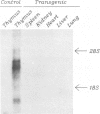
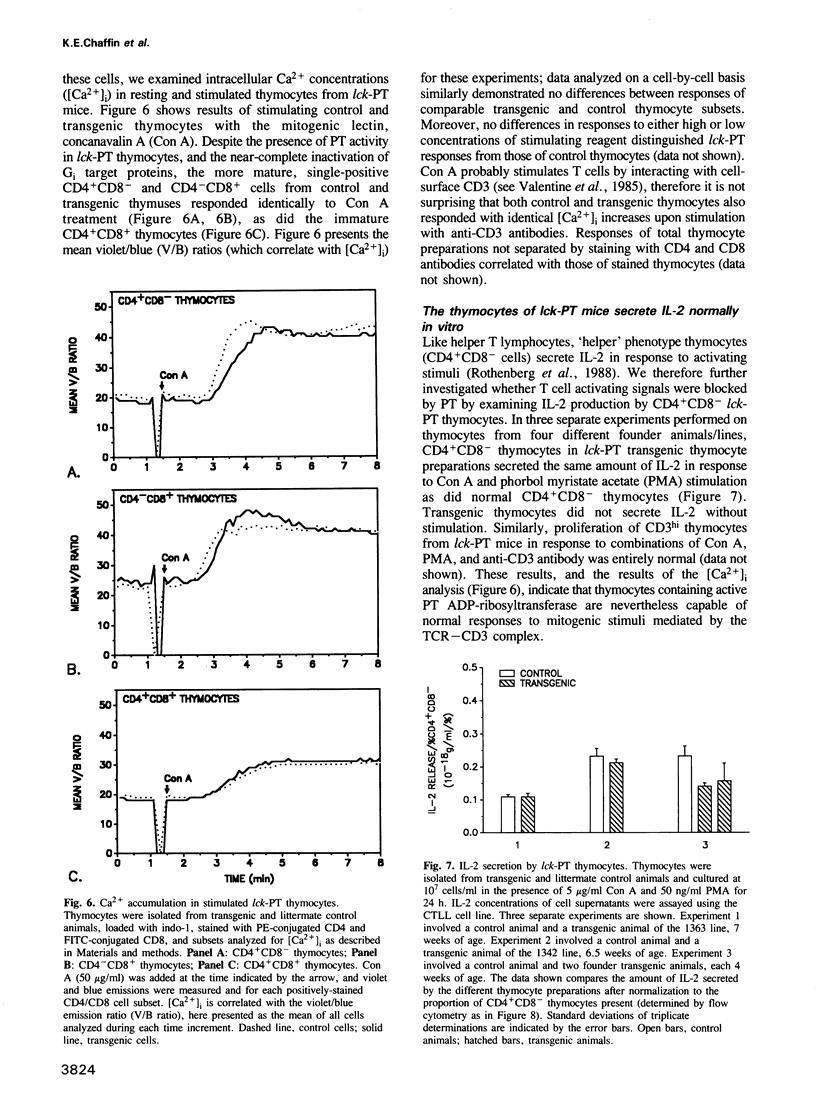
Abstract
Stimulation of the T lymphocyte antigen receptor-CD3 complex (TCR-CD3) causes T cell activation by a process associated with increased phosphatidylinositol-specific phospholipase C (PI-PLC) activity. Evidence exists suggesting that GTP-binding (G) proteins, particularly the pertussis toxin (PT)-sensitive Gi proteins, participate in this signal transduction pathway. To clarify the role of Gi proteins in TCR-CD3 signaling, and to investigate other possible functions of Gi molecules in T cells, we expressed the S1 subunit of PT in the thymocytes of transgenic mice using the lymphocyte-specific lck promoter. Transgenic thymocytes contained S1 activity and exhibited profound depletion of Gi protein PT substrates in a manner suggesting their inactivation by S1 in vivo. Nevertheless, treatment of transgenic thymocytes with mitogenic stimuli provoked normal increases in intracellular free Ca2+ concentrations and IL-2 secretion, indicating that Gi proteins are not required for T cell activation. These normal signaling responses notwithstanding, mature thymocytes accumulated in lck-PT mice and did not appear in secondary lymphoid organs or in the circulation. Viewed in the context of the known features of Bordetella pertussis infection, our results suggest that a PT-sensitive signaling process, probably involving Gi proteins, regulates thymocyte emigration.
Full text
PDF

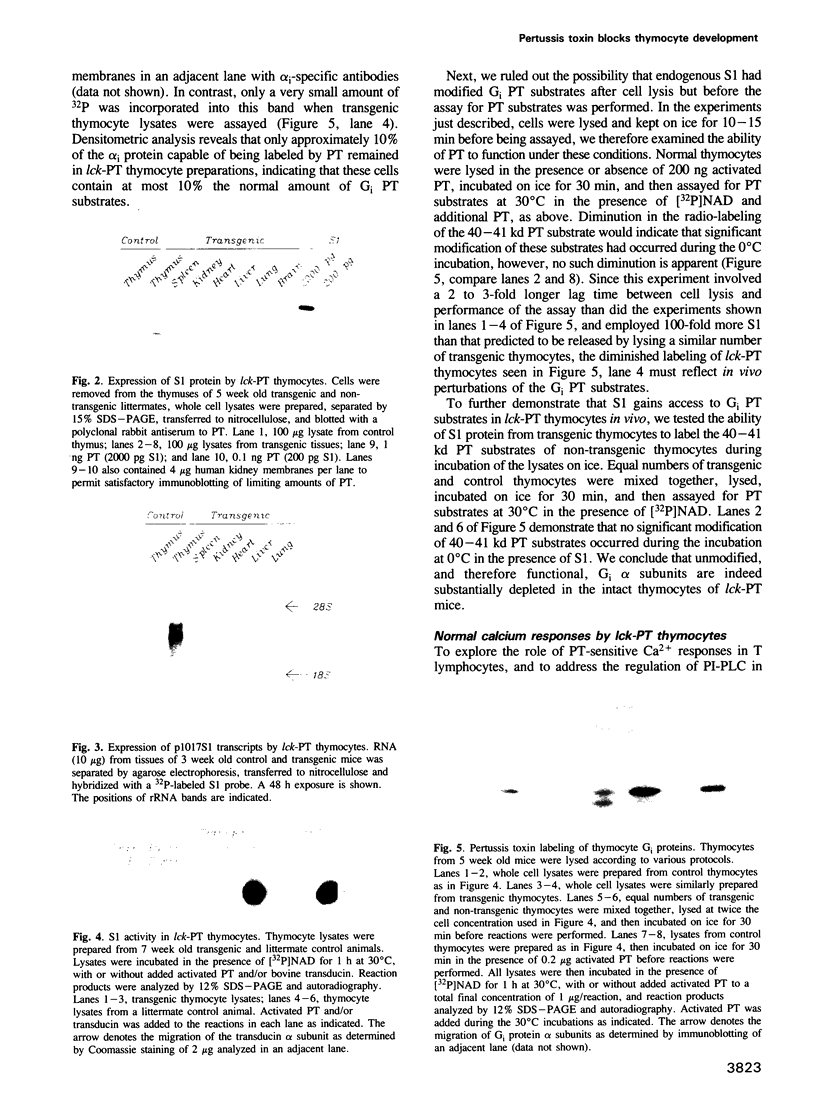




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Aussel C., Mary D., Peyron J. F., Pelassy C., Ferrua B., Fehlmann M. Inhibition and activation of interleukin 2 synthesis by direct modification of guanosine triphosphate-binding proteins. J Immunol. 1988 Jan 1;140(1):215–220. [PubMed] [Google Scholar]
- Backlund P. S., Jr, Meade B. D., Manclark C. R., Cantoni G. L., Aksamit R. R. Pertussis toxin inhibition of chemotaxis and the ADP-ribosylation of a membrane protein in a human-mouse hybrid cell line. Proc Natl Acad Sci U S A. 1985 May;82(9):2637–2641. doi: 10.1073/pnas.82.9.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri J. T., Rappuoli R., Collier R. J. Expression of the S-1 catalytic subunit of pertussis toxin in Escherichia coli. Infect Immun. 1987 May;55(5):1321–1323. doi: 10.1128/iai.55.5.1321-1323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals C. R., Wilson C. B., Perlmutter R. M. A small multigene family encodes Gi signal-transduction proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7886–7890. doi: 10.1073/pnas.84.22.7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales R., Eastman J., Kaplan J. Quantitation of circulating T and B lymphocytes in children with whooping cough. Pediatr Res. 1976 Dec;10(12):965–967. doi: 10.1203/00006450-197612000-00003. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Black W. J., Munoz J. J., Peacock M. G., Schad P. A., Cowell J. L., Burchall J. J., Lim M., Kent A., Steinman L., Falkow S. ADP-ribosyltransferase activity of pertussis toxin and immunomodulation by Bordetella pertussis. Science. 1988 Apr 29;240(4852):656–659. doi: 10.1126/science.2896387. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Burns D. L., Hausman S. Z., Lindner W., Robey F. A., Manclark C. R. Structural characterization of pertussis toxin A subunit. J Biol Chem. 1987 Dec 25;262(36):17677–17682. [PubMed] [Google Scholar]
- Buss J. E., Mumby S. M., Casey P. J., Gilman A. G., Sefton B. M. Myristoylated alpha subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Crispe I. N., Bevan M. J. Expression and functional significance of the J11d marker on mouse thymocytes. J Immunol. 1987 Apr 1;138(7):2013–2018. [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Wallace M. A., Wojcikiewicz R. J. Evidence for involvement of guanine nucleotide-binding regulatory proteins in the activation of phospholipases by hormones. FASEB J. 1988 Jul;2(10):2569–2574. doi: 10.1096/fasebj.2.10.2838362. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Garvin A. M., Abraham K. M., Forbush K. A., Farr A. G., Davison B. L., Perlmutter R. M. Disruption of thymocyte development and lymphomagenesis induced by SV40 T-antigen. Int Immunol. 1990;2(2):173–180. doi: 10.1093/intimm/2.2.173. [DOI] [PubMed] [Google Scholar]
- Garvin A. M., Pawar S., Marth J. D., Perlmutter R. M. Structure of the murine lck gene and its rearrangement in a murine lymphoma cell line. Mol Cell Biol. 1988 Aug;8(8):3058–3064. doi: 10.1128/mcb.8.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gray L. S., Huber K. S., Gray M. C., Hewlett E. L., Engelhard V. H. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J Immunol. 1989 Mar 1;142(5):1631–1638. [PubMed] [Google Scholar]
- Havran W. L., Poenie M., Kimura J., Tsien R., Weiss A., Allison J. P. Expression and function of the CD3-antigen receptor on murine CD4+8+ thymocytes. Nature. 1987 Nov 12;330(6144):170–173. doi: 10.1038/330170a0. [DOI] [PubMed] [Google Scholar]
- Huang K., Im S. Y., Samlowski W. E., Daynes R. A. Molecular mechanisms of lymphocyte extravasation. III. The loss of lymphocyte extravasation potential induced by pertussis toxin is not mediated via the activation of protein kinase C. J Immunol. 1989 Jul 1;143(1):229–238. [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Kim S. Y., Ang S. L., Bloch D. B., Bloch K. D., Kawahara Y., Tolman C., Lee R., Seidman J. G., Neer E. J. Identification of cDNA encoding an additional alpha subunit of a human GTP-binding protein: expression of three alpha i subtypes in human tissues and cell lines. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4153–4157. doi: 10.1073/pnas.85.12.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linch D. C., Wallace D. L., O'Flynn K. Signal transduction in human T lymphocytes. Immunol Rev. 1987 Feb;95:137–159. doi: 10.1111/j.1600-065x.1987.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Locht C., Cieplak W., Marchitto K. S., Sato H., Keith J. M. Activities of complete and truncated forms of pertussis toxin subunits S1 and S2 synthesized by Escherichia coli. Infect Immun. 1987 Nov;55(11):2546–2553. doi: 10.1128/iai.55.11.2546-2553.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORSE S. I. STUDIES ON THE LYMPHOCYTOSIS INDUCED IN MICE BY BORDETELLA PERTUSSIS. J Exp Med. 1965 Jan 1;121:49–68. doi: 10.1084/jem.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchitto K. S., Munoz J. J., Keith J. M. Detection of subunits of pertussis toxin in Tn5-induced Bordetella mutants deficient in toxin biological activity. Infect Immun. 1987 May;55(5):1309–1313. doi: 10.1128/iai.55.5.1309-1313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Lewis D. B., Wilson C. B., Gearn M. E., Krebs E. G., Perlmutter R. M. Regulation of pp56lck during T-cell activation: functional implications for the src-like protein tyrosine kinases. EMBO J. 1987 Sep;6(9):2727–2734. doi: 10.1002/j.1460-2075.1987.tb02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Moriarty T. M., Padrell E., Carty D. J., Omri G., Landau E. M., Iyengar R. Go protein as signal transducer in the pertussis toxin-sensitive phosphatidylinositol pathway. Nature. 1990 Jan 4;343(6253):79–82. doi: 10.1038/343079a0. [DOI] [PubMed] [Google Scholar]
- Morris C., Ling L. State of the art: poison centers in Minnesota. Minn Med. 1988 Nov;71(11):698-9, 706. [PubMed] [Google Scholar]
- Morse S. I., Barron B. A. Studies on the leukocytosis and lymphocytosis induced by Bordetella pertussis. 3. The distribution of transfused lymphocytes in pertussis-treated and normal mice. J Exp Med. 1970 Oct 1;132(4):663–672. doi: 10.1084/jem.132.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. I., Riester S. K. Studies on the leukocytosis and lymphocytosis induced by Bordetella pertussis. I. Radioautographic analysis of the circulating cells in mice undergoing pertussis-induced hyperleukocytosis. J Exp Med. 1967 Mar 1;125(3):401–408. doi: 10.1084/jem.125.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Bartoloni A., Perugini M., Rappuoli R. Expression and immunological properties of the five subunits of pertussis toxin. Infect Immun. 1987 Apr;55(4):963–967. doi: 10.1128/iai.55.4.963-967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J. J., Urdahl K. B., Luong H. T., Chused T. M., Samelson L. E., Klausner R. D. Aluminum fluoride induces phosphatidylinositol turnover, elevation of cytoplasmic free calcium, and phosphorylation of the T cell antigen receptor in murine T cells. J Immunol. 1987 Nov 15;139(10):3463–3469. [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Reichert R. A., Weissman I. L., Butcher E. C. Phenotypic analysis of thymocytes that express homing receptors for peripheral lymph nodes. J Immunol. 1986 May 15;136(10):3521–3528. [PubMed] [Google Scholar]
- Rothenberg E. V., McGuire K. L., Boyer P. D. Molecular indices of functional competence in developing T cells. Immunol Rev. 1988 Aug;104:29–53. doi: 10.1111/j.1600-065x.1988.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Runeberg-Nyman K., Engström O., Löfdahl S., Ylöstalo S., Sarvas M. Expression and secretion of pertussis toxin subunit S1 in Bacillus subtilis. Microb Pathog. 1987 Dec;3(6):461–468. doi: 10.1016/0882-4010(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Sartor O., Gregory F. S., Templeton N. S., Pawar S., Perlmutter R. M., Rosen N. Selective expression of alternative lck mRNAs in human malignant cell lines. Mol Cell Biol. 1989 Jul;9(7):2983–2988. doi: 10.1128/mcb.9.7.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrezenmeier H., Ahnert-Hilger G., Fleischer B. Inactivation of a T cell receptor-associated GTP-binding protein by antibody-induced modulation of the T cell receptor/CD3 complex. J Exp Med. 1988 Aug 1;168(2):817–822. doi: 10.1084/jem.168.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H. The human growth hormone gene family: nucleotide sequences show recent divergence and predict a new polypeptide hormone. DNA. 1982;1(3):239–249. doi: 10.1089/dna.1.1982.1.239. [DOI] [PubMed] [Google Scholar]
- Simpson L. L., Stiles B. G., Zepeda H., Wilkins T. D. Production by Clostridium spiroforme of an iotalike toxin that possesses mono(ADP-ribosyl)transferase activity: identification of a novel class of ADP-ribosyltransferases. Infect Immun. 1989 Jan;57(1):255–261. doi: 10.1128/iai.57.1.255-261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Braaten B. A., Daynes R. A. Molecular mechanisms of lymphocyte extravasation. I. Studies of two selective inhibitors of lymphocyte recirculation. J Immunol. 1984 Jan;132(1):354–362. [PubMed] [Google Scholar]
- Stanley J. B., Gorczynski R. M., Delovitch T. L., Mills G. B. IL-2 secretion is pertussis toxin sensitive in a T lymphocyte hybridoma. J Immunol. 1989 May 15;142(10):3546–3552. [PubMed] [Google Scholar]
- Sugimoto M., Nakanishi Y., Otokawa M., Uchida N., Yasuda T., Sato H., Sato Y. Effect of Bordetella pertussis leukocytosis (lymphocytosis)-promoting factor (LPF) on the physical lymphoepithelial-cell association studied with the use of an in vitro model of mouse thymus. J Immunol. 1983 Jun;130(6):2767–2774. [PubMed] [Google Scholar]
- Sussman J. J., Bonifacino J. S., Lippincott-Schwartz J., Weissman A. M., Saito T., Klausner R. D., Ashwell J. D. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell. 1988 Jan 15;52(1):85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]
- Thom R. E., Casnellie J. E. Pertussis toxin activates protein kinase C and a tyrosine protein kinase in the human T cell line Jurkat. FEBS Lett. 1989 Feb 13;244(1):181–184. doi: 10.1016/0014-5793(89)81188-3. [DOI] [PubMed] [Google Scholar]
- Tsoukas C. D., Valentine M., Lotz M., Vaughan J. H., Carson D. A. The role of the T3 molecular complex on human T lymphocyte-mediated cytotoxicity. Adv Exp Med Biol. 1985;184:365–385. doi: 10.1007/978-1-4684-8326-0_25. [DOI] [PubMed] [Google Scholar]
- Wang P., Toyoshima S., Osawa T. Properties of a novel GTP-binding protein which is associated with soluble phosphoinositides-specific phospholipase C. J Biochem. 1988 Jan;103(1):137–142. doi: 10.1093/oxfordjournals.jbchem.a122219. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Yatani A., Mattera R., Codina J., Graf R., Okabe K., Padrell E., Iyengar R., Brown A. M., Birnbaumer L. The G protein-gated atrial K+ channel is stimulated by three distinct Gi alpha-subunits. Nature. 1988 Dec 15;336(6200):680–682. doi: 10.1038/336680a0. [DOI] [PubMed] [Google Scholar]