Abstract
Introduction
To further investigate manifestations of episodic memory impairments in adolescents, we examined the role of encoding on recognition of stimuli in conditions designed to emphasize their item-specific versus relational characteristics in a group of 12–18 year olds with autism spectrum disorders (ASD). We also examined how strategic learning and memory processes, verbal abilities, attention, and age were associated with recognition in this group.
Materials and method
Twenty two high functioning adolescents with ASD (mean age = 15 years; SD = 1.8; range = 12.2–17.9), and 26 age, gender, and IQ-matched adolescents with typical development (TYP) (mean age = 14.7 years; SD = 1.9; range = 12.3–17.8) completed the Relational and Item-Specific Encoding task (RiSE), the California Verbal Learning Test-Children’s Version (CVLT-C), the Wechsler Abbreviated Scales of Intelligence, and the Connors’ Parent Rating Scale-Revised. Univariate statistical analyses were performed.
Results
The ASD group showed poorer performance on strategic memory assessed by the CVLT-C. Surprisingly, on the RiSE, ASD showed poorer discriminability for objects encoded in item-specific versus relational encoding conditions and were more impaired in familiarity (after relational encoding) than in recollection. ASD also did not show the hypothesized association between item and associative recognition and CVLT-C performance found in TYP. Instead, in the ASD group recognition was associated with increased age.
Conclusions
Findings from the RiSE task demonstrated that adolescents with ASD do not always exhibit impaired memory for relational information as commonly believed. Instead, memory was worse when cognitive control demands were high, when encoding focused on specific item features, and when familiarity was used to retrieve relational information. Recognition also was better in older participants. This suggests that learning and memory deficits in adolescents with ASD, may not be due primarily to failed relational binding processes in the hippocampus but, rather to disrupted strategic memory and familiarity processes associated with the prefrontal and perirhinal cortices. These findings demonstrate the importance and utility of using well-validated cognitive neuroscience tasks and of considering the ages of participants when comparing the neural underpinnings of different memory processes in both typical and atypical populations.
Keywords: Autism spectrum disorders, Adolescence, Episodic memory, Learning, Item-specific encoding, Relational encoding, Cognitive control, Recollection, Familiarity
1. Introduction
1.1. Episodic memory in typical development and ASD
Contemporary cognitive neuroscience theories suggest there are two neurally-dissociable memory systems – an implicit or non-declarative system, and an explicit or declarative system (Cohen et al., 1985; Eichenbaum, 2004; Schacter and Tulving, 1994; Squire, 2004). Declarative memory has been further divided into semantic (knowledge about language and the world) and episodic (memory for events within a specific temporal or spatial context) components (Tulving, 2002; Zola-Morgan et al., 1983). Successful retrieval of episodic memories is related to the encoding of distinctive features of individual items, as well as encoding relations between items(Anderson and Bower, 1972; Hunt and Einstein, 1981), which both rely on the medial temporal lobe (MTL) including the hippocampus as well as two anatomically dissociable cortical systems that converge there. The first of these – the anterior temporal system (ATS) – is comprised of brain regions including the perirhinal and lateral entorhinal cortices, which are involved in item and object recognition (Haskins et al., 2008) and the formation of multidimensional object representations (Taylor et al., 2006). The second cortical system – the posterior medial system (PMS) – consists of the parahippocampal and retrospenial cortices. The PMS plays a strong role in episodic retrieval, through the matching of incoming cues with their spatial, temporal, or environmental contexts (Ranganath and Ritchey, 2012).
When considering the case of individuals with autism spectrum disorders (ASD), the most influential view of their memory profile is that they exhibit intact semantic memory and impaired episodic memory (Bowler et al., 2000; Ben Shalom, 2003). Indeed, there have been multiple demonstrations that semantic memory (Bowler et al., 2000, 2009; Minshew and Goldstein, 1993; Salmond et al., Aug, 2005; Toichi and Kamio, 2002) is largely spared in individuals with ASD; although they exhibit deficits in episodic memory related to contextual details of events (Bowler et al., 2011, 2004; Gaigg et al., 2008; Hala et al., 2005; Lind and Bowler, Sep, 2009; O’Shea et al., 2005), the spatio-temporal sequence of events (Poirier et al., 2011), and contextual information that is autobiographical or more self-referential (Brezis, 2015; Crane and Goddard, 2008; Henderson et al., 2009; Lind and Bowler, 2008; Maister et al., 2013). Based on the aforementioned findings, and on early neuropathology studies (Bauman and Kemper, 1985), it is widely believed that ASD is a disorder of the hippocampus (Salmond et al., Aug, 2005) involving impairment in the ability to flexibly process episodically-defined associations between items. However, an episodic versus semantic memory deficit does not necessarily equate to hippocampal dysfunction given the other regions of the MTL, the prefrontal cortex (PFC), and the rest of the brain that mediate episodic memory (Ritchey et al., 2015).
1.2. Recollection and familiarity in typical development and ASD
Episodic memory retrieval is generally thought to be supported by the dual processes of recollection and familiarity (Eichenbaum et al., 1994, 2007; Yonelinas, 1994) (but see (Squire, 1994) for the single process view). Recollection is a threshold-based experience of retrieving qualitative information about items and related aspects of the encoding context (e.g., “I remember meeting Mary last month at the school concert”), whereas familiarity can be based on a sense of “knowing” based on the strength of the memory trace (e.g., “I know I met this woman somewhere before, what is her name? ”). This is an important distinction because recollection requires hippocampal and PMS involvement whereas familiarity can be accomplished in the ATS through the perirhinal cortex, without hippocampal involvement (Eichenbaum et al., 2007; Brown and Aggleton, 2001; Diana et al., 2007).
In the case of individuals with ASD, there have been very few empirical studies of recollection and familiarity in adolescents, and none using the receiver operating characteristic (ROC) curve methodology that permits ready parsing of the contributions of the parahippocampal and perirhinal cortices to these two processes (Yonelinas, 1994). The only study in children that assessed recollection and familiarity found that low functioning children and adolescents with ASD showed deficits in both processes relative to TYP and a developmentally delayed control group, while high functioning children with ASD showed comparable familiarity to TYP with mildly poorer recollection. Bigham et al. (2010) These findings in children with higher cognitive abilities were consistent with studies by Bowler et al. (2000), who used a remember-know paradigm in adults, and found that those with ASD showed less remembering (comparable to recollection) than TYP alongside similar levels of knowing (comparable to familiarity). The contention that ASD involves impaired recollection and intact familiarity also has been indirectly supported by studies showing there are general impairments in the single process of recollection (Bennetto et al., 1996; Bowler et al., 2014), as well as a study using a self-ordered pointing task in children with ASD which concluded their deficits might originate from spared familiarity and impaired recollection performance although these constructs were not directly assessed by the authors (Joseph et al., 2005). In sum, while there is a general consensus that ASD involves relative strengths in familiarity-based episodic memory for items mediated by the perirhinal cortex, alongside pathology of relational binding mechanisms of the hippocampus thought to support recollection (Gaigg et al., 2008; Joseph et al., 2005; Davachi, 2006; Mayes et al., 2007), there have been very few actual studies of the dual processes of recollection and familiarity in adolescents with ASD and none using the ROC curve methodology that could help shed light on the MTL pathophysiology underlying ASD-related memory impairments.
1.3. Cognitive control of memory in typical development and ASD
Episodic memory also is sub-served by cognitive control processes governed by the prefrontal cortex. These permit strategic context-relevant stimulus processing, retrieval of online goal representations, and transformation of stimuli in accordance with such goals (Ranganath and Knight, 2003; Wagner et al., 2004). Ventrolateral aspects of the PFC are recruited during memory maintenance of semantic information, while the dorsolateral PFC is brought online to process relations between items (Blumenfeld and Ranganath, 2006; Blumenfeld et al., 2011) that may eventually constitute contextually-laden episodic memories (Preston and Eichenbaum, 2013).
Given their deficits in cognitive control and executive functions (Solomon et al., 2008; Solomon et al., 2009), it is not surprising that individuals with ASD have memory problems related to poor strategic information processing (Minshew and Goldstein, 2001; Southwick et al., 2011) and impaired inhibition of prepotent response tendencies (Maister et al., 2013; Bennetto et al., 1996), which can be ameliorated by providing contextual cues at recall (i.e., task support (Bowler et al., 2004, 1997., 2015; Solomon et al., 2015)). In fact, it has been suggested that individuals with ASD exhibit heterogeneity and fractionation in the functioning of general cognitive systems (Bennetto et al., 1996), and that those with more intact executive control (Maister et al., 2013) and verbal abilities (Goddard et al., 2014) are better able to compensate for MTL deficits (Ben Shalom, 2003).
The goal of the present study was to help illuminate the underlying neural mechanisms of episodic memory in adolescence by studying adolescents with ASD compared to those with TYP. To do so, we examined recognition for items encoded in both an item-specific and a relational manner; assessed the relative contributions of recollection and familiarity to recognition using ROC curves – which could shed light on pathophysiology; and evaluated the use of potentially compensatory processes. We used a new well-validated task developed through the Cognitive Neuroscience Test Reliability and Clinical applications for Schizophrenia (CNTRaCS) Consortium (CNTRACs, 2011) called the Relational and Item-specific Encoding Task (RiSE) designed to examine effects of both item-specific and relational encoding on episodic memory as well as to disentangle the contributions of specific prefrontal and medial temporal lobe regions to episodic memory impairment when using neuroimaging (Ragland et al., 2012). We also administered a more traditional neuropsychological task called the California Verbal Learning Task Children’s Version (Delis et al., 1994) to assess strategic learning and memory processes in both groups; and investigated the influence of other factors including age, verbal IQ, and attentional abilities on RiSE task performance.
Based on the extant literature on episodic memory impairment in ASD, one would predict superior recognition for stimuli that have undergone item-specific encoding, worse performance for information that has been encoded relationally, and more severe deficits in recollection than in familiarity during episodic retrieval. Based on previous studies using the CVLT-C in ASD (Bennetto et al., 1996; Phelan et al., 2011), we also predicted that adolescents with ASD would demonstrate impaired deployment of learning and memory strategies including poorer use of semantic clustering, a flatter learning slope, a greater buildup of proactive interference, and less consistent recall performance than TYP, but that recall would be improved when compensatory cues (task support) were provided at retrieval. Consistent with this, our final hypothesis was that, for the ASD group, performance on the summary measures of the RiSE (d’ in both the item and relational encoding conditions) would be predicted by summary performance on the CVLT-C (Trial 5 short delay free recall), suggesting that the ability of this group to complete tasks involving strategic executive control processes would predict recognition performance as suggested by Maister et al. (2013). We also predicted that other potential compensatory mechanisms including better verbal and attentional abilities (Goddard et al., 2014), and chronological age also would be related to RiSE performance for both groups.
2. Material and methods
2.1. Participants
Twenty-eight individuals with ASD and 26 individuals with TYP development were recruited through psychiatrists, psychologists, speech and language pathologists, advocacy groups, state-funded centers for persons with developmental disabilities, and MIND Institute’s Subject Tracking System database and were enrolled in the study. All participants had a Full Scale IQ > 70 on the Wechsler Abbreviated Scales of Intelligence (Wechsler, 2011). Participants with ASD had scores in the autism spectrum range on the Autism Diagnostic Observation Schedule-2 (Lord et al., 2000), the Social Communication Questionnaire (Rutter et al., 2003), and met diagnostic criteria based on a checklist of items from the DSM-5 (APA, 2013). Exclusion criteria for ASD subjects included diagnoses with known genetic etiologies, and current parent-reported diagnoses of depression, anxiety disorders, or psychosis.
Participants taking antipsychotic medications were excluded, and there were no participants in the included sample taking psychostimulants. Six subjects with ASD were removed from data analysis due to below-chance performance on RiSE item recognition discriminability, typically due to their high false alarm rates. These six subjects scored lower on the Verbal Comprehension. Index from the abbreviated Wechsler scales than the subjects with ASD included in the sample (t(26) = 2.30, p=.03) and were all male. This left a sample where n = 12 members of the ASD group (54%) met criteria for an attention deficit hyperactivity disorder (ADHD) diagnosis as assessed by the Conners Parent Rating Scale-Revised (Conners et al., 1998), whereas the TYP group had a rate of 4%. The final sample included 22 adolescents with ASD (mean age=15 years; SD = 1.8; range=12.2–17.9) and 26 with typical development (mean age = 14.7 years; SD = 1.9; range=12.3–17.8), who were matched on age, gender, and IQ. Five ASD and four TD participants were women (Nyden et al., 2000). See Table 1. After receiving a complete description, parents of all participants gave written consent and participants gave assent to participate in the study, which was approved by the University of California, Davis Institutional Review Board.
Table 1.
Participant characteristics.
| ASD (n = 22) | TYP (n = 26) | |
|---|---|---|
| Males | 17 (77%) | 22 (85%) |
| Females (%) | 5 (23%) | 4 (15%) |
| Chronological age (years) | 14.98 (1.82) | 14.71 (1.91) |
| Comorbid ADHD | 12 (54%) | 1 (4%) |
| Verbal IQ | 102.23 (11.73) | 105.62 (11.17) |
| Nonverbal IQ | 103.73 (13.02) | 104.46 (11.57) |
| Full-Scale IQ | 103.43 (11.09) | 104.85 (11.71) |
| ADOS-2 Total | 10.00 (2.00) | NA |
| SCQ Total | 22.27 (5.86) | 2.88 (1.90) |
Note: Data are summarized as mean (SD) for continuous variables and frequency (%) for categorical ones. ASD=Autism Spectrum Disorder, TYP=Typically Developing, ADOS=Autism Diagnostic Observation Schedule, Second Edition SCQ=Social Communication Questionnaire
2.2. ASD diagnostic and qualification measures
In addition to meeting criteria on a checklist of DSM-5 diagnostic criteria, the following were administered to participants:
Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) (Lord et al., 2012). The ADOS-2 is a semi-structured interactive session and interview protocol that provides opportunities for the child to display a number of social and communicative behaviors. Module 3 was administered to 16 younger participants; Module 4 was administered to 9 older participants. Module 3 Comparison scores and Module 4 Calibrated Severity Scores (CSS) (Hus and Lord, 2014) were collapsed into one reported ADOS-2 score. An assessor trained to reliability with the MIND Research Core provided all assessments. The ADOS-2 only was administered to participants with ASD. See Table 1.
Social Communication Questionnaire, Lifetime Version (SCQ) (Berument et al., 1999). The SCQ is a parent-report questionnaire with 40 yes-or-no questions that are taken from the Autism Diagnostic Interview-Revised (Lord et al., 1994), the gold standard parent interview, about the child’s social and communicative behaviors over the child’s lifetime. It is used to screen for autism spectrum disorders, with a total score ≥ 15 indicating the presence of an autism spectrum disorder. The SCQ also was given to participants with TYP who needed to score ≤ 11 to be included.
Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II) (Wechsler, 2011). The WASI–II is an individually-administered measure of full-scale IQ designed for individuals 6–90 years of age. The WASI–II produces a nonverbal IQ index (Perceptual Reasoning Index; PRI), a verbal IQ index (Verbal Comprehension Index; VCI), in addition to a full-scale IQ. Standard scores have a population mean of 100 and standard deviation of 15.
Conners’ Parent Rating Scale – Revised, Long Version CPRS-R (L) (Conners et al., 1998). The CPSR-R (L) is a parent-report questionnaire with 80 items designed to identify ADHD and other behavior problems in children. Internal reliability for the CPRS-R is good (.75–.94) and test-retest reliability is high (Conners et al., 1998).
2.3. Memory measures
We used the Relational and Item-Specific Encoding task (RiSE), (Ragland et al., 2012) presented using E-Prime® (Version 2.0), to examine recognition performance in both conditions as well as recollection and familiarity. Item specific memory processing involves forming an episodic memory based upon some isolated feature of that item, whereas relational memory processing requires thinking about that item in relation to other items that are part of that encoding event or episode. The RiSE consists of both encoding and recognition phases (See Fig. 1). Participants first performed two incidental encoding tasks (Fig. 1, panel A). In the first of these – the Item-Specific Encoding task – they were shown 36 images presented for 2 s each, with a 1-s inter-stimulus interval (ISI), and were asked to make a 2-button “yes/no” response indicating whether an object was “living” or not. They next performed the Relational Encoding condition which involved 18 pairs of objects that were each presented for 4 s with a 1-s ISI. They were asked to make a 2-button “yes/no” response indicating whether 1 item could fit inside the other. Given the potential confound of task-switching, encoding conditions were alternated in a pseudo-random block design. (3 item-specific blocks with 12 trials each and 3 relational blocks with 6 trials each). Three second instruction screens announced changes between encoding blocks and encoding decisions (“living? ” or “inside? ”) and remained visible to remind participants of the current encoding condition. Task instructions also clarified that these decisions were to be made about the objects “in real life”, not just in the pictorial representations.
Fig. 1.
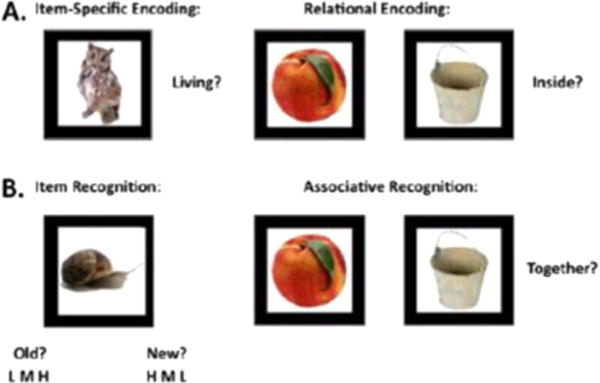
Examples of encoding and retrieval conditions from the RiSE task. For the Item Specific Encoding Condition, subjects were asked to indicate if the object was living. For the Relational Encoding Condition, subjects were asked if one object could fit inside the other. Both item recognition, using confidence intervals, and associative recognition, with yes/no, were assessed. Adapted from Ragland et al. (2012).
After the encoding phase of the task, 2 retrieval tasks were administered (Fig. 1, panel B). The first task assessed item recognition. All 72 studied objects (36 items that had been presented in the item-specific condition, and 36 that had been relationally presented) were randomly intermixed and presented with 72 new unstudied foils, and participants were asked to indicate whether each item was “old” or “new.” They also rated their level of confidence in their response using 1 of 3 options (high, medium, and low). The second task assessed associative recognition. All 18 object pairs studied during relational encoding were randomly intermixed and presented with 18 rearranged object pairs consisting of items presented on different trials during relational encoding and not originally paired together. Since the goal was to control for item memory and only test memory for the associations between the items, no new foils were included. Participants made 2-button “yes/no” judgments indicating whether items in each pair had presented “together.” Both retrieval tasks were self-paced and participants were instructed to “Work as quickly and accurately as you can.” We examined performance for items for which an overly literal interpretation could confound results and found none. Dependent variables produced from this task from both encoding conditions included reaction times, hits, false alarms, discrimin-ability (d’). To derive recollection and familiarity estimates, as specified by the dual process signal detection theory (Yonelinas, 1994), subject ROC curves were constructed by calculating the ratio between the hit and false alarm rates at each confidence level to describe use of recollection which reflected highly confident and highly accurate responses calculated from the y-intercept of the constructed curve, and familiarity, reflecting recognition across a wider range of confidence responses calculated by the degree of curvilinearity for the fitted ROC curve.
Verbal learning strategies, recall, and inhibition of prepotent response tendencies were investigated using the CVLT-C (Delis et al., 1994). The task involved reading a 15 word shopping list to participants over 5 learning trials, proceeded by a new “B” list consisting of new items as well as distractors, from the same categories as the original items, on the subsequent trial. Subjects were asked to recall the lists immediately after each of the 5 learning trials, after the B list, and after a longer delay (approximately 20 min). This measure provides information about short delay and long delay recall in both cued and free recall conditions. Trial 5 short delay free recall represents the best overall index of task performance and was thus used as a summary measure. In addition, the test also allows examinations of percent recall consistency and learning slope as measures of organization in learning. The learning slope is calculated as the slope of a least-squares regression line fitted to the correct response scores across the first five immediate recall trials. The unstandardized slopes are measured as the amount of new words learned per trial on average. The CVLT-C was standardized to a nationally representative sample of children aged from 5 to 16. The test also demonstrates adequate reliability, with reliability coefficients ranging from .64 to .80, with an average of .72 on all metrics.
3. Results
All statistical analyses were performed using SPSS version 21, SAS version 9.4, R (Team, 2015) (http://www.R-project.org), and custom Matlab scripts. The main dependent variable, discrimin-ability (d’), was calculated by subtracting the z-scored hit rate from the z-scored false alarm rate. Table 2 contains the information about both groups’ performance on the RiSE task. Unlike the validation study where performance on recognition was the same for both encoding conditions (Ragland et al., 2012), the ASD and TYP groups showed better recognition for items that had been encoded in an item-specific versus a relational manner (TYP: F(1, 25) = 19.42, p < .01; ASD: F(1, 21) = 8.71, p < .01).
Table 2.
Descriptive statistics for performance on item recognition RiSE task for the two groups.
| Item specific encoding
|
Relational encoding
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ASD (n = 22) | TYP (n = 26) | P value | Cohen’s D | ASD (n=22) | TYP (n = 26) | P Value | Cohen’s D | |
| Hit rate | .89 | .92 | .27 | .31 | .85 | .86 | .75 | .09 |
| Encoding response Time | .98 | .97 | .85 | −.05 | 1.85 | 1.65 | .01* | −.77 |
| Discriminability (d’) | 3.10 | 3.71 | .02 | .66 | 2.91 | 3.25 | .17 | .41 |
| Retrieval response time | 1.14 | 1.06 | .27 | −.32 | 1.16 | 1.07 | .19 | −.38 |
| Recollection | .71 | .74 | .96 | .13 | .65 | .63 | .76 | −.09 |
| Familiarity | 1.39 | 1.69 | .39 | .24 | 1.15 | 1.70 | .03* | .63 |
| False alarm rate (total) | NA | NA | NA | NA | .06 | .03 | .11 | −.48 |
Note: Response Times reported in seconds.
p < .05.
Contrary to predictions, the ASD group did more poorly on recognition for objects that had been encoded in the item-specific condition, compared to the TYP group F(1, 46)=4.12, p=.048, whereas the two groups performed comparably on items encoded relationally F(1, 46) = 1.97, p=.17. There was also no group difference in associative recognition for items that were encoded in the relational memory condition (F(1, 46) = .46, p=.50). In order to ensure that the differences between the groups were not due to fatigue, discriminability on the first half of the test was compared to the second half using a repeated measures ANOVA, and there was no main effect of diagnostic group (F(1, 46)=2.08, p=.15), no main effect of time (F(1, 46) = .70, p=.40), nor a significant interaction between group and time (F(1, 46) = 1.73, p=.19).
We next produced estimates of familiarity and recollection from confidence ratings using ROC curves. Participants from each group were able to rate their confidence and appeared to use the scales similarly (see Fig. 2). Group differences in the use of familiarity and recollection also were examined in one-way ANOVAs conducted for each encoding condition (see Fig. 3). Contrary to predictions, there were no significant differences between the two groups for estimates of recollection following either item-specific, F(1, 46) = .21, p=.65, or relational encoding conditions, F(1, 46) = .09, p=.76 (Fig. 3, panel B). There were, however, some group differences found for familiarity. There were no significant differences between the groups with ASD and TYP for familiarity in the item-specific encoding condition, (F(1, 46) = .70, p = .41), but there was a significant difference between the groups in their familiarity performance following relational encoding, F(1, 46)=4.71, p=.03 (Fig. 3, panel A), suggesting that the ASD group relied relatively less on familiarity during item recognition following relational encoding than did TYP.
Fig. 2.
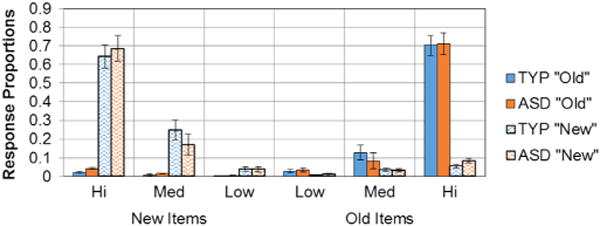
Response proportions by confidence intervals. Proportion of confidence responses for both old and new items were calculated in each group. Participants in each group used the scales similarly.
Fig. 3.
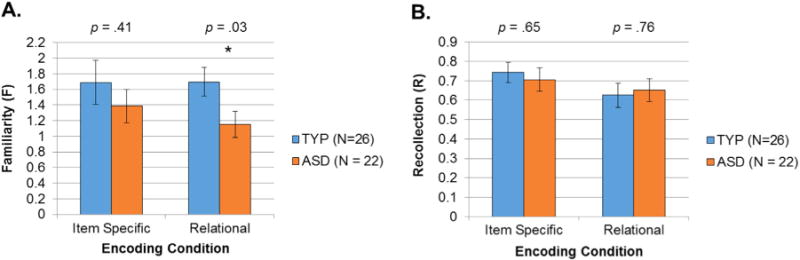
Recollection and Familiarity. Recollection and Familiarity estimates for stimuli encoded in the item-specific and relational conditions. Recollection and familiarity estimates were obtained by fitting the dual-process signal detection model (DPSD; Yonelinas, 1994). In the DPSD model, recollection is expressed as a probability, and familiarity is expressed as the discrimination index or d’.
To test the third hypothesis, group differences on CVLT-C performance on trials 1–5, learning slope, semantic clustering, and percent recall consistency, were examined using one-way ANOVAs. There were no group differences in recall performance between adolescents with ASD and TYP on Trial 1, F(1, 46) = .04, p=.84. However, impairments in the ASD group became evident on Trials 2–5 (Trial 5 difference=F(1, 46)=4.43, p=.04), suggesting the ASD group experienced interference from previously presented materials. Trend level poorer performance by the ASD group on the first presentation of the B List suggests that this interference was likely due to distraction by categories they had seen before (Flist × group (1, 46) = 1.56, p = .09). Adolescents with TYP development learned more new words per trial, as indicated by significant differences in the learning slopes, F(1, 46)=4.67, p=.04. In addition, adolescents with ASD did not consistently remember the same words trial-to-trial, as indicated by reduced percent recall consistency, F(1, 46) = 11.76, p=.001. Although the ASD group performed more poorly, there were no significant group differences in semantic clustering scores (F(1, 46) = .42, p=.52). See Fig. 4.
Fig. 4.
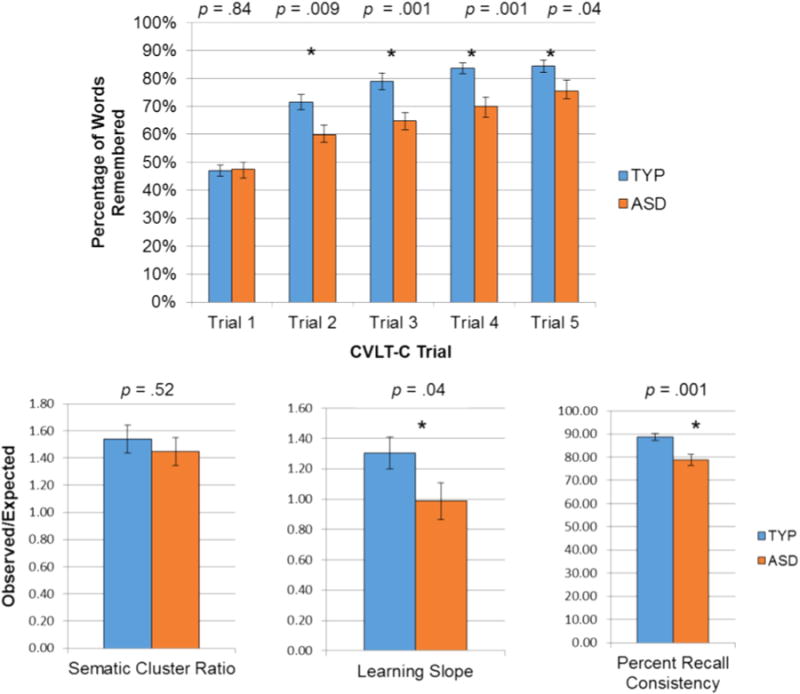
Performance on the CVLT-C. The group with ASD recalled significantly fewer words on Trials 2–5, learned less new words per trial (Learning Slope), and were not as consistent in words recalled (Percent Recall Consistency). There were no group differences on Trial 1, or on semantic clustering.
To examine group differences in performance on Trial 5 after both long and short delays, we conducted a 2 × 2 ANOVA. There was a main effect of group (F(1, 46) = 12.94, p < .001), a main effect of delay type (F(1, 46) = 7.70, p < .01), and a significant group by delay type interaction (F(1, 46) = 6.95, p < .05). As done previously in the literature, due to lower recall in the short delay condition for the ASD group on Trial 5, we standardized both short and long delayed performance scores by dividing delayed recall by each individual’s Trial 5 performance (Bennetto et al., 1996) when examining free and cued recall performance. After this adjustment, there were no significant differences between the groups on both free and cued conditions for short and long delayed recall. Repeated measures ANOVAs revealed significantly better performances on the cued conditions after a short delay, F(1, 21) = 7.45, p < .05, and long delay, F(1, 21) = 11.54, p < .01 in the ASD group, suggesting that cueing provided beneficial task support as hypothesized. There were no significant differences between free and cued recall in the TYP developing group (short delay, F(1, 25) = 1.49, p = .23; long delay, F(1, 25) = .03, p = .86).
To test our final hypothesis, we conducted regression analysis to examine the factors associated with RiSE discriminability performance following item-specific and relational encoding for the ASD and TYP groups. Given the suspicion of heteroscedasticity across the two groups and to protect against the influence of outliers, robust linear regressions were run as an alternative to ordinary least squares. We used MM estimation (Yohai, 1987) which combines a high-breakdown point (i.e., an ability to cope with a large number of outliers while maintaining its robustness) with the popular M estimation (Huber, 1973). Analyses were implemented in SAS PROC ROBUSTREG. Due to sample size limitations, we first examined interactions between diagnostic group and VCI, age, ADHD symptoms, and performance on CVLT-C Trial 5 in independent (unadjusted) models to investigate which were potential predictors in the presence of the diagnostic group variable. If a variable had a p-value < .20 or the p-value for the interaction of the variable and diagnostic group was < .20, we retained that variable for inclusion in a multiple model. Variables were tested and kept in the final multiple models only if they added significantly to those models. The results of the independent models predicting performance on item specific discriminability revealed different patterns of associations with discriminability across groups for performance on CVLT-C Trial 5 (interaction β =−.33, standard error=.10, p=.001) and age (interaction β=.25, standard error=.13, p=.06), but not for VCI (interaction β =−.01, standard error = .02, p=.75) and ADHD symptoms (interaction β =.02, standard error=.03, p=.57). The terms corresponding to the association with the dependent variable in the TD group were non-significant for ADHD symptoms (β = −.03, standard error=.03, p=.24), but approached significance for VCI (β = .03, standard error = .02, p=.06). R2 values for these models ranged between .12 and .24. Table 3 presents the results of the final model predicting item specific discriminability, which included terms for performance on CVLT-C Trial 5, age, and their interactions with group. The significant effect of group indicated that participants with ASD performed worse than TD participants with similar age and performance on CVLT-C Trial 5. The significant age by group interaction and a nonsignificant age effect indicate that age had a positive relation only in the ASD group. The CVLT-C by group interaction indicates that there was a positive relation between performance on CVLT-C and performance on item specific discriminability only in the TYP group. The estimated effects (after accounting for the effects of the other) were comparable in magnitude to those in the unadjusted models, suggesting that performance on CVLT-C Trial 5 and age, independently predicted performance on item specific discriminability.
Table 3.
Summary (parameter estimates, SE) of the robust regression models examining predictors of discriminability.
| Item specific discriminability | Relational discriminability | |||
|---|---|---|---|---|
|
|
|
|||
| Estimate, β | SE | Estimate, β | SE | |
| Group | −.61*** | .17 | −.33 | .14 |
| Age (years) | −.05 | .41 | .04 | .07 |
| Age × Group | .31*** | .09 | .24* | .10 |
| CVLT-C Trial 5 Performance | .29*** | .06 | .19** | .07 |
| CVLT-C × Group | −.39 | .08 | −.34*** | .09 |
| Summary | R2 | .38 | R2 | .33 |
Note: SE=standard error.
p<.05,
p< .01,
p <.001.
Categorical variable Group was coded as 0=TYP, 1 = ASD. Continuous predictors were centered at their mean.
The results of the independent models predicting discrimin-ability for relationally encoded items also showed different patterns of associations with the dependent variable across groups for performance on CVLT-C Trial 5 (interaction β =−.30, standard error=.11, p < .01) and age (interaction β =.20, standard error = .13, p = .12). For VCI, neither the effect in the TD group (β=.002, standard error = .02, p = .92), nor the interaction with group (β=.01, standard error=.02, p = .69) were significant. The same was true for ADHD symptoms (β = .01, standard error=.03, p = .86; interaction −β = .03, standard error=.03, p = .47). R2 values for these models ranged between .04 and .15. For the final model predicting discriminability for relationally encoded items, CVLT-C performance, the group by age interaction, and the group by CVLT-C performance were all significant. The group by age interaction indicates that there was a positive relation between age and relationally encoded discriminability in the ASD group, and the group by CVLT-C interaction indicates a significant positive relation between relational discriminability and CVLT-C performance only in the TYP group. See Table 3.
4. Discussion
This study extends what is known about the development of episodic memory in adolescents with ASD and TYP by examining the effects of item-specific and relational encoding, recollection and familiarity, cognitive control-related strategic learning processes, and the compensatory use of verbal abilities, attention, and age on recognition memory. As predicted, the ASD group exhibited weaker cognitive control-related strategic memory processes that produced less overall learning characterized by proactive interference and inconsistency than the TYP group. Unexpectedly, individuals with ASD demonstrated intact associative recognition and recollection, but showed poorer discriminability for objects that had been encoded in the item-specific condition and reduced familiarity for items that had been encoded relationally than the TYP group. Performance on item and associative recognition was predicted by age for the ASD group only, while performance on item and associative recognition was predicted by a summary measure of strategic learning processes (CVLTC-C Trial 5) for those with TYP only.
Findings of the current study are at odds with the widely-held view that individuals with ASD exhibit better memory performance for items versus relations between items. We propose three explanations for this. First, the current study suggests that characteristics of specific encoding conditions can alter the recognition memory profile of individuals with ASD. For example, the item-specific encoding condition of the RiSE involved incidental encoding while individuals made judgments related to a relatively abstract stimulus feature. Abstraction is known to be difficult for those with ASD (Solomon et al., 2011). Thus, encoding while making this form of judgment may have contributed less effectively to the otherwise typical deep encoding found in individuals with ASD (Toichi and Kamio, 2002; Mottron et al., 2001). Findings of intact recognition of items encoded relationally, on the other hand, suggests that those with ASD were relatively better at recognizing items under conditions involving their strong visuospatial information processing abilities (Joseph et al., 2005; Roser et al., 2015; Caron et al., 2006). We are not the first to observe that stimulus characteristics influence how effectively items can be encoded (Gaigg et al., 2008).
Related to this, a second explanation for why findings on the RiSE did not conform to expectations is that studies of episodic memory vary widely with respect to the level of directly self-referential information processing (e.g. autobiographical memory, metamemory, episodic future thinking) they investigate (Tulving, 1985). It is this directly self-referential information processing that is most compromised in ASD (Cheng et al., 2015). Not surprisingly, episodic memory studies finding impairments in children, adolescents and adults with ASD generally test this type of information processing (Lind et al., 2014, 2013; Brezis et al., 2014), which is not required in the RiSE.
Finally, group differences in adolescent development of memory mechanisms also may help put our findings into perspective, and suggest potential directions for future research. Although previously believed to reach adult levels by middle childhood, newer studies illustrate that episodic memory develops considerably through adolescence, such that the full integration of the capabilities of the PFC and the anterior and posterior networks of the MTL does not occur until young adulthood (Ghetti and Angelini, 2008; Ghetti and Bunge, 2012; Brainerd et al., 2004; Ghetti and Bauer, 2011; Ghetti et al., 2011). This integration has been credited to the maturation of cognitive control processes, including those involved in context maintenance and response inhibition (Jaeger et al., 2012; O’Connor et al., 2010), semantic organization of items (Schwenck et al., 2009), and the ability to monitor memory accuracy (Ghetti and Alexander, 2004; Ghetti and Castelli, 2006; Ghetti et al., 2008; DeMarie and Ferron, 2003), that occurs during the teen years. Binding mechanisms of the hippocampus that integrate information about events with information about their spatio-temporal contextual details also undergo more protracted development after childhood than previously thought, and together with prefrontal development, produce improvements in episodic memory and recollection in typical development (DeMaster et al., 2013, 2014). In addition to these direct memory-related processes, the adolescent maturation of verbal abilities and attentional control also contribute to more effective information processing (Boudewyn et al., 2015) and recognition memory characteristic of adolescence versus childhood (Quamme et al., 2004). Therefore, it is possible that the failure to confirm findings from the largely adult ASD episodic memory literature stems from the fact that typical adolescence, is a time of significant development of relational versus item-specific memory processing. If this is the case, then group differences might not become evident until the memory processing of the TYP group reaches adult levels, while that of the ASD group does not. This would produce recognition deficits under both encoding conditions of the RiSE. According to this logic, item and familiarity based memory impairments observed in adolescence may constitute an early marker of episodic memory dysfunction that was detected by our task. In fact, this is not the first time we have observed this pattern of adult deficits and an absence of developmental ones. In a behavioral study of young adults completing a transitive inference learning paradigm (Solomon et al., 2011), we found strategic information processing related deficits in adults with ASD versus TYP, which we did not replicate in an fMRI study of adolescents (Solomon et al., 2015). This explanation of continued adolescent development of relational encoding and recollection also is supported by the fact that studies to date using the RiSE with adult samples show that difficulty between the two conditions is either matched, or d’ is better following relational versus item encoding (Ragland et al., 2012, 2015), while in the current study, discriminability and reaction times suggest that the item condition is easier for both groups.
We did not confirm hypotheses related to item-specific and relational encoding, but did find that those with ASD quickly fell behind their TYP counterparts on the CVLT-C, and showed inconsistent learning with the acquisition of fewer new words per list. This, combined with trend level findings of proactive interference on List B, suggests that cognitive control problems related to response competition and the ability to maintain a clear sense of temporal context during the task was most problematic for those with ASD. Taken in the context of their weak, cognitive control individuals with ASD may make use of their relatively stronger recollection versus familiarity when encoding visuo-spatial information, and these intact relational memories may be more resistant to interference. A better understanding of the mechanisms underlying the encoding and retrieval of such robust memories could shed light on the origins of the strong networks of memories related to the circumscribed interests relatively unique to ASD.
Contrary to the widely-held neurobiological view of hippocampal dysfunction driving memory impairments in ASD as noted in the introduction (Salmond et al., 2005), findings of the current study raise the possibility that those with ASD may be relatively worse at learning items when cognitive control is necessary or involves areas of the ATS; and relatively better at recognizing items that have been relationally encoded under the right conditions (e.g., being given a strategy that enables them to encode items using their relatively strong visuospatial processing abilities), which are sub-served by a relatively intact PMS. In fact, under such conditions, persons with ASD may become more reliant on such relational recollections that are devoid of familiarity. Consistent with this second view, some have argued that the hippocampus proper may be spared or even used in a compensatory manner in ASD given their strengths in rote memory (Solomon et al., 2011). Since the hippocampus is enlarged in both children and adolescents with ASD, it may exhibit use-dependent plasticity (Schumann et al., 2004; Barnea-Goraly et al., 2014) or increased size could be a consequence of PFC deficits that produce a pattern of elevated usage of subcortical brain structures (Friederici et al., 2013). This latter interpretation is consistent with recent findings that recruitment of both the hippocampus and the caudate was associated with better transitive inference learning in adolescents with ASD who engaged the PFC less than individuals with TYP (Solomon et al., 2015). Interestingly, another recent study of memory for scenes and scene details reached a similar conclusion that a hippocampal binding deficit was not sufficient to account for episodic memory impairments (Cooper et al., 2015) in individuals with ASD.
In addition to generating testable hypotheses about the neurobiology of memory in ASD, the current study has important clinical implications. As has been found by others, the need to recall over a series of trials was particularly problematic for those with ASD (Poirier et al., 2011), due to proactive interference and the inability to inhibit prepotent response tendencies. This impairment in the ability to learn items of information and the relations between them could have far-reaching academic and vocational consequences when affected persons are required to remember materials encoded over a temporal sequence. This form of challenge is encountered in reading comprehension, in complex math problem solving, and in following directions. Given that verbal abilities can scaffold and compensate for fractionation of cognitive systems and attention deficits, speech and language therapy could have important benefits beyond just improving communication. Finally, as commonly recommended (Jordan, 2008), visuospatial methods of learning associations may provide a compensatory scaffold for many individuals with ASD given their ability to produce perceptual unity among the to be remembered items (as supported by their unimpaired relational recollection) and/or stable representations that are resistant to interference (Bower, 1970). In sum, individuals with ASD should be treated using psychopharmacological, psychosocial, or neural retraining interventions that improve cognitive control; and that bolster verbal and visuo-spatial learning mechanisms. Furthermore, findings of an association between age and improvements in recognition in individuals with ASD suggest there is hope for maturation with age.
It could be argued that autism and ADHD are distinct categorical disorders with discrete phenotypes, and that the presence of ADHD symptoms in a high proportion of participants with autism confounds results of our study. The debate about whether mental disorders are best conceptualized as categorical or dimensional is longstanding (Lilienfeld and Treadway, 2016). Most recently, the National Institute of Mental Heath (NIMH) has articulated an alternative to the categorical diagnosis of the DSM, called the Research Domain Criteria project (RDoC). RDoC seeks to characterize mental disorders as products of overlapping dimensional perturbations in genes, molecules, cells, and neural circuits that govern functioning across core dimensions of cognition and emotion processing. Consistent with this premise, there is growing support for the notion that autism is not a single biological entity, but a dimensional disturbance, which manifests with significant heterogeneity across a wide range of symptoms (Geschwind and Levitt, 2007), suggesting it is unlikely that inattention symptoms found in ASD would manifest exactly like those found in ADHD. Our study supports this contention in several ways. First, there were no significant group by symptom interactions between inattention, hyperactivity, or total ADHD symptoms in the univariate analyses we conducted. Second, CVLT-C findings in our study did not show the pattern of generalized deficits across all measures which would be expected if individuals with ASD exhibited categorical ADHD-like memory problems. Instead, individuals with ASD were only impaired on those measures of the CVLT-C that tapped strategic memory processes. Similarly, performance on sub-scales of the CVLT-C, and RiSE discriminability was only impaired following item (and not relational) encoding. Participants also were impaired on familiarity and not on recollection suggesting they followed an ASD-specific pattern, which has not been seen in schizophrenia (Ragland et al., 2012, 2015). In sum, while some authors (Lundervold et al., 2012) have reported that both working memory and response inhibition abilities were very important to consider when assessing performance on the CVLT in adults with ADHD, our study suggests that these cognitive processes involved in ADHD do not operate the same way in adolescents with ASD, and cannot explain the current pattern of findings.
Although this study had considerable strengths in that the patients were well characterized, medication-free, and memory encoding in ASD is relatively under-studied, it was limited in several respects. First, the sample was relatively small and consisted of the narrow group of high functioning adolescents with ASD who could complete the task without high false alarm rates. It is not clear whether these results would generalize beyond this select group. Second, relatively limited assessments of executive control and other cognitive processes were conducted. Future studies should include more extensive measurements of the components of executive control including working memory/context maintenance, and cognitive flexibility/task switching, and of response inhibition. Third, the study was cross-sectional. There is a great need for more longitudinal behavioral and functional neuroimaging (fMRI) studies of adolescents with ASD that focus on memory-related neural circuits involving the hippocampus, ATS, and PMS to help specify the mechanisms underlying their development. It also bears mention that our findings illustrate the potential utility of comparing performance on mechanistically-informed cognitive tasks which can help illuminate similarities and differences in the pathophysiology of various neurodevelopmental disorders. For example, results of studies using the RiSE in schizophrenia suggest there may be a dissociation between schizophrenia and ASD with respect to the relative impairments of the PMS versus the ATS (Ragland et al., 2015).
In conclusion, this work suggests a new way to understand memory in ASD that may inform treatment and can be investigated in future studies. Maister et al. (2013) argue that because associative memory is weaker, individuals with ASD need to draw more on the executive resources of the PFC. Instead we would argue that it would be worthwhile to use fMRI in adolescents with ASD to examine a related but inverse proposition. Due to sub-optimal prefrontal functioning, those with ASD may need to rely more on the flexible relational processing of the hippocampus, related subcortical brain structures, and the PMS to achieve comparable task performance to TYP. In fact, several recent studies using evoked response potentials (ERPs) have argued a similar position asserting that qualitatively and quantitatively different patterns of neural recruitment with activity centered in the posterior versus prefrontal plus posterior activity found in TYP, characterize successful recognition memory in those with ASD (Massand et al., 2013; Massand and Bowler, 2015). These findings also highlight the importance and utility of using well-validated cognitive neuroscience tasks and of considering the role of development when comparing the neural underpinnings of different memory processes in both typical and atypical populations.
Acknowledgments
The authors would like thank the participants and their families. The authors also would like to acknowledge Jonathan Beck, John Matter, and Leslie Gilhooly, who served as research assistants on the study.
Support
During this work, Dr. Solomon was supported by an R21 from the National Institute of Mental Health (1R21 MH099250-01). Dr. Carter was supported by the National Institute of Mental Health (2R01 MH059883-05A1) and (1R24MH081807). Dr. Ragland was supported by the National Institutes of Mental Health (R01MH084895, Ragland, PI). Statistical support was provided by the MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125).
Footnotes
Conflict of interest and disclosures
Drs. Solomon, Iosif, Carter, & Ragland and Mr. McCauley report no biomedical financial interests or potential conflicts of interest.
References
- Anderson JR, Bower GH. Recognition and retrieval processes in free recall. Psychol Rev. 1972;79(2):97. [Google Scholar]
- APA. DSM-5. American Psychiatric Association; Washington DC: 2013. [Google Scholar]
- Barnea-Goraly N, Frazier TW, Piacenza L, et al. A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog Neuropsychopharmacol Biol Psychiatry. 2014;3:124–128. doi: 10.1016/j.pnpbp.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35(6):866–866. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Ben Shalom D. Memory in autism: review and synthesis. Cortex. 2003;39(4–5):1129–1138. doi: 10.1016/s0010-9452(08)70881-5. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Rogers SJ. Intact and impaired memory functions in autism. Child Dev. 1996;67(4):1816–1835. [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Rogers SJ. Intact and impaired memory functions in autism. Child Dev. 1996;67(4):1816–1835. [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175(5):444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bigham S, Boucher J, Mayes A, Anns S. Assessing recollection and familiarity in autistic spectrum disorders: Methods and findings. J Autism Dev Disord. 2010;40(7):878–889. doi: 10.1007/s10803-010-0937-7. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;18(3):916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Parks CM, Yonelinas AP, Ranganath C. Putting the pieces together: the role of dorsolateral prefrontal cortex in relational memory encoding. J Cognit Neurosci. 2011;23(1):257–265. doi: 10.1162/jocn.2010.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudewyn MA, Long DL, Traxler MJ, et al. Sensitivity to referential ambiguity in discourse: the role of attention, working memory, and verbal ability. J Cognit Neurosci. 2015 doi: 10.1162/jocn_a_00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower GH. Organizational factors in memory. Cognit Psychol. 1970;1(1):18–46. [Google Scholar]
- Bowler DM, Matthews NJ, Gardiner JM. Asperger’s syndrome and memory: Similarity to autism but not amnesia. Neuropsychologia. 1997;35(1):65–70. doi: 10.1016/s0028-3932(96)00054-1. [DOI] [PubMed] [Google Scholar]
- Bowler DM, Gardiner JM, Grice SJ. Episodic memory and remembering in adults with Asperger syndrome. J Autism Dev Disord. 2000;30(4):295–304. doi: 10.1023/a:1005575216176. [DOI] [PubMed] [Google Scholar]
- Bowler DM, Gardiner JM, Berthollier N. Source memory in adolescents and adults with Asperger’s syndrome. J Autism Dev Disord. 2004;34(5):533–542. doi: 10.1007/s10803-004-2548-7. [DOI] [PubMed] [Google Scholar]
- Bowler DM, Gaigg SB, Gardiner JM. Binding of multiple features in memory by high-functioning adults with autism spectrum disorder. J Autism Dev Disord. 2014;44(9):2355–2362. doi: 10.1007/s10803-014-2105-y. [DOI] [PubMed] [Google Scholar]
- Bowler DM, Gaigg SB, Gardiner JM. Brief report: the role of task support in the spatial and temporal source memory of adults with autism spectrum disorder. J Autism Dev Disord. 2015:1–5. doi: 10.1007/s10803-015-2378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler DM, Limoges E, Mottron L. Different verbal learning strategies in autism spectrum disorder: evidence from the rey auditory verbal learning test. J Autism Dev Disord. 2009;39(6):910–915. doi: 10.1007/s10803-009-0697-4. [DOI] [PubMed] [Google Scholar]
- Bowler D, Gaigg S, Lind S. Memory in Autism: Binding, Self, and Brain 2011 [Google Scholar]
- Brainerd CJ, Holliday RE, Reyna VF. Behavioral measurement of remembering phenomenologies: so simple a child can do it. Child Dev. 2004;75(2):505–522. doi: 10.1111/j.1467-8624.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- Brezis RS. Memory integration in the autobiographical narratives of individuals with autism. Front Hum Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezis RS, Galili T, Wong T, Piggot JI. Impaired social processing in autism and its reflections in memory: a deeper view of encoding and retrieval processes. J Autism Dev Disord. 2014;44(5):1183–1192. doi: 10.1007/s10803-013-1980-y. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2(1):51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Caron MJ, Mottron L, Berthiaume C, Dawson M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain. 2006;129(Pt 7):1789–1802. doi: 10.1093/brain/awl072. [DOI] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Gu H, Zhang J, Feng J. Autism: reduced connectivity between cortical areas involved in face expression, theory of mind, and the sense of self. Brain. 2015;2015:awv051. doi: 10.1093/brain/awv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognitive Neuroscience Test Reliability and Clinical applications for Schizophrenia (CNTRACs) Consortium. Cognitive Neuroscience Test Reliability and Clinical applications for Schizophrenia 2011. 2015 ( http://cntracs.ucdavis.edu)
- Cohen NJ, Eichenbaum H, Deacedo BS, Corkin S. Different memory systems underlying acquisition of procedural and declarative knowledgea. Ann N Y Acad Sci. 1985;444(1):54–71. doi: 10.1111/j.1749-6632.1985.tb37579.x. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised conners’ parent rating scale (CPRS-R): factor structure, reliability, and criterion. J Abnorm Child Psychol. 1998;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Cooper RA, Plaisted-Grant KC, Hannula DE, Ranganath C, Baron-Cohen S, Simons JS. Impaired recollection of visual scene details in adults with autism spectrum conditions. J Abnorm Psychol. 2015;124(3):565–575. doi: 10.1037/abn0000070. [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L. Episodic and semantic autobiographical memory in adults with autism spectrum disorders. J Autism Dev Disord. 2008;38(3):498–506. doi: 10.1007/s10803-007-0420-2. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer J, Kaplan E, Ober BA. The California Verbal Learning Test-Children’s Version. Psychological Corporation; New York: 1994. [Google Scholar]
- DeMarie D, Ferron J. Capacity, strategies, and metamemory: tests of a three-factor model of memory development. J Exp Child Psychol. 2003;84(3):167–193. doi: 10.1016/s0022-0965(03)00004-3. [DOI] [PubMed] [Google Scholar]
- DeMaster D, Pathman T, Ghetti S. Development of memory for spatial context: hippocampal and cortical contributions. Neuropsychologia. 2013;51(12):2415–2426. doi: 10.1016/j.neuropsychologia.2013.05.026. [DOI] [PubMed] [Google Scholar]
- DeMaster D, Pathman T, Lee JK, Ghetti S. Structural development of the hippocampus and episodic memory: developmental differences along the anterior/posterior axis. Cereb Cortex. 2014;24(11):3036–3045. doi: 10.1093/cercor/bht160. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cognit Sci. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. An information processing framework for memory representation by the hippocampus. Cogn Neurosci. 2004;3:679–690. [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav Brain Sci. 1994;17(03):449–472. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Mueller JL, Sehm B, Ragert P. Language learning without control: the role of the PFC. J Cognit Neurosci. 2013;25(5):814–821. doi: 10.1162/jocn_a_00350. [DOI] [PubMed] [Google Scholar]
- Gaigg SB, Gardiner JM, Bowler DM. Free recall in autism spectrum disorder: the role of relational and item-specific encoding. Neuropsychologia. 2008;7(4):983–992. doi: 10.1016/j.neuropsychologia.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Alexander KW. If it happened, I would remember it: strategic use of event memorability in the rejection of false autobiographical events. Child Dev. 2004;75(2):542–561. doi: 10.1111/j.1467-8624.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Castelli P. Developmental differences in false-event rejection: effects of memorability-based warnings. Memory. 2006;14(6):762–776. doi: 10.1080/09658210600648548. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from the dual-process signal detection model. Child Dev. 2008;79(2):339–358. doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Bauer PJ. Origins and Development of Recollection: Perspectives from Psychology and Neuroscience. Oxford University Press; United Kingdom: 2011. [Google Scholar]
- Ghetti S, Bunge SA. Neural changes underlying the development of episodic memory during middle childhood. Dev Cogn Neurosci. 2012;2(4):381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, Lyons KE, Lazzarin F, Cornoldi C. The development of metamemory monitoring during retrieval: the case of memory strength and memory absence. J Exp Child Psychol. 2008;99(3):157–181. doi: 10.1016/j.jecp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Mirandola C, Angelini L, Cornoldi C, Ciaramelli E. Development of subjective recollection: understanding of and introspection on memory states. Child Dev. 2011;82(6):1954–1969. doi: 10.1111/j.1467-8624.2011.01645.x. [DOI] [PubMed] [Google Scholar]
- Goddard L, Dritschel B, Robinson S, Howlin P. Development of autobiographical memory in children with autism spectrum disorders: deficits, gains, and predictors of performance. Dev Psychopathol. 2014;26(1):215–228. doi: 10.1017/S0954579413000904. [DOI] [PubMed] [Google Scholar]
- Hala S, Rasmussen C, Henderson AM. Three types of source monitoring by children with and without autism: The role of executive function. J Autism Dev Disord. 2005;35(1):75–89. doi: 10.1007/s10803-004-1036-4. [DOI] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59(4):554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Zahka NE, Kojkowski NM, et al. Self-referenced memory, social cognition, and symptom presentation in autism. J Child Psychol Psychiatry. 2009;50(7):853–861. doi: 10.1111/j.1469-7610.2008.02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber PJ. Robust regression: asymptotics, conjectures and Monte Carlo. Ann Stat. 1973:799–821. [Google Scholar]
- Hunt RR, Einstein GO. Relational and item-specific information in memory. J Verbal Learn Verbal Behav. 1981;20(5):497–514. [Google Scholar]
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord. 2014;44(8):1996–2012. doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger A, Selmeczy D, O’Connor AR, Diaz M, Dobbins IG. Prefrontal cortex contributions to controlled memory judgment: fMRI evidence from adolescents and young adults. Neuropsychologia. 2012;50(14):3745–3756. doi: 10.1016/j.neuropsychologia.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan RR. Practical implications of memory characteristics in autistic spectrum disorders. Mem Autism. 2008;293 [Google Scholar]
- Joseph RM, Steele SD, Meyer E, Tager-Flusberg H. Self-ordered pointing in children with autism: failure to use verbal mediation in the service of working memory? Neuropsychologia. 2005;43(10):1400–1411. doi: 10.1016/j.neuropsychologia.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Treadway MT. Clashing diagnostic approaches: DSM-ICD versus RDoC. Annu Rev Clin Psychol. 2016;12:1. doi: 10.1146/annurev-clinpsy-021815-093122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind SE, Bowler DM. Recognition memory, self-other source memory, and theory-of-mind in children with autism spectrum disorder. J Autism Dev Disord. 2009;39(9):1231–1239. doi: 10.1007/s10803-009-0735-2. [DOI] [PubMed] [Google Scholar]
- Lind SE, Williams DM, Bowler DM, Peel A. Episodic memory and episodic future thinking impairments in high-functioning autism spectrum disorder: an underlying difficulty with scene construction or self-projection? Neuropsychology. 2014;28(1):55. doi: 10.1037/neu0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind SE, Williams DM, Raber J, Peel A, Bowler DM. Spatial navigation impairments among intellectually high-functioning adults with autism spectrum disorder: exploring relations with theory of mind, episodic memory, and episodic future thinking. J Abnorm Psychol. 2013;122(4):1189. doi: 10.1037/a0034819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind S, Bowler D. Episodic Memory and Autonoetic Consciousness in Autism Spectrum Disorders: The Roles of Self-awareness, Representational Abilities, and Temporal Cognition 2008 [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule: ADOS-2. Western Psychological Services; Torrance: 2012. [Google Scholar]
- Lundervold AJ, Stickert M, Hysing M, Sorensen L, Gillberg C, Posserud MB. Attention deficits in children with combined autism and ADHD: a CPT study. J Atten Disord. 2012;1087054712453168 doi: 10.1177/1087054712453168. [DOI] [PubMed] [Google Scholar]
- Maister L, Simons JS, Plaisted-Grant K. Executive functions are employed to process episodic and relational memories in children with autism spectrum disorders. Neuropsychology. 2013;27(6):615–627. doi: 10.1037/a0034492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massand E, Bowler DM. Atypical neurophysiology underlying episodic and semantic memory in adults with autism spectrum disorder. J Autism Dev Disord. 2015;45(2):298–315. doi: 10.1007/s10803-013-1869-9. [DOI] [PubMed] [Google Scholar]
- Massand E, Bowler DM, Mottron L, Hosein A, Jemel B. ERP correlates of recognition memory in autism spectrum disorder. J Autism Dev Disord. 2013;43(9):2038–2047. doi: 10.1007/s10803-012-1755-x. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cognit Sci. 2007;11(3):126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G. Is autism an amnesic disorder? Evidence from the california verbal learning test. Neuropsychology. 1993;7(2):209–216. [Google Scholar]
- Minshew NJ, Goldstein G. The pattern of intact and impaired memory functions in autism. J Child Psychol Psychiatry. 2001;42(8):1095–1101. doi: 10.1111/1469-7610.00808. [DOI] [PubMed] [Google Scholar]
- Mottron L, Morasse K, Belleville S. A study of memory functioning in individuals with autism. J Child Psychol Psychiatry. 2001;42(02):253–260. [PubMed] [Google Scholar]
- Nyden A, Hjelmquist E, Gillberg C. Autism spectrum and attention-deficit disorders in girls. Some neuropsychological aspects. Eur Child Adolesc Psychiatry. 2000;9(3):180–185. doi: 10.1007/s007870070041. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Han S, Dobbins IG. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? J Neurosci. 2010;30(8):2924–2934. doi: 10.1523/JNEUROSCI.4225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea AG, Fein DA, Cillessen AH, Klin A, Schultz RT. Source memory in children with autism spectrum disorders. Dev Neuropsychol. 2005;27(3):337–360. doi: 10.1207/s15326942dn2703_3. [DOI] [PubMed] [Google Scholar]
- Phelan HL, Filliter JH, Johnson SA. Brief report: memory performance on the California verbal learning test-children’s version in autism spectrum disorder. J Autism Dev Disord. 2011;41(4):518–523. doi: 10.1007/s10803-010-1069-9. [DOI] [PubMed] [Google Scholar]
- Poirier M, Martin JS, Gaigg SB, Bowler DM. Short-term memory in autism spectrum disorder. J Abnorm Psychol. 2011;120(1):247. doi: 10.1037/a0022298. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;9(17):R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, Widaman KF, Kroll NE, Sauvé MJ. Recall and recognition in mild hypoxia: using covariance structural modeling to test competing theories of explicit memory. Neuropsychologia. 2004;42(5):672–691. doi: 10.1016/j.neuropsychologia.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Ranganath C, Barch DM, et al. Relational and item-specific encoding (RISE): task development and psychometric characteristics. Schizophr Bull. 2012;38(1):114–124. doi: 10.1093/schbul/sbr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Ranganath C, Harms MP, et al. Functional and neuroanatomic specificity of episodic memory dysfunction in schizophrenia: a functional magnetic resonance imaging study of the relational and item-specific encoding task. JAMA Psychiatry. 2015;72(9):909–916. doi: 10.1001/jamapsychiatry.2015.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13(10):713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Knight R. Memory Encoding And Retrieval: A Cognitive Neuroscience Perspective 2003 [Google Scholar]
- Research Domain Criteria (RDoC) Constructs. ( www.nimh.nih.gov/research-priorities/rdoc/constructs/rdoc-matrix.shtml)
- Ritchey M, Libby LA, Ranganath C. Cortico-hippocampal systems involved in memory and cognition: the PMAT framework. Prog Brain Res. 2015 doi: 10.1016/bs.pbr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Roser ME, Aslin RN, McKenzie R, Zahra D, Fiser J. Enhanced visual statistical learning in adults with autism. Neuropsychology. 2015;29(2):163. doi: 10.1037/neu0000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. SCQ: Social Communication Questionnaire. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Salmond CH, Ashburner J, Connelly A, Friston KJ, Gadian DG, Vargha-Khadem F. The role of the medial temporal lobe in autistic spectrum disorders. Eur J Neurosci. 2005;22(3):764–772. doi: 10.1111/j.1460-9568.2005.04217.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Tulving E. Memory Systems 1994. Mit Press; United States: 1994. [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;14(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenck C, Bjorklund DF, Schneider W. Developmental and individual differences in young children’s use and maintenance of a selective memory strategy. Dev Psychol. 2009;45(4):1034–1050. doi: 10.1037/a0015597. [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, et al. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47(12):2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Buaminger N, Rogers SJ. Abstract reasoning and friendship in high functioning preadolescents with autism spectrum disorders. J Autism Dev Disord. 2011;41(1):32–43. doi: 10.1007/s10803-010-1017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ragland JD, Niendam TA, et al. Atypical learning in autism spectrum disorders: a functional magnetic resonance imaging study of transitive inference. J Am Acad Child Adolesc Psychiatry. 2015 doi: 10.1016/j.jaac.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Cummings N, Carter CS. Cognitive control in autism spectrum disorders. Int J Dev Neurosci. 2008;26(2):239–247. doi: 10.1016/j.ijdevneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Frank MJ, Smith AC, Ly S, Carter CS. Transitive inference in adults with autism spectrum disorders. Cogn Affect Behav Neurosci. 2011;11(3):437–449. doi: 10.3758/s13415-011-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Smith AC, Frank MJ, Ly S, Carter CS. Probabilistic reinforcement learning in adults with autism spectrum disorders. Autism Res. 2011;4(2):109–120. doi: 10.1002/aur.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick JS, Bigler ED, Froehlich A, et al. Memory functioning in children and adolescents with autism. Neuropsychology. 2011;25(6):702–710. doi: 10.1037/a0024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and forgetting: long-term and gradual changes in memory storage. Int Rev Neurobiol. 1994;37:243. [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82(3):171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proc Natl Acad Sci USA. 2006;103(21):8239–8244. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC. R: A language and environment for statistical computing. Vienna, Austria: 2015. p. 2014. URL ( http://www.R-project.org) [Google Scholar]
- Toichi M, Kamio Y. Long-term memory and levels-of-processing in autism. Neuropsychologia. 2002;40(7):964–969. doi: 10.1016/s0028-3932(01)00163-4. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53(1):1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of Episodic Memory 1985 [Google Scholar]
- Wagner AD, Bunge SA, Badre D. Cognitive control, semantic memory, and priming: Contributions from prefrontal cortex. Cognit Neurosci. 2004;III:709–726. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. second. Pearson; New York: 2011. Manual. [Google Scholar]
- Yohai VJ. High breakdown-point and high efficiency robust estimates for regression. Ann Stat. 1987:642–656. [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychology: Learn Mem Cogn. 1994;20(6):1341. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Cohen NJ, Squire LR. Recall of remote episodic memory in amnesia. Neuropsychologia. 1983;21(5):487–500. doi: 10.1016/0028-3932(83)90005-2. [DOI] [PubMed] [Google Scholar]


