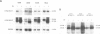Abstract
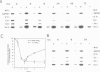
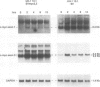
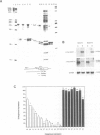
Negative feedback regulation of c-myc gene expression has been observed in some, but not all, cell types. In order to demonstrate conclusively the existence of this mechanism and gain insight into the cause of its inactivation, we have directly examined its function in B cells and then investigated its activity in a number of cell types. We demonstrate the existence of negative c-myc autoregulation by showing the rapid, dose dependent and reversible suppression of endogenous c-myc expression in EBV-immortalized B lymphoblastoid cells transfected with a c-myc gene expressed under the control of a heavy metal inducible promoter. Autoregulation occurs at the level of transcriptional initiation and is mediated by at least one stable intermediate or cofactor molecule. The c-myc autoregulatory mechanism was found operative in all (11 of 11) non-tumorigenic cells tested, including normal and immortalized lymphocytes and fibroblasts. However, this mechanism was found to be inactive in all (10 of 10) tumor cell lines derived from a variety of tissues including those carrying normal and oncogenically activated c-myc genes. These data establish the existence of an important regulatory circuit modulating c-myc expression in normal cells and suggest that its inactivation may represent a general regulatory disturbance of transformed cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Harris A. W., Pinkert C. A., Corcoran L. M., Alexander W. S., Cory S., Palmiter R. D., Brinster R. L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985 Dec 12;318(6046):533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Bernard O., Cory S., Gerondakis S., Webb E., Adams J. M. Sequence of the murine and human cellular myc oncogenes and two modes of myc transcription resulting from chromosome translocation in B lymphoid tumours. EMBO J. 1983;2(12):2375–2383. doi: 10.1002/j.1460-2075.1983.tb01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E., Mathieson B. J., Varesio L., Cleveland J. L., Borchert P. A., Rapp U. R. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature. 1985 Dec 19;318(6047):667–670. doi: 10.1038/318667a0. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Venturelli D., Kaczmarek L., Narni F., Talpaz M., Anderson B., Beran M., Baserga R. Altered expression of G1-specific genes in human malignant myeloid cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1495–1498. doi: 10.1073/pnas.83.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Casali P., Inghirami G., Nakamura M., Davies T. F., Notkins A. L. Human monoclonals from antigen-specific selection of B lymphocytes and transformation by EBV. Science. 1986 Oct 24;234(4775):476–479. doi: 10.1126/science.3020687. [DOI] [PubMed] [Google Scholar]
- Cesarman E., Dalla-Favera R., Bentley D., Groudine M. Mutations in the first exon are associated with altered transcription of c-myc in Burkitt lymphoma. Science. 1987 Nov 27;238(4831):1272–1275. doi: 10.1126/science.3685977. [DOI] [PubMed] [Google Scholar]
- Cleveland J. L., Huleihel M., Bressler P., Siebenlist U., Akiyama L., Eisenman R. N., Rapp U. R. Negative regulation of c-myc transcription involves myc family proteins. Oncogene Res. 1988;3(4):357–375. [PubMed] [Google Scholar]
- Clynes R., Wax J., Stanton L. W., Smith-Gill S., Potter M., Marcu K. B. Rapid induction of IgM-secreting murine plasmacytomas by pristane and an immunoglobulin heavy-chain promoter/enhancer-driven c-myc/v-Ha-ras retrovirus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6067–6071. doi: 10.1073/pnas.85.16.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Collins S., Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982 Aug 12;298(5875):679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Cory S., Bernard O., Bowtell D., Schrader S., Schrader J. W. Murine c-myc retroviruses alter the growth requirements of myeloid cell lines. Oncogene Res. 1987 Jun;1(1):61–76. [PubMed] [Google Scholar]
- Crews S., Barth R., Hood L., Prehn J., Calame K. Mouse c-myc oncogene is located on chromosome 15 and translocated to chromosome 12 in plasmacytomas. Science. 1982 Dec 24;218(4579):1319–1321. doi: 10.1126/science.7146913. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Martinotti S., Franchini G., Papas T. S., Gallo R. C., Wong-Staal F. Cloning and characterization of different human sequences related to the onc gene (v-myc) of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1982 Nov;79(21):6497–6501. doi: 10.1073/pnas.79.21.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dmitrovsky E., Kuehl W. M., Hollis G. F., Kirsch I. R., Bender T. P., Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986 Aug 21;322(6081):748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Avian acute leukemia viruses MC29 and MH2 share specific RNA sequences: evidence for a second class of transforming genes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1633–1637. doi: 10.1073/pnas.76.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisman M. D., Rothberg P. G., Diehl R. E., Morse C. C., Spandorfer J. M., Astrin S. M. Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol Cell Biol. 1985 Aug;5(8):1969–1976. doi: 10.1128/mcb.5.8.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisman M. D., Scott J. K., Astrin S. M. Evidence that the familial adenomatous polyposis gene is involved in a subset of colon cancers with a complementable defect in c-myc regulation. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4264–4268. doi: 10.1073/pnas.86.11.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Eisenman R. N. Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol. 1984 Nov;4(11):2486–2497. doi: 10.1128/mcb.4.11.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Inghirami G., Nakamura M., Balow J. E., Notkins A. L., Casali P. Model for studying virus attachment: identification and quantitation of Epstein-Barr virus-binding cells by using biotinylated virus in flow cytometry. J Virol. 1988 Jul;62(7):2453–2463. doi: 10.1128/jvi.62.7.2453-2463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keath E. J., Caimi P. G., Cole M. D. Fibroblast lines expressing activated c-myc oncogenes are tumorigenic in nude mice and syngeneic animals. Cell. 1984 Dec;39(2 Pt 1):339–348. doi: 10.1016/0092-8674(84)90012-6. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Larsson L. G., Ivhed I., Gidlund M., Pettersson U., Vennström B., Nilsson K. Phorbol ester-induced terminal differentiation is inhibited in human U-937 monoblastic cells expressing a v-myc oncogene. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2638–2642. doi: 10.1073/pnas.85.8.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Liu E., Santos G., Lee W. M., Osborne C. K., Benz C. C. Effects of c-myc overexpression on the growth characteristics of MCF-7 human breast cancer cells. Oncogene. 1989 Aug;4(8):979–984. [PubMed] [Google Scholar]
- Lombardi L., Newcomb E. W., Dalla-Favera R. Pathogenesis of Burkitt lymphoma: expression of an activated c-myc oncogene causes the tumorigenic conversion of EBV-infected human B lymphoblasts. Cell. 1987 Apr 24;49(2):161–170. doi: 10.1016/0092-8674(87)90556-3. [DOI] [PubMed] [Google Scholar]
- Mango S. E., Schuler G. D., Steele M. E., Cole M. D. Germ line c-myc is not down-regulated by loss or exclusion of activating factors in myc-induced macrophage tumors. Mol Cell Biol. 1989 Aug;9(8):3482–3490. doi: 10.1128/mcb.9.8.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B. Regulation of expression of the c-myc proto-oncogene. Bioessays. 1987 Jan;6(1):28–32. doi: 10.1002/bies.950060108. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Hartley J. W., Fredrickson T. N., Yetter R. A., Majumdar C., Cleveland J. L., Rapp U. R. Recombinant murine retroviruses containing avian v-myc induce a wide spectrum of neoplasms in newborn mice. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6868–6872. doi: 10.1073/pnas.83.18.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougneau E., Cerni C., Tillier F., Cuzin F. Tumorigenic transformation of rat FR3T3 fibroblasts carrying an activated myc oncogene requires subsequent mutational events. Oncogene Res. 1988;2(2):177–188. [PubMed] [Google Scholar]
- Mullins J. I., Brody D. S., Binari R. C., Jr, Cotter S. M. Viral transduction of c-myc gene in naturally occurring feline leukaemias. 1984 Apr 26-May 2Nature. 308(5962):856–858. doi: 10.1038/308856a0. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Nishikura K., ar-Rushdi A., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the normal and of the translocated human c-myc oncogenes in B cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4822–4826. doi: 10.1073/pnas.80.15.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn L. J., Brooks M. W., Laufer E. M., Land H. Negative autoregulation of c-myc transcription. EMBO J. 1990 Apr;9(4):1113–1121. doi: 10.1002/j.1460-2075.1990.tb08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Mushinski J. F., Mushinski E. B., Brust S., Wax J. S., Wiener F., Babonits M., Rapp U. R., Morse H. C., 3rd Avian v-myc replaces chromosomal translocation in murine plasmacytomagenesis. Science. 1987 Feb 13;235(4790):787–789. doi: 10.1126/science.3810165. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Cole M. D. Posttranscriptional regulation of cellular gene expression by the c-myc oncogene. Mol Cell Biol. 1989 Jan;9(1):124–134. doi: 10.1128/mcb.9.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G. C., Ziff E. B. DNA-binding motif. Nature. 1989 Oct 5;341(6241):392–392. doi: 10.1038/341392a0. [DOI] [PubMed] [Google Scholar]
- Prochownik E. V., Kukowska J. Deregulated expression of c-myc by murine erythroleukaemia cells prevents differentiation. 1986 Aug 28-Sep 3Nature. 322(6082):848–850. doi: 10.1038/322848a0. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Brightman K., Scott A., Ihle J. N. Abrogation of IL-3 and IL-2 dependence by recombinant murine retroviruses expressing v-myc oncogenes. Nature. 1985 Oct 3;317(6036):434–438. doi: 10.1038/317434a0. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. C., Stanton L. W., Riley S. C., Marcu K. B., Witte O. N. Synergism of v-myc and v-Ha-ras in the in vitro neoplastic progression of murine lymphoid cells. Mol Cell Biol. 1986 Sep;6(9):3221–3231. doi: 10.1128/mcb.6.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Keath E. J., Piccoli S. P., Cole M. D. Novel myc oncogene RNA from abortive immunoglobulin-gene recombination in mouse plasmacytomas. Cell. 1982 Dec;31(2 Pt 1):443–452. doi: 10.1016/0092-8674(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Chen H. Y., Brinster R. L., Palmiter R. D. A 12-base-pair DNA motif that is repeated several times in metallothionein gene promoters confers metal regulation to a heterologous gene. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7318–7322. doi: 10.1073/pnas.81.23.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott S. J., Davis R. L., Thayer M. J., Cheng P. F., Weintraub H., Lassar A. B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988 Oct 21;242(4877):405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneguzzo F., Hayday A. C., Keating A. Electric field-mediated DNA transfer: transient and stable gene expression in human and mouse lymphoid cells. Mol Cell Biol. 1986 Feb;6(2):703–706. doi: 10.1128/mcb.6.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg R., Noordermeer I. A., Krüse-Wolters M., Ruiter D. J., Schrier P. I. c-myc down-regulates class I HLA expression in human melanomas. EMBO J. 1988 Apr;7(4):1023–1029. doi: 10.1002/j.1460-2075.1988.tb02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares R., Cabrera C. V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987 Jul 31;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- Zerlin M., Julius M. A., Cerni C., Marcu K. B. Elevated expression of an exogenous c-myc gene is insufficient for transformation and tumorigenic conversion of established fibroblasts. Oncogene. 1987 Mar;1(1):19–27. [PubMed] [Google Scholar]