INTRODUCTION
During the past few decades, research has provided breakthroughs that have enhanced our understanding of the mechanisms and pathways that regulate the immune system’s response to cancer.1 However, despite these advances, obstacles still exist for the field of cancer immunotherapy.1 These include the inability to predict treatment efficacy and patient response; the need for additional biomarkers; the development of resistance to cancer immunotherapies; the lack of clinical study designs that are optimized to determine efficacy; and high treatment costs.2–10 Future advances in cancer immunotherapy are expected to overcome and resolve many of these challenges. Anticipated innovations include more targeted treatments; the development of personalized biomarker profiles; drug combination therapies that will improve efficacy and reduce toxicity; and immunopreventive strategies that will diminish cancer incidence and recurrence and associated treatment costs.11–14
CANCER IMMUNOTHERAPY CHALLENGES
Efficacy Is Often Unpredictable
A major challenge for cancer immunotherapies is the need to develop agents that are consistently effective in a majority of patients and cancer types.2 Dramatic results have been observed in some patients treated with cancer immunotherapies, indicating that it is feasible to restore effective antitumor immune surveillance.2 However, to date, many immunotherapy treatments have demonstrated efficacy in only a select group of cancers and usually in a minority of patients with those cancers.2,3,9
Reasons for the variability in patient response to cancer immunotherapies have been proposed, including the need to identify additional biomarkers and cancer pathways, tumor heterogeneity, variability in cancer type and stage, treatment history, and the underlying immunosuppressive biology of the cancer.2,4 Treatments that target single molecular mutations or cancer pathways have only modestly affected survival in some cancers.5 This approach, which has been described as “reductionist,” might be improved by administering drug combinations that target multiple mutations and cancer pathways. 5 In addition, a large number of the mutations found in human tumors do not occur with meaningful regularity among different patients.15 Therefore, immunotherapies directed at molecular mutations most likely need to be customized and patient-specific in order to be more effective.15
Even the durable clinical benefits that have been observed with immune checkpoint blockers (ICBs) in some tumor types have been seen only in a minority of patients.2 Clinical trial data have shown that approximately 15% to 25% (although sometimes more) of patients with various types of cancer respond to cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) receptor or programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) ICBs.1,5,9,10 One theory regarding the variability in patient response to ICBs is that additional checkpoints that play a significant role in inhibiting the body’s anticancer immune defenses need to be identified.9 Researchers are working to discover additional genetic mutations, cancer pathways, and immune checkpoints and to develop new drugs to target them.3,9 Although challenging, these efforts will likely lead to more promising targeted cancer treatments.5
An additional issue that may be impeding greater efficacy with cancer immunotherapies is the longstanding use of conventional chemotherapy as first-line cancer treatment.16 Consequently, because cancer immunotherapies are not yet widely indicated as first-line treatments, they are typically given to patients whose immune systems are already compromised due to advanced disease and/or previous therapies. The ability of cancer immunotherapies to restore antitumor immune function under these conditions is challenging; therefore, higher efficacy rates might be achieved in a greater percentage of patients if personalized cancer immunotherapies were administered earlier to restore a robust antitumor response while the immune system can still recover.16
Difficulty Identifying Clinically Significant Biomarkers
One major limitation of cancer immunotherapy is the availability of known targetable tumor-specific antigens (TSAs), also called “neoantigens,” that are solely expressed by tumor cells.1,2 Tumor-associated antigens, which are expressed by both tumor and normal tissues, also provide an option for immunotherapy, but targeting them is likely to cause off-target toxicities and has achieved little success.1
In tumor models, a single-driver mutation is capable of conferring distinct biological properties and powering oncogenic capabilities, making tumor cells strongly dependent on that genomic alteration for survival.5 Such driver mutations are found in small subsets of patients across different solid tumors.17,18 The immune system can control malignancies by targeting the genetic mutations that lead to oncogenic outgrowth. 15 It is therefore important to develop cancer immunotherapies that enhance TSA-specific T-cell reactivity.15 Because TSAs are expressed only by tumors, this approach offers the potential of high specificity, which will likely enhance both efficacy and safety.15
Cancer testis antigens (CTAs) have also been extensively investigated and are thought to be a promising category of immunotherapy targets.2,4 The characteristics that make CTAs potentially optimal biomarkers for cancer immunotherapy include highly selective expression in tumor versus normal tissues; broad expression in a variety of human cancers of different histological origin; and remarkable “immunogenicity” allowing the induction of humoral and/or cellular immune responses in cancer patients.2,4 CTAs may also be optimal targets for cancer immunotherapy directed at cancer stem cells (CSCs).2 CTAs are expressed by CSCs and play a role in CSC differentiation and biology.2 Most cells comprising a tumor mass are thought to result from the differentiation and cloning of a small number of CSCs that maintain and constantly “feed” the growth of the tumor.2 With evidence that CSCs exist in many different tumors, it is imperative to identify and understand tumor antigens expressed by CSCs.2
Need for More Predictive Biomarkers
Clinical biomarkers may have diagnostic, predictive, prognostic, or pharmacogenomic value.5 Predictive biomarkers are the most useful in daily practice because they enable selection of patients who will obtain the greatest benefits from a treatment, as well as exclusion of patients who are unlikely to respond.5 Prognostic biomarkers are predictive of patient outcomes irrespective of treatment, and they are therefore used less frequently for treatment decisions.5 The successful development of clinically significant biomarkers depends upon three features: their biological role with respect to malignant transformation and tumor progression; the ability to detect them with robust, reliable, and clinically applicable analytical genomic tests; and their prognostic or predictive value, as validated in clinical trials.5
Identifying biomarkers that have predictive or prognostic value for use in selecting patients who will benefit from treatment with cancer immunotherapy is a lengthy and difficult process.5 To date, few predictive biomarkers for cancer immuno therapy treatments have been robustly validated.5 Still, a predictive benefit has been observed for certain biomarkers with respect to response rate in patients with oncogene-addicted tumors when those patients receive matched targeted immuno therapies.5 For example, human epidermal growth factor receptor 2 (HER2) amplification has been found in 20% of patients with gastric cancer; these patients have been found to exhibit a response rate of 40% to 50% when treated with the monoclonal antibody trastuzumab.5
PD-L1 has been perhaps the most investigated biomarker with regard to potential predictive capabilities; it has been studied in numerous randomized controlled trials.5 Evidence in different tumor types has suggested that the higher the PD-L1 expression by the tumor, the better the response rate and survival rates with PD-1/PD-L1 ICB treatment.4,5 Interestingly, however, it has been found that treatment benefits with PD-1/PD-L1 ICBs are not solely restricted to PD-L1–positive patients.4,5 In two pivotal trials that studied nivolumab (Opdivo, Bristol-Myers Squibb) in the treatment of metastatic melanoma (MM), 20% to 30% of PD-L1–negative patients responded to treatment, compared with 50% of PD-L1–positive subjects.19,20 In the Checkmate-066 trial, improved one-year overall survival (OS) was reported for PD-L1–negative patients with MM treated with nivolumab compared with those treated with dacarbazine.20 These findings suggest that there may be currently unknown biomarkers other than PD-1/PD-L1 that are predictive of response to these agents. The interpretability of PD-L1 expression levels across clinical trials may also be limited by assay-related inconsistencies and the fact that PD-L1 is an inducible marker that may vary in different tumor contexts.5
Tumor mutational load could also be a potential predictive biomarker for PD-1/PD-L1 blockade therapy.15,21 Patients with tumors that have a high mutational burden have been found to demonstrate better OS when treated with PD-1/PD-L1 ICBs, compared to patients with tumors that have a low mutational burden.5 This has been shown for both carcinogen-induced tumors (melanoma, non–small-cell lung cancer [NSCLC]), and in tumors developed in a context of DNA repair deficiency.5 It has been proposed that tumors with high mutational loads are likely to be more immunogenic; therefore, they persistently stimulate the activation of neoantigen-specific CD4+ and CD8+ T cells.21 Pre-existing CD8+ T cells located at the invasive tumor margin have also been associated with tumor regression with PD-1/PD-L1 blockade in MM.5,21 Interferon (IFN)-based transcriptomic signatures are another potentially predictive marker for PD-1/PD-L1 ICB treatment.5 They have been observed in MM; however, they may also be applicable to other tumor types.5 These features may potentially be predictive markers because they are all representative of a tumor’s immunogenicity (with respect to mutational burden) or antitumor immunity (with respect to tumor-infiltrating lymphocytes or IFN signature).5
For the CTLA-4 ICB ipilimumab (Yervoy, Bristol-Myers Squibb), several serum markers such as C-reactive protein, lactate dehydrogenase, soluble CD25, and vascular endothelial growth factor have been associated with positive clinical outcomes in advanced melanoma patients.21 In addition, a variety of assays are available to measure changes in target immune cell populations (such as myeloid-derived suppressor cells), assess tumor-associated antigen-specific responses, and evaluate the functionality and gene expression profile of antigen-specific T-cell populations.21 These assays have led to the preliminary identification of potential biomarkers for CTLA-4 ICB therapy, such as absolute lymphocyte count (in melanoma and other solid tumors); CD4+/inducible costimulator-positive T cells (in bladder cancer, breast cancer, and mesothelioma); CD4+ and CD8+ antigen-specific T-cell response (in melanoma, ovarian, and prostate cancers); or CTA NY-ESO-1 and IFN-gamma responsive genes (in melanoma patients).21 However, these potential biomarkers for CTLA-4 ICB treatment were identified in small cohort studies and need to be validated in larger cohorts of patients in prospective clinical trials.21 Long-term benefit in patients with melanoma treated with ipilimumab has also been associated with a higher mutational load, although this effect appears to be less profound than that observed for patients with NSCLC who had a high mutational load and were treated with PD-1 ICBs.15
The need to identify additional, more robust predictive biomarkers poses considerable technical challenges. Clinically predictive genomic mutations are uncommon, diverse, and distributed across many cancer types.5 Therefore, the identification of these mutations requires highly sensitive, multiplexed, comprehensive sequencing techniques, even for routine clinical care.5 Classic Sanger genomic sequencing lacks sufficient sensitivity, cannot test multiple genomic mutations simultaneously, only detects point mutations or small insertions or deletions, and is not cost-effective.5 Therefore, high-throughput next-generation sequencing (NGS) technologies are needed to fulfill the requirements to enable routine biomarker screening for clinical care.5
Two strategies involving the application of NGS technologies have been developed for this purpose: the utilization of customized gene panels, and whole-exome, genome, or transcriptome panels.5 Gene panels can detect multiple potentially relevant mutations across several cancer genes at once and can be scaled to clinical requirements.5 Based on early experiences, customized gene panel genotyping has been found to be feasible and adaptable to the challenges inherent in clinical settings, which include limited biopsy tissue; low tumor cellularity; formalin-fixed paraffin-embedded tissue samples; and rapid turnaround time required for therapeutic decisions.22 This technique is expected to be clinically useful for routine cancer care or prompt selection of patients for biomarker-driven clinical trials.23,24 Customized gene panels are increasingly being used in academic centers for routine genotyping and patient care.5 However, this technology is currently limited to large academic centers and is not yet widely available in other clinical settings.5
Whole-exome and genome, or RNA sequencing platforms, are mostly used for molecular prescreening in research and are rarely applied in clinical genotyping.5 Several limitations restrict the clinical use of this technology.5 One issue is the need to manage massive amounts of genomic data, which is not compatible with the short time frame required for therapeutic decision-making.5 A second issue is the difficulty in interpreting the large quantity of genomic data that is generated, much of which has unknown clinical significance.5 A third issue is the cost associated with this approach.6,22,25 Whichever NGS platform is used, the issues regarding the clinical interpretation of the genomic results present important challenges regarding the wide applicability of NGS technologies.5 It is therefore evident that multidisciplinary teams of expert geneticists, molecular biologists, bioinformaticians, and oncologists will be needed for adequate data analysis, interpretation, and therapeutic decision-making regarding biomarker data.5
Tumor Heterogeneity Impedes Efficacy
Tumor heterogeneity is another obstacle to the success of cancer immunotherapy.5 During cancer development, cancerous cells acquiring certain genetic mutations have a survival advantage, so they dominate local areas of the tumor by displacing cells that lack these alterations; this effect is enhanced by clonal expansion.5 According to this “Darwinian” model, all cells within a tumor should therefore be biologically similar.5 However, rather than clonal expansion, evidence indicates that tumor initiation and progression may instead rely on a relatively minor population of self-renewing CSCs.5 Two CSC models for tumor initiation and progression have been proposed: a strictly hierarchical model, where CSCs are the only cell population within the tumor that self-renew and have tumorigenic potential; and a nonhierarchical model, where every tumor cell has inherent plasticity and potential to de-differentiate and revert to the CSC state in response to intrinsic or microenvironmental factors.5
However, both the Darwinian and CSC models may be overly simplistic: If they were to hold true, tumor masses would be dominated by homogeneous cellular clones.5 Instead, it has been found that genomic instability that generates new mutations inherently occurs in cancer cells, as well as in response to exogenous carcinogens (such as sunburn and smoking).4,5 Rather than tumor progression being linear, it may occur in a branched model, with tumor masses composed of an increasing quantity of genetically distinct subclones.5,21 This model has been evidenced by several high-throughput whole-genome sequencing studies of different tumor types, which found high genomic variability within both primary tumors and metastatic regions.4,5 In one study, genomic characterization of multiple renal cancer primary and metastatic lesions found genomic, transcriptomic, and functional heterogeneity within separate tumor sites, indicating the presence of different tumor subclones. 5 A high level of intratumor heterogeneity has also been confirmed in other tumor types, such as lung cancer.5 Whole-exome sequencing of 11 localized adenocarcinomas revealed high levels of intratumor heterogeneity, with the majority of mutations (76%) present in all tumor regions.4
Similar reports regarding other solid tumors have suggested that despite high levels of tumor heterogeneity, recurrent driver mutations (which are truncal and tend to occur early in tumorigenesis) dominate.26 These mutations are present in every tumor clone and tumor region, potentially representing robust therapeutic targets.5 This finding means that a single-site tumor biopsy may be sufficient to detect driver mutations that can be targeted by treatment.5 This is significant because it suggests that multiple biopsies to check for heterogeneity may not be necessary for therapeutic decision-making.5 However, accumulating evidence suggests that subclonal passenger mutation cellular populations may also be a factor in supporting the growth and survival of neighboring clonally dominant cells, causing the growth of some regions to become more aggressive than others.5 Subclonal populations are also thought to be responsible for acquired oncogene resistance.27
Development of Resistance to Drug Treatment
Tumor heterogeneity and the development of resistant cancer cell clones are closely related, with both issues contributing to the therapeutic failures observed with cancer immunotherapy in clinical practice.5 Cancer signaling networks are remarkably flexible and adaptive, so resistance is likely to develop to any single targeted cancer treatment.28 Subclonal cancer cell populations, as well as branched clonal evolution, are thought to be partly responsible for the development of drug resistance.5,27
Mechanisms of acquired resistance can generally be classified into three groups: those that involve secondary genomic mutations in the drug target; those that cause the reactivation of a cancer pathway; and those that activate alternative signaling pathways.27 Less-established mechanisms, such as nongenomic alterations (epigenetic and transcriptional changes) and intratumoral immunity, are also thought to cause acquired resistance to targeted therapies.5,29 In a recent study, several patients with advanced melanoma who had developed acquired treatment resistance to the ICB pembrolizumab (Keytruda, Merck) were studied.30,31 Tumor biopsies taken before treatment and after the patients had relapsed were compared to identify genetic alterations that developed after treatment, which may have contributed to resistance.30,31 The tumors in two patients had developed mutations in the JAK1 or JAK2 gene, disrupting the IFN-gamma signaling pathway and reducing the expression of genes involved in T-cell recognition and elimination of cancer cells.30,31 A third patient had developed a mutation in the B2M gene, which is involved in the expression of cell-surface proteins that immune cells use to recognize and eliminate cancer cells.30,31 The researchers are now studying cell line and mouse models of these mutations and testing other treatments or combinations of PD-1 ICB therapies to overcome the resistance.31
As this example illustrates, biopsies upon disease progression are needed to determine resistance mechanisms and targets that may potentially inhibit them.5 However, repeatedly acquiring biopsies can be a challenge in routine clinical practice because it may increase morbidity, and therefore it may not be feasible in all cases.5 Liquid biopsies, which involve the genomic analysis of circulating free DNA or tumor cells (such as extracellular vesicles or CSCs), could minimize these issues.5 Such platforms are being studied and developed in resistance settings and next-generation biomarker-driven clinical trials.5 Liquid biopsies could also be helpful in characterizing the level of tumor heterogeneity in resistant patients, enabling the personalization of treatment.5 This approach to assessing tumor heterogeneity and monitoring resistance is potentially revolutionary; however, whether it will be scalable for clinical purposes is yet to be determined.5
Need for Distinct Clinical Study Designs to Evaluate Efficacy
The criteria used to assess cancer immunotherapies should be distinct from those used to assess response to chemotherapy and other cytotoxic agents.6 Because immunotherapies rely on the activation of the immune system instead of directly attacking tumor cells, they can have delayed antitumor activities.32 Therefore, the endpoints traditionally used in clinical trials of cytotoxic anticancer drugs may be inadequate in determining the efficacy of cancer immunotherapy treatments.32
The need for distinct clinical trial designs for the evaluation of cancer immunotherapies became clear when a clinical trial of the anti-CTLA-4 ICB tremelimumab (investigational, AstraZeneca) was prematurely terminated due to “lack of improvement.”33 In a follow-up evaluation, it was found that a separation in survival curves for the study population in this trial occurred at 24 months.33 Based on this observation, the endpoints for two subsequent phase 3 trials of ipilimumab were extended, allowing enough time for improvement in OS to be demonstrated, which was the basis for subsequent Food and Drug Administration (FDA) approval of this agent for the treatment of melanoma.33 Other immune-related criteria, including evaluation of the phenotype and functions of immune cells, such as tumor-specific cytotoxic T lymphocytes, in patients’ blood must be taken into account when defining clinical study endpoints for evaluating cancer immunotherapy treatments.1 Endpoints should also be incorporated to assess long-term disease-free survival due to the immune memory that occurs with some cancer immunotherapies.1
Another challenge with respect to the design of clinical trials for the study of cancer immunotherapy agents involves the difficulty of studying drug efficacy in the small cohorts of patients with specific biomarkers. When positive results are observed in early clinical trials, they must usually be confirmed in large phase 3, randomized clinical trials to generate the data that must be submitted for FDA review and approval.5 However, genomic alterations that can be targeted with medications are very diverse and are usually represented in only small subsets of patients with certain tumor types.5 Conducting large phase 3, randomized studies in patient cohorts with mutations that in some instances account for 1% to 5% of overall cases might take many years, or even be impossible, with respect to rare tumor types.5 Next-generation clinical trial designs are being developed that take into account small cohorts of patients with specific biomarkers due to tumor heterogeneity.5
Cancer Immunotherapy Drugs Are Expensive
In recent years, the economic sustainability of health care systems has become a worldwide concern.8 The introduction of immunotherapy drugs and novel molecularly targeted agents to the therapeutic armamentarium has been a “game changer” for cancer patients in terms of OS and quality of life.8 However, these agents are very expensive, and the effects of these costs on the health care system need to be considered carefully.8 In 2014, U.S. spending on oncology drugs reached $42.4 billion, according to IMS Health.7
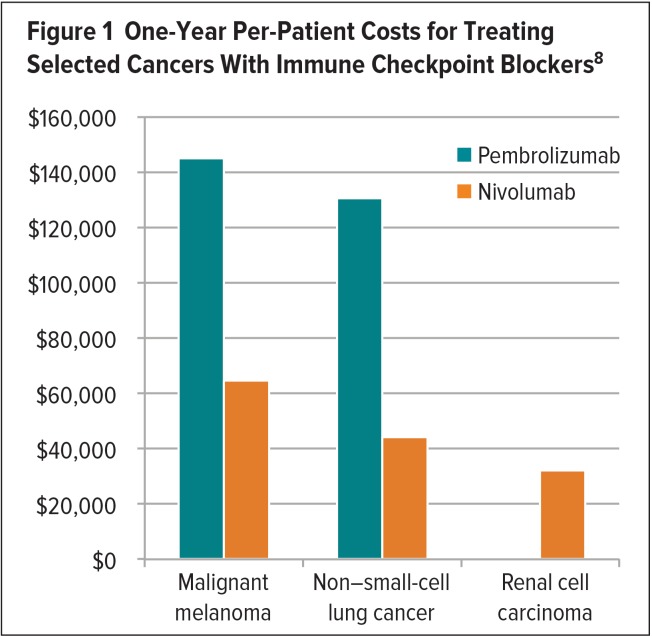
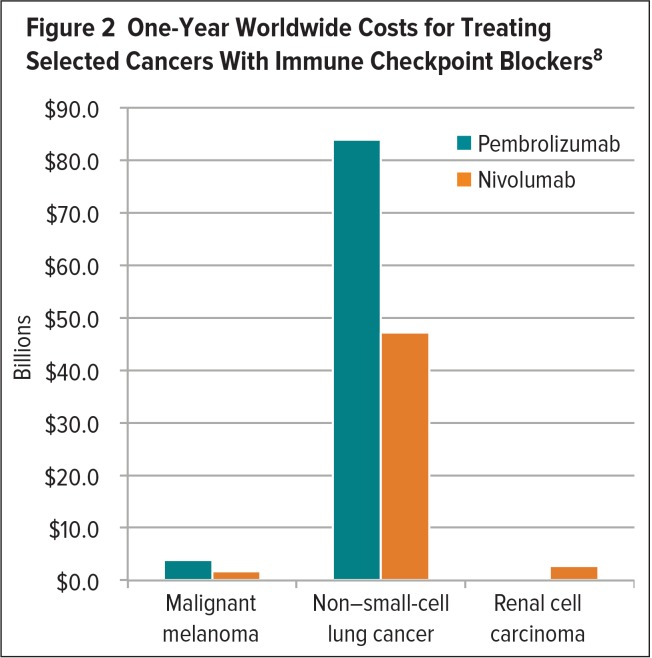
The cost of targeted anticancer agents and immunotherapies varies among different tumor types.8 Positive results obtained with nivolumab or pembrolizumab in patients with MM, NSCLC, and renal cell carcinoma (RCC) have spurred the evaluation of these agents in additional cancers.8 Although a proliferation of new indications would be welcome, these very expensive treatments may yield an unsustainable cost burden.7,8 In 2016, a study evaluated the one-year per-patient and worldwide costs associated with the use of these ICBs in patients with MM, NSCLC, and RCC.8 According to this study, pembrolizumab incurred one-year per-patient costs of $145,010 and $130,511 for the treatment of MM and NSCLC, respectively, and achieved median progression-free survival (PFS) of 6.3 months for each type of cancer.8 The one-year worldwide cost for treating these cancers with pembrolizumab was estimated to be $3.8 billion and $83.9 billion, respectively.8 The one-year per-patient cost for treating MM and NSCLC with nivolumab was estimated to be $64,680 and $44,100, achieving a median PFS of 5.1 months and 3.5 months, respectively.8 The one-year worldwide cost of treating MM and NSCLC with nivolumab was $1.7 billion and $47.2 billion, respectively.8 The large difference in worldwide drug costs for the treatment of these cancers can be explained by the higher global incidence of NSCLC compared with MM.8 The one-year per-patient cost for treating RCC with nivolumab was estimated to be $32,130 to achieve a median PFS of 4.6 months, at a worldwide cost of $2.7 billion (Figures 1 and 2).8
Figure 1.
One-Year Per-Patient Costs for Treating Selected Cancers With Immune Checkpoint Blockers8
Figure 2.
One-Year Worldwide Costs for Treating Selected Cancers With Immune Checkpoint Blockers8
In addition to burdening health care systems, prohibitively expensive cancer immunotherapy drugs can strain patient finances to the point that the medications are inaccessible to those who need them.9 Even if insurers cover FDA-approved treatments, patient copayments can be very high for expensive drugs.9 Costs may be covered for patients who volunteer for clinical trials of new agents or novel drug combinations.9 However, not everyone can enter or wishes to enter a study. Racial minorities, for instance, are underrepresented in these clinical studies for reasons that are unclear.9 The high cost of developing and administering some personalized forms of cancer immunotherapy also limits their use to certain patient populations.1 To counteract this, more cost-effective, high-throughput genetic screening and other techniques are being researched and applied to make cancer immunotherapies accessible to a broader range of patients.1
The high treatment costs for cancer suggest the need for close cooperation and coordination among the medical community, corporations, and economists to preserve the sustainability of the global health system and continued patient access to care.8 The identification of novel clinical and molecular predictive biomarkers that can be applied to select patients who will benefit from expensive targeted and immunotherapy treatments is among the measures that may reduce costs.8 Cost-effectiveness, cost–benefit, and quality-adjusted life year analyses, as well as different drug reimbursement modalities, may also be useful.8 Although ICBs have improved median PFS or OS by just several months in some clinical trials, some costly chemotherapy treatments add only weeks of life.1,5,8 However, a minority of patients receiving ICB treatment have achieved long-term survival benefits, so this potential outcome may justify treatment costs.5,8,9
A summary of the challenges for cancer immunotherapy, the problems associated with them, and the proposed solutions is presented in Table 1.
Table 1.
| Problem | Challenge | Solution |
|---|---|---|
| Unpredictable Efficacy | ||
| Cancer immunotherapies are effective only in subsets of patients with select cancers |
|
|
| Biomarker Identification | ||
| Difficulty identifying clinically significant biomarkers among the increasing number of genetic mutations detected across tumor types |
|
|
| Need to identify more predictive biomarkers for cancer immunotherapies |
|
|
| Tumor Heterogeneity | ||
| High level of heterogeneity found in tumor and metastatic lesion genetic mutations |
|
|
| Acquired Treatment Resistance | ||
| Emergence of resistance to single-target cancer immunotherapy treatments |
|
|
| Clinical Trial Design | ||
| Distinct clinical criteria needed for cancer immunotherapy evaluation |
|
|
| Low prevalence of some biomarkers in patient cohorts |
|
|
| Cost | ||
| Cancer immunotherapy drugs are expensive |
|
|
CSCs = cancer stem cells; NGS = next-generation sequencing; QoL = quality of life; SOC = standard of care.
FUTURE TRENDS FOR CANCER IMMUNOTHERAPY
More Targeted Approaches to Enhance Efficacy and Reduce Toxicity
Work must still be done to establish immunotherapy as the standard of care for immune-sensitive tumors, to broaden its applicability across a variety of cancers, and to enhance its efficacy for a wider range of patients.1,11 The field of cancer immunotherapy is expected to advance rapidly in the coming years, moving away from cancer immunotherapies that broadly activate the immune system toward more targeted approaches that enhance efficacy and reduce toxicity.1,11 To achieve this, additional tumor antigens need to be defined as targets for cancer immunotherapies.11 The identification of additional prognostic and predictive biomarkers will also provide benefits in predicting and improving patient survival.11
These biomarkers will likely be identified through research that integrates conventional immunological approaches along with high-throughput genomic and proteonomic screening.1 The characterization of additional cancer genes and biomarkers using high-throughput technologies is already occurring as a result of national and international efforts by The Cancer Genome Atlas (funded by the National Cancer Institute and National Human Genome Research Institute) and the International Cancer Genome Consortium.5 Several publicly available databases have also been proposed to track the clinical significance and actionability of genetic abnormalities.5
Advancements in high-throughput technologies have made it possible to analyze the mutation antigen profile, genetic signature, and epigenetic modification of immune and tumor cells; the scope of antibody response; and the homing capacity, cytotoxic function, magnitude, and characterization of the T-cell receptors of T lymphocytes.21 These technologies will allow the customized identification of predictive genetic markers, immunologic signatures, and TSAs for individual patients, providing a personalized biomarker profile.21 This approach is expected to help patients avoid immune-related and other adverse events and to reduce treatment costs associated with lack of response.21
The replacement of potentially more-toxic and less-effective first-line chemotherapy treatments with relatively benign ICBs may be a future trend that improves efficacy and safety.16 In order for more cancer immunotherapies to be considered the standard of care and indicated as first-line treatment, they will have to demonstrate comparable or superior efficacy and reduced toxicity in head-to-head clinical trials versus current first-line agents.16 This will likely require targeting the correct biomarkers in the patient’s tumor microenvironment based on early immunogenetic analysis.
In an initial move in this direction, the FDA approved the ICB pembrolizumab in October 2016 as a first-line treatment in patients with metastatic NSCLC who have high PD-L1 expression (a tumor proportion score of more than 50%) and who do not have targetable EGFR or ALK mutations.34 This approval was based on clinical study data demonstrating that the efficacy and safety of pembrolizumab in such previously untreated patients were superior to investigator-chosen, standard platinum-containing chemotherapy doublets (pemetrexed, gemcitabine, or paclitaxel in combination with carboplatin or cisplatin).34
Personalized Drug Combination Therapies to Enhance Efficacy
The current approach of targeting single molecular abnormalities or cancer pathways is described by some experts as “reductionist” and unlikely to lead to a cancer cure.5 Drug combinations that target several molecular alterations or cancer pathways, similar to the strategy for human immuno deficiency virus treatment, might enhance the efficacy of cancer treatments. 5,11 Personalized drug combinations based on the specific biomarkers or pathways that drive the biology of each patient’s tumor are expected to be among the most promising strategies for the future.5,12
Recent insights regarding the immune regulation of cancer are expected to provide the basis for the development of more potent cancer immunotherapy combinations.4 Given the success of ICBs, these agents may constitute a basic component for drug combination strategies that may include multiple checkpoint inhibitors or other anticancer agents.5,11 However, toxicity would be expected to be a limiting factor with drug combination strategies, so the recognition and management of adverse events will be critical for treatment success.5
Immunoprevention Strategies to Prevent Cancer and Its Recurrence
Although the role of immunotherapy in the treatment of cancer is increasing rapidly, progress regarding its use in preventive strategies has been slower.14 Theoretically, immunoprevention has the potential to head off cancer (“primary prevention”) or its recurrence (“secondary prevention”) in patients with minimal residual disease.14 The use of immunotherapy for cancer prevention has largely been experimental.14 However, for melanoma, which is one of the more immunogenic tumor types, the FDA has approved IFN-alpha-2b as adjuvant immunotherapy for patients with a high risk of recurrence.14 To date, this is the only cancer immunotherapy that has been approved for this purpose.14
Cancer immunoprevention has gained increasing attention because vaccines against hepatitis B and human papilloma-virus have succeeded in preventing the cancers associated with these viruses.14 These vaccines are effective due to their ability to prevent viral infections, thereby eliminating their oncogenic potential.14 However, the development of nonviral cancers involves progressive genetic alterations that accumulate over time, driving the transition from normal tissue through premalignant transformation to the eventual development of a malignancy.14 This process provides a window of time for preventive intervention, especially for patients who are known to be at high risk.14
Ideally, preventive cancer vaccines would target antigens that are specific to cancer cells and essential for tumor survival, such as driver mutations, and would be available to a carefully selected high-risk population.14 Identification of a premalignant marker would allow preventive therapy to be initiated within an effective time frame.14 Current efforts in the field of cancer immunoprevention are primarily focused on identifying tumor antigens that are expressed on early cancers or premalignant lesions.13 These antigens could be incorporated into vaccines for treatment of patients with these malignancies, then administered at an earlier stage in high-risk populations for cancer prevention.13
Although there has been little progress to date in developing successful primary prevention strategies using vaccines, some secondary prevention vaccination strategies to prevent cancer recurrence after adjuvant treatment have shown promise.12,14 In one clinical trial, patients (n = 22) with stage 3 melanoma who had an unfavorable prognosis received a vaccine that consisted of bone marrow and peripheral blood mononuclear cell-derived dendritic cells loaded with melanoma-associated peptides (tyrosinase, Melan A/MART-1, gp100, MAGE-1, and/or MAGE-3) or autologous tumor cell lysate, and keyhole limpet hemocyanin.35 Although three-year disease-free survival (DFS) demonstrated a trend toward improvement compared to pair-matched historical controls (40.9% versus 14.5%, respectively), only three-year OS improved significantly (68.2% versus 25.7%).35
Several trials investigating the secondary prevention of breast cancer with vaccines have targeted the oncogenic antigen Her-2/NEU (HER2).14 Currently, trastuzumab, an HER2-specific monoclonal antibody, is the mainstay of adjuvant treatment for HER2-positive breast cancer.14 However, research has also identified a number of tumor antigens present in HER2-positive breast cancer that could also be included in a preventive vaccine.13 In a recent clinical trial that enrolled 106 patients with lymph-node-positive or high-risk lymph-node-negative breast cancer, a synthetic E75 peptide combined with granulocyte-macrophage colony-stimulating factor was administered after completion of surgery, radiation, and chemotherapy.36 A trend toward improvement in two-year DFS in the vaccinated group was found compared with 76 control patients (94.3% versus 86.8%, respectively; P = 0.08).36 Furthermore, there was no disease recurrence in the group of patients with tumors that had high HER2 expression who received the vaccine in addition to trastuzumab, compared with 20% in the group of patients receiving the vaccine alone, suggesting that these agents should be administered concomitantly.36 Other peptides are also being evaluated as vaccine targets for the secondary prevention of breast cancer, NSCLC, and ovarian cancer.14
The road to successful cancer prevention through immunization for nonvirally induced cancer is long. Successful strategies will require the development of high-throughput methods for antigen and biomarker identification, the development of proven vaccine constructs, and careful clinical trial design.14 Although data in support of cancer immunoprevention is accumulating, prevention of nonvirally induced cancers is in the very early stages of development; data have been evaluated from only a few clinical trials.13,14
CONCLUSION
During recent decades, our understanding of cancer immunology has advanced dramatically.1 Many obstacles still impede the success of cancer immunotherapies in a wider variety of malignancies and patients.1 However, the rapid progress that has led to the present era of cancer immunotherapy is expected to continue. Current obstacles will likely be surmounted through the implementation of available and potential solutions, including the development of more targeted cancer immunotherapies; personalized treatment with cancer immunotherapy drug combinations; cancer immunoprevention strategies; and additional important innovations.1,5,11,12,14
ABBREVIATIONS
- CSC
cancer stem cell
- CTA
cancer testis antigen
- CTLA-4
cytotoxic T-lymphocyte–associated protein 4
- ICB
immune checkpoint blocker
- IFN
interferon
- MM
metastatic melanoma
- NGS
next-generation sequencing
- NSCLC
non–small-cell lung cancer
- OS
overall survival
- PD-1
programmed death-1
- PD-L1
programmed death ligand-1
- PFS
progression-free survival
- RCC
renal cell carcinoma
- TSA
tumor-specific antigen
Despite dramatic breakthroughs, obstacles remain for the field of immunotherapy in cancer. These include the inability to predict treatment efficacy and patient response; the need for improved biomarkers; the development of resistance to immunotherapies; the lack of optimized clinical study designs; and high costs. Are solutions in sight?
Footnotes
This is the last in a series of three articles about cancer immunotherapy. The first article discussed cancer immunotherapy strategies and agents, and the second article discussed efficacy, safety, and other clinical considerations.
REFERENCES
- 1.Alatrash G, Jakher H, Stafford PD, Mittendorf EA. Cancer immuno therapies, their safety and toxicity. Expert Opin Drug Saf. 2013;12(5):631–645. doi: 10.1517/14740338.2013.795944. [DOI] [PubMed] [Google Scholar]
- 2.Chiriva-Internati M, Bot A. A new era in cancer immunotherapy: discovering novel targets and reprogramming the immune system. Int Rev Immunol. 2015;34(2):101–103. doi: 10.3109/08830185.2015.1015888. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoli D. Cancer and the immune system: basic concepts and targets for intervention. Semin Oncol. 2015;42(4):523–538. doi: 10.1053/j.seminoncol.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zugazagoitia J, Guedes C, Ponce S, et al. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551–1566. doi: 10.1016/j.clinthera.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Wayteck L, Breckpot K, Demeester J, et al. A personalized view on cancer immunotherapy. Cancer Lett. 2014;352(1):113–125. doi: 10.1016/j.canlet.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Adams KT. Cancer immunotherapies—and their cost—take center stage at ASCO’s 2015 annual meeting. Manag Care. 2015;24(7):30–32. [PubMed] [Google Scholar]
- 8.Tartari F, Santoni M, Burattini L, et al. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: recent insights. Cancer Treat Rev. 2016;48:20–24. doi: 10.1016/j.ctrv.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Grady D. Harnessing the immune system to fight cancer. The New York Times. Jul 30, 2016. [Accessed August 17, 2016]. Available at: www.nytimes.com/2016/07/31/health/harnessing-the-immune-system-to-fight-cancer.html.
- 10.National Cancer Institute. Checking in on cancer checkpoint inhibitors. Dec 18, 2015. [Accessed October 26, 2016]. Available at: www.cancer.gov/news-events/cancer-currents-blog/
- 11.Karlitepe A, Ozalp O, Avci CB. New approaches for cancer immunotherapy. Tumour Biol. 2015;36(6):4075–4078. doi: 10.1007/s13277-015-3491-2. [DOI] [PubMed] [Google Scholar]
- 12.Klener P, Jr, Otahal P, Lateckova L, Klener P. Immuno therapy approaches in cancer treatment. Curr Pharm Biotechnol. 2015;16(9):771–781. doi: 10.2174/1389201016666150619114554. [DOI] [PubMed] [Google Scholar]
- 13.Finn OJ, Beatty PL. Cancer immunoprevention. Curr Opin Immunol. 2016;39:52–58. doi: 10.1016/j.coi.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smit MA, Jaffee EM, Lutz ER. Cancer immunoprevention—the next frontier. Cancer Prev Res. 2014;7(11):1072–1080. doi: 10.1158/1940-6207.CAPR-14-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 16.West H. Nivolumab as first-line monotherapy for advanced non-small cell lung cancer; could we replace first-line chemotherapy with immunotherapy? Transl Lung Cancer Res. 2014;3(6):400–402. doi: 10.3978/j.issn.2218-6751.2014.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roychowdhury S, Chinnaiyan AM. Translating genomics for precision cancer medicine. Annu Rev Genomics Hum Genet. 2014;15:395–415. doi: 10.1146/annurev-genom-090413-025552. [DOI] [PubMed] [Google Scholar]
- 18.Arnedos M, Soria JC, André F, Tursz T. Personalized treatments of cancer patients: a reality in daily practice, a costly dream, or a shared vision of the future from the oncology community? Cancer Treat Rev. 2014;40:1192–1198. doi: 10.1016/j.ctrv.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 20.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J, Hegde PS, Clynes R, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4(3):1–26. doi: 10.1186/s40425-016-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagan J, Van Allen EM. Next-generation sequencing to guide cancer therapy. Genome Med. 2015;7(1):80. doi: 10.1186/s13073-015-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodon J, Saura C, Dienstmann R, et al. Molecular prescreening to select patient population in early clinical trials. Nat Rev Clin Oncol. 2012;9:359–366. doi: 10.1038/nrclinonc.2012.48. [DOI] [PubMed] [Google Scholar]
- 24.Hollebecque A, Massard C, Soria JC. Implementing precision medicine initiatives in the clinic: a new paradigm in drug development. Curr Opin Oncol. 2014;26:340–346. doi: 10.1097/CCO.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 25.Heuckmann JM, Thomas RK. A new generation of cancer genome diagnostics for routine clinical use: overcoming the roadblocks to personalized cancer medicine. Ann Oncol. 2015;26:1830–1837. doi: 10.1093/annonc/mdv184. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Fujimoto J, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346:256–259. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumors: learning from lung cancer. Nat Rev Clin Oncol. 2014;11:473–481. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 28.Weiner LM. Cancer immunology for the clinician. Clin Adv Hematol. 2015;13(5):299–306. [PubMed] [Google Scholar]
- 29.Hugo W, Shi H, Sun L, et al. Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell. 2015;162:1271–1285. doi: 10.1016/j.cell.2015.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Cancer Institute. Mutations linked to immunotherapy resistance. Aug 5, 2016. [Accessed March 10, 2017]. Available at: www.cancer.gov/news-events/cancer-currents-blog/2016/immunotherapy-resistance-melanoma.
- 32.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoos A, Britten C. The immunooncology framework: enabling a new era of cancer therapy. Oncoimmunology. 2012;1:334–339. doi: 10.4161/onci.19268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keytruda. Efficacy data: 1st line treatment. Merck & Company, Inc.; [Accessed March 6, 2017]. Available at: www.keytruda.com/hcp/nsclc/first-line-monotherapy. [Google Scholar]
- 35.Markowicz S, Nowecki ZI, Rutkowski P, et al. Adjuvant vaccination with melanoma antigen-pulsed dendritic cells in stage III melanoma patients. Med Oncol. 2012;29:2966–2977. doi: 10.1007/s12032-012-0168-1. [DOI] [PubMed] [Google Scholar]
- 36.Mittendorf EA, Clifton GT, Holmes JP, et al. Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: from U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2012;118:2594–2602. doi: 10.1002/cncr.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]