Abstract
Background
South Carolina (SC) ranks 10th in opioid prescriptions per capita - 33% higher than the national average. SC is also home to a large military and veteran population, and prescription opioid use for chronic pain is alarmingly common among veterans, especially those returning from Afghanistan and Iraq. This paper describes the background and development of an Academic Detailing (AD) educational intervention to improve use of a Prescription Drug Monitoring Program (PDMP) among SC physicians who serve military members and veterans. The aim of this intervention was to improve safe opioid prescribing practices and prevent prescription opioid misuse among this high-risk population.
Methods
A multidisciplinary study team of physicians, pharmacists, psychologists, epidemiologists, and representatives from the SCs Prescription Monitoring Program (PMP) utilized the Medical Research Council (MRC) complex interventions framework to guide the development of the educational intervention. The theoretical and modelling phases of the AD intervention development are described and preliminary evidence of feasibility and acceptability is provided.
Results
Ninety-three physicians consented to the study from 2 practice sites. Eighty-seven academic detailing visits were completed, and 59 one-month follow-up surveys were received. Participants rated the academic detailing intervention high in helpfulness of information, intention to use information, and overall satisfaction with the intervention. The component of the intervention felt to be most helpful was the academic detailing visit itself. Characteristics of the participants and the intervention, as well as anticipated barriers to behavior change are detailed.
Conclusions
Preliminary results support the feasibility of AD delivery to veteran and community patient settings, the feasibility of facilitating PDMP registration during an AD visit, and that AD visits were generally found satisfying to participants and helpful in improving knowledge and confidence about safe opioid prescribing practices. The component of the intervention felt to be most helpful to the participants was the actual academic detailing visit, and most participants rated their intentions high to use the information and tools from the visit. Intervention key messages, preliminary outcome measures, as well as successes and challenges in developing and delivering this intervention are discussed in order to advance best practices in developing educational interventions in this important area of public health.
Keywords: Academic detailing, Opioid, Prescribing, Prescription drug monitoring program
Introduction
Background
The Centers for Disease Control (CDC) and Prevention have declared abuse of prescription opioids a national epidemic.1 Although the US population represents only 4.6% of the global population, Americans consume 80% of the global opioid supply and 99% of the hydrocodone supply.2 More people die in the U.S. as a result of prescription opioid overdose than from heroin and cocaine overdose combined1, with more than three times as many people dying of prescription opioid overdoses in 2014 (16,000) than in 1999 (4,000).3,4 For young adults, prescription opioids have now become the drugs of choice for illicit use, second only to marijuana.3,5 Prescription opioid abuse and misuse also places a heavy burden on the health care system, accounting for almost a half million visits to emergency departments in 20094 and costing health insurers $72.5 billion annually in direct health care costs.6
While approximately 20% of the general population suffers from chronic non-cancer pain, an estimated 40% to 50% of Operation Enduring Freedom and Operation Iraqi Freedom OEF/OIF veterans report such pain.7 Studies document a historical over-reliance on opioid pain medications for treatment of combat veterans,8–10 and concerns that opioid medications are not ideal for long-term treatment of certain combat-related pain.11,12–16 More than one third of veterans are estimated to have misused substances to manage their pain.17 Thus, the Army’s Office of the Surgeon General’s Pain Management Task Force recommended mitigating the risk of prescription drug misuse among pain patients,18 and an Institute of Medicine Committee report on Substance Use Disorders in the U.S. Armed Forces called for the Department of Defense (DoD) to proactively prevent the misuse and abuse of prescription medications.19
For both military and non-military patient populations, primary care physicians are on the front lines of chronic pain management and are in a prime position for prevention efforts. However, training in pain management and safe opioid prescribing provided to physicians during medical school and residency remains minimal, leading to noted deficiencies in newly trained physicians in the management of chronic pain20 and calls for improvement in the scope, content, and duration of training in pain management in medical education.21 Although the Association of American Medical Colleges estimates that 93% of US medical schools planned or implemented changes in curriculum to address opiates, pain and substance abuse in the last 5 years,22 less than 20% of practicing primary care physicians consider themselves prepared to identify substance use disorders23 and less than half consider themselves sufficiently trained in prescribing opioids.24 This reveals an important educational gap for currently practicing primary care physicians who are on the front lines of this epidemic.
In response to this educational gap, the recent CDC guidelines for the management of chronic pain, 25 targeted at primary care physicians, sent a clear message about the risks of opioids and the lack of evidence for long-term use of opioids for chronic pain. The guideline recommends several strategies for monitoring patients on prescription opioids, including the use of state-operated prescription monitoring program (PMP) data to identify and prevent opioid misuse.26 PMP data provides a list of potentially abusable prescriptions dispensed to each patient, including opioid pain medications (e.g. oxycodone), benzodiazepines (e.g. lorazepam), and stimulants (e.g. amphetamines; Appendix A). PMPs were created as a tool to improve patient safety by fostering communication between patient and physician, to identify “doctor shopping, “ and to identify high-risk co-prescriptions (especially those that increase risk of overdose, such as benzodiazepines).27–30 PMPs have been shown to effectively reduce prescription drug use by modifying physician prescribing habits and reducing the surplus of abusable drugs.31,32 However, PMP data alone will not prevent opioid overdoses or improve clinical practice.33,34 It is known that clinicians have not consistently used PMPs,26 and when underutilized by prescribers, PMPs fall short of their intended role of providing information on patients’ recent opioid prescription (and other controlled substance) histories.35 To have a real impact, PMP data must be accessible and useful to prescribers who are engaged in opioid prescribing, and prescribers must increase both their knowledge and utilization of PDMP databases to accurately and effectively address issues of diversion, doctor-shopping, and high-risk medication combinations. As more PMPs have been launched and utilized, there have been calls for increased prescriber outreach, training and technical assistance in PMP use, including defining best practices, in order to improve prescriber awareness, utilization and interpretation of PMP data. 32, 36, 37–40
There have been no studies to date describing or evaluating PMP educational interventions for prescribers. Given the concentration of military members and veterans in SC, we developed a pilot project with funding from the National Institute on Drug Abuse that aimed to create a novel prevention program based on educational best practices in order to increase physician use of safe opioid prescribing practices, including routine use of a patient’s prescription history through a PMP. The specific aims of the project were to: (1) Design an educational intervention for physician prescribers applicable to environments treating military members, families, veterans; (2) Enroll 112 physicians in a pilot study of the intervention; and (3) Evaluate the feasibility and effectiveness of the pilot educational intervention. We describe the methods and outcomes of this study below.
Methods
Development of the Educational Intervention
In developing the educational intervention, the study team adhered closely to the Medical Research Council (MRC) complex intervention framework, which provides guidance on using a stepped approach to the development and evaluation of complex interventions (Figure 1).41 This phased approach separates the different questions being asked and helps researchers establish the probable active components of the intervention. Given that the proposed intervention would have several active educational components and would need to be adapted for delivery to physicians in various practice settings (VA, military, and community practices), it was felt that the MRC framework would assist the developers in tailoring the intervention to each local context while providing feasible solutions to individual barriers to behavior change (a key feature of Academic Detailing,42 described below). While the project started with NIH funding in September 2013, project partners began planning stages in 2012.
FIGURE 1.
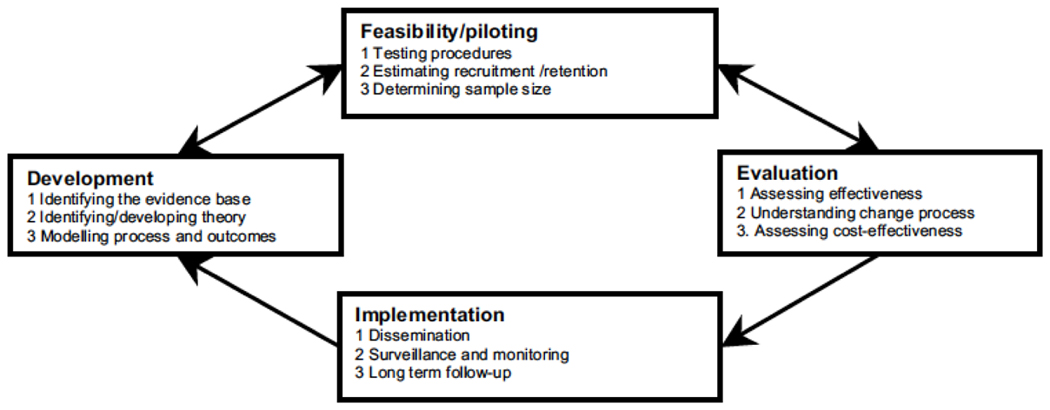
Key elements of the development and evaluation process. Used with permission from BMJ.
Theoretical Phase
This first phase of the MRC framework involves identifying the evidence that an educational intervention might have the desired effect. For this project, the planning partners gathered an expert panel on key topics and conducted a formal literature review of educational interventions for practicing physicians and chronic pain guidelines related to safe opioid prescribing. It was decided to further evaluate Academic Detailing (AD) as an educational outreach intervention. AD, which provides face-to-face, interactive education of prescribers by trained health care professionals, was felt to be the optimal educational intervention to bridge the logistical and educational gap in prescriber’s effective use of PMP data34,43 as it could assess and address the physician’s prescribing concerns while providing training on safe practices for optimal pain management.44,45 Additionally, face-to-face AD interventions have demonstrated success in enhancing physician prescribing behavior and changing health care professionals’ practice patterns,46–49 and economic analysis has found that for each $1 spent on AD programs, $2 was saved in Medicaid expenditures.31,45 Additionally, although AD is recognized as an effective intervention to change and reinforce prescribing behavior and has a role in preventive care, there are no studies evaluating use of AD to facilitate integration of PMP systems into primary care practice.
Academic Detailing (AD) Intervention Development
Development and Review of Key Messages
After reviewing all available pain guidelines, applicable literature, and principles of academic detailing, the SCOSI-M clinical team, consisting of physicians (addiction, internal medicine, psychiatry), pharmacists, trained academic detailers and psychologists met to consolidate literature review to inform selected key educational messages. Acknowledging the time restraints of any educational intervention, the team chose three preliminary key messages (with the acronym “S.O.S.”):
Share a patient provider agreement.
Optimize patient treatment using a multi-dimensional pain rating scale.
Screen for appropriate opioid use, including accessing PMP data.
The team then undertook further review of each of the chosen key messages to delineate the best standard of care recommendations for each key message, using the principles of the AD to ensure the messages were practical as well as patient-and practice-oriented. The ADs also ensured that the intervention and messages would be collaborative, solution-oriented, and geared towards having the learners “learning by doing” by having the prescribers sign in to the PMP and utilize the system during the session. This allowed ADs to immediately troubleshoot any barriers to signing into the system as well as provide education in the interpretation of real-time PMP data.
Modelling Phase
The next phase of the MRC framework delineates component parts of the educational intervention and how the active components of the package may relate to final outcomes. Phase I included a focus group with prescribers serving military and non-military patients, interactive workshop sessions between detailers and physician consultants, development of educational materials to support and reinforce the key messages of the intervention, and live testing of the AD intervention.
Prescriber Focus Group
Facilitated discussions with practicing physicians (n=4) on opioid prescribing for pain provided a ‘real time’ needs assessment, fine-tuning of key messages, a discussion of perceived benefits and barriers for each key message, and a reminder of the importance of empathic AD delivery.
The major barriers identified in implementing the key messages were insufficient prescriber knowledge combined with insufficient time and resources to learn and implement safe opioid prescribing practices (including cumbersome sign-up process for PMP, and lack of patient-provider agreements and pain/risk scales)
The team also worked with the focus group to identify potential facilitators to implementing key messages. Prescribers were not knowledgeable about AD, but were open to this educational option if it could be time-efficient (i.e. about 20 minutes), personalized, clinically relevant and meaningful to their practice. All providers believed chronic pain management was a topic worthy of learning. They also voiced a need for in-office facilitators for screening and educating patients, such as easy-to use patient educational handouts and screening tests and assistance with PMP registration.
Interactive workshop sessions
Workshops allowed the detailers and physician consultants to collectively identify: (1) components of the key messages on safe opioid use that were most likely to change physician behavior with regards to safe opioid prescribing, (2) barriers to acceptance of the key messages, and (3) enablers (e.g,. education, tools) to overcome barriers. Pre-workshop reading assignments allowed for collective literature evaluation and advanced the core clinical training of each detailer. The end result was refinement of key messages, identification of component parts of the intervention, identification of support materials and physician/patient tools to include in physician packets, and mentoring for each detailer.
Component Parts
Based on the focus groups, workshops, and the literature review, the following 5 component parts of the AD intervention were identified:
Patient-Provider Agreement (PPA): The team vetted and consolidated all available published PPAs, creating one patient-centered, carbon-copied, PPA that included patient education and informed consent. This agreement was reviewed and modified based on feedback from four prescribers.
Validated Pain Scale: The study team reviewed all validated pain rating scales appropriate for primary care. Based upon its validation in primary care populations and the efficiency with which it can be administered, the team chose the PEG scale,50 a 3-item abbreviated scale of the Brief Pain Inventory that includes one intensity item and two interference items (Pain intensity during the past week, pain interference with Enjoyment of life, and pain interference with General activity, scored 0–10).
Facilitated PMP registration: The study team collaborated with SC DHEC to develop a protocol for facilitated PMP registration. To ensure all mandatory privacy requirements were met, as they enrolled on-line for the visit, physicians (1) read and provided acknowledgement of the DHEC privacy statement; (2) provided practice address and DEA number; and (3) gave consent for immediate registration. The study team sent this information to DHEC prior to the visit; the new PMP account was activated in real-time during the AD visit and DHEC provided the PMP user ID and password. This facilitated registration streamlined several routine steps identified as barriers. It replaced the need to complete the on-line training and the need for a signature on the privacy statement observed by a notary. While SC Department of Health and Environmental Control (DHEC) usually requested 2-week lead time to register a new physician for the PMP, this process allowed the registration to be streamlined to two days. The detailer then provided a real-time tutorial on the use of PMP. This process was refined for physicians at the VA.
Visit Protocol: Face-to-face meetings between the detailer and physicians provide individualized educational outreach (1) to help with safe and evidence-based opioid prescribing and (2) to support patient care decisions that balance risks and benefits of opioids in chronic pain. The visit content and protocol is detailed below.
Incentive: The study team worked to ensure that the AD visit would contribute to required hours and that enrolled physicians could earn up to 2 credits towards Continuing Medical Education through The University of South Carolina School of Medicine – Palmetto Health CME Organization.
AD Visit Content and Training
Our lead Academic Detailer trained five additional detailers. An Advanced AD Skills Workshop was held and a detailed internal document was developed as a reference and pre-visit refresher.
Live test visits with physicians replaced the role plays normally used to prepare for AD visits. The AD visits included a brief introduction, rapport building, and use of open-ended questions to assess the physician’s perceived interest in each key message and possible barriers to change in practice and prescribing. Detailers addressed key messages using interactive techniques, and contacted DHEC during the visit to complete the physician’s PMP registration. If the physician had interest and time, the pharmacist had the physician log onto the PMP with his or her own credentials and search for a known patient using name and date of birth information (when this could not be done, a hypothetical record was shown). The detailer then explained appropriate use of the information on the patient record, using examples from the physician package. The intervention was semi-structured with flexibility with regard to the pace and content emphasis a particular physician may need. The facilitated PMP registration allowed for on-site training in generating and interpreting PMP reports, which is an efficient, practical, and practice-oriented educational best practice that optimizes active learning while saving the physician time. During the live tests, detailers ensured they assessed the learner’s needs, engaged in collaborative problem-solving learning activities, tested the clinical support tools/materials, and allowed the “learners to do the work of learning” as they signed in and navigated the PMP. Any barriers to implementing the intervention were noted and addressed, and take-aways from live visits were shared among the AD team.
Preparation for project launch and recruitment
With a goal of recruiting 100 participants (110 with expected 10% attrition), the project developed a study website to facilitate recruitment, written consent, enrollment and confidential data collection. A participant application was developed which included screening questions, informed consent (including education and consent specific to the PMP), and a confidential baseline questionnaire. Participation was voluntary and was not based on provider characteristics such as prescribing patterns. The study’s human subjects procedures were approved by the Brandeis University Institutional Review Board and are also governed by a Data Safety Monitoring Plan. SC DHEC was a partner in project development, and data release was governed by a Data Sharing Agreement.
Results
Of 116 physician applications received for the study, 93 were complete, 21 either failed screening or did not offer informed consent, one was a duplicate subject, and one was a ‘test’ case.
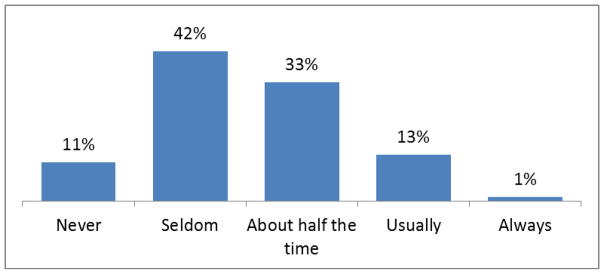
All physicians provided informed consent and completed a baseline questionnaire (Table 1; Figure 2). The project successfully recruited a diverse group of physician participants (Table 1). The majority had been in medical practice for more than 10 years; a substantial minority claimed they had registered in the past for the PMP; and the majority treated more than 40 patients with chronic, non-cancer pain. The physician applicants were also heterogeneous in their self-reported prescribing of Schedule II opioids (opioids with higher levels of abuse potential) for patients with chronic pain (Figure 2).
Table 1.
Participants recruited to educational intervention (n=93)
| Physician Participant Characteristic | N (%) |
|---|---|
| Race/ethnicity | |
| Black, non-Hispanic | 16 (20.0%) |
| White, non-Hispanic | 51 (63.8%) |
| Other, non-Hispanic | 13 (16.2%) |
| Hispanic (any race) | 9 (9.7%) |
| Not reported | 4 (4.3%) |
| Gender | |
| Female | 47 (50.5%) |
| Male | 46 (49.5%) |
| Setting | |
| Veterans Administration | 49 (52.7%) |
| Community clinic | 44 (47.3%) |
| Years of Medical Practice | |
| Under 5 | 15 (16.1%) |
| 5–10 | 15 (16.1%) |
| 11–20 | 25 (26.9%) |
| More than 20 | 38 (40.9%) |
| Area of practice | |
| General, internal, family | 87 (93.6%) |
| Other (oncologists not eligible) | 6 (6.4%) |
| Registered PMP User, Self-report | |
| Yes, prior to visit | 43 (46.2%) |
| Not prior to visit | 50 (53.8%) |
| Number of patients under treatment for chronic, non-cancer pain | |
| 40 or more | 56 (60.2%) |
| Under 40 | 37 (39.8%) |
PMP=Prescription Monitoring Program
FIGURE 2.
How frequently do you prescribe a Schedule II opioid to patients with chronic, non-cancer pain (n=93)
Visit Completion Results
The academic detailing visits occurred between September 23 and November 20, 2015, which required substantial coordination of the schedules of the part-time project pharmacists to accommodate driving times to distant clinic offices, to group physicians in similar locations, and to match physician schedules. Five project-trained pharmacists completed 87 visits (93.5% of applicants) and successfully submitted study documentation for 86 visits (one missing). The remaining six applicants either completed their application after the pharmacist visitor had traveled to a distant location and could not return to that location, or submitted their application too late for DHEC staff to ‘open’ a PMP account prior to a visit. After the launch of the intervention, we learned that the PMP data vendor would change, and this would result in a new registration process, new interface, and new PMP patient record. Thus, the ending of the PMP contract became a non-negotiable end date for all pharmacist visits.
The pharmacist detailer completed a 24-item data collection form after each visit including closed- and open-ended items that captured the training conditions, start and end time for visit (for CME credits), number of physicians educated, other staff in attendance, areas where the physician had additional questions, anticipated barriers to implementation, and administrative items about materials and follow-up address (Table 2; Figure 3).
Table 2.
Characteristics of Academic Detailing Visits (n=87)
| Visit Characteristic | Measurement |
|---|---|
|
| |
| Mean visit length, min | 62.3 |
|
| |
| Mean number of physicians in visit (range) | 1.3 (1–3) |
|
| |
| Number of physicians in visit (%) | |
| 1 | 59 (69%) |
| 2 | 24 (28%) |
| 3 | 3 (3%) |
|
| |
| Visits with clinical/administrative staff present (%) | 11 (13%) |
|
| |
| Location of visit in physician’s office (%) | 44 (50%) |
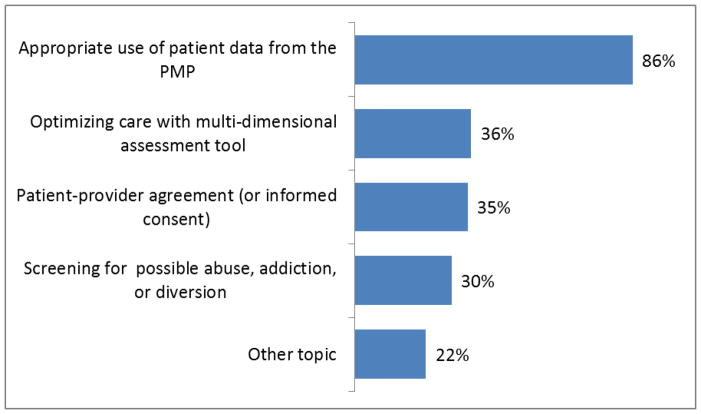
Figure 3.
Topic about which the physician had further questions
Characteristics of Visits
While the projected visit time was only 20–30 minutes, the mean visit length with VA physicians was 59.9 minutes (median 57.5) and for community physicians was 64.8 minutes (median 60.0), demonstrating a high level of engagement in the visit. We assessed that the optimal strategy was one physician in each visit, however, we tailored the visit to the requirements of the clinic and physician’s desires and a substantial proportion were conducted with pairs or trios of physicians (Table 2). Thirteen percent of the visits (n=11) also had other clinical or administrative staff in attendance; these participants included office administrator, nurse, nurse practitioner, physician assistant, office manager, and clinic pharmacist. Thus, team members of the physician were sometimes invited to “listen in”. One half of visits occurred in the physician’s own office, as intended, but other visits were held in a conference room, an empty exam room, or break room.
PMP Log-on
The pharmacist detailer was not always successful in having the physician actually log onto the PMP database and accomplish “real time” experience with this skill. Based on the visit report form, 7 participants (8%) did not complete this task, which was a critical component of the education. The reasons were somewhat idiosyncratic and demonstrate the multiple steps necessary for ‘successful completion’. For 3 of the 7 unsuccessful visits, the noted reason was that the physician did not have a DEA number (which was voluntary for VA physicians) and this precluded DHEC from opening an account for the physician. For the other 4 unsuccessful visits it was a time constraint and discussion of other key messages. However, 3 of these physicians who did not log into the PMP were already registered and had used the PMP in the past. One time constraint occurred because a pair of physicians was being trained and time permitted one to log into the PMP and not the second physician. In this instance, the physician who received the practice experience offered to sit with the untrained physician after the visit. In sum, the visits were highly tailored to the expressed needs of the physician participant and respectful to the time they had available.
Additional questions and anticipated barriers
On the visit report form, the pharmacist also reported which areas, if any, the physician showed engagement by asking questions to elicit more information. Figure 2 below shows the areas where the physician had follow-up questions that demonstrated interest in learning more about a key message, which was predominantly about the appropriate use of PMP data (86%).
Finally, the pharmacist detailer reported open-ended observations about the types of barriers the physician expressed about using the PMP or implementing aspects of the three SOS key messages (Table 3). Although many participants anticipated no barriers to implementing the SOS messages, difficulty running or remembering to run PMP reports and having time for implementation of the key messages (including PMP use) were the most frequently cited.
TABLE 3.
Anticipated barriers to practice change
| PMP Use (n=75) | N |
|---|---|
| No barriers | 32 |
| Time constraints | 19 |
| Remembering to run/how to run reports; need practice | 11 |
| Concerns about data (accuracy; interpretation; consequences; need data from border state) | 10 |
| Difficult to use; not “user-friendly” | 6 |
| Concern about incorporating into workflow | 5 |
| Computer/connection issues | 2 |
| Liability concerns | 2 |
| Other (don’t write controlled substances; switching to new vendor; want/have delegate; unsure how to document) | 6 |
| Other “SOS” Messages (n=71) | N |
| No barriers | 35 |
| Time constraints to using SOS approach | 16 |
| Concern for push-back from patients using SOS approach | 6 |
| Issues locating electronic health records | 3 |
| Concern for liability issues with Patient-Provider Agreement | 1 |
| Unclassified | 4 |
PMP=Prescription Monitoring Program; DEA= Drug Enforcement Administration; SOS=Share a patient provider agreement; Optimize patient treatment using a multi-dimensional pain rating scale; Screen for appropriate opioid use.
Discussion
The SCOSI-M initiative brought together stakeholders from across the country to develop a state-wide educational intervention for physicians aimed at improving the utilization of standard-of-care risk reduction measures for patients on opioid pain medications. Given the multi-faceted nature of chronic pain, the varying challenges of safe opioid prescribing for patients in various practice settings (military and non-military), and the rapidly evolving legislative environment regarding opioid prescribing and education, this project necessitated a complex and flexible educational intervention that comprised multiple interacting components to optimize the likelihood of behavioral change and adhere to best educational practices (e.g. using a variety of educational methods).
In this study, the MRC framework and principles of AD were used to identify and clarify potential component parts of the intervention and how the active components might relate to the expected outcome of behavioral change. The SCOSI-M intervention utilized active learning methods (Academic Detailing) and facilitated PDMP registration to improve the acceptance and integration of a prevention technology (PMP use) into routine primary care practice in order to address a major public health issue. The MRC framework allowed us to identify and address development and implementation challenges and enablers in order to adapt the intervention to various practice settings and in response to barriers such as evolving legislative changes.
These results support the feasibility of AD delivery to physicians in veteran and community patient settings, the feasibility of facilitating PDMP registration during an AD visit and that participants were actually engaged in AD visit, asking follow-up questions, spending on average an hour with the visitor, and having discussions of perceived barriers. However, at the time of the visit, these findings identified that one of the major anticipated barriers to behavior change involved time constraint and difficulty running or remembering to run PMP reports. Further, we are currently analyzing follow-up survey data from participants and these data indicate that the AD visit was helpful and that most participants were able to identify informational components and tools that they intended to use when prescribing opioids.
There are several limitations to this study. Although the 93 completed applications exceeded recruitment goals for two of the three recruitment settings (VA and community physicians), we fell short of the desired 112 applications which we believed would yield 100 completed visits. We received no applications from physicians at military treatment facilities, and we underestimated the challenges involved in recruitment from military and VA facilities. While there was some expressed interest among leadership individuals at one local military program, the project was unable to address all administrative concerns about participation in the training intervention, in part because of the data collection that would permit us to evaluate the effect of the pilot intervention. The success in gaining access to the VA came after many contacts and conversations with the facility, ultimately identifying a VA champion with mutual interest in PMP training, which provided common ground for collaboration.
Second, throughout the intervention development and launch, the VA’s and SC’s controlled substance policy environment was evolving, including legislative changes to permit delegate use of PMP, mandated controlled substance education in SC, and mandated use of PMP use when prescribing a controlled substance in SC. Additionally, the SC PMP changed its data vendor at the end of project AD visits, which improved the registration process and improved the format of the patient record. Thus, while we may have been successful in orienting new users to the system, we don’t know if this is sufficient for them to remain users when the system changed. These system-wide changes have disrupted components of our planned evaluation by introducing a discontinuity in the system that both interrupted use of a system that we trained on, and also greatly increased the number of new PMP users by requiring its use. Further, some critical data elements we anticipated receiving to compare physician requests for PMP data before and after training may now be inaccessible because the previous vendor data were archived. Lastly, physicians were not randomly selected, so generalizability is unknown. Last, although this educational intervention focused on academic detailing, the facilitated PMP registration could be considered a form of practice facilitation.51 In keeping with the MRC framework, future versions of this intervention should evaluate these components separately to determine the differential effects on outcomes, if any. Additionally, future versions of this intervention could incorporate performance measurement and feedback,52 which is often used with academic detailing, as well as estimates of cost during evaluation.
Some advantages of this study are the adherence of the intervention to the principles of academic detailing,42 and the use of a thorough needs assessment through the MRC framework. Additionally, we were able to recruit participants from two different practice environments (VA and community). Another strength was the detailed implementation record-keeping maintained by the AD visitors which permitted clear documentation of time spent, topics pursued by physicians, and key barriers discussed. This information provides valuable data for implementation of future interventions. Finally, our follow-up survey data now under analysis indicates we were successful in recontacting and obtaining surveys to evaluate self-report change in key behaviors from 68 participants, or over 78% of physicians visited.
As more PMPs are launched and utilized, and as mandated use of PMPs increase, there will be a continued need for prescriber education to improve awareness, utilization and interpretation of PDMP data,32, 37–40 and evaluation of the best training methods. Given its feasibility and acceptability to the target audience, academic detailing with facilitated PDMP registration is a feasible and promising approach that should be considered to address this training need. Pre- and post- outcome data in future studies should further evaluate whether this intervention changes provider behavior, is cost-effective, and ultimately improves patient outcomes.
Appendix: Prescription drug monitoring program
Lessons for Practice.
Physicians cite insufficient time and resources to learn and implement safe opioid prescribing practices
Academic detailing can address barriers to safe opioid prescribing
State-wide academic detailing interventions are feasible and acceptable to military and non-military physicians
Acknowledgments
Authors acknowledge Christie Frick, RPH and Tracie Anderson, RPH, from South Carolina Department of Health and Environmental Control for assistance with developing the educational intervention. We also acknowledge Crystal Endsley, PharmD at the Dorn VAMC for assistance with launching the intervention at the VA site.
Funding: Dr. Wooten’s effort is funded by the National Institute on Drug Abuse (K01DA037412). Dr. Larson, Dr. Adams and Ruslan Nikitin’s effort is funded in part National Institute on Drug Abuse (R34 DA037039). Dr. Barth served as a consultant for the R34 (DA037039).
Footnotes
Author Contribution: Mary Jo Larson, Kelly S. Barth and Sarah Ball contributed to study design. All authors interpreted the data and contributed in manuscript writing. Kelly S. Barth, Rachel Sayko Adams, Ruslan Nikitin, Sarah Ball, and Mary Jo Larson wrote the iterative drafts of the manuscripts. All authors read, contributed to, and approved the final manuscript.
Contributor Information
Kelly S. Barth, Associate Professor, Department of Psychiatry and Behavioral Sciences, College of Medicine, Medical University of South Carolina, Charleston, SC
Sarah Ball, Research Assistant Professor, Division of General Internal Medicine and Geriatrics, Medical University of South Carolina, Charleston, SC.
Rachel Sayko Adams, Scientist, Institute for Behavioral Health, The Heller School for Social Policy and Management, Brandeis University, Waltham, MA.
Ruslan Nikitin, Research Associate, Institute for Behavioral Health, Heller School for Social Policy and Management, Brandeis University, Waltham, MA, USA.
Nikki R. Wooten, Assistant Professor, College of Social Work, University of South Carolina, Chair, Military Specialization, Lieutenant Colonel, Army National Guard.
Zaina P. Qureshi, Assistant Professor, Dept. of Health Services Policy and Management, University of South Carolina, Columbia, SC AND Adjunct Professor, Clinical Pharmacy and Outcomes Sciences, South Carolina College of Pharmacy, Columbia, SC.
Mary Jo Larson, Senior Scientist and Lecturer, Heller School for Social Policy and Management, Brandeis University, Waltham, MA, USA.
References
- 1.Vital Signs: Overdoses of prescription opioid pain relievers — United States, 1999—2008. MMWR. 2011 Nov 4;60(43):1487–1492. [PubMed] [Google Scholar]
- 2.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11:S63–S88.1. [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. The TEDS Report: Substance Abuse Treatment Admissions Involving Abuse of Pain Relievers: 1998 and 2008. Rockville, MD: Jul 15, 2010. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Number and age-adjusted rates of drug-poisoning deaths involving opioid analgesics and heroin: United States, 2000–2014 2015 [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: volume 1: summary of national findings. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2010. [Accessed June 2, 2016]. at: http://oas.samhsa.gov/nsduh/2k9nsduh/2k9resultsp.pdf. [Google Scholar]
- 6.Coalition Against Insurance Fraud. Prescription for peril: how insurance fraud finances theft and abuse of addictive prescription drugs. Washington, DC: Coalition Against Insurance Fraud; 2007. [Accessed September 26, 2011]. Available at http://www.insurancefraud.org/downloads/drugDiversion.pdf. [Google Scholar]
- 7.Stecker T, Fortney J, Owen R, McGovern MP, Williams S. Co-Occurring Medical, Psychiatric, and Alcohol-Related Disorders Among Veterans Returning From Iraq and Afghanistan. Psychosomatics. 2010;51(6):503–507. doi: 10.1176/appi.psy.51.6.503. [DOI] [PubMed] [Google Scholar]
- 8.Office of the Army Surgeon General. Pain Management Task Force: Providing a Standardized DoD and VHA Vision and Approach to Pain Management to Optimize the Care for Warriors and their Families. Final Report. 2010 Sep 26; www.armymedicine.army.mil/reports/Pain_Management_Task_Force.pdf. 2013.
- 9.Kim HM, Smith EG, Ganoczy D, et al. Predictors of suicide in patient charts among patients with depression in the Veterans Health Administration health system: importance of prescription drug and alcohol abuse. J Clin Psychiatry. 2012;73(10):e1269–1275. doi: 10.4088/JCP.12m07658. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen CJ, Stander VA, McWhorter SK, Rabenhorst MM, Milner JS. Effects of combat deployment on risky and self-destructive behavior among active duty military personnel. J Psychiatr Res. 2011;45(10):1321–1331. doi: 10.1016/j.jpsychires.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Hooten WM, Bruce BK. Beliefs and attitudes about prescribing opioids among healthcare providers seeking continuing medical education. J Opioid Manag. 2011;7(6):417–424. doi: 10.5055/jom.2011.0082. [DOI] [PubMed] [Google Scholar]
- 12.Morasco B, Duckart J, Dobscha S. Adherence to Clinical Guidelines for Opioid Therapy for Chronic Pain in Patients with Substance Use Disorder. J Gen Intern Med. 2011;26(9):965–971. doi: 10.1007/s11606-011-1734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 14.Maxwell SR, McQueen DS, Ellaway R. eDrug: a dynamic interactive electronic drug formulary for medical students. British journal of clinical pharmacology. 2006;62(6):673–681. doi: 10.1111/j.1365-2125.2006.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) Guidelines. Pain physician. 2008;11(2 Suppl):S5–S62. [PubMed] [Google Scholar]
- 16.Department of Veterans Affairs, Department of Defense. [Accessed June 2, 2016];VA/DoD Clinical Practice Guideline for the Management of Opioid Therapy for Chronic Pain. 2010 http://www.healthquality.va.gov/cot/cot_310_full.pdf.
- 17.Goebel JR, Compton P, Zubkoff L, et al. Prescription sharing, alcohol use, and street drug use to manage pain among veterans. J Pain Symptom Manage. 2011 doi: 10.1016/j.jpainsymman.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Office of the Army Surgeon General. Pain Management Task Force: Providing a Standardized DoD and VHA Vision and Approach to Pain Management to Optimize the Care for Warriors and their Families. 2010 [Google Scholar]
- 19.IOM (Institute of Medicine) Substance Use Disorders in the US Armed Forces. Washington, DC: The National Academies Press; 2012. http://www.nap.edu/openbook.php?record_id=13441. [PubMed] [Google Scholar]
- 20.Crosson FJ, Leu J, Roemer BM, Ross MN. Gaps in residency training should be addressed to better prepare doctors for a twenty-first-century delivery system. Health Affairs. 2011;30(11):2142–8. doi: 10.1377/hlthaff.2011.0184. [DOI] [PubMed] [Google Scholar]
- 21.Lippe PM, Brock C, David J, Crossno R, Gitlow S. The First National Pain Medicine Summit—Final Summary Report. Pain Medicine. 2010;11(10):1447–1468. doi: 10.1111/j.1526-4637.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 22.O’Rourke J. BU Today. Boston University Office of Marketing & Communications; Boston, MA: 2016. New MED Curriculum Aimed at Stemming Explosive Rise in Opioid Misuse. [Google Scholar]
- 23.CASA. The National Center on Substance Abuse at Columbia University. Missed opportunity: national survey of primary care physicians and patients on substance abuse. National Center on Substance Abuse at Columbia University; New York: 2000. [Google Scholar]
- 24.Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag. 2014;10(6):375–82. doi: 10.5055/jom.2014.0234. [DOI] [PubMed] [Google Scholar]
- 25.Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016a;315(15):1624–45. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain-United States, 2016. MMWR Recomm Rep. 2016b;65(RR-1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 27.Prescription Drug Monitoring Program Center of Excellence at Brandeis University. Notes from the Field, NF 3.2 Project Lazarus: Using PDMP Data to Mobilize and Measure Community Drug Abuse Prevention. Waltham, MA: Brandeis University, Prescription Drug Monitoring Center of Excellence; 2012. p. 74. [Google Scholar]
- 28.Prescription Drug Monitoring Program Center of Excellence at Brandeis University. Notes from the Field, NF 1.1 Trends in Wyoming PMP Prescription History Reporting: Evidence for a Decrease in Doctor Shopping? Waltham, MA: Brandeis University, Prescription Drug Monitoring Center of Excellence; 2010. [Google Scholar]
- 29.Prescription Drug Monitoring Program Center of Excellence at Brandeis University. Notes from the Field, NF 2.6 Drug-Related Deaths in Virginia: Medical Examiner Use of PMP Data. Waltham, MA: Brandeis University, Prescription Drug Monitoring Center of Excellence; 2011. [Google Scholar]
- 30.Prescription Drug Monitoring Program Center of Excellence at Brandeis University. Notes from the Field, NF 3.1 Real Time Reporting: Oklahoma’s Pioneering PMP. Waltham, MA: Brandeis University, Prescription Drug Monitoring Center of Excellence; 2012. [Google Scholar]
- 31.Simeone R, Holland L. An Evaluation of Prescription Drug Monitoring Programs. 2006 Available on the Web: http://www.simeoneassociates.com/simeone3.pdf.
- 32.Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation of Prescription Drug Monitoring Programs associated with reductions in opioid-related death rates. Health Affairs. 2016;35(7):1324–1332. doi: 10.1377/hlthaff.2015.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011 May;12(5):747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 34.Reifler LM, Droz D, Bailey JE, et al. Do Prescription Monitoring Programs Impact State Trends in Opioid Abuse/Misuse? Pain Medicine. 2012;13(3):434–442. doi: 10.1111/j.1526-4637.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- 35.Deyo RA, Irvine JM, Millet LM, et al. Measures Such As Interstate Cooperation Would Improve The Efficacy Of Programs To Track Controlled Drug Prescriptions. Health Affairs March 1, 2013. 2013;32(3):603–613. doi: 10.1377/hlthaff.2012.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carnevale Associates L. Leveraging Prescription Drug Monitoring Programs to Reduce Drug Use and its Damaging Consequences. Gaithersburg, MD: 2011. [Google Scholar]
- 37.Rutkow L, Turner L, Lucas E, Hwang C, Alexander GC. Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health Affairs. 2015;34(3):484–92. doi: 10.1377/hlthaff.2014.1085. [DOI] [PubMed] [Google Scholar]
- 38.Barrett K, Watson A. Physician perspectives on a pilot prescription monitoring program. J Pain Palliat Care Pharmacother. 2005;19(3):5–13. [PubMed] [Google Scholar]
- 39.Irvine JM, Hallvik SE, Hildebran C, Marino M, Beran T, Deyo RA. Who uses a prescription drug monitoring program and how? Insights from a statewide survey of Oregon clinicans. J Pain. 2014;15(7):744–55. doi: 10.1016/j.jpain.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman L, Williams KS, Coates J, Knox M. Awareness and utilization of a prescription monitoring program among physicians. J Pain Palliat Care Pharmacother. 2011;25(4):313–7. doi: 10.3109/15360288.2011.606292. [DOI] [PubMed] [Google Scholar]
- 41.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeh JS, Van Hoof TJ, Fischer MA. Key Features of Academic Detailing: Development of an expert consensus using the Delphi method. Am Health Drug Benefits. 2016;9(1):42–50. [PMC free article] [PubMed] [Google Scholar]
- 43.Katz N, Panas L, Kim M, et al. Usefulness of prescription monitoring programs for surveillance—analysis of Schedule II opioid prescription data in Massachusetts, 1996–2006. Pharmacoepidemiol Drug Saf. 2010;19(2):115–123. doi: 10.1002/pds.1878. [DOI] [PubMed] [Google Scholar]
- 44.Clow PW, Dunst CJ, Trivette CM, Hamby DW. Educational Outreach (Academic Detailing) and Physician Prescribing Practices. [Accessed on June 2, 2016];Cornerstones. 1(1):1–19. 200. at http://www.tracecenter.info/cornerstones/cornerstones_vol1_no1.pdf. [Google Scholar]
- 45.Lu CY, Ross-Degnan D, Soumerai SB, Pearson SA. Interventions designed to improve the quality and efficiency of medication use in managed care: a critical review of the literature - 2001–2007. BMC Health Serv Res. 2008;8:75. doi: 10.1186/1472-6963-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avorn J, Soumerai SB. Improving drug-therapy decisions through educational outreach. A randomized controlled trial of academically based “detailing”. N Engl J Med. 1983;308(24):1457–1463. doi: 10.1056/NEJM198306163082406. [DOI] [PubMed] [Google Scholar]
- 47.Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21(3):380–385. doi: 10.1017/s026646230505049x. [DOI] [PubMed] [Google Scholar]
- 48.May FW, Rowett DS, Gilbert AL, McNeece JI, Hurley E. Outcomes of an educational-outreach service for community medical practitioners: non-steroidal anti-inflammatory drugs. Med J Aust. 1999;170(10):471–474. [PubMed] [Google Scholar]
- 49.O’Brien M, Rogers S, Jamtvedt G, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD000409.pub2. Art. No.: CD000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland J, Asch SM, Kroenke K. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24:733–738. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Hoof TJ, Grant R, Cambpell C, et al. Society for Continuing Medical Education Intervention Guideline Series: Guideline 2, Practice Facilitation. JCEHP. 2015;35(Suppl 2):S55–S59. doi: 10.1097/CEH.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 52.Van Hoof TJ, Grant RE, Miller NE, et al. Society for Academic Continuing Medical Education Intervention Guideline Series: Guideline 1, Performance measurement and feedback. JCEHP. 2015;35(Suppl 2):S51–S54. doi: 10.1097/CEH.0000000000000013. [DOI] [PubMed] [Google Scholar]