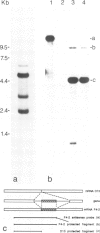
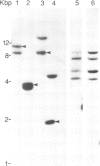
Abstract
The lamin LIII gene of Xenopus laevis has been characterized. The gene is duplicated in the Xenopus genome. The transcribed region spreads over 22 kb of genomic DNA encoding 12 exons. Two alternatively spliced mRNAs are observed which encode LIII isoforms that differ only by the 12 C-terminal amino acids which, however, both contain the CaaX motif known to be the target of post-translational modifications. The intron pattern of the lamin LIII gene is strikingly similar to that of an invertebrate intermediate filament (IF) gene over the entire protein coding sequence. The similarity in gene structure is restricted to the rod domain when compared with vertebrate types I-III IF genes. Our data suggest a model of how IF proteins evolved from a lamin-like ancestor by deletion of two signal sequences; the nuclear localization signal and the C-terminal ras-related CaaX motif. The data rule out the previously proposed hypothesis that IF proteins evolved from an intronless ancestor with an early divergence of neuronal and non-neuronal IF proteins. Together with the data presented in the accompanying paper by Dodemond et al. it can be concluded that the tail domains of lamins and invertebrate IF proteins, but not those of vertebrate IF proteins, are homologous. Thus, the different vertebrate IF proteins probably evolved by combination of the central rod domain with different tail domains by exon shuffling.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986 Oct 9;323(6088):560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- Balcarek J. M., Cowan N. J. Structure of the mouse glial fibrillary acidic protein gene: implications for the evolution of the intermediate filament multigene family. Nucleic Acids Res. 1985 Aug 12;13(15):5527–5543. doi: 10.1093/nar/13.15.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente R., Krohne G., Franke W. W. Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell. 1985 May;41(1):177–190. doi: 10.1016/0092-8674(85)90072-8. [DOI] [PubMed] [Google Scholar]
- Bisbee C. A., Baker M. A., Wilson A. C., Haji-Azimi I., Fischberg M. Albumin phylogeny for clawed frogs (Xenopus). Science. 1977 Feb 25;195(4280):785–787. doi: 10.1126/science.65013. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Dessev G., Goldman R. The oocyte lamin persists as a single major component of the nuclear lamina during embryonic development of the surf clam. Int J Dev Biol. 1990 Jun;34(2):267–274. [PubMed] [Google Scholar]
- Dodemont H., Riemer D., Weber K. Structure of an invertebrate gene encoding cytoplasmic intermediate filament (IF) proteins: implications for the origin and the diversification of IF proteins. EMBO J. 1990 Dec;9(12):4083–4094. doi: 10.1002/j.1460-2075.1990.tb07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W. On the occurrence of a fibrous lamina on the inner aspect of the nuclear envelope in certain cells of vertebrates. Am J Anat. 1966 Jul;119(1):129–145. doi: 10.1002/aja.1001190108. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher D. Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Lamin B constitutes an intermediate filament attachment site at the nuclear envelope. J Cell Biol. 1987 Jul;105(1):117–125. doi: 10.1083/jcb.105.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Maroulakou I., Blobel G. Lamin A, lamin B, and lamin B receptor analogues in yeast. J Cell Biol. 1989 Jun;108(6):2069–2082. doi: 10.1083/jcb.108.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerace L., Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Graf J. D. Genetic mapping in Xenopus laevis: eight linkage groups established. Genetics. 1989 Oct;123(2):389–398. doi: 10.1093/genetics/123.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Landesman Y., Drees B., Bare J. W., Saumweber H., Paddy M. R., Sedat J. W., Smith D. E., Benton B. M., Fisher P. A. Drosophila nuclear lamin precursor Dm0 is translated from either of two developmentally regulated mRNA species apparently encoded by a single gene. J Cell Biol. 1988 Mar;106(3):585–596. doi: 10.1083/jcb.106.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hedberg K. K., Chen L. B. Absence of intermediate filaments in a human adrenal cortex carcinoma-derived cell line. Exp Cell Res. 1986 Apr;163(2):509–517. doi: 10.1016/0014-4827(86)90081-9. [DOI] [PubMed] [Google Scholar]
- Holtz D., Tanaka R. A., Hartwig J., McKeon F. The CaaX motif of lamin A functions in conjunction with the nuclear localization signal to target assembly to the nuclear envelope. Cell. 1989 Dec 22;59(6):969–977. doi: 10.1016/0092-8674(89)90753-8. [DOI] [PubMed] [Google Scholar]
- Hosbach H. A., Wyler T., Weber R. The Xenopus laevis globin gene family: chromosomal arrangement and gene structure. Cell. 1983 Jan;32(1):45–53. doi: 10.1016/0092-8674(83)90495-6. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Wood D., Simons J. P., Kay R. M., Williams J. G. Linkage of adult alpha- and beta-globin genes in X. laevis and gene duplication by tetraploidization. Cell. 1980 Sep;21(2):555–564. doi: 10.1016/0092-8674(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W. UV crosslinking of RNA to nylon membrane enhances hybridization signals. Mol Biol Rep. 1986;11(2):107–115. doi: 10.1007/BF00364822. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Krohne G., Benavente R. The nuclear lamins. A multigene family of proteins in evolution and differentiation. Exp Cell Res. 1986 Jan;162(1):1–10. doi: 10.1016/0014-4827(86)90421-0. [DOI] [PubMed] [Google Scholar]
- Krohne G., Waizenegger I., Höger T. H. The conserved carboxy-terminal cysteine of nuclear lamins is essential for lamin association with the nuclear envelope. J Cell Biol. 1989 Nov;109(5):2003–2011. doi: 10.1083/jcb.109.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G., Wolin S. L., McKeon F. D., Franke W. W., Kirschner M. W. Nuclear lamin LI of Xenopus laevis: cDNA cloning, amino acid sequence and binding specificity of a member of the lamin B subfamily. EMBO J. 1987 Dec 1;6(12):3801–3808. doi: 10.1002/j.1460-2075.1987.tb02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., Kurer V., Eppenberger H. M., Nigg E. A. The nuclear lamin protein family in higher vertebrates. Identification of quantitatively minor lamin proteins by monoclonal antibodies. J Biol Chem. 1986 Oct 5;261(28):13293–13301. [PubMed] [Google Scholar]
- Lehner C. F., Stick R., Eppenberger H. M., Nigg E. A. Differential expression of nuclear lamin proteins during chicken development. J Cell Biol. 1987 Jul;105(1):577–587. doi: 10.1083/jcb.105.1.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Cowan N. J. Anomalous placement of introns in a member of the intermediate filament multigene family: an evolutionary conundrum. Mol Cell Biol. 1986 May;6(5):1529–1534. doi: 10.1128/mcb.6.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewinger L., McKeon F. Mutations in the nuclear lamin proteins resulting in their aberrant assembly in the cytoplasm. EMBO J. 1988 Aug;7(8):2301–2309. doi: 10.1002/j.1460-2075.1988.tb03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Baglia F. A., Newmeyer D. D., Ohlsson-Wilhelm B. M. The major 67 000 molecular weight protein of the clam oocyte nuclear envelope is lamin-like. J Cell Sci. 1984 Apr;67:69–85. doi: 10.1242/jcs.67.1.69. [DOI] [PubMed] [Google Scholar]
- McKeon F. D., Kirschner M. W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986 Feb 6;319(6053):463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- Müller W. P. The lampbrush chromosomes of Xenopus laevis (Daudin). Chromosoma. 1974;47(3):283–296. doi: 10.1007/BF00328862. [DOI] [PubMed] [Google Scholar]
- PAPPAS G. D. The fine structure of the nuclear envelope of Amoeba proteus. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):431–434. doi: 10.1083/jcb.2.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Kitten G. T., Lehner C. F., Vorburger K., Bailer S. M., Maridor G., Nigg E. A. Cloning and sequencing of cDNA clones encoding chicken lamins A and B1 and comparison of the primary structures of vertebrate A- and B-type lamins. J Mol Biol. 1989 Aug 5;208(3):393–404. doi: 10.1016/0022-2836(89)90504-4. [DOI] [PubMed] [Google Scholar]
- Quax W., Egberts W. V., Hendriks W., Quax-Jeuken Y., Bloemendal H. The structure of the vimentin gene. Cell. 1983 Nov;35(1):215–223. doi: 10.1016/0092-8674(83)90224-6. [DOI] [PubMed] [Google Scholar]
- Quax W., van den Broek L., Egberts W. V., Ramaekers F., Bloemendal H. Characterization of the hamster desmin gene: expression and formation of desmin filaments in nonmuscle cells after gene transfer. Cell. 1985 Nov;43(1):327–338. doi: 10.1016/0092-8674(85)90038-8. [DOI] [PubMed] [Google Scholar]
- Röber R. A., Weber K., Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989 Feb;105(2):365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Stewart C., Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987 Nov 6;51(3):383–392. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- Stick R., Angres B., Lehner C. F., Nigg E. A. The fates of chicken nuclear lamin proteins during mitosis: evidence for a reversible redistribution of lamin B2 between inner nuclear membrane and elements of the endoplasmic reticulum. J Cell Biol. 1988 Aug;107(2):397–406. doi: 10.1083/jcb.107.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stick R., Hausen P. Changes in the nuclear lamina composition during early development of Xenopus laevis. Cell. 1985 May;41(1):191–200. doi: 10.1016/0092-8674(85)90073-x. [DOI] [PubMed] [Google Scholar]
- Stick R., Schwarz H. Disappearance and reformation of the nuclear lamina structure during specific stages of meiosis in oocytes. Cell. 1983 Jul;33(3):949–958. doi: 10.1016/0092-8674(83)90038-7. [DOI] [PubMed] [Google Scholar]
- Stick R. cDNA cloning of the developmentally regulated lamin LIII of Xenopus laevis. EMBO J. 1988 Oct;7(10):3189–3197. doi: 10.1002/j.1460-2075.1988.tb03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F., Spohr G. Isolation and characterization of sarcomeric actin genes expressed in Xenopus laevis embryos. J Mol Biol. 1986 Feb 5;187(3):349–361. doi: 10.1016/0022-2836(86)90438-9. [DOI] [PubMed] [Google Scholar]
- Thiébaud C. H., Fischberg M. DNA content in the genus Xenopus. Chromosoma. 1977 Feb 3;59(3):253–257. doi: 10.1007/BF00292781. [DOI] [PubMed] [Google Scholar]
- Tymowska J., Fischberg M. A comparison of the karyotype, constitutive heterochromatin, and nucleolar organizer regions of the new tetraploid species Xenopus epitropicalis Fischberg and Picard with those of Xenopus tropicalis Gray (Anura, Pipidae). Cytogenet Cell Genet. 1982;34(1-2):149–157. doi: 10.1159/000131803. [DOI] [PubMed] [Google Scholar]
- Venetianer A., Schiller D. L., Magin T., Franke W. W. Cessation of cytokeratin expression in a rat hepatoma cell line lacking differentiated functions. Nature. 1983 Oct 20;305(5936):730–733. doi: 10.1038/305730a0. [DOI] [PubMed] [Google Scholar]
- Vorburger K., Kitten G. T., Nigg E. A. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal CXXM motif. EMBO J. 1989 Dec 20;8(13):4007–4013. doi: 10.1002/j.1460-2075.1989.tb08583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorburger K., Lehner C. F., Kitten G. T., Eppenberger H. M., Nigg E. A. A second higher vertebrate B-type lamin. cDNA sequence determination and in vitro processing of chicken lamin B2. J Mol Biol. 1989 Aug 5;208(3):405–415. doi: 10.1016/0022-2836(89)90505-6. [DOI] [PubMed] [Google Scholar]
- Weber K. Link between lamins and intermediate filaments. Nature. 1986 Apr 3;320(6061):402–402. doi: 10.1038/320401a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Dodemont H., Kossmagk-Stephan K. Amino acid sequences and homopolymer-forming ability of the intermediate filament proteins from an invertebrate epithelium. EMBO J. 1988 Oct;7(10):2995–3001. doi: 10.1002/j.1460-2075.1988.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Ulrich W. Cytoplasmic intermediate filament proteins of invertebrates are closer to nuclear lamins than are vertebrate intermediate filament proteins; sequence characterization of two muscle proteins of a nematode. EMBO J. 1989 Nov;8(11):3221–3227. doi: 10.1002/j.1460-2075.1989.tb08481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]