Abstract
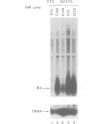
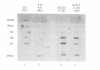
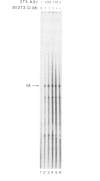
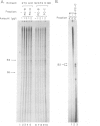
Transcription by RNA polymerase III of the B2 family of middle-repetitive elements is activated in response to transformation by a variety of agents, including DNA tumour viruses, RNA tumour viruses and chemical carcinogens. We have investigated the mechanism of activation in SV40-transformed cells and we find that the effect is due to an increase in the activity of the general class III transcription factor TFIIIC, achieved both by an increase in factor abundance and by a change in its phosphorylation state. SV40 transformation also stimulates transcription of other genes by RNA polymerase III but the effect may be balanced by compensatory post-transcriptional changes. TFIIIC may mediate the stimulation of polymerase III transcription by a range of transforming viruses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aufiero B., Schneider R. J. The hepatitis B virus X-gene product trans-activates both RNA polymerase II and III promoters. EMBO J. 1990 Feb;9(2):497–504. doi: 10.1002/j.1460-2075.1990.tb08136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K. L., Hill R. E., Pietras D. F., Woodworth-Gutai M., Kane-Haas C., Houston J. M., Heath J. K., Hastie N. D. Most highly repeated dispersed DNA families in the mouse genome. Mol Cell Biol. 1984 Aug;4(8):1561–1571. doi: 10.1128/mcb.4.8.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L., Folk W. R. Differential activation of RNA polymerase III-transcribed genes by the polyomavirus enhancer and the adenovirus E1A gene products. Nucleic Acids Res. 1985 Feb 25;13(4):1413–1428. doi: 10.1093/nar/13.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. Activation of a Qa/Tla class I major histocompatibility antigen gene is a general feature of oncogenesis in the mouse. Nature. 1983 Dec 22;306(5945):756–760. doi: 10.1038/306756a0. [DOI] [PubMed] [Google Scholar]
- Carey M. F., Singh K., Botchan M., Cozzarelli N. R. Induction of specific transcription by RNA polymerase III in transformed cells. Mol Cell Biol. 1986 Sep;6(9):3068–3076. doi: 10.1128/mcb.6.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M. F., Singh K. Enhanced B2 transcription in simian virus 40-transformed cells is mediated through the formation of RNA polymerase III transcription complexes on previously inactive genes. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7059–7063. doi: 10.1073/pnas.85.19.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J. A potential role for RNA transcribed from B2 repeats in the regulation of mRNA stability. Cell. 1987 Apr 24;49(2):157–158. doi: 10.1016/0092-8674(87)90553-8. [DOI] [PubMed] [Google Scholar]
- Dean N., Berk A. J. Ordering promoter binding of class III transcription factors TFIIIC1 and TFIIIC2. Mol Cell Biol. 1988 Aug;8(8):3017–3025. doi: 10.1128/mcb.8.8.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Parfett C. L., Denhardt D. T. Transcriptional regulation of two serum-induced RNAs in mouse fibroblasts: equivalence of one species to B2 repetitive elements. Mol Cell Biol. 1985 Nov;5(11):3280–3288. doi: 10.1128/mcb.5.11.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Mitchell J. B. Induction of B2 RNA polymerase III transcription by heat shock: enrichment for heat shock induced sequences in rodent cells by hybridization subtraction. Nucleic Acids Res. 1986 Jul 25;14(14):5793–5811. doi: 10.1093/nar/14.14.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G., Hofstetter H., Birnstiel M. L. Two conserved sequence blocks within eukaryotic tRNA genes are major promoter elements. Nature. 1981 Dec 17;294(5842):626–631. doi: 10.1038/294626a0. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Feldman L. T., Berk A. J. Transcription of class III genes activated by viral immediate early proteins. Science. 1985 Oct 25;230(4724):447–450. doi: 10.1126/science.2996135. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Gokal P. K., Cavanaugh A. H., Thompson E. A., Jr The effects of cycloheximide upon transcription of rRNA, 5 S RNA, and tRNA genes. J Biol Chem. 1986 Feb 25;261(6):2536–2541. [PubMed] [Google Scholar]
- Hilberg F., Stocking C., Ostertag W., Grez M. Functional analysis of a retroviral host-range mutant: altered long terminal repeat sequences allow expression in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5232–5236. doi: 10.1073/pnas.84.15.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler W. K., Kovelman R., Roeder R. G. Activation of transcription factor IIIC by the adenovirus E1A protein. Cell. 1988 Jun 17;53(6):907–920. doi: 10.1016/s0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Hoeffler W. K., Roeder R. G. Enhancement of RNA polymerase III transcription by the E1A gene product of adenovirus. Cell. 1985 Jul;41(3):955–963. doi: 10.1016/s0092-8674(85)80076-3. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Kramerov D. A., Lekakh I. V., Samarina O. P., Ryskov A. P. The sequences homologous to major interspersed repeats B1 and B2 of mouse genome are present in mRNA and small cytoplasmic poly(A) + RNA. Nucleic Acids Res. 1982 Dec 11;10(23):7477–7491. doi: 10.1093/nar/10.23.7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerov D. A., Tillib S. V., Lekakh I. V., Ryskov A. P., Georgiev G. P. Biosynthesis and cytoplasmic distribution of small poly(A)-containing B2 RNA. Biochim Biophys Acta. 1985 Feb 20;824(2):85–98. doi: 10.1016/0167-4781(85)90084-3. [DOI] [PubMed] [Google Scholar]
- Kramerov D. A., Tillib S. V., Ryskov A. P., Georgiev G. P. Nucleotide sequence of small polyadenylated B2 RNA. Nucleic Acids Res. 1985 Sep 25;13(18):6423–6437. doi: 10.1093/nar/13.18.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayev A. S., Markusheva T. V., Kramerov D. A., Ryskov A. P., Skryabin K. G., Bayev A. A., Georgiev G. P. Ubiquitous transposon-like repeats B1 and B2 of the mouse genome: B2 sequencing. Nucleic Acids Res. 1982 Dec 11;10(23):7461–7475. doi: 10.1093/nar/10.23.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Simanis V., Bartsch R., Yewdell J., Gannon J., Mole S. Cellular targets for SV40 large T-antigen. Proc R Soc Lond B Biol Sci. 1985 Oct 22;226(1242):25–42. doi: 10.1098/rspb.1985.0077. [DOI] [PubMed] [Google Scholar]
- Lania L., Pannuti A., La Mantia G., Basilico C. The transcription of B2 repeated sequences is regulated during the transition from quiescent to proliferative state in cultured rodent cells. FEBS Lett. 1987 Jul 27;219(2):400–404. doi: 10.1016/0014-5793(87)80260-0. [DOI] [PubMed] [Google Scholar]
- Loeken M., Bikel I., Livingston D. M., Brady J. trans-activation of RNA polymerase II and III promoters by SV40 small t antigen. Cell. 1988 Dec 23;55(6):1171–1177. doi: 10.1016/0092-8674(88)90261-9. [DOI] [PubMed] [Google Scholar]
- Majello B., La Mantia G., Simeone A., Boncinelli E., Lania L. Activation of major histocompatibility complex class I mRNA containing an Alu-like repeat in polyoma virus-transformed rat cells. Nature. 1985 Apr 4;314(6010):457–459. doi: 10.1038/314457a0. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Murphy D., Brickell P. M., Latchman D. S., Willison K., Rigby P. W. Transcripts regulated during normal embryonic development and oncogenic transformation share a repetitive element. Cell. 1983 Dec;35(3 Pt 2):865–871. doi: 10.1016/0092-8674(83)90119-8. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Chia W., Clayton C. E., Lovett M. The structure and expression of the integrated viral DNA in mouse cells transformed by simian virus 40. Proc R Soc Lond B Biol Sci. 1980 Nov 19;210(1180):437–450. doi: 10.1098/rspb.1980.0145. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Ivanov P. L., Tokarskaya O. N., Kramerov D. A., Grigoryan M. S., Georgiev G. P. Major transcripts containing B1 and B2 repetitive sequences in cytoplasmic poly(A)+RNA from mouse tissues. FEBS Lett. 1985 Mar 11;182(1):73–76. doi: 10.1016/0014-5793(85)81156-x. [DOI] [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Iversen P., Rechsteiner M. The turnover of tRNAs microinjected into animal cells. Nucleic Acids Res. 1978 Oct;5(10):3715–3729. doi: 10.1093/nar/5.10.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. B., Sklar V. E., Jaehning J. A., Weinmann R., Roeder R. G. Isolation and partial characterization of the multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in the mouse myeloma, MOPC 315. J Biol Chem. 1974 Sep 25;249(18):5889–5897. [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Segall J., Matsui T., Roeder R. G. Multiple factors are required for the accurate transcription of purified genes by RNA polymerase III. J Biol Chem. 1980 Dec 25;255(24):11986–11991. [PubMed] [Google Scholar]
- Simanis V., Lane D. P. An immunoaffinity purification procedure for SV40 large T antigen. Virology. 1985 Jul 15;144(1):88–100. doi: 10.1016/0042-6822(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Singh K., Carey M., Saragosti S., Botchan M. Expression of enhanced levels of small RNA polymerase III transcripts encoded by the B2 repeats in simian virus 40-transformed mouse cells. Nature. 1985 Apr 11;314(6011):553–556. doi: 10.1038/314553a0. [DOI] [PubMed] [Google Scholar]
- White R. J., Stott D., Rigby P. W. Regulation of RNA polymerase III transcription in response to F9 embryonal carcinoma stem cell differentiation. Cell. 1989 Dec 22;59(6):1081–1092. doi: 10.1016/0092-8674(89)90764-2. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S., Dean N., Han M., Berk A. J. Adenovirus stimulation of transcription by RNA polymerase III: evidence for an E1A-dependent increase in transcription factor IIIC concentration. EMBO J. 1986 Feb;5(2):343–354. doi: 10.1002/j.1460-2075.1986.tb04218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]