Abstract
Objectives:
Existing research comparing hospital length of stay for patients treated with non–vitamin K oral anticoagulants or parenteral bridging to warfarin has been conducted primarily with the agent rivaroxaban. The objective of this study was to compare hospital length of stay between patients initiated on the non–vitamin K oral anticoagulants, apixaban or rivaroxaban, and patients initiated on parenteral anticoagulation agents plus warfarin for the treatment of venous thromboembolism.
Methods:
A retrospective cohort study was conducted at an 859-bed, not-for-profit, teaching hospital. Adult patients admitted for a primary diagnosis of venous thromboembolism between 1 November 2012 and 31 August 2015 and treated with apixaban or rivaroxaban or a parenteral anticoagulant plus warfarin were included in the study. Eligible patients were identified using International Classification of Diseases, Ninth Revision codes for a primary diagnosis of acute thromboses and emboli and medication administration record data. Individuals using anticoagulation therapy prior to admission, released from the emergency department, or treated with thrombectomy or fibrinolytic therapy were excluded.
Results:
A total of 152 patients were included in this study. Patient characteristics, including renal function, were similar between study arms. Venous thromboembolism treatment with apixaban or rivaroxaban compared to a parenteral anticoagulant plus warfarin was associated with a reduced hospital length of stay (2.63 vs 5.33 days; p < 0.05) and decreased total hospital cost adjusted to 2015 dollars (US$21,694 vs US$38,851; p = 0.013).
Conclusion:
These results suggest that treatment with a non–vitamin K anticoagulant may significantly reduce hospital length of stay and total hospital cost compared to a parenteral anticoagulant plus warfarin for patients admitted for venous thromboembolism.
Keywords: Adult medicine, anticoagulants, anticoagulation, cardiology, clinical practice, cost benefit, disease management, hematology, warfarin
Background
Anticoagulation therapy with the non–vitamin K oral anticoagulants (NOACs) is now recommended as the preferred treatment for venous thromboembolism (VTE) per the 2016 CHEST guidelines.1 Compared to the previously recommended first-line combination therapy of a parenteral anticoagulant and a vitamin K antagonist, the NOACs have many potential benefits including quicker onsets of action, shorter half-lives, and fewer food and drug interactions.2–7 Two of the NOACs, apixaban and rivaroxaban, were Food and Drug Administration (FDA)-approved for the treatment of VTE without the use of parenteral bridging, and none of the NOACs require therapeutic drug monitoring throughout the course of therapy.4–8 In addition, the NOACs have also been shown to reduce hospital length of stay (LOS).9–24
Early studies examining the impact of the NOACs on hospital LOS were primarily conducted in patients with nonvalvular atrial fibrillation.9–12 In recent years, additional studies have evaluated the impact of the NOACs on hospital LOS in patients with VTE.13–24 Among the initial studies conducted in the setting of VTE treatment is a post hoc analysis of data from the EINSTEIN-DVT and EINSTEIN-PE trials which demonstrated an association between the use of rivaroxaban for VTE treatment and reduction in hospital LOS.13 Specifically, the authors found a 3-day reduction in LOS for patients with deep vein thrombosis (DVT) (p < 0.0001) and a 1-day reduction in LOS for patients with pulmonary embolism (PE) (p < 0.0001) with rivaroxaban compared to enoxaparin plus a vitamin K antagonist.13
A limitation of the existing literature evaluating hospital LOS in the setting of VTE treatment is that the majority of these studies have only compared rivaroxaban with traditional parenteral bridging methods.13–23 It is still unclear whether the other NOACs consistently demonstrate similar reductions in hospital LOS for VTE patients. Specifically, the authors identified only one study evaluating the impact of apixaban and no studies evaluating dabigatran or edoxaban in this patient population.24 As rivaroxaban and apixaban are the two most commonly utilized NOACs in practice, additional studies would provide valuable information for practitioners about whether apixaban shares similar benefits as rivaroxaban with regard to LOS in VTE patients. This study compared hospital LOS for patients treated with apixaban or rivaroxaban versus parenteral bridging plus warfarin for VTE.
Methods
Patient selection and study design
This retrospective cohort study was conducted at a single, 859-bed, not-for-profit, academic hospital in the United States. The protocol was reviewed and approved by all affiliated institutional review boards.
Eligible patients were identified using an Epic® informatics report. The report criteria included dates between November 2012 and August 2015, International Classification of Diseases, Ninth Revision (ICD-9) codes for acute thromboses and emboli (415.1, 415.11, 415.13, 415.19, 453.4, 453.40, 453.41, 453.42, 453.8, 453.89, 453.9), and medication administration record data showing administration of at least one dose of any of the following medications: apixaban, enoxaparin, fondaparinux, rivaroxaban, warfarin, or unfractionated heparin. The resulting report listed patients who were admitted to the study site between November 2012 and August 2015 for a primary diagnosis of VTE and received at least one dose of therapeutic anticoagulation.
Adults between 18 and 89 years of age who were admitted for a primary diagnosis of VTE and received VTE-approved treatment doses of apixaban or rivaroxaban as monotherapy or a combination of a parenteral agent (enoxaparin, fondaparinux, or unfractionated heparin) and warfarin were included in the study. Individuals who were already prescribed anticoagulation therapy prior to admission, pregnant, actively bleeding, had a creatinine clearance less than 30 mL/min, or had a contraindication to any of the study medications were excluded. Individuals who received treatment with fibrinolytic therapy, thrombectomy, or a vena cava filter were also excluded. Individuals who received less than 48 h of parenteral therapy prior to transitioning to an NOAC were included in their respective NOAC study groups, whereas individuals who received greater than 48 h of parenteral therapy prior to transitioning to an NOAC were excluded from the study.
To limit inter-investigator variability during data collection, the research protocol included pre-specified definitions for identifying the appropriate data points within the patient’s electronic medical record.
Outcomes
The primary outcome was the difference in hospital LOS in days between patients with VTE who were treated with the NOACs apixaban or rivaroxaban versus parenteral bridging plus warfarin. Secondary outcomes were the results of the primary outcome stratified by primary admission diagnosis of DVT alone, PE alone, or DVT and PE; the difference in total hospital cost adjusted to 2015 dollars; and the difference in the Pulmonary Embolism Severity Index (PESI) score among patients diagnosed with PE.
For the purpose of this study, the total hospital cost is a measure of the cumulative charges accrued during a patient’s hospitalization and billed by the hospital to the patient’s insurance or to the patient following hospital discharge. Itemized treatment costs and actual reimbursement values for the study population were not available to the investigators.
Statistical analysis
The sample size estimate was based on results from a post hoc analysis of the EINSTEIN trials which showed an average 2-day reduction in hospital LOS for patients treated with rivaroxaban versus enoxaparin plus a vitamin K antagonist.9 We estimated that an enrollment of 78 patients per study group would be needed to provide a power of 80% at a two-sided significance level of 0.05. Study results were analyzed via Microsoft Excel. Specifically, the student’s t test and analysis of variance (ANOVA) were used for continuous data, and chi-square and Fisher’s exact tests were used for nominal data.
Results
Patient characteristics
Of the 699 patients who were screened for study inclusion, 152 patients were included in the study with 74 patients assigned to the bridging study group and 78 patients assigned to the NOAC study group (77 received rivaroxaban and 1 received apixaban). The baseline characteristics of these study groups are summarized in Table 1. These characteristics were similar between study groups, with the exception of the mean age, heart failure history, mean diastolic blood pressure, and mean weight. The majority of patients included in the study were admitted for a primary diagnosis of PE (68%). Smoking status and histories of cancer and thrombophilic conditions were similar between groups.
Table 1.
Baseline characteristics.
| Characteristic | Bridging group (N = 74) | NOAC group (N = 78) | p value |
|---|---|---|---|
| Mean age (years) | 64 | 57 | 0.009 |
| Male (n (%)) | 35 (47) | 36 (46) | 0.784 |
| Race | 0.431 | ||
| Caucasian (n (%)) | 65 (87.8) | 65 (83.3) | |
| African American (n (%)) | 7 (9.5) | 12 (15.4) | |
| Other (n (%)) | 2 (2.7) | 1 (1.3) | |
| Comorbidities | |||
| Cancer (n (%)) | 24 (32.4) | 20 (25.6) | 0.344 |
| Chronic lung disease (n (%)) | 14 (18.9) | 11 (14.1) | 0.412 |
| Thrombophilic condition (n (%)) | 5 (6.8) | 6 (7.7) | 0.809 |
| Heart failure (n (%)) | 14 (18.9) | 5 (6.4) | 0.018 |
| Smoking history | 0.535 | ||
| Never (n (%)) | 41 (55.4) | 47 (60.3) | |
| Former (n (%)) | 24 (32.4) | 19 (24.4) | |
| Current (n (%)) | 9 (12.2) | 12 (15.4) | |
| Primary diagnosis | 0.566 | ||
| DVT (n (%)) | 18 (24.3) | 14 (17.9) | |
| PE (n (%)) | 49 (66.2) | 55 (70.5) | |
| DVT + PE (n (%)) | 7 (9.5) | 9 (11.5) | |
| Baseline vitals (mean) | |||
| Weight (kg) | 87 | 94 | 0.049 |
| Height (in) | 67 | 69 | 0.076 |
| BMI (kg/m2) | 29.55 | 31.29 | 0.159 |
| SBP (mm Hg) | 133 | 139 | 0.081 |
| DBP (mm Hg) | 78 | 84 | 0.043 |
| Heart rate (beats/min) | 87 | 90 | 0.360 |
| Temperature (°C) | 37.5 | 36.8 | 0.340 |
| Respiratory rate (breaths/min) | 19 | 19 | 0.744 |
| Altered mental status (n (%)) | 1 (1.4) | 2 (2.6) | 0.585 |
| Oxygen saturation (%) | 96 | 96 | 0.618 |
| Baseline labs (mean) | |||
| Hemoglobin (g/dL) | 12.8 | 13.2 | 0.151 |
| Hematocrit (%) | 38.5 | 39.9 | 0.081 |
| Platelet count (K cells/mm3) | 225 | 231 | 0.657 |
| Serum creatinine (mg/dL) | 0.97 | 0.93 | 0.237 |
| Albumin (g/dL) | 3.9 | 4.0 | 0.227 |
| PT (s) | 14.1 | 14.2 | 0.632 |
| INR | 1.1 | 1.1 | 0.314 |
| aPTT (s) | 34.8 | 36.7 | 0.751 |
NOAC: non–vitamin K anticoagulant; DVT: deep vein thrombosis; PE: pulmonary embolism; PT: prothrombin time; INR: international normalized ratio; aPTT: activated partial thromboplastin time; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index.
Outcomes and analysis
The mean hospital LOS was 2.63 days in the NOAC group and 5.33 days in the bridging group (difference: 2.7 days; p < 0.05). Figure 1 illustrates the results of the primary outcome. In addition to a significant reduction in hospital LOS, treatment of VTE with NOACs was also associated with a significant reduction in total hospital cost adjusted to 2015 dollars (US$21,694 vs US$38,851; p = 0.013).
Figure 1.
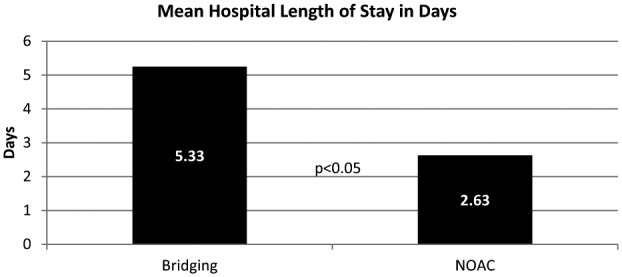
Result of primary outcome.
When the results were stratified according to the primary admission diagnoses of DVT, PE, and DVT with PE, there was no significant difference in hospital LOS between treatment groups for patients admitted with the primary diagnoses of DVT (p = 0.159) or DVT with PE (p = 0.155). There was, however, a significant difference in hospital LOS for patients treated with NOACs (2.63 days) and bridging (6 days) for the primary admission diagnoses of PE (difference: 3.37 days; p < 0.05). Further analysis of the mean PESI score between groups revealed a difference between patients assigned to VTE treatment with NOACs versus bridging (76 vs 87; p = 0.040). Table 2 outlines the results of secondary outcomes.
Table 2.
Results of secondary outcomes.
| Secondary outcome | Bridging group (N = 74) | NOAC group (N = 78) | p value |
|---|---|---|---|
| Hospital LOS in patients admitted for DVT (mean days) | 3.71 | 2.63 | 0.159 |
| Hospital LOS in patients admitted for PE (mean days) | 6 | 2.63 | < 0.05 |
| Hospital LOS in patients admitted for DVT plus PE (mean days) | 4.58 | 2.75 | 0.155 |
| Cost of hospitalization, mean amount in 2015 US dollars | US$38,851 | US$21,694 | 0.013 |
| PESI score in patients with PE (mean score) | 87 | 76 | 0.040 |
NOAC: non–vitamin K anticoagulant; LOS: length of stay; DVT: deep vein thrombosis; PE: pulmonary embolism; PESI: Pulmonary Embolism Severity Index.
Discussion
This retrospective cohort study involving 152 patients with VTE illustrated that treatment with the NOACs, apixaban and rivaroxaban, versus traditional bridging methods was associated with a reduced hospital LOS and total cost of hospitalization. These results were consistent with previously published studies which found that VTE treatment with rivaroxaban was associated with a reduction in hospital LOS between 1 and 4 days.13–23
Because existing literature of hospital LOS in VTE has primarily evaluated rivaroxaban, we sought to investigate the impact of the other NOACs on LOS in the setting of VTE. We opted to investigate apixaban and rivaroxaban because these are the only two available NOACs that do not require a period of parenteral therapy prior to the initiation of the oral agent and are the two most commonly used NOACs for VTE treatment. While we had hoped this study would provide additional information about the impact of apixaban on hospital LOS, there was only one patient who received apixaban included in our study. This is partially due to the fact that our pre-specified study inclusion criteria limited study enrollment to patients who were admitted to our institution between November 2012 and August 2015, and apixaban did not receive an FDA-labeled indication for VTE treatment until 2014. Following this initial approval and the subsequent inclusion of apixaban on our hospital formulary, gradual physician acceptance based on limited safety data likely affected the utilization of apixaban within our facility during the time frame set for patient enrollment. Use of apixaban for the treatment of VTE is of particular interest in the setting of renal disease. The package insert for rivaroxaban recommends to avoid use for VTE treatment in patients with a creatinine clearance less than 30 mL/min, whereas the package insert for apixaban does not recommend against use for VTE treatment in patients with renal impairment but notes that such patients were excluded from clinical trials.4,5 For these reasons, the lack of data for apixaban can be considered a limitation of this study, and additional research is still necessary to determine the impact of apixaban on hospital LOS and in the setting of renal dysfunction.
While the average wholesale costs of apixaban and rivaroxaban are greater than that of enoxaparin and warfarin, it has been suggested that the lack of laboratory monitoring could lead to significant reductions in indirect treatment costs for patients prescribed these agents. Examples of indirect costs that need to be considered include point-of-care international normalized ratio (INR) levels, venipuncture laboratory draws, anticoagulation clinic visits, provider telephone calls, and hospital readmissions related to adverse effects or treatment failures. Additional research is needed to quantify these indirect treatment costs and to compare these costs with the wholesale costs of the NOACs.
There was a significant difference in hospital LOS between treatment groups for patients who were admitted with a primary diagnosis of PE alone, but not for DVT alone or DVT and PE, possibly due to small sample size. A comparison of the mean PESI score between groups revealed that the bridging group had a higher mean PESI score than the NOAC group. The mean PESI score of 87 found in the bridging group is associated with an intermediate risk of mortality or severe morbidity, whereas the mean PESI score of 76 in the NOAC group is associated with a low risk of mortality or severe morbidity. It is important to recognize that in addition to the selected treatment agents, the lower severity of disease found in patients treated with an NOAC may have positively impacted the benefit found in this group with regard to total hospital LOS due to the recommendation for outpatient management of patients with low-risk PESI scores.
Limitations of this study include the retrospective design, incomplete documentation in the patient charts, the potential for inter-investigator variability in data collection methods, and the potential for confounding factors that can impact hospital LOS. Examples of confounding factors that could prolong a patient’s hospital LOS include the presence of multiple comorbidities and discharge delays with difficulty securing placement for patients at skilled nursing facilities or long-term care centers. An additional limitation of this study is the inability to quantify prevented hospital admissions for patients who were not admitted to the hospital for treatment but were instead discharged directly from the emergency department with a prescription for outpatient treatment. Patients discharged directly from the emergency department were excluded from the study, and the authors identified a total of 28 patients that met this exclusion criterion.
Conclusion
Our study results suggest that the use of an NOAC, specifically rivaroxaban, for the treatment of VTE is associated with reduced hospital LOS and total cost of hospitalization. This study further supports the aforementioned trials showing benefit with rivaroxaban and apixaban with regard to hospital LOS as well as the latest clinical practice guidelines which recommend the NOACs as efficacious first-line agents for the treatment of VTE.1
Acknowledgments
This research has been previously presented as a poster/poster abstract at the American College of Clinical Pharmacy (ACCP) annual meeting in October 2016. The poster abstract was subsequently published in Pharmacotherapy (Volume 36, Number 12, 2016).
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Z.A. Stacy is a consultant and speaker for Janssen Pharmaceuticals, Inc. Other authors declared no potential conflicts of interest.
Ethical approval: Ethical approval for this study was obtained from Mercy Hospital St. Louis IRB (828890-1).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The publication of this article was supported by St. Louis College of Pharmacy.
References
- 1. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149(2): 315–352. [DOI] [PubMed] [Google Scholar]
- 2. Guyatt GH, Akl EA, Crowther M, et al. Antithrombotic therapy and prevention of thrombosis. 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141(2 Suppl): 7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lexi-Comp Online. Lexi-drugs. Hudson, OH: Lexi-Comp, http://online.lexi.com.ezproxy.ttuhsc.edu/lco/action/home/switch (accessed 21 December 2016). [Google Scholar]
- 4. Janssen Pharmaceuticals. Xarelto (package insert). Raritan, NJ: Janssen Pharmaceuticals, 2011. [Google Scholar]
- 5. Bristol-Myers Squibb Company. Eliquis (package insert). New York: Bristol-Myers Squibb Company, 2015. [Google Scholar]
- 6. Boehringer Ingelheim Pharmaceuticals. Pradaxa (package insert). Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, 2015. [Google Scholar]
- 7. Daiichi Sankya Co., Ltd. Savaysa (package insert). Tokyo, Japan: Daiichi Sankya Co., Ltd, 2015. [Google Scholar]
- 8. Merli GJ, Hollander JE, Lefebvre P, et al. Rates of hospitalization among patients with deep vein thrombosis before and after the introduction of rivaroxaban. Hosp Pract 2015; 43(2): 86–93. [DOI] [PubMed] [Google Scholar]
- 9. Laliberté F, Pilon D, Raut MK, et al. Hospital length of stay: is rivaroxaban associated with shorter inpatient stay compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin 2014; 30(4): 645–653. [DOI] [PubMed] [Google Scholar]
- 10. Laliberté F, Cloutier M, Crivera C, et al. Effects of rivaroxaban versus warfarin on hospitalization days and other health care resource utilization in patients with nonvalvular atrial fibrillation: an observational study from a cohort of matched users. Clin Ther 2015; 37(3): 554–562. [DOI] [PubMed] [Google Scholar]
- 11. Farr AM, Jing Y, Johnston S, et al. Comparison of hospital length of stay between hospitalized non-valvular atrial fibrillation patients treated with either apixaban or warfarin. Hosp Pract 2015; 43(3): 172–179. [DOI] [PubMed] [Google Scholar]
- 12. Xie L, Vo L, Keshishian A, et al. Comparison of hospital length of stay and hospitalization costs among patients with non-valvular atrial fibrillation treated with apixaban or warfarin: an early view. J Med Econ 2016; 19(8): 769–776. [DOI] [PubMed] [Google Scholar]
- 13. Bookhart BK, Haskell L, Bamber L, et al. Length of stay and economic consequences with rivaroxaban vs enoxaparin/vitamin K antagonist in patients with DVT and PE: findings from the North American EINSTEIN clinical trial program. J Med Econ 2014; 17(10): 691–695. [DOI] [PubMed] [Google Scholar]
- 14. Van Bellen B, Bamber L, Correa de Carvalho F, et al. Reduction in the length of stay with rivaroxaban as a single-drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Curr Med Res Opin 2014; 30(5): 829–837. [DOI] [PubMed] [Google Scholar]
- 15. Matsuo H, Prins M, Lensing AW, et al. Shortened length of hospital stay with rivaroxaban in patients with symptomatic venous thromboembolism in Japan: the J-EINSTEIN pulmonary embolism and deep vein thrombosis program. Curr Med Res Opin 2015; 31(6): 1057–1061. [DOI] [PubMed] [Google Scholar]
- 16. Roberts KM, Knight TB, Padilla-Tolentino E, et al. Length of stay comparison between rivaroxaban and warfarin in the treatment of pulmonary embolism: results from a real-world observational cohort study. Thrombosis 2015; 2015: 414523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desai A, Desai A, Calixte R, et al. Comparing length of stay between patients taking rivaroxaban and conventional anticoagulants for treatment of venous thromboembolism. Lung 2016; 194(4): 605–611. [DOI] [PubMed] [Google Scholar]
- 18. Coleman CI, Fermann GJ, Weeda ER, et al. Is rivaroxaban associated with shorter hospital stays and reduced costs versus parenteral bridging to warfarin among patients with pulmonary embolism? Clin Appl Thromb Hemost. Epub ahead of print 1 January 2016. DOI: 10.1177/1076029616661415. [DOI] [PubMed] [Google Scholar]
- 19. Weeda ER, Kohn CG, Peacock WF, et al. Rivaroxaban versus heparin bridging to warfarin therapy: impact on hospital length of stay and treatment costs for low-risk patients with pulmonary embolism. Pharmacotherapy 2016; 36(10): 1109–1115. [DOI] [PubMed] [Google Scholar]
- 20. Margolis JM, Deitelzweig S, Kline J, et al. Pulmonary embolism inpatients treated with rivaroxaban had shorter hospital stays and lower costs compared with warfarin. Clin Ther 2016; 38(11): 2496–2503. [DOI] [PubMed] [Google Scholar]
- 21. Weeda ER, Wells PS, Peacock WF, et al. Hospital length-of-stay and costs among pulmonary embolism patients treated with rivaroxaban versus parenteral bridging to warfarin. Intern Emerg Med 2017; 12: 311–318. [DOI] [PubMed] [Google Scholar]
- 22. Margolis JM, Deitelzweig S, Kline J, et al. Shorter hospital stays and lower costs for rivaroxaban compared with warfarin for venous thrombosis admissions. J Am Heart Assoc 2016; 5(10): e003788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paczyńska M, Kurnicka K, Lichodziejewska B, et al. Acute pulmonary embolism treatment with rivaroxaban results in a shorter duration of hospitalisation compared to standard therapy: an academic centre experience. Kardiol Pol 2016; 74(7): 650–656. [DOI] [PubMed] [Google Scholar]
- 24. Liu X, Johnson M, Mardekian J, et al. Apixaban reduces hospitalizations in patients with venous thromboembolism: an analysis of the apixaban for the initial management of pulmonary embolism and deep-vein thrombosis as first-line therapy (AMPLIFY) trial. J Am Heart Assoc 2015; 4(12): e002340. [DOI] [PMC free article] [PubMed] [Google Scholar]


