Abstract
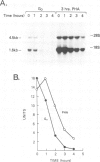
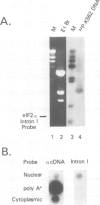
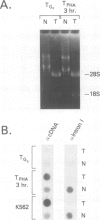
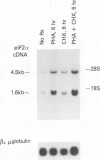
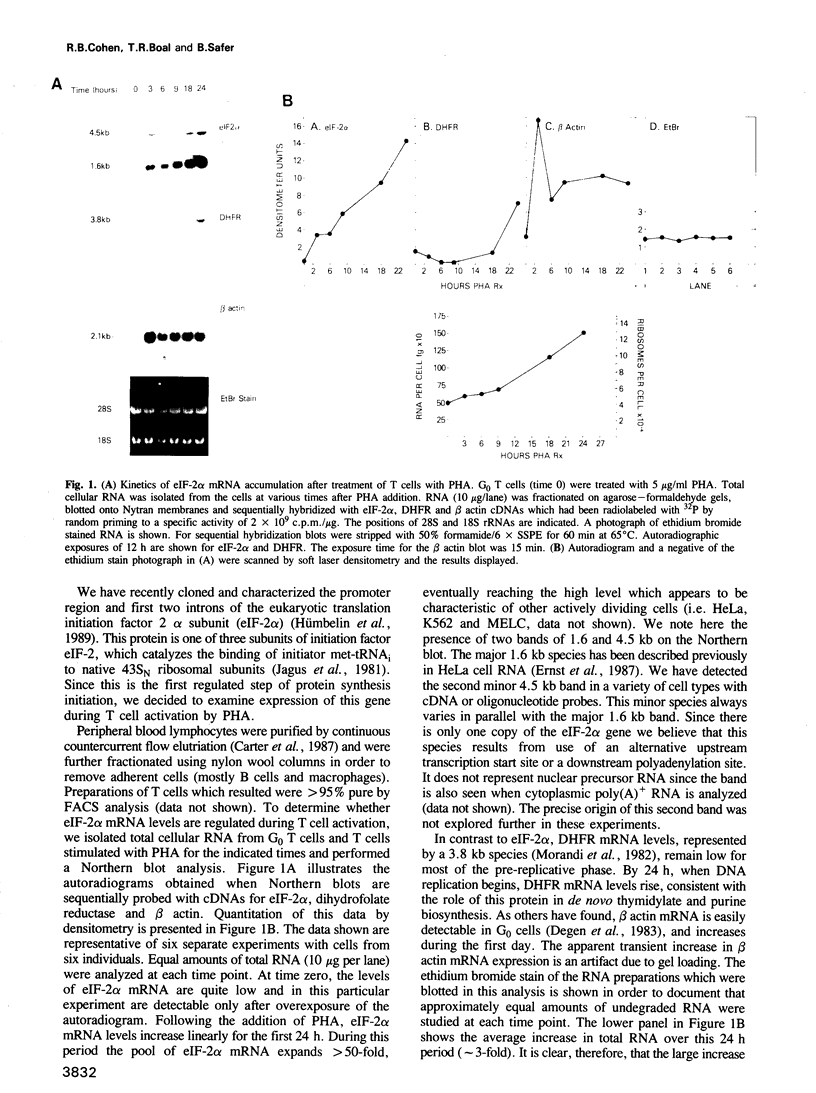
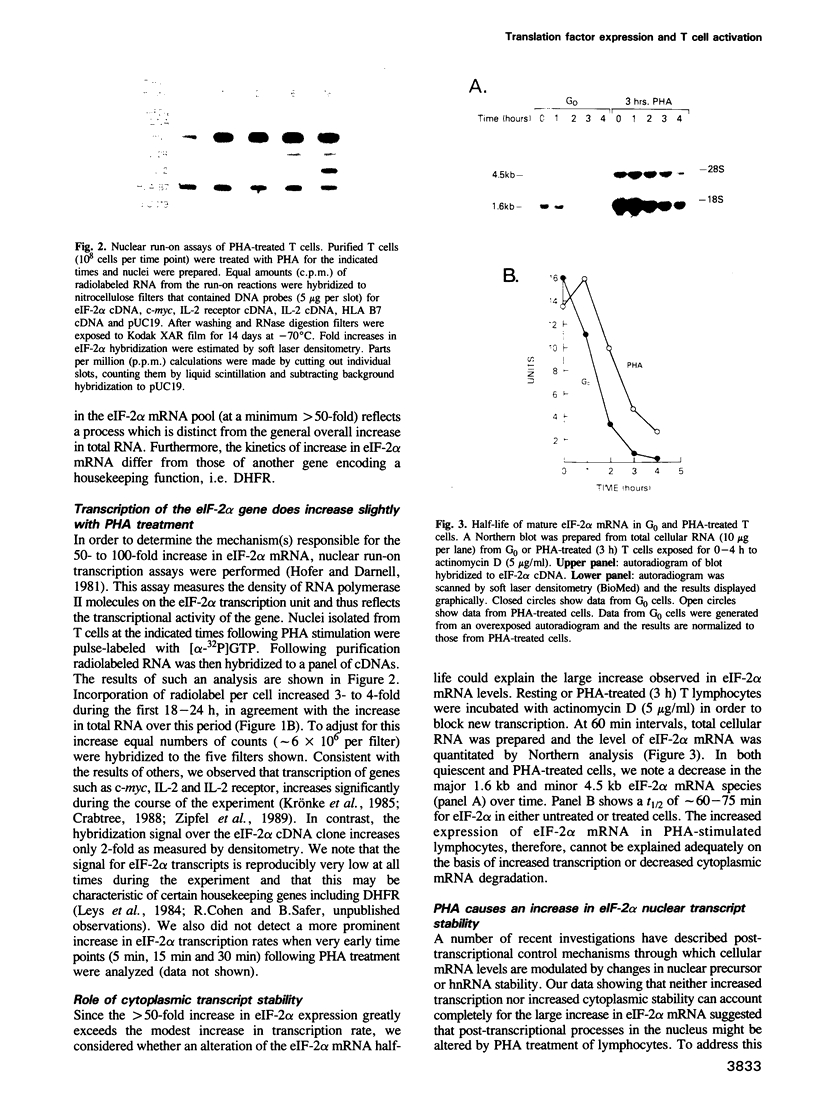
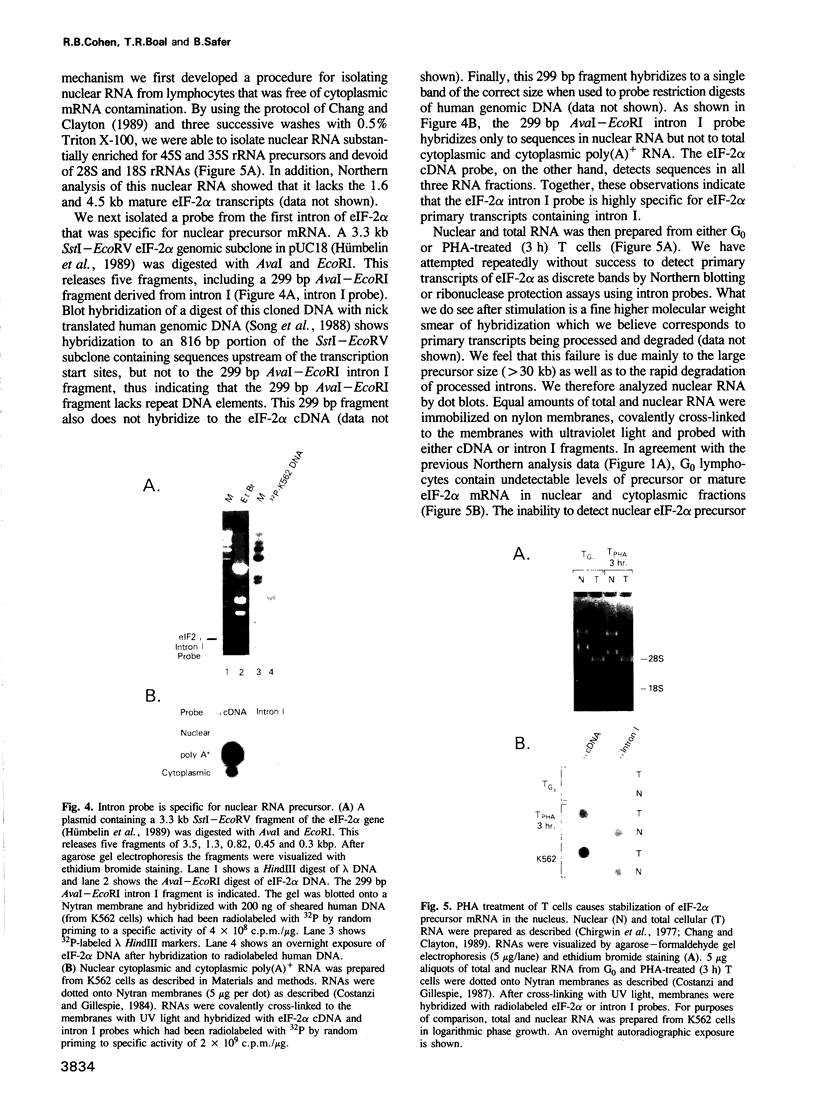
G0 human T cells synthesize protein at low rates and contain very low levels of eIF-2 alpha mRNA. eIF-2 alpha plays a pivotal role in the earliest regulated steps of translation initiation. We examined eIF-2 alpha gene expression in normal human T cells stimulated with PHA. Nuclear run-on assays indicate low rates of eIF-2 alpha gene transcription in G0 cells and these change 2-fold with PHA treatment. Actinomycin D chase experiments show that the t1/2 of eIF-2 alpha mRNA is similar in G0 and PHA-treated T cells. Analysis of nuclear RNA with probes specific for eIF-2 alpha intron sequences shows that increased eIF-2 alpha expression after PHA treatment is largely due to intranuclear stabilization of the primary transcript. The increase in eIF-2 alpha mRNA does not require new protein synthesis. Hence, expression of this gene appears to be a part of the primary response program of T cells when they are exposed to mitogen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahern T., Kay J. E. Protein synthesis and ribosome activation during the early stages of phytohemagglutinin lymphocyte stimulation. Exp Cell Res. 1975 May;92(2):513–515. doi: 10.1016/0014-4827(75)90410-3. [DOI] [PubMed] [Google Scholar]
- Ahern T., Sampson J., Kay J. E. Initiation of protein synthesis during lymphocyte stimulation. Nature. 1974 Apr 5;248(448):519–521. doi: 10.1038/248519a0. [DOI] [PubMed] [Google Scholar]
- Amaldi F., Bozzoni I., Beccari E., Pierandrei-Amaldi P. Expression of ribosomal protein genes and regulation of ribosome biosynthesis in Xenopus development. Trends Biochem Sci. 1989 May;14(5):175–178. doi: 10.1016/0968-0004(89)90269-7. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Cooper H. L. The relationship between hnRNA+-poly(A) and mRNA+-poly (A) in non-dividing human lymphocytes. Evidence for distinct synthetic pathways for mRNA precursor- and nonprecursor-hnRNA. Biochim Biophys Acta. 1978 Jan 26;517(1):84–98. doi: 10.1016/0005-2787(78)90036-9. [DOI] [PubMed] [Google Scholar]
- Carneiro M., Schibler U. Accumulation of rare and moderately abundant mRNAs in mouse L-cells is mainly post-transcriptionally regulated. J Mol Biol. 1984 Oct 5;178(4):869–880. doi: 10.1016/0022-2836(84)90316-4. [DOI] [PubMed] [Google Scholar]
- Carter C. S., Leitman S. F., Cullis H., Muul L. M., Nason-Burchenal K., Rosenberg S. A., Klein H. G. Use of a continuous-flow cell separator in density gradient isolation of lymphocytes. Transfusion. 1987 Jul-Aug;27(4):362–365. doi: 10.1046/j.1537-2995.1987.27487264750.x. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Mouse RNAase MRP RNA is encoded by a nuclear gene and contains a decamer sequence complementary to a conserved region of mitochondrial RNA substrate. Cell. 1989 Jan 13;56(1):131–139. doi: 10.1016/0092-8674(89)90991-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. B., Sheffery M. Nucleosome disruption precedes transcription and is largely limited to the transcribed domain of globin genes in murine erythroleukemia cells. J Mol Biol. 1985 Mar 5;182(1):109–129. doi: 10.1016/0022-2836(85)90031-2. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., Braverman R. Close correlation between initiator methionyl-tRNA level and rate of protein synthesis during human lymphocyte growth cycle. J Biol Chem. 1981 Jul 25;256(14):7461–7467. [PubMed] [Google Scholar]
- Cooper H. L., Braverman R. Free fibosomes and growth stimulation in human peripheral lymphocytes: activation of free ribisomes as an essential event in growth induction. J Cell Physiol. 1977 Nov;93(2):213–225. doi: 10.1002/jcp.1040930207. [DOI] [PubMed] [Google Scholar]
- Costanzi C., Gillespie D. Fast blots: immobilization of DNA and RNA from cells. Methods Enzymol. 1987;152:582–587. doi: 10.1016/0076-6879(87)52065-1. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Ernst H., Duncan R. F., Hershey J. W. Cloning and sequencing of complementary DNAs encoding the alpha-subunit of translational initiation factor eIF-2. Characterization of the protein and its messenger RNA. J Biol Chem. 1987 Jan 25;262(3):1206–1212. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferrer M., Burrone O. R., Algranati I. D. Inhibition of polypeptides synthesis by a factor isolated from ribosomes of resting human lymphocytes: studies on the mechanism of action. FEBS Lett. 1980 Dec 1;121(2):203–206. doi: 10.1016/0014-5793(80)80342-5. [DOI] [PubMed] [Google Scholar]
- Green R. C. Changes in acid ribonuclease and other acid hydrolases during lymphocyte stimulation. Exp Cell Res. 1977 Nov;110(1):215–223. doi: 10.1016/0014-4827(77)90287-7. [DOI] [PubMed] [Google Scholar]
- Gudas J. M., Knight G. B., Pardee A. B. Nuclear posttranscriptional processing of thymidine kinase mRNA at the onset of DNA synthesis. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4705–4709. doi: 10.1073/pnas.85.13.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Hraba-Renevey S., Türler H., Kress M., Salomon C., Weil R. SV40-induced expression of mouse gene 24p3 involves a post-transcriptional mechanism. Oncogene. 1989 May;4(5):601–608. [PubMed] [Google Scholar]
- Hümbelin M., Safer B., Chiorini J. A., Hershey J. W., Cohen R. B. Isolation and characterization of the promoter and flanking regions of the gene encoding the human protein-synthesis-initiation factor 2 alpha. Gene. 1989 Sep 30;81(2):315–324. doi: 10.1016/0378-1119(89)90192-3. [DOI] [PubMed] [Google Scholar]
- Jagus R., Anderson W. F., Safer B. The regulation of initiation of mammalian protein synthesis. Prog Nucleic Acid Res Mol Biol. 1981;25:127–185. doi: 10.1016/s0079-6603(08)60484-5. [DOI] [PubMed] [Google Scholar]
- Jagus R., Kay J. E. Distribution of lymphocyte messenger RNA during stimulation by phytohaemagglutinin. Eur J Biochem. 1979 Oct 15;100(2):503–510. doi: 10.1111/j.1432-1033.1979.tb04195.x. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Williams J. G., Abelson H. T., Green H., Penman S. Changes in RNA in relation to growth of the fibroblast. III. Posttranscriptional regulation of mRNA formation in resting and growing cells. Cell. 1975 Jan;4(1):69–75. doi: 10.1016/0092-8674(75)90135-x. [DOI] [PubMed] [Google Scholar]
- Kao S. Y., Calman A. F., Luciw P. A., Peterlin B. M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987 Dec 3;330(6147):489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Benzie C. R., Dicker P., Lindahl-Kiessling K. Inhibition of initiation of protein synthesis in rabbit reticulocyte lysates by a factor present in lymphocyte cytoplasm. FEBS Lett. 1978 Jul 1;91(1):40–44. doi: 10.1016/0014-5793(78)80012-x. [DOI] [PubMed] [Google Scholar]
- Kraft N., Shortman K. A suggested control function for the animal tissue ribonuclease-ribonuclease inhibitor system, based on studies of isolated cells and phytohaemagglutinin-transformed lymphocytes. Biochim Biophys Acta. 1970 Sep 17;217(1):164–175. doi: 10.1016/0005-2787(70)90133-4. [DOI] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Sequential expression of genes involved in human T lymphocyte growth and differentiation. J Exp Med. 1985 Jun 1;161(6):1593–1598. doi: 10.1084/jem.161.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys E. J., Crouse G. F., Kellems R. E. Dihydrofolate reductase gene expression in cultured mouse cells is regulated by transcript stabilization in the nucleus. J Cell Biol. 1984 Jul;99(1 Pt 1):180–187. doi: 10.1083/jcb.99.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton L. B., Lakkis F. G., Deloria D., Williams J. W., Strom T. B. Requirements for primary T cell activation. Transplant Proc. 1987 Feb;19(1 Pt 1):331–332. [PubMed] [Google Scholar]
- Morandi C., Masters J. N., Mottes M., Attardi G. Multiple forms of human dihydrofolate reductase messenger RNA. Cloning and expression in Escherichia coli of their DNA coding sequence. J Mol Biol. 1982 Apr 15;156(3):583–607. doi: 10.1016/0022-2836(82)90268-6. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Allen M. L., Rabinovitch P. S., Kuepfer C. A., White M. W. Mitogenic signaling pathways regulating expression of c-myc and ornithine decarboxylase genes in bovine T-lymphocytes. Biochemistry. 1988 Nov 15;27(23):8689–8693. doi: 10.1021/bi00423a027. [DOI] [PubMed] [Google Scholar]
- Resch K., Wood T., Cooper H. L. Demonstration of free dissociation factor activity in the cytoplasm of lymphocytes. FEBS Lett. 1980 Aug 11;117(1):284–288. doi: 10.1016/0014-5793(80)80963-x. [DOI] [PubMed] [Google Scholar]
- Song M. K., Dozin B., Grieco D., Rall J. E., Nikodem V. M. Transcriptional activation and stabilization of malic enzyme mRNA precursor by thyroid hormone. J Biol Chem. 1988 Dec 5;263(34):17970–17974. [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler A., Miller C. W., Johnson K. R., Selsted M. E., Rovera G., Koeffler H. P. Regulation of gene expression of myeloperoxidase during myeloid differentiation. J Cell Physiol. 1988 Aug;136(2):215–225. doi: 10.1002/jcp.1041360203. [DOI] [PubMed] [Google Scholar]
- Udey M. C., Parker C. W. Membrane protein synthesis in mitogen-stimulated human T lymphocytes. J Immunol. 1981 Mar;126(3):1106–1113. [PubMed] [Google Scholar]
- Vaessen R. T., Houweling A., van der Eb A. J. Post-transcriptional control of class I MHC mRNA expression in adenovirus 12-transformed cells. Science. 1987 Mar 20;235(4795):1486–1488. doi: 10.1126/science.3823900. [DOI] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varesio L., Holden H. T. Mechanisms of lymphocyte activation: linkage between early protein synthesis and late lymphocyte proliferation. J Immunol. 1980 May;124(5):2288–2294. [PubMed] [Google Scholar]
- Wettenhall R. E., London D. R. Evidence for translational control of "early" protein synthesis in lymphocytes stimulated with concanavaline A. Biochim Biophys Acta. 1974 May 17;349(2):214–225. doi: 10.1016/0005-2787(74)90082-3. [DOI] [PubMed] [Google Scholar]
- Wettenhall R. E., Slobbe A., Higgins T. J. Evidence for the presence of mRNA in the post-ribosomal cytoplasm of sheep lymphocytes. Biochim Biophys Acta. 1976 May 19;432(3):312–322. doi: 10.1016/0005-2787(76)90140-4. [DOI] [PubMed] [Google Scholar]
- Wettenhall R. E., Slobbe A. Rate-limiting factors for lymphocyte protein synthesis. Ribosome commitment and the capacity of lymphocyte cell-free systems to translate exogenous mRNAs. Biochim Biophys Acta. 1979 Jul 26;563(2):400–412. doi: 10.1016/0005-2787(79)90059-5. [DOI] [PubMed] [Google Scholar]
- White M. W., Oberhauser A. K., Kuepfer C. A., Morris D. R. Different early-signaling pathways coupled to transcriptional and posttranscriptional regulation of gene expression during mitogenic activation of T lymphocytes. Mol Cell Biol. 1987 Aug;7(8):3004–3007. doi: 10.1128/mcb.7.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel P. F., Irving S. G., Kelly K., Siebenlist U. Complexity of the primary genetic response to mitogenic activation of human T cells. Mol Cell Biol. 1989 Mar;9(3):1041–1048. doi: 10.1128/mcb.9.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]