Abstract
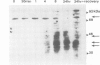
The role played by protein kinase C (PKC) in TH gene regulation was investigated at transcriptional and post-transcriptional levels using PC12 cells. The cells were treated with the phorbol ester TPA, which not only activates PKC but also causes down-regulation. PKC levels were monitored by [3H]PDBU binding assay and by using an anti-PKC antibody that detected intact PKC (79 kd) as well as its catalytic and regulatory domains. The [3H]PDBU binding to the membrane-associated PKC increased within 15-30 min of TPA treatment; thereafter total cellular [3H]PDBU binding decreased to a minimum of 20% of the control at 8 h. The rate of decrease in binding was greater than the decrease in the intensity of the staining of PKC holo enzyme visualized by anti-PKC antibody. TH mRNA levels, measured over the same time period, rose within 15 min of TPA treatment to peak at 4 h and subsequently declined below control level, paralleling the depletion of PKC. If cells depleted of PKC were reincubated in the normal medium, a recovery in PKC level was seen and, in parallel, TH mRNA levels increased to above control level. Furthermore, if down-regulation of PKC was prevented by incubating the cells with the protease inhibitor leupeptin, a decrease beyond control level in TH mRNA was not observed. TPA rapidly induced TH gene transcription; a maximal increase of two-fold was observed at 15 min, but the transcriptional rate then declined although it did not decrease beyond control values after 8 and 24 h of TPA treatment.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
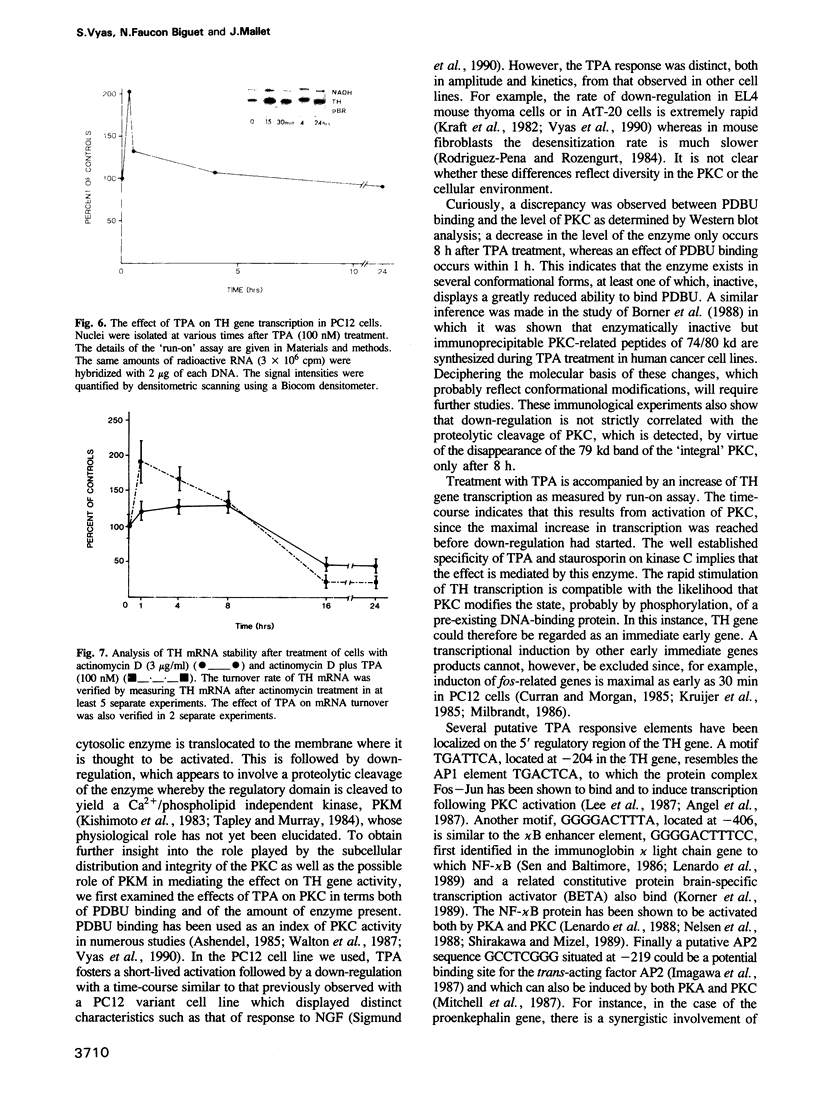


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Ashendel C. L. The phorbol ester receptor: a phospholipid-regulated protein kinase. Biochim Biophys Acta. 1985 Sep 9;822(2):219–242. doi: 10.1016/0304-4157(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Biguet N. F., Buda M., Lamouroux A., Samolyk D., Mallet J. Time course of the changes of TH mRNA in rat brain and adrenal medulla after a single injection of reserpine. EMBO J. 1986 Feb;5(2):287–291. doi: 10.1002/j.1460-2075.1986.tb04211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Adler J. E., Dreyfus C. F., Friedman W. F., LaGamma E. F., Roach A. H. Biochemistry of information storage in the nervous system. Science. 1987 Jun 5;236(4806):1263–1268. doi: 10.1126/science.2884727. [DOI] [PubMed] [Google Scholar]
- Borner C., Eppenberger U., Wyss R., Fabbro D. Continuous synthesis of two protein-kinase-C-related proteins after down-regulation by phorbol esters. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2110–2114. doi: 10.1073/pnas.85.7.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. J., Niedel J. E., Bell R. M., Young W. S., 3rd Distinct patterns of expression of different protein kinase C mRNAs in rat tissues. Cell. 1987 Apr 10;49(1):57–63. doi: 10.1016/0092-8674(87)90755-0. [DOI] [PubMed] [Google Scholar]
- Cahill A. L., Horwitz J., Perlman R. L. Phosphorylation of tyrosine hydroxylase in protein kinase C-deficient PC12 cells. Neuroscience. 1989;30(3):811–818. doi: 10.1016/0306-4522(89)90172-3. [DOI] [PubMed] [Google Scholar]
- Comb M., Mermod N., Hyman S. E., Pearlberg J., Ross M. E., Goodman H. M. Proteins bound at adjacent DNA elements act synergistically to regulate human proenkephalin cAMP inducible transcription. EMBO J. 1988 Dec 1;7(12):3793–3805. doi: 10.1002/j.1460-2075.1988.tb03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Fernyhough P., Mill J. F., Roberts J. L., Ishii D. N. Stabilization of tubulin mRNAs by insulin and insulin-like growth factor I during neurite formation. Brain Res Mol Brain Res. 1989 Nov;6(2-3):109–120. doi: 10.1016/0169-328x(89)90044-2. [DOI] [PubMed] [Google Scholar]
- Griffith L. C., Schulman H. The multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca2+-dependent phosphorylation of tyrosine hydroxylase. J Biol Chem. 1988 Jul 5;263(19):9542–9549. [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housey G. M., O'Brian C. A., Johnson M. D., Kirschmeier P., Weinstein I. B. Isolation of cDNA clones encoding protein kinase C: evidence for a protein kinase C-related gene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1065–1069. doi: 10.1073/pnas.84.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. P., Nakabayashi H., Huang F. L. Isozymic forms of rat brain Ca2+-activated and phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8535–8539. doi: 10.1073/pnas.83.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. P. The mechanism of protein kinase C activation. Trends Neurosci. 1989 Nov;12(11):425–432. doi: 10.1016/0166-2236(89)90091-x. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Korner M., Rattner A., Mauxion F., Sen R., Citri Y. A brain-specific transcription activator. Neuron. 1989 Nov;3(5):563–572. doi: 10.1016/0896-6273(89)90266-3. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Labatut R., Buda M., Bérod A. Long-term changes in rat brain tyrosine hydroxylase following reserpine treatment: a quantitative immunochemical analysis. J Neurochem. 1988 May;50(5):1375–1380. doi: 10.1111/j.1471-4159.1988.tb03019.x. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Fan C. M., Maniatis T., Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989 Apr 21;57(2):287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- Lewis E. J., Harrington C. A., Chikaraishi D. M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. J., Tank A. W., Weiner N., Chikaraishi D. M. Regulation of tyrosine hydroxylase mRNA by glucocorticoid and cyclic AMP in a rat pheochromocytoma cell line. Isolation of a cDNA clone for tyrosine hydroxylase mRNA. J Biol Chem. 1983 Dec 10;258(23):14632–14637. [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Preparation of pancreatic mRNA: cell-free translation of an insulin-immunoreactive polypeptide. Nucleic Acids Res. 1976 Feb;3(2):381–391. doi: 10.1093/nar/3.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Moriyama Y., Hulmes J. D., Pan Y. C., Nelson H., Nelson N. cDNA sequence encoding the 16-kDa proteolipid of chromaffin granules implies gene duplication in the evolution of H+-ATPases. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5521–5524. doi: 10.1073/pnas.85.15.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies H. J., Palfrey H. C., Hirning L. D., Miller R. J. Down regulation of protein kinase C in neuronal cells: effects on neurotransmitter release. J Neurosci. 1987 Apr;7(4):1198–1206. doi: 10.1523/JNEUROSCI.07-04-01198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S. The calpains. Trends Neurosci. 1989 Nov;12(11):438–444. doi: 10.1016/0166-2236(89)90093-3. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor rapidly induces c-fos mRNA in PC12 rat pheochromocytoma cells. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4789–4793. doi: 10.1073/pnas.83.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Nelsen B., Hellman L., Sen R. The NF-kappa B-binding site mediates phorbol ester-inducible transcription in nonlymphoid cells. Mol Cell Biol. 1988 Aug;8(8):3526–3531. doi: 10.1128/mcb.8.8.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Kawasaki H., Imajoh S., Suzuki K., Inagaki M., Yokokura H., Sakoh T., Hidaka H. Tissue-specific expression of three distinct types of rabbit protein kinase C. Nature. 1987 Jan 8;325(7000):161–166. doi: 10.1038/325161a0. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Patel J., Marangos P. J., Contel N., Gardner G., Post R. M. Increased phosphorylation of a membrane protein consequent to amygdaloid kindling. J Neurochem. 1984 Jul;43(1):169–173. doi: 10.1111/j.1471-4159.1984.tb06693.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Schweizer G., Cadepond-Vincent F., Baulieu E. E. Nuclear synthesis of egg white protein messenger ribonucleic acids in chick oviduct: effects of the anti-estrogen tamoxifen on estrogen-, progesterone-, and dexamethasone-induced synthesis. Biochemistry. 1985 Mar 26;24(7):1742–1749. doi: 10.1021/bi00328a026. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Berry N., Oda T., Ase K., Kikkawa U., Nishizuka Y. Isolation of protein kinase C subspecies from a preparation of human T lymphocytes. FEBS Lett. 1988 Jul 18;234(2):387–391. doi: 10.1016/0014-5793(88)80122-4. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989 Jun;9(6):2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund O., Naor Z., Anderson D. J., Stein R. Effect of nerve growth factor and fibroblast growth factor on SCG10 and c-fos expression and neurite outgrowth in protein kinase C-depleted PC12 cells. J Biol Chem. 1990 Feb 5;265(4):2257–2261. [PubMed] [Google Scholar]
- Summerhill E. M., Wood K., Fishman M. C. Regulation of tyrosine hydroxylase gene expression during differentiation of neuroblastoma cells. Brain Res. 1987 Jul;388(2):99–103. doi: 10.1016/s0006-8993(87)80002-1. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tapley P. M., Murray A. W. Modulation of Ca2+-activated, phospholipid-dependent protein kinase in platelets treated with a tumor-promoting phorbol ester. Biochem Biophys Res Commun. 1984 Jul 18;122(1):158–164. doi: 10.1016/0006-291x(84)90453-4. [DOI] [PubMed] [Google Scholar]
- Vyas S., Bishop J. F., Gehlert D. R., Patel J. Effects of protein kinase C down-regulation on secretory events and proopiomelanocortin gene expression in anterior pituitary tumor (AtT-20) cells. J Neurochem. 1990 Jan;54(1):248–255. doi: 10.1111/j.1471-4159.1990.tb13308.x. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Bertics P. J., Hudson L. G., Vedvick T. S., Gill G. N. A three-step purification procedure for protein kinase C: characterization of the purified enzyme. Anal Biochem. 1987 Mar;161(2):425–437. doi: 10.1016/0003-2697(87)90471-4. [DOI] [PubMed] [Google Scholar]
- Young S., Rothbard J., Parker P. J. A monoclonal antibody recognising the site of limited proteolysis of protein kinase C. Inhibition of down-regulation in vivo. Eur J Biochem. 1988 Apr 5;173(1):247–252. doi: 10.1111/j.1432-1033.1988.tb13991.x. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Schwarzschild M. A., Rittenhouse A. R. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]