Summary
While highly active anti-retroviral therapy has dramatically improved the survival of HIV-infected individuals, there is an increased risk for other co-morbidities, such as COPD, manifesting as emphysema. Given that emphysema orginates around the airways, and that human airway basal cells (BC) are adult airway stem/progenitor cells, we hypothesized that HIV reprograms BCs to a distinct phenotype that contribute to the development of emphysema. Our data indicate that HIV binds to, but does not replicate, in BC. HIV binding to BCs induces them to acquire an invasive phenotype, mediated by up-regulation of MMP-9 expression through activation of MAPK signaling pathways. This HIV-induced “destructive” phenotype may contribute to degradation of extracellular matrix and tissue damage relevant to the development of emphysema commonly seen in HIV+ individuals.
Graphical abstract

Introduction
With the advent of combination antiviral therapy for HIV+ individuals, the incidence of opportunistic infections has been markedly reduced, and the disease has become a treatable disorder with a significantly increased life span (Murphy et al., 2001; Palella et al., 1998). However, as HIV-1+ individuals are living longer, new comorbidities have emerged and HIV-1 infection is now associated with a variety of systemic disorders, including a high incidence of chronic obstructive pulmonary disease (COPD), manifesting as emphysema (Bhatia and Chow, 2016; Bhatia et al., 2012; Crothers et al., 2006; Crothers et al., 2011; Diaz et al., 1992; Diaz et al., 2000; Diaz et al., 2003; Giantsou, 2011; Kalim et al., 2008; Morris et al., 2011a; Morris et al., 2011b; Naicker et al., 2015; Palella and Phair, 2011; Petrache et al., 2008). HIV-1 associated emphysema was originally linked to opportunistic lung infections and intravenous drug use (Beck et al., 2001; Morris et al., 2000). A number of studies, however, have clearly demonstrated that HIV-1+ individuals who have no prior history of pulmonary infection have a higher incidence of emphysema than that of the general population and develop emphysema at an early age, i.e., infection with HIV-1 is a cofactor in the development of COPD (Crothers et al., 2006; Crothers et al., 2011; Diaz et al., 1992; Diaz et al., 2000; Diaz et al., 2003; Giantsou, 2011; Morris et al., 2011a; Morris et al., 2011b; Petrache et al., 2008). The pathogenesis of HIV-associated emphysema is not understood, with some evidence pointing to alveolar macrophage (AM) and CD8 T cell mediators (Buhl et al., 1993; Plata et al., 1987; Twigg et al., 1999), direct effects on the lung of HIV proteins such as Env and Tat (Green et al., 2014; Kanmogne et al., 2005; Park et al., 2001) and adverse effects of anti-retroviral therapies (George et al., 2009).
Since the first pathologic manifestations of COPD are in the small airway epithelium (SAE), and lung destruction that characterizes emphysema begins in the alveoli surrounding the SAE (Crystal, 2014; Hogg et al., 2004; Hogg et al., 1968), we hypothesized that HIV-1 is capable of interacting directly with the SAE, initiating pathologic programming of the SAE to acquire a “destructive phenotype” that initiates the emphysematous process. To assess this hypothesis, we focused on human airway basal cells (BC), the airway epithelial stem/progenitor cells responsible for differentiation into all of the airway epithelial cell types. We focused on the cellular receptors involved in interaction of HIV-1 with airway BCs and the biological consequences and sunsequently activated signaling pathways, and examined HIV-induced reprogramming of BCs toward a destructive phenotype relevant to emphysema. Our data argue that HIV can bind to BCs through heparan sulfate proteoglycan receptors, but does not replicate in normal human airway BCs. Strikingly, HIV binding to the BC initiates a cascade of events mediated through MAPK signaling pathways and induces increased expression and secretion of metalloproteases (MMP), particularly MMP-9, capable of inducing tissue destruction. Consistent with the report that Zika virus can infect, and reprogram neural progenitors (Tang et al., 2016), the observations in the present study may represent another example of how a virus can induce disease by adversely affecting the normal function of adult stem/progenitor cells in a specific organ.
Experimental Procedures
All subjects were evaluated at the Weill Cornell NIH Clinical and Translational Science Center and Department of Genetic Medicine Clinical Research Facility, using Weill Cornell Institutional Review Board-approved clinical protocols and written informed consent obtained. Small airway epithelial cells were collected by fiberoptic bronchoscopy by brushing as previously described (Harvey et al., 2007, Hackett et al., 2012, Buro-Auriemma et al., 2013). Basal cells were isolated from the airway epithelium of healthy nonsmokers (n = 10) as previously described (Hackett et al., 2011). RNA sequencing was carried out using Illumina protocols and with paired-end sequencing (Illumina HiSeq 2000; Illumina, San Diego, CA). BC cultures were carried out on plastic flasks in BEGM medium (GIBCO-Life Technologies, Grand Island, NY) as previously described.
All BC preparations were >95% positive for BC markers and negative for markers of other airway epithelium cell types. All individuals had no significant past medical history, and physical examination, chest imaging and lung function was normal. RNA sequencing was performed as previously described (Hackett et al., 2011; Ryan et al., 2014). HIV-1 stocks (X4-tropic NL4-3) were generated by calcium phosphate transfection of HEK293T cells (MBS mammalian transfection kit, Stratagene) with the proviral vector, pNL-4-3 (NIH AIDS Research and Reference Reagent Program). To assess HIV interaction with BC, binding assays and proliferation assays were used ± presence of heparan sulfate or herparinase. Flow cytometry was used to evaluate surface expression of heparan sulfate proteoglycans and syndecans 1–4. Light miscroscopy was used to assess the overall effect on BC cultures. AM quantitative invasion assays was u sed to examine the “descrtructive” phenotype of BC exposed to HIV. The supernantants of HIV+BC were assessed for the presence of proteases with the Human Protease Array kit (R&D Systems), as was MMP-9 expression using TaqMan PCR and zymography. TIMP-1 leves were evaluated by ELISA. The Human Phoso-MAPK Array kit (R&D Systems) was used to identify pathways activated by HIV reprogrammed BC and phosphorylated Erk1/2 quanitfied by Western analysis. To further examine these pathways, MEK inhibitor or JNK/NFκB inhibitor was used. Statistical comparisons were calculated using an unpaired two-tailed Student’s t-test with unequal variance; p<0.05 was considered significant. For complete details of all these assays, see Supplemental Methods.
Results
HIV Receptors Expressed by BC
RNA-Seq analysis revealed that human large airway BC expressed several HIV-1 receptors including heparan sulfate proteoglycan 2 and syndecan 1–4 (HSPG2 and SDC1-4), nicotinic acetylcholine receptor α7, toll-like receptor (TLR) 2 and 4 and integrin α4β7 but not classical HIV-1 receptors, CD4 and co-receptors, CCR5 and CXCR4 (Table S1). Of note, syndecan 1 and 4 were highly expressed.
HIV Binds to Human Airway Basal Cells Through a Trypsin-sensitive Cell Surface Receptor
To investigate if HIV-1 interacts with human airway BCs, viral binding assays were performed. Primary human BC were exposed to HIV-1NL4-3 for 3 and 24 hr, washed to remove unbound virus and then lysed to release HIV capsid protein, p24 bound to the cell surface. Virus binding was measured by HIV-1 p24 ELISA. HIV-1NL4-3 bound to human BC after incubation for 3 and 24 hr whereas heat-inactivated HIV did not (Figure 1A). Treatment of HIV-bound BC with trypsin significantly reduced HIV binding by 88%, suggesting that HIV binding was mediated by a trypsin-sensitive cell surface receptor (p<0.02; Figure 1B).
Figure 1.

Binding of HIV to human airway basal cells. A. Binding of HIV-1NL4-3. BC were exposed to the virus, washed and then lysed in 0.1% Triton-X. Virus binding was measured by HIV-1 p24 levels after 3 and 24 hr. B. Sensitivity of HIV binding to the BC to trypsin. After 3 hr of incubation, cells were incubated with 0.05% trypsin/EDTA for 5 min, washed, lysed and analyzed by HIV-1 p24 levels. C. Flow cytometry assessment of cell surface expression of heparan sulfate proteoglycans (HSPGs) in untreated and trypsin-treated basal cells. The solid line represents staining with monoclonal antibody against the indicated HSPG. The broken line represents staining with corresponding isotype control. Shown are the histograms of heparan sulfate and syndecans 1–4, each from one representative experiment of 3 independent experiments. D. Heparan sulfate inhibition of HIV binding to BC. Pretreatment of HIV with heparan sulfate at different concentrations inhibits HIV binding to airway BC in a dose-dependent manner. Results shown are the average of three independent experiments using cells from 3 different individuals. E. Effect of heparinase III pretreatment of BC on HIV binding. Prior heparinase III treatment on BC significantly abolishes HIV binding. F. HIV binds to, but does not replicate in airway BC. Shown is a time-course of BC levels of p24 following addition of HIV-1 to airway BC. Cell lysates were collected at the indicated time points and used for quantification of HIV-1 p24 levels. *p<0.05, **p<0.01, NL4-3 vs NL4-3 + heparan sulfate.
Human Airway BC Capture HIV via Cell Surface Heparan Sulfate
Heparan sulfate proteoglycans serve as attachment receptors for various viruses, including HIV (Bobardt et al., 2003; de Witte et al., 2007; Gallay, 2004; Jiang et al., 2015a; Jiang et al., 2015b; Saphire et al., 1999; Saphire et al., 2001). To determine if HSPGs were involved in HIV binding to BC, cells were first stained with antibodies against heparan sulfate and syndecans (SDC-1 to -4) and analyzed by flow cytometry. The results demonstrated that heparan sulfate and all 4 syndecans were expressed in human airway BC (Figure 1C, left panel). Treatment of BC with 0.05% trypsin/EDTA significantly decreased the BC expression of surface heparan sulfate and syndecans (Figure 1C, right panel) compared to untreated BC.
Based on these data, studies were carried out to assess virus binding to BC in the presence of heparan sulfate. Pretreatment of virus with increasing concentrations of heparan sulfate (from 0.1 to 200 μg/ml) blocked HIV binding to BC in a dose-dependent manner (Figure 1D). In addition, removal of cell surface heparan sulfate moieties using heparinase III at 0.5 mIU/ml and 1 mIU/ml decreased virus binding to BC by 40% and 49% respectively (p<0.02; Figure 1E). Overall, these data suggest that HIV binding to BC is mediated by heparan sulfate expressed on the cell surface.
To examine if HIV could replicate in human BCs, these cells were exposed to HIV over-night, washed and then cultured in BEGM for 9 days. Cell lysates were collected at indicated time points and assayed for HIV-1 p24 levels. The cellular p24 levels were decreased throughout the culture period, indicating that virus did not propagate in BC (Figure 1F). Consistent with these data, heparan sulfate inhibited virus binding and the p24 level was markedly reduced and maintained a similar level throughout the culture period.
HIV Induces BC Morphological Changes and to Adopt a Cell Invasion Phenotype
We next investigated if HIV binding to the BC induced morphological changes. At day 0, BC displayed a healthy morphology in both control and HIV-treated BC (Figure 2A, upper panel). At day 5, elongated cells and disruption of the cell monolayer with “holes” were observed in HIV-treated BC (Figure 2A, lower panel). The number of “holes” in HIV-treated BC culture is significantly higher than untreated BC (29± 9 vs 2 ± 1 holes/monolayer, p < 0.0002, Figure S1). These findings suggested that HIV binding to BC evokes a destructive phenotype, possibly by secretion of mediators (e.g. proteases) that disrupt cell junctions.
Figure 2.

HIV induced BC to acquire a destructive phenotype. Shown are in vitro assessments of HIV-induced BC morphological changes and cell invasion through connective tissue. A. Morphology of BC after exposure to HIV. At day 5 there are “holes” in the BC culture. B. HIV induces BC invasion through connective tissue. BC were plated onto matrigel-coated chambers. Data shown is the stained migratory cells at the bottom of the chambers in control and HIV-treated culture from one representative of 3 independent experiments. Bar = 100 μm.
To assess if HIV binding to BC programmed the BC to destroy connective tissue, HIV bound to BC were assessed in a cell invasion assay through matrigel. Cells were seeded on matrigel-coated culture chamber and treated with HIV. After 5 days, cells on the bottom side were fixed, stained and viewed under a microscope. Results from three experiments demonstrated that there was increase in the number of migratory cells in HIV-treated BC as compared to untreated control (42 cells vs 3 cells; Figure 2B and Figures S2A and S2B).
HIV Modulates MMP-9 Expression in Human Airway BC
Metalloproteinases (MMPs) mediate degradation of extracellular matrix, cell invasion, remodeling and tissue damage (Atkinson and Senior, 2003; Elkington and Friedland, 2006; Grzela et al., 2016). To evaluate the hypothesis that the HIV reprogramming of normal BC to take on a destructive phenotype is mediated by HIV inducing the BC to express matrix metalloproteases, the cell lysates of BC + HIV were assessed using a human protease array. MMP-7, -8, -9 and -12 were expressed in BC (Figure 3A), but among all MMPs, MMP-9 was induced most dramatically compared to control (Figure 3B). Based on this data, and the knowledge that MMP-9 plays an important role in cigarette smoke induced emphysema (Churg et al., 2007; Selman et al., 2003) and MMP-9 levels are elevated in lung epithelial lining fluid of HIV+ individuals (Kaner et al., 2009), we focused subsequent studies on HIV induction of MMP-9 expression in airway BC.
Figure 3.

Expression of matrix metalloproteinases (MMPs) in mock and HIV-treated BC. BC were exposed to HIVNL4-3 (p24 at 200 ng/ml) for 48 hr, and cell lysates were assessed using a human protease array to detect the expression of MMPs. A. The locations of each MMP on the array membrane are identified. The array images were obtained from 1 min exposure to X-ray film. B. Quantification of the expression of the pixel density of each MMP (in duplicate) was measured. The data represents the average of each MMP after subtraction of the background signal.
Based on the protease array findings, we sought to investigate if HIV could modulate MMP-9 mRNA expression in BC. Cells were exposed to increasing HIV input (p24 from 5 to 200 ng/ml) for 2 days, and total RNA and culture supernatants were collected for MMP-9 gene expression and activity assays. Taqman quantitative PCR showed that HIV induced MMP-9 mRNA expression in a dose-dependent fashion (all p<0.05 vs no viral input; Figure 4A). Treatment of BC with either heat-inactivated HIV and cigarette smoke extract (CSE at 3 and 6%) alone had no effect on MMP-9 gene expression (Figure 4B). However, comparison of HIV + CSE vs CSE alone showed a significant induction in MMP-9 mRNA expression (p<0.002 for 3% CSE and p<0.003 for 6% CSE; Figure 4B). Consistent with the gene expression data, there was an increased secretion of MMP-9 from HIV-exposed BC vs untreated (18.7 vs 8 ng/ml, p<0.002) and HIV + CSE-exposed BC vs CSE alone (for 3% CSE, 12.6 vs 5.9 ng/ml, p<0.0001 and for 6% CSE, 10 vs 4.1 ng/ml, p<0.001) as confirmed by ELISA (Figure 4C). Heat-inactivated HIV had no effect on MMP-9 secretion and MMP-9 level was comparable to untreated BC. Zymography analysis demonstrated that there was an increase in MMP-9 activity in HIV- and HIV + CSE-treated BC (lanes 4, 8 and 9; Figure 4D) when compared to untreated, heat–inactivated HIV-treated and CSE-treated BC (lanes 3, 5, 6 and 7; Figure 4D). Active form of MMP-9 was observed in HIV and HIV + CSE-treated groups (lanes 4, 8 and 9; Figure 4D). Similarly, primary small airway basal cells obtained and purified from bronchoscopic brushing of healthy nonsmokers demonstrated heparan sulfate –dependent HIV binding (Figure S3A), upregulation of MMP-9 (Figure S3B) and increased level of secreted MMP-9 (Figure S3C).
Figure 4.

HIV modulation of MMP-9 expression in BC. A. HIV induces MMP-9 expression in BC in a dose-dependent manner. BC were exposed to increasing viral input (p24 from 5 to 200 ng/ml) for 2 days and MMP-9 expression quantified by TaqMan PCR. The data is normalized to 18s RNA. B. Assessment of expression of MMP-9 in HIV-treated and HIV + cigarette smoke extract (CSE)-treated BC. C. Quantification of MMP-9 in culture supernatants of untreated, CSE, HIV and HIV+CSE treated BC cultures. MMP-9 levels were quantified by ELISA. D. Gelatin zymography analysis of MMP-9 activity in cylture supernatants from treated BC. Lane 1 - pro-MMP-9 standard; lane 2 - active MMP-9 standard; lane 3 - untreated, lane 4 - HIV-treated BC; lane 5 - heat-inactivated HIV-treated BC; lane 6 - 3% CSE-treated BC; lane 7 - 6% CSE-treated BC; lane 8 - HIV+ 3% CSE-treated BC; lane 9 - HIV+ 6% CSE-treated BC.
In COPD, there was an imbalance between proteases/anti-protease level leading to proteolysis and matrix degradation within the lung compartments. In separate experiments, both MMP-9 and TIMP-1 levels were measuresd. There was an increase level of MMP-9, TIMP-1 and the ratio of MMP-9 to TIMP-1 level in HIV-treated BC vs untreated BC (for MMP-9, 6924 pg/ml vs 693 pg/ml, p<0.0003, for TIMP-1, pg/ml 44929 vs 22768 pg/ml, p<0.001 and for MMP-9:TIMP-1 ratio, 0.15 vs 0.03, p<0.001; Figure S4).
HIV Binding to BC Induces MAPK Phosphorylation
To investigate which signaling pathway(s) were activated in HIV-exposed BC, starved BC were either stimulated with basal medium or HIV-containing basal medium for 15 min and the cell lysates were analyzed using a MAPK phosphorylation array. The data indicated that HIV binding to BC increased phosphorylation of p38 MAPK, Erk1/2, CREB, JNK, GSK-3α/β and HSP27 when compared to control (Figures S5 and S6A). Western blot analysis also confirmed that there was increased phosphorylated Erk in HIV-treated BC as compared to BC treated with basal medium. Pretreatment of virus with heparan sulfate at 30 μg/ml significantly blocked HIV-induced Erk phosphorylation (Figure S6B).
Phosphorylation of p38 MAPK, Erk and JNK results in activation of downstream signaling cascades that are closely linked to activation of transcription factors including AP-1, SP-1 and NFκB implicated in regulation of MMP-9 gene expression (Bond et al., 1998; Gum et al., 1997; Karin, 1995; Labrie and St-Pierre, 2013; McCawley et al., 1999; Reddy et al., 1999; Sato and Seiki, 1993; Simon et al., 1998).
The involvement of phosphorylated kinases in the expression of MMP-9 was confirmed with inhibitors of MEK (PD98059, 20 M), NFκB and JNK (resveratrol, 70 M) at both transcriptional and secretion levels. Cells were pretreated with inhibitors for 1 hr prior to HIV treatment. Both inhibitors significantly suppressed HIV-induced MMP-9 mRNA (PD98059, p<0.03 and resveratrol, p<0.05; Figure 5A) and secreted MMP-9 in culture supernatants (PD98059, p<0.03 and resveratrol, p<10−5; Figure 5B). Both inhibitors alone did not have any effects on BC and were comparable to untreated control (medium) and vehicle control (DMSO). Zymography analysis showed that there was a reduction in intensity corresponding to pro-MMP-9 in the inhibitor treated-BC (PD98059 treatment: lane 6 vs lane 7; resveratrol treatment: lane 13 vs lane 14; Figure 5C). Overall, these results suggest that HIV-induced MMP-9 expression is mediated through Erk, NFκB and JNK pathways.
Figure 5.
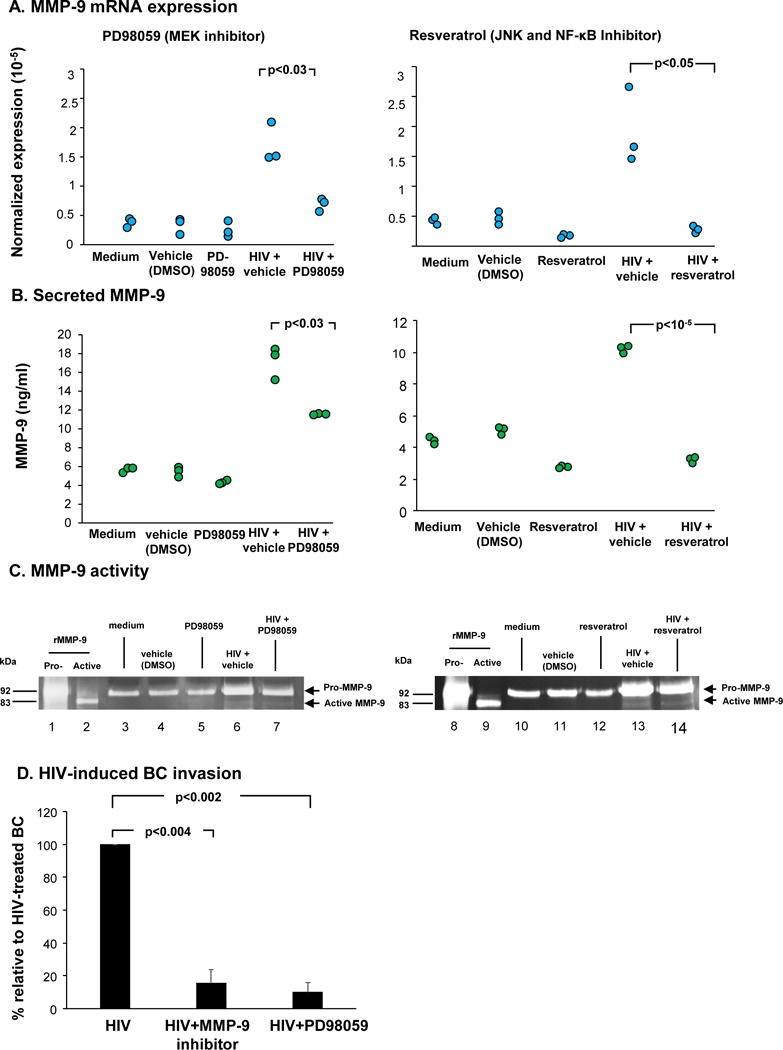
MEK inhibitor (PD98059) and resveratrol suppress HIV-induced MMP-9 expression in BC. BC were treated with PD98059 (20 M) or resveratrol (75 M) for 1 hr prior to virus exposure. BC were collected and analyzed. A. MMP-9 mRNA expression by Taqman PCR. B. Level of secreted MMP-9 in culture supernatants assessed by ELISA from BC culture treated with HIV in the absence and presence of inhibitors. C. MMP-9 activity in culture supernatants assessed by gelatin zymography analysis. Lanes 1, 8 - pro-MMP-9 standard; lane 2, 9 - active MMP-9 standard; lanes 3, 10 - untreated BC; lanes 4, 11 - vehicle control; lane 5 - PD98059 alone; lanes 6, 13 - HIV+vehicle; lane 7 - HIV+PD98059; lane 12- resveratrol; lane 14 - HIV+resveratrol. D. Suppression of invasion of HIV-bound airway BC through connective tissue by MEK and MMP-9 inhibitors. The number of migratory cells in HIV-treated in the absence and presence of inhibitors were quantified. Data is presented as the percentage of cell invasion relative to HIV-treated BC from three independent experiments.
HIV-induced BC Invasion is Associated with Erk Signaling and MMP-9
Given that HIV induces BC invasion and MMP-9 expression, we investigated if the invasive phenotype was mediated by MMP-9 and Erk signaling. Cells were seeded on matrigel-coated culture chamber and treated with HIV in the presence of MMP-9 and MEK inhibitors. After 5 days, cells on the bottom side were fixed, stained and viewed under a microscope. Relative to HIV-treated BC, both MMP-9 and MEK inhibitors significantly suppressed HIV-induced BC invasion (MMP-9 inhibitor, p<0.004; PD98059, p<0.002; Figure 5D). Taken together, HIV binding to BC induced MMP-9 and acquired “destructive phenotype” to invade through connective tissues.
Discussion
With the development of effective anti-retroviral therapy (ART), the survival of individuals infected with HIV-1 has been dramatically improved (Murphy et al., 2001; Palella et al., 1998). However, chronic HIV-1 infection is associated with several comorbid disorders, including a high incidence of chronic obstructive pulmonary disease manifesting as emphysema (Bhatia and Chow, 2016; Bhatia et al., 2012; Crothers et al., 2006; Crothers et al., 2011; Diaz et al., 1992; Diaz et al., 2000; Diaz et al., 2003; Giantsou, 2011; Kalim et al., 2008; Morris et al., 2011a; Morris et al., 2011b; Naicker et al., 2015; Palella and Phair, 2011; Petrache et al., 2008). The pathogenesis of HIV-associated emphysema is not well understood, with some evidence implicating lung destruction mediated by alveolar macrophages, CD8 T cells, HIV envelope proteins and possibly anti-retroviral drugs (Buhl et al., 1993; George et al., 2009; Green et al., 2014; Kanmogne et al., 2005; Park et al., 2001; Plata et al., 1987; Twigg et al., 1999). In this study, we hypothesized that the HIV virions may modulate cellular functions of lung parenchymal cells, mediating pathological programming contributing to lung destruction through multiple mechanisms. Given that human airway basal cells are the adult stem/progenitor cells that differentiate into specialized airway epithelial ciliated and secretory cells during normal turn over and repair, we focused on whether HIV reprograms BC to distinctive phenotypes that contribute to the development of emphysema. Strikingly, the data demonstrate that HIV binds to but does not replicate in BC, HIV binding to BC induces morphological changes and the BC acquire an invasive phenotype, mediated by up-regulation of MMP-9 expression through activation of MAPK signaling pathways.
There is evidence from a number of studies demonstrating that, despite effective systemic therapy, there is still a burden of HIV in the lung. For example, persistent HIV-1 in alveolar macrophages is observed in HIV-1 infected individual with systemic viral suppression (Costiniuk and Jenabian, 2014; Cribbs et al., 2015). Consistent with these observations, several reports have shown that HIV-infected AM release various cytokines that induce inflammation, immune activation and infiltration of neutrophils, and produce proteases (Costiniuk and Jenabian, 2014; Kaner et al., 2009). Yearsley et al (Yearsley et al., 2005) reported that HIV-infected macrophage-like cells and uninfected MMP-9 expressing neighboring cells are localized in emphysematous area of lung. This suggests that HIV-infected macrophages may spread viral particles and mediators that influence neighboring cells in the airway epithelium, resulting in emphysema. While the basal cells attached to basement membrane, it has been shown that rat tracheal basal cells can extend cytoplasmic projections to apical surface of the airway epithelium when they could be exposed to HIV on the epithelial surface (Shum et al., 2008). It is possible that BC can sample pathogens such as virus and viral proteins released from other neighboring cells and damaged vascular tissues in apical environement.
Matric Metalloproteinases
Matrix metalloproteinases (MMPs) are proteolytic enzymes capable of degrading the extracellular matrix, facilitating cell migration and tissue remodeling (Atkinson and Senior, 2003; Elkington and Friedland, 2006; Grzela et al., 2016; Legrand et al., 1999). MMPs also regulate cytokines and chemokines activity, influencing the recruitment and functions of inflammatory cells (Parks et al., 2004). Previous studies have shown that MMPs released by multiple cell types including macrophages, epithelial cells, T cells and neutrophils play an important role in COPD (Demedts et al., 2005; Gueders et al., 2006; Navratilova et al., 2016; Owen, 2008). MMP-9 has been of interest in the pathogenesis of cigarette smoking-associated emphysema (Atkinson and Senior, 2003; Churg et al., 2007; Selman et al., 2003). In patients with COPD, elevated MMP-9 levels are observed in the lung (Kang et al., 2003; Ohnishi et al., 1998), alveolar macrophages (Russell et al., 2002) and induced sputum (Beeh et al., 2003; Cataldo et al., 2000; Culpitt et al., 2005). Alveolar macrophages from normal smokers express more MMP-9 than those from normal individuals (Lim et al., 2000). Mouse models with transgenic expression of MMP-9 in alveolar macrophages have adult-onset emphysema (Foronjy et al., 2008), and there is up-regulation of MMP-9 in the airways of mice with chronic airway inflammation and remodeling (Yu et al., 2012).
Consistent with the observations in this study, HIV-1+ smokers with emphysema showed increased levels of MMP-9 in lung epithelial lining fluid compared to HIV‾ smokers (Kaner et al., 2009) and there is increased MMP-9 expression in the lung of AIDS patient with emphysema (Yearsley et al., 2005). Similarly, HIV-1 gp120 induces MMP-9 expression via binding to mannose receptor in vaginal epithelial cells in vitro (Fanibunda et al., 2011). HIV-1 binding to mannose receptor induces production of matrix metalloproteinase 2 (MMP-2) through human mannose receptor-mediated intracellular signaling in human astrocytes (Lopez-Herrera et al., 2005). These studies support our hypothesis that HIV can reprogram epithelial cells to destructive phenotypes by induction of proteases. Consistent with our observations, a recent study demonstrated that X4-tropic HIV can be internalized by airway epithetlial cells and then impair lung epithelial permeability and promote inflammation without direct infection (Brune et al., 2016). Apart from HIV itself, Gundavarapu et al (Gundavarapu et al., 2013) has demonstrated that HIV gp120 promotes mucus production in bronchial epithelial cells via CXCR4/α7-nicotinic acetylcholine receptors. These studies support our hypothesis that HIV can reprogram epithelial cells to destructive phenotypes by induction of proteases in different tissues.
HIV-modulated BC Reprogramming
In the present study, we have identified a possible mechanism for the HIV-mediated up-regulation of MMP-9 in the lung. HIV binds to BC resulting in morphological changes, with the BC acquiring a cell invasion phenotype and up-regulation of MMP-9 expression. Specific inhibitors of MEK (PD98059) and NFκB and JNK (resveratrol) suppress HIV-induced MMP-9 expression and migration in BC. It is reasonable to infer that the cellular damage in HIV-bound BC is caused, at least in part, by the release of proteases such as MMP-9. In a cell invasion assay, HIV-induced MMP-9 was shown to degrade extracellular matrix components to allow BC to migrate through matrigel. To our knowledge, this is the first demonstration that HIV can reprogram a non-hematopoietic adult stem/progenitor cell, in this case to acquire destructive phenotypes that contribute to tissue destruction and damage that are relevant to emphysema commonly seen in HIV+ individuals. The concept that virus-induced disease pathogenesis can be mediated by virus-adult stem/progenitor cell interactions is consistent with the recent observation that Zika virus can infect human neural progenitor cell, leading to attenuated population growth through virus induced apoptosis and cell cycle dysregulation (Tang et al., 2016).
Supplementary Material
Acknowledgments
We thank Mattew S. Walters for helpful discussions; and Nahla Mohamed for help preparing this manuscript. These studies were supported, in part, by R01HL107882, R01HL118857 and U01HL121828.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession numbers. The data are publically available in the Gene Expression Omnibus (GEO) site (http://www.ncbi.nlm.nih.gov/geo), curated by the National Center for Bioinformatics. Accession number for the data is GSE85538.
Author contributions. N.P.C. conceived of the study, and performed research, data analysis and manuscript writing. X.O. performed research and data analysis. K.M.K. performed research and data analysis. J.S. performed RNA-seq analysis. R.J.K. conceived of the study and manuscript writing. R.G.C. conceived of the study, and performed data analysis and manuscript writing.
References
- Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- Beck JM, Rosen MJ, Peavy HH. Pulmonary complications of HIV infection. Report of the Fourth NHLBI Workshop. Am J Respir Crit Care Med. 2001;164:2120–2126. doi: 10.1164/ajrccm.164.11.2102047. [DOI] [PubMed] [Google Scholar]
- Beeh KM, Beier J, Kornmann O, Buhl R. Sputum matrix metalloproteinase-9, tissue inhibitor of metalloprotinease-1, and their molar ratio in patients with chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis and healthy subjects. Respir Med. 2003;97:634–639. doi: 10.1053/rmed.2003.1493. [DOI] [PubMed] [Google Scholar]
- Bhatia NS, Chow FC. Neurologic Complications in Treated HIV-1 Infection. Curr Neurol Neurosci Rep. 2016;16:62. doi: 10.1007/s11910-016-0666-1. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Ryscavage P, Taiwo B. Accelerated aging and human immunodeficiency virus infection: emerging challenges of growing older in the era of successful antiretroviral therapy. J Neurovirol. 2012;18:247–255. doi: 10.1007/s13365-011-0073-y. [DOI] [PubMed] [Google Scholar]
- Bobardt MD, Saphire AC, Hung HC, Yu X, Van der Schueren B, Zhang Z, David G, Gallay PA. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity. 2003;18:27–39. doi: 10.1016/s1074-7613(02)00504-6. [DOI] [PubMed] [Google Scholar]
- Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- Brune KA, Ferreira F, Mandke P, Chau E, Aggarwal NR, D’Alessio FR, Lambert AA, Kirk G, Blankson J, Drummond MB, et al. HIV Impairs Lung Epithelial Integrity and Enters the Epithelium to Promote Chronic Lung Inflammation. PLoS One. 2016;11:e0149679. doi: 10.1371/journal.pone.0149679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl R, Jaffe HA, Holroyd KJ, Borok Z, Roum JH, Mastrangeli A, Wells FB, Kirby M, Saltini C, Crystal RG. Activation of alveolar macrophages in asymptomatic HIV-infected individuals. J Immunol. 1993;150:1019–1028. [PubMed] [Google Scholar]
- Cataldo D, Munaut C, Noel A, Frankenne F, Bartsch P, Foidart JM, Louis R. MMP-2- and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2000;123:259–267. doi: 10.1159/000024452. [DOI] [PubMed] [Google Scholar]
- Churg A, Wang R, Wang X, Onnervik PO, Thim K, Wright JL. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax. 2007;62:706–713. doi: 10.1136/thx.2006.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costiniuk CT, Jenabian MA. The lungs as anatomical reservoirs of HIV infection. Rev Med Virol. 2014;24:35–54. doi: 10.1002/rmv.1772. [DOI] [PubMed] [Google Scholar]
- Cribbs SK, Lennox J, Caliendo AM, Brown LA, Guidot DM. Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. AIDS Res Hum Retroviruses. 2015;31:64–70. doi: 10.1089/aid.2014.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC, Veterans Aging Cohort 5 Project, T Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, Oursler KK, Rimland D, Gibert CL, Butt AA, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal RG. Airway basal cells. The “smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:1355–1362. doi: 10.1164/rccm.201408-1492PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpitt SV, Rogers DF, Traves SL, Barnes PJ, Donnelly LE. Sputum matrix metalloproteases: comparison between chronic obstructive pulmonary disease and asthma. Respir Med. 2005;99:703–710. doi: 10.1016/j.rmed.2004.10.022. [DOI] [PubMed] [Google Scholar]
- de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TB, Gallay P. Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proc Natl Acad Sci U S A. 2007;104:19464–19469. doi: 10.1073/pnas.0703747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demedts IK, Brusselle GG, Bracke KR, Vermaelen KY, Pauwels RA. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol. 2005;5:257–263. doi: 10.1016/j.coph.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Diaz PT, Clanton TL, Pacht ER. Emphysema-like pulmonary disease associated with human immunodeficiency virus infection. Ann Intern Med. 1992;116:124–128. doi: 10.7326/0003-4819-116-2-124. [DOI] [PubMed] [Google Scholar]
- Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, Drake J, Clanton TL. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132:369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- Diaz PT, Wewers MD, Pacht E, Drake J, Nagaraja HN, Clanton TL. Respiratory symptoms among HIV-seropositive individuals. Chest. 2003;123:1977–1982. doi: 10.1378/chest.123.6.1977. [DOI] [PubMed] [Google Scholar]
- Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–266. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanibunda SE, Modi DN, Gokral JS, Bandivdekar AH. HIV gp120 binds to mannose receptor on vaginal epithelial cells and induces production of matrix metalloproteinases. PLoS One. 2011;6:e28014. doi: 10.1371/journal.pone.0028014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foronjy R, Nkyimbeng T, Wallace A, Thankachen J, Okada Y, Lemaitre V, D’Armiento J. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1149–1157. doi: 10.1152/ajplung.00481.2007. [DOI] [PubMed] [Google Scholar]
- Gallay P. Syndecans and HIV-1 pathogenesis. Microbes Infect. 2004;6:617–622. doi: 10.1016/j.micinf.2004.02.004. [DOI] [PubMed] [Google Scholar]
- George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4:e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giantsou EPD. Chronic obstructive pulmonary disease in adults with human immunodeficiency virus infection: a systematic review. Health. 2011;3:218–227. [Google Scholar]
- Green LA, Yi R, Petrusca D, Wang T, Elghouche A, Gupta SK, Petrache I, Clauss M. HIV envelope protein gp120-induced apoptosis in lung microvascular endothelial cells by concerted upregulation of EMAP II and its receptor, CXCR3. Am J Physiol Lung Cell Mol Physiol. 2014;306:L372–382. doi: 10.1152/ajplung.00193.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzela K, Litwiniuk M, Zagorska W, Grzela T. Airway Remodeling in Chronic Obstructive Pulmonary Disease and Asthma: the Role of Matrix Metalloproteinase-9. Arch Immunol Ther Exp (Warsz) 2016;64:47–55. doi: 10.1007/s00005-015-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueders MM, Foidart JM, Noel A, Cataldo DD. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases. Eur J Pharmacol. 2006;533:133–144. doi: 10.1016/j.ejphar.2005.12.082. [DOI] [PubMed] [Google Scholar]
- Gum R, Wang H, Lengyel E, Juarez J, Boyd D. Regulation of 92 kDa type IV collagenase expression by the jun aminoterminal kinase- and the extracellular signal-regulated kinase-dependent signaling cascades. Oncogene. 1997;14:1481–1493. doi: 10.1038/sj.onc.1200973. [DOI] [PubMed] [Google Scholar]
- Gundavarapu S, Mishra NC, Singh SP, Langley RJ, Saeed AI, Feghali-Bostwick CA, McIntosh JM, Hutt J, Hegde R, Buch S, et al. HIV gp120 induces mucus formation in human bronchial epithelial cells through CXCR4/alpha7-nicotinic acetylcholine receptors. PLoS One. 2013;8:e77160. doi: 10.1371/journal.pone.0077160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, Witover B, Salit J, Crystal RG. The human airway epithelial basal cell transcriptome. PLoS One. 2011;6:e18378. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- Jiang AP, Jiang JF, Guo MG, Jin YM, Li YY, Wang JH. Human Blood-Circulating Basophils Capture HIV-1 and Mediate Viral trans-Infection of CD4+ T Cells. J Virol. 2015a;89:8050–8062. doi: 10.1128/JVI.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang AP, Jiang JF, Wei JF, Guo MG, Qin Y, Guo QQ, Ma L, Liu BC, Wang X, Veazey RS, et al. Human Mucosal Mast Cells Capture HIV-1 and Mediate Viral trans-Infection of CD4+ T Cells. J Virol. 2015b;90:2928–2937. doi: 10.1128/JVI.03008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalim S, Szczech LA, Wyatt CM. Acute kidney injury in HIV-infected patients. Semin Nephrol. 2008;28:556–562. doi: 10.1016/j.semnephrol.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaner RJ, Santiago F, Crystal RG. Up-regulation of alveolar macrophage matrix metalloproteinases in HIV1(+) smokers with early emphysema. J Leukoc Biol. 2009;86:913–922. doi: 10.1189/jlb.0408240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MJ, Oh YM, Lee JC, Kim DG, Park MJ, Lee MG, Hyun IG, Han SK, Shim YS, Jung KS. Lung matrix metalloproteinase-9 correlates with cigarette smoking and obstruction of airflow. J Korean Med Sci. 2003;18:821–827. doi: 10.3346/jkms.2003.18.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun. 2005;333:1107–1115. doi: 10.1016/j.bbrc.2005.05.198. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Labrie M, St-Pierre Y. Epigenetic regulation of mmp-9 gene expression. Cell Mol Life Sci. 2013;70:3109–3124. doi: 10.1007/s00018-012-1214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand C, Gilles C, Zahm JM, Polette M, Buisson AC, Kaplan H, Birembaut P, Tournier JM. Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol. 1999;146:517–529. doi: 10.1083/jcb.146.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Roche N, Oliver BG, Mattos W, Barnes PJ, Chung KF. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am J Respir Crit Care Med. 2000;162:1355–1360. doi: 10.1164/ajrccm.162.4.9910097. [DOI] [PubMed] [Google Scholar]
- Lopez-Herrera A, Liu Y, Rugeles MT, He JJ. HIV-1 interaction with human mannose receptor (hMR) induces production of matrix metalloproteinase 2 (MMP-2) through hMR-mediated intracellular signaling in astrocytes. Biochim Biophys Acta. 2005;1741:55–64. doi: 10.1016/j.bbadis.2004.12.001. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Li S, Wattenberg EV, Hudson LG. Sustained activation of the mitogen-activated protein kinase pathway. A mechanism underlying receptor tyrosine kinase specificity for matrix metalloproteinase-9 induction and cell migration. J Biol Chem. 1999;274:4347–4353. doi: 10.1074/jbc.274.7.4347. [DOI] [PubMed] [Google Scholar]
- Morris A, Crothers K, Beck JM, Huang L, American Thoracic Society Committee on, H.I.V.P.D An official ATS workshop report: Emerging issues and current controversies in HIV-associated pulmonary diseases. Proc Am Thorac Soc. 2011a;8:17–26. doi: 10.1513/pats.2009-047WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A, George MP, Crothers K, Huang L, Lucht L, Kessinger C, Kleerup EC, Lung HIVS. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc. 2011b;8:320–325. doi: 10.1513/pats.201006-045WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AM, Huang L, Bacchetti P, Turner J, Hopewell PC, Wallace JM, Kvale PA, Rosen MJ, Glassroth J, Reichman LB, et al. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. The Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 2000;162:612–616. doi: 10.1164/ajrccm.162.2.9912058. [DOI] [PubMed] [Google Scholar]
- Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, Flanigan TP, Kumar PN, Mintz L, Wallach FR, Nemo GJ, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- Naicker S, Rahmanian S, Kopp JB. HIV and chronic kidney disease. Clin Nephrol. 2015;83:32–38. doi: 10.5414/CNP83S032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova Z, Kolek V, Petrek M. Matrix Metalloproteinases and Their Inhibitors in Chronic Obstructive Pulmonary Disease. Arch Immunol Ther Exp (Warsz) 2016;64:177–193. doi: 10.1007/s00005-015-0375-5. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Takagi M, Kurokawa Y, Satomi S, Konttinen YT. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest. 1998;78:1077–1087. [PubMed] [Google Scholar]
- Owen CA. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:253–268. doi: 10.2147/copd.s2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Phair JP. Cardiovascular disease in HIV infection. Curr Opin HIV AIDS. 2011;6:266–271. doi: 10.1097/COH.0b013e328347876c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IW, Ullrich CK, Schoenberger E, Ganju RK, Groopman JE. HIV-1 Tat induces microvascular endothelial apoptosis through caspase activation. J Immunol. 2001;167:2766–2771. doi: 10.4049/jimmunol.167.5.2766. [DOI] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Petrache I, Diab K, Knox KS, Twigg HL, 3rd, Stephens RS, Flores S, Tuder RM. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax. 2008;63:463–469. doi: 10.1136/thx.2007.079111. [DOI] [PubMed] [Google Scholar]
- Plata F, Autran B, Martins LP, Wain-Hobson S, Raphael M, Mayaud C, Denis M, Guillon JM, Debre P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987;328:348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Reddy KB, Krueger JS, Kondapaka SB, Diglio CA. Mitogen-activated protein kinase (MAPK) regulates the expression of progelatinase B (MMP-9) in breast epithelial cells. Int J Cancer. 1999;82:268–273. doi: 10.1002/(sici)1097-0215(19990719)82:2<268::aid-ijc18>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Russell RE, Culpitt SV, DeMatos C, Donnelly L, Smith M, Wiggins J, Barnes PJ. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2002;26:602–609. doi: 10.1165/ajrcmb.26.5.4685. [DOI] [PubMed] [Google Scholar]
- Ryan DM, Vincent TL, Salit J, Walters MS, Agosto-Perez F, Shaykhiev R, Strulovici-Barel Y, Downey RJ, Buro-Auriemma LJ, Staudt MR, et al. Smoking dysregulates the human airway basal cell transcriptome at COPD risk locus 19q13.2. PLoS One. 2014;9:e88051. doi: 10.1371/journal.pone.0088051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphire AC, Bobardt MD, Gallay PA. Host cyclophilin A mediates HIV-1 attachment to target cells via heparans. EMBO J. 1999;18:6771–6785. doi: 10.1093/emboj/18.23.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphire AC, Bobardt MD, Zhang Z, David G, Gallay PA. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol. 2001;75:9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- Selman M, Cisneros-Lira J, Gaxiola M, Ramirez R, Kudlacz EM, Mitchell PG, Pardo A. Matrix metalloproteinases inhibition attenuates tobacco smoke-induced emphysema in Guinea pigs. Chest. 2003;123:1633–1641. doi: 10.1378/chest.123.5.1633. [DOI] [PubMed] [Google Scholar]
- Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell. 2008;135:1108–1117. doi: 10.1016/j.cell.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Goepfert H, Boyd D. Inhibition of the p38 mitogen-activated protein kinase by SB 203580 blocks PMA-induced Mr 92,000 type IV collagenase secretion and in vitro invasion. Cancer Res. 1998;58:1135–1139. [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg HL, 3rd, Spain BA, Soliman DM, Knox K, Sidner RA, Schnizlein-Bick C, Wilkes DS, Iwamoto GK. Production of interferon-gamma by lung lymphocytes in HIV-infected individuals. Am J Physiol. 1999;276:L256–262. doi: 10.1152/ajplung.1999.276.2.L256. [DOI] [PubMed] [Google Scholar]
- Yearsley MM, Diaz PT, Knoell D, Nuovo GJ. Correlation of HIV-1 detection and histology in AIDS-associated emphysema. Diagn Mol Pathol. 2005;14:48–52. doi: 10.1097/01.pas.0000142168.72253.11. [DOI] [PubMed] [Google Scholar]
- Yu Y, Sakai H, Misawa M, Chiba Y. Matrix Metalloproteinases-9 (MMPs-9) and -12 Are Upregulated in the Airways of Mice with Chronic Airway Inflammation and Remodeling. ISRN Pulmonology. 2012;2012:7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


