5.091 (95% confidence interval [95% CI] 2.955-8.773); natural direct effect: P = 0.5622, OR 0.763 (95% CI 0.305-1.907); natural indirect effect: P <0.0001, OR 5.563 (95% CI 3.222-9.604); total effect: P <0.0001, OR 14.463 (95% CI 8.300-25.200)
Estimated ORs for clinical hand OA as a function of AUSCAN for hand OA pain subscale scores of 0, 5, 15, and 25 were 5.091 (95% CI 2.955-8.773), 4.016 (95% CI 2.533-6.366), 2.498 (95% CI 1.657-3.767), 1.554 (95% CI 0.906-2.665), respectively.
Figure 1.
Association of the AUStralian/CANadian Osteoarthritis Hand Index (AUSCAN) for hand osteoarthritis (OA) physical function subscale score with clinical hand OA and specific comorbidities. A multivariable logistic regression analysis was performed controlling for sex, age, country of origin, and education level. Subjects defined as having more impairment (those with AUSCAN for hand OA physical function subscale scores in the highest quartile) were compared with subjects with scores in the lower quartiles. Odds ratios (ORs) with 95% confidence intervals (95%Cis) are shown for having more impairment versus not having more impairment. Possible AUSCAN for hand OA pain subscale scores range from 0 to 100, with 0 indicating no pain.
Clinical hand OA and grip strength
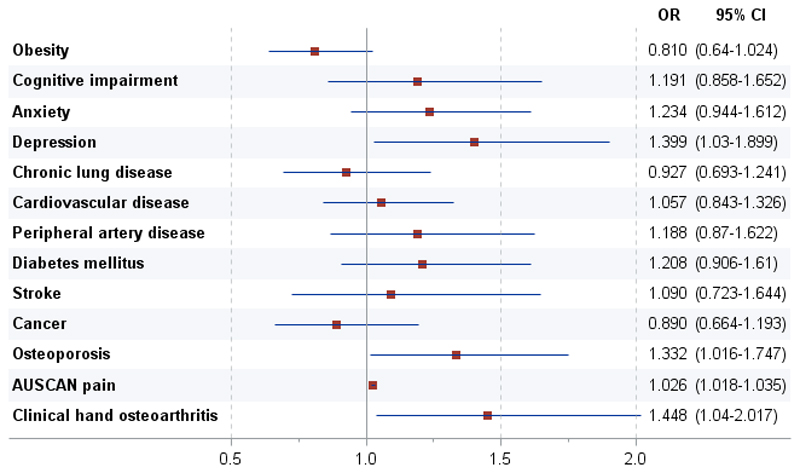
Multivariable logistic regressions analyses to evaluate the effect of hand OA on grip strength, controlling for confounding and assessing mediation effects, are outlined in Table 4. When we compared the analysis adjusted for sex, age, country of origin, and education level (model 1) with the analysis also adjusted for comorbidity (model 2), we found no difference in the effect of hand OA, which suggests that clinical hand OA was significantly associated with less grip strength, and that comorbidity was not a confounder. When the pain score was included (model 3) there was evidence that part but not all of the effect of hand OA works through pain. The most important interaction was between age and sex (model 4). The method used for testing mediation confirmed that pain was responsible for 75% of the effect of clinical hand OA on grip strength. When individual comorbidities were considered (see Supplementary Table 2), only depression and osteoporosis were associated with less grip strength (Figure 2).
Table 4.
Multivariable logistic regression analyses for the association of grip strength with clinical hand OA*
| Model | Clinical hand osteoarthritis | β | Standard Error | P | Δβ%b | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| 1 | Plus sex, age, country of origin, education level (less than secondary) | 1.1066 | 0.1282 | <0.0001 | 3.024 | 2.352-3.888 | |
| 2 | Plus sex, age, country of origin, education level (less than secondary), and comorbidity (≥ 3 diseases) | 1.0655 | 0.1291 | <0.0001 | 3.9 | 2.902 | 2.254-3.738 |
| 3 | Plus sex, age, country of origin, education level (less than secondary), comorbidity (≥ 3 diseases), and AUSCAN for hand OA pain subscale scorec | 0.3990 | 0.1686 | 0.0180 | 167.0 | 1.490 | 1.071-2.074 |
| 4 | Plus sex, age, country of origin, education level (less than secondary), comorbidity (≥ 3 diseases), AUSCAN for hand OA pain subscale scorec, and interaction of sex with age | 0.3837 | 0.1678 | 0.0222 | 1.468 | 1.056-2.039 |
The outcome being measured was the likelihood of having less grip strength (a grip strength score in the lowest tertile of subsample distributions stratified by sex and country of origin) versus not having less grip strength (a score in the higher tertiles)
Estimated coefficients.
Amount of adjustment = 100 x (β previous Model – β Model) / β Model.
Possible AUStralian/CANadian Osteoarthritis Hand Index (AUSCAN) for hand osteoarthritis (OA) pain subscale scores range from 0 to 100, with 0 indicating no pain.
Controlled direct effect and natural direct effect: P = 0.0222, odds ratio (OR) 1.468 ((95% confidence interval [95% CI] 1.056-2.039); natural indirect effect: P <0.0001, OR 1.953 (95% CI 1.571-2.429); total effect P <0.0001, OR 2.867 (95% CI 2.212-3.715).
Figure 2.
Association of grip strength with clinical hand OA and specific comorbidities. A multivariable logistic regression analysis was performed controlling for sex, age, country of origin, and education level. Subjects defined as having less grip strength (those with grip strength scores at the lowest tertile of subsample distribution stratified by sex and country of origin) were compared with subjects with scores in the higher tertiles. Odds ratios (ORs) with 95% confidence intervals (95%Cis) are shown for having less grip strength versus not having less grip strength. Possible AUSCAN for hand OA pain subscale scores range from 0 to 100, with 0 indicating no pain.
Discussion
The present study shows that comorbidity is independently associated with more impaired self-reported and performance-based functional limitations, without confounding the association of hand OA with physical function. Pain is a mediator of hand OA with regard to both measures, but it only decreases, without cancelling, the association of OA with the study outcomes that remain significant even after multivariable adjustments for conditions normally considered associated with functional limitation.
In accordance with international guidelines (13,14), all participants underwent a standardized clinical assessment of OA. The AUSCAN for hand OA physical function subscale, which is a validated, reliable, standardized and patient-centered index for physical function, was used to assess the self-reported functional status of all the participants. Since it is possible that self-reported measures of physical function could be affected by cultural, educational, psychological, health, and cognitive variables, we also used hand grip strength as a performance-based physical function measure (18).
Anxiety and symptoms of depressive, as well as stroke and osteoporosis, are independently associated with more impaired self-reported physical function, but only symptoms of depression and osteoporosis are significantly associated with less grip strength. These results warrant careful consideration, because patients’ emotional health might affect the perception of being able to function. We believe that patient-reported outcome tell us more about the impact of hand OA than grip strength, which measures only a single task. Indeed, while grip strength measures the capacity and the willingness of a person to perform a task, the AUSCAN for hand OA physical function subscale measures function from the perspective of a person’s daily life, in which environmental and personal factors might interact.
The association of higher anxiety with more impaired self-reported function could be due to anxiety associated with anticipation of pain, and depressed individuals’ lack of perceived self-efficacy could lead to their avoiding or decreasing daily activities. This is consistent with prior reports that focused on hip and knee OA (19–21) and supports the importance of emotional status in the assessment of patients with OA. Moreover, when treatment is being evaluated, it is important to remember that the emotional status of the patient will affect both the adherence to and outcome of such treatment. In contrast to some previous reports (21,22), symptoms of depression in our study was associated to more impaired self-reported physical functioning and grip strength performance. This could be explained by the negative attitude of depressed individuals affecting the perception of their health and functional status and also affecting their performance during the muscle strength test, but further studies are needed to clarify this relationship.
We have demonstrated here that hand OA affects the functional ability of participants even after adjusting for pain. Indeed, it is well known that musculoskeletal performance is affected by multiple factors besides pain, such as nutritional status and anthropometric characteristics. Interestingly, obesity was not associated to impairment in upper limb function, confirming that in these older individuals, the detrimental inflammatory and hormonal profile of obesity, which often contributes to negative overall health outcomes in younger adults (23), was not demonstrated. These results are highly relevant, because they underline the fact that obesity in older individuals seems related only to OA affecting weight-bearing joints, as we have recently reported (24), and thus not to hand OA.
Our study has many strengths. First, we had a large sample selected from older community-dwelling Europeans who can be considered representative of the general population of elderly persons. OA was assessed in the same way across all participating countries, and its clinical diagnosis was formulated in accordance with standardized international guidelines (13,14). It is also important to point out that standardized, validated measures were used to evaluate the participants’ self-reported and performance-based physical function.
The study has some limitations as well. Since it was cross-sectional it was impossible to verify the association between predictive variables and outcomes. As the population samples were drawn from selected areas in Europe, they may not be representative of the national population of the countries included. If we want to generalize the results, we can do so only with regard to the clinical form of the disease and not the radiographic one, because radiographs were not used in this study. Comorbidity was assessed on the basis of self-reported diseases and selected screening tests, and was not ascertained clinically. Nevertheless, the self-reported diagnoses, can be considered reliable, since they were confirmed by the patients’ medication use and the screening instruments that were used. The AUSCAN, which is only rarely included in the battery used in population-based studies, was used to assess both pain and physical function. It is important to emphasize that the pain assessed by the AUSCAN also served to diagnose hand OA, although several other factors, mostly based on the physical examination, were also taken into consideration (13,14).
In conclusion, this cross-sectional study demonstrates that the association of hand OA with physical function is not affected by comorbidity which is, instead, strongly and independently associated with functional limitations. Moreover, the association of hand OA with physical functional limitations as evaluated by self-reported as well as performance-based measures was mediated, but not cancelled, by pain. The added value of this study is that the results support the hypothesis that emotional and somatic comorbidities can influence both the perception and the objective functional ability of individuals with hand OA. Finally, these findings shed light on the controversial risk factor of obesity, which does not seem to be associated with hand OA. Longitudinal investigations are needed to determine whether these results hold true in prospective follow-up evaluations.
Supplementary Material
Acknowledgements
We acknowledge the major contributions to this project to Thorsten Nikolaus, MD of the Bethesda Geriatric Clinic of the University of Ulm, Germany, who died in September 2013.
Our appreciation also goes to all of the individuals who participated in any way in the EPOSA study.
Funding
A non-commercial private funder supported the study.
The Indicators for Monitoring COPD and Asthma - Activity and Function in the Elderly in Ulm study (IMCA - ActiFE) was supported by the European Union (No.: 2005121) and the Ministry of Science, Baden-Württemberg. The Italian cohort study is part of the National Research Council Project on Aging (PNR). The Longitudinal Aging Study Amsterdam (LASA) is financially supported by the Dutch Ministry of Health, Welfare and Sports. The Peñagrande study was partially supported by the National Fund for Health Research (Fondo de Investigaciones en Salud) of Spain (project numbers FIS PI 05/1898; FIS RETICEF RD06/0013/1013 and FIS PS09/02143). The Swedish Twin Registry is supported in part by the Swedish Ministry of Higher Education. The Hertfordshire Cohort Study is funded by the Medical Research Council of Great Britain, Arthritis Research UK, the British Heart Foundation and the International Osteoporosis Foundation.
Footnotes
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Siviero had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Zambon, Castell, Cooper, Deeg, Denkinger, Dennison, Edwards, Otero, Pedersen, Peter, van Schoor, Maggi.
Acquisition of data. Siviero, Zambon, Limongi, Castell, Cooper, Deeg, Denkinger, Dennison, Edwards, Gesmundo, Otero, Pedersen, Peter, Queipo, Timmermans, van Schoor, Maggi.
Analysis and interpretation of data. Siviero, Zambon, Castell, Cooper, Deeg, Denkinger, Dennison, Edwards, Pedersen, Peter, Timmermans, van Schoor, Maggi.
Conflicts of Interest
Dr. Cooper has received consulting fees, speaking fees, and/or honoraria from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda, and UCB (less than $ 10,000 each).
Dr. Dennison was the recipient of speaking fees from Lilly (less than $ 10,000).
No conflicting interests were reported by the other authors.
The funders were not involved in designing/executing the study, in analyzing/interpreting the data, or in drafting the manuscript.
References
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Scientific Group. WHO technical report series; 919. Geneva, Switzerland: Wold Health Organization; 2003. The burden of musculoskeletal conditions at the start of the new millennium; pp. 1–218. [PubMed] [Google Scholar]
- 3.Hochberg MC, Vignon E, Maheu E. Session 2: clinical aspects. Clinical assessment of hand OA. Osteoarthritis Cartilage. 2000;8(Suppl A):S38–40. doi: 10.1053/joca.2000.0335. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Niu J, Kelly-Hayes M, Chaisson CE, Aliabadi P, Felson DT. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. Am J Epidemiol. 2002;156:1021–1027. doi: 10.1093/aje/kwf141. [DOI] [PubMed] [Google Scholar]
- 6.Kloppenburg M, Kwok WY. Hand osteoarthritis—a heterogeneous disorder. Nat Rev Rheumatol. 2011;8:22–31. doi: 10.1038/nrrheum.2011.170. [DOI] [PubMed] [Google Scholar]
- 7.Dziedzic K, Thomas E, Hill S, Wilkie R, Peat G, Croft PR. The impact of musculoskeletal hand problems in older adults: findings from the North Staffordshire Osteoarthritis Project (NorStOP) Rheumatology (Oxford) 2007 Jun;46(6):963–7. doi: 10.1093/rheumatology/kem005. [DOI] [PubMed] [Google Scholar]
- 8.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamm T, Mathis M, Aletaha D, Kloppenburg M, Machold K, Smolen J. Mapping hand functioning in hand osteoarthritis: comparing self-report instruments with a comprehensive hand function test. Arthritis Rheum. 2007 Oct 15;57(7):1230–7. doi: 10.1002/art.22989. [DOI] [PubMed] [Google Scholar]
- 10.van der Pas S, Castell MV, Cooper C, et al. European project on osteoarthritis: design of a six-cohort study on the personal and societal burden of osteoarthritis in an older European population. BMC Musculoskelet Disord. 2013;14(1):138–148. doi: 10.1186/1471-2474-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellamy N, Campbell J, Haraoui B, Gerecz-Simon E, Buchbinder R, Hobby K, et al. Clinimetric properties of the AUSCAN Osteoarthritis Hand Index: an evaluation of reliability, validity and responsiveness. Osteoarthritis Cartilage. 2002;10(11):863–9. doi: 10.1053/joca.2002.0838. [DOI] [PubMed] [Google Scholar]
- 12.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 13.Altman RD. Classification of disease: Osteoarthritis. Semin Arthritis Rheum. 1991;20(6 Suppl2):40–7. doi: 10.1016/0049-0172(91)90026-v. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68(1):8–17. doi: 10.1136/ard.2007.084772. [DOI] [PubMed] [Google Scholar]
- 15.Tombaugh TN, McIntyre NJ. The mini mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Valeri L, VanderWeele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser AW, Bøyesen P, Haugen IK, Schoones JW, van der Heijde DM, Rosendaal FR, et al. Instruments Measuring Pain, Physical Function, or Patient's Global Assessment in Hand Osteoarthritis: A Systematic Literature Search. J Rheumatol. 2015;42(11):2118–34. doi: 10.3899/jrheum.141228. [DOI] [PubMed] [Google Scholar]
- 19.Sharma L, Cahue S, Song J, Hayes KC, Pai YC, Dunlop DD. Physical functioning over three years in knee osteoarthritis: role of psychological, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48(12):3359–3370. doi: 10.1002/art.11420. [DOI] [PubMed] [Google Scholar]
- 20.Creamer P, Lethbridge-Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology (Oxford) 2000;39(5):490–6. doi: 10.1093/rheumatology/39.5.490. [DOI] [PubMed] [Google Scholar]
- 21.Maly MR, Costigan PA, Olney SJ. Determinants of self-report outcome measures in people with knee osteoarthritis. Arch Phys Med Rehabil. 2006;87(1):96–104. doi: 10.1016/j.apmr.2005.08.110. [DOI] [PubMed] [Google Scholar]
- 22.Scopaz KA, Piva SR, Wisniewski S, Fitzgerald GK. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Arch Phys Med Rehabil. 2009 Nov;90(11):1866–73. doi: 10.1016/j.apmr.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veronese N, Cereda E, Solmi M, Fowler SA, Manzato E, Maggi S, et al. Inverse relationship between body mass index and mortality in older nursing home residents: a meta-analysis of 19,538 elderly subjects. Obes Rev. 2015 Nov;16(11):1001–15. doi: 10.1111/obr.12309. [DOI] [PubMed] [Google Scholar]
- 24.Zambon S, Siviero P, Denkinger M, Limongi F, Castell MV, van der Pas S, et al. Osteoarthritis, comorbidity and pain: Their role in determining functional limitations in older populations (European project on Osteoarthritis) Arthritis Care Res (Hoboken) 2015 Oct 16; doi: 10.1002/acr.22755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.