Abstract
Event-related oscillations (EROs) are rhythmic changes that are evoked by a sensory and/or cognitive stimulus that can influence the dynamics of the EEG. EROs are defined by the decomposition of the EEG signal into magnitude (energy) and phase information and can be elicited in both humans and animals. EROs have been linked to several relevant genes associated with ethanol dependence phenotypes in humans and are altered in selectively bred alcohol-preferring rats. However, pharmacological studies are only beginning to emerge investigating the impact low intoxicating doses of ethanol can have on event-related neural oscillations. The main goal of the present study was to investigate the effects of low levels of voluntary consumption of ethanol, in rats, on phase locking of EROs in order to give further insight into the acute intoxicating effects of ethanol on the brain. To this end, we allow rats to self-administer unsweetened 20% ethanol over 15 intermittent sessions. This method results in a stable low-dose consumption of ethanol. Using an auditory event-related potential “oddball” paradigm, we investigated the effects of alcohol on the phase variability of EROs from electrodes implanted into the frontal cortex, dorsal hippocampus, and amygdala. We found that intermittent ethanol self-administration was sufficient to produce a significant reduction in overall intraregional synchrony across all targeted regions. These data suggest that phase locking of EROs within brain regions known to be impacted by alcohol may represent a sensitive biomarker of low levels of alcohol intoxication.
Keywords: EEG phase synchrony, event-related oscillations, ethanol, voluntary consumption, rat
Moderate drinking levels, such as those achieved by 1–2 drinks per day, are perceived to have some health benefits including a reduction in anxiety, feelings of conviviality, and improved cardiovascular health [1]. However, the potential risks associated with daily drinking might offset these benefits. In addition to increasing the likelihood of some cancers and stroke, moderate consumption may potentiate a shift towards heavier drinking in at risk individuals [1]. While numerous translational studies have used relatively high concentrations of ethanol, seen with alcohol use disorders (AUD), to obtain maximal and consistent ethanol effects in animal models [2], far fewer studies have focused on the physiological effects of low-dose alcohol consumption. Neuroelectrophysiology is a sensitive tool for determining the impact of acute and chronic ethanol in both humans and rodent models [3, 4]. By identifying electrophysiological signatures of moderate drinking, this technique may prove to be an effective, non-invasive method for identifying individuals at risk for developing AUDs.
An event-related potential (ERP) is a neurophysiological response to a time-locked sensory or cognitive event resulting in a sequence of negative and positive voltage deflections in the electroencephalogram (EEG). This neurological response to a salient event can also modify oscillatory activity by “phase re-ordering” or realignment of background EEG frequency bands [5, 6]. This time-locked synchronization of oscillatory activity is termed an event-related oscillation (ERO) [7]. Prior research has demonstrated that measures of phase synchrony in EROs is sensitive to the effects of ethanol on local and global neural networks in both rodents [4, 8–10] and in humans [4, 11]. Additionally, ERO measures may serve as biomarkers for neuropsychiatric diseases including alcohol use disorders [11, 12], and is associated with increased susceptibility to ethanol dependence in rodent models [9, 13].
Although substantial information has been gained on neurobiological mechanisms contributing to high-levels of alcohol consumption seen in alcohol use disorders, significant challenges remain in understanding how the brain responds to low levels of alcohol intoxication and what neural network may mediate the behavioral effects previously seen. To this end, we allow rats to self-administer unsweetened 20% ethanol over 15 intermittent sessions. This method of administration results in a stable low-dose self-administration. Using an auditory event-related potential “oddball” paradigm, we investigated the effects of low dose alcohol on the phase variability of EROs recorded from electrodes implanted into the frontal cortex, dorsal hippocampus, and amygdala, in freely moving rats.
Twenty Male Wistar rats were obtained from Charles River (USA). Rats were pair-housed in standard plastic cages in a temperature controlled room with a 12h light/dark cycle (lights on at 8:00 am). All testing was conducted at the onset of the light cycle. All experimental protocols were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996).
Rats were surgically implanted with bipolar recording electrodes in the amygdala (AMYG, AP: −1.0 mm, ML: ± 4.5 mm, DV: −9.5 mm, anterior amygdaloid complex) and dorsal hippocampus (DHPC, AP: −3.0 mm, ML: ± 3.0 mm, DV: −2.5 mm, CA2 field). A unipolar surface electrode implanted in the frontal cortex (FCTX, AP: 3.0 mm, ML: ± 3.0 mm, FR1) with a corresponding reference electrode in the skull overlying the cerebellum [see 7, 8]. Electrode connections were made to a multi-pin PlasticsOne® connector and the assembly was anchored to the skull with dental acrylic and anchor screws. EEG signals were recorded, using a unipolar montage, with a band pass of 0.5–70 Hz with a 60-Hz notch filter in. ERPs trials were digitized at a rate of 256 Hz.
After at least 8 days of recovery from surgery, rats were given 15, 24h sessions of intermittent 2-bottle choice across 33 days (PD92-125). At the start of each session, a clear Plexiglas® divider was used to separate the home cage into two individual compartments for the duration of each individual session. Each animal was given one bottle of tap water and one bottle of either 20% ethanol (EtOH group, n=8) or a second bottle of water (Control group, n=9). Bottles were weighted beforehand, 30m after onset, and at the end of the session at 24h. Two “leak” bottles (one ethanol, one tap water) were used to control for accidental sipper leakage during the experiment. These were placed in an identical plastic cage and weighed at the same time points.
Event-related potential (ERP) recordings occurred at three time points: (a) before the start of the 2-bottle choice sessions (baseline), (b) 1h after the start of the 2-bottle choice on the 11th or 12th session (1h test), and (c) at the end of the 2-bottle choice on the 14th or 15th session (24h test). Rats were initially placed in the apparatus and connected to the cables to habituate the animal 24h prior to baseline testing. ERPs were elicited passively with an acoustic “oddball” paradigm similar to those described previously [10, 14]. This task consisted of 312 individual tone presentations with three tone types, generated by a programmable multiple-tone generator. Rare tones (2000 Hz square wave, 20 ms duration, 85 dB, 10% probability) were interspersed with standard tones (1000 Hz square wave, 20 ms duration, 70 dB, 84% probability) and occasional noise tones (white noise, 20 ms duration, 100 dB, 6% probability) so that no two rare tones occurred successively. Each auditory stimulus trial was 1000 ms in duration (200 ms pre- stimulus+800 ms post-stimulus) and separated by variable intervals ranging from 750 to 1500 ms. Potential artifacts were identified by computer software and confirmed by visual analysis of raw EEG.
Energy and phase-locking index (PLI) analysis has been previously described [10]. Briefly, stimulus-related trials were entered into the time frequency analyses algorithm. The S-transform, a generalization of the Gabor transform [15], was used (see [16]). The S-transform was simplified by performing first a forward Fourier transform. Then, for each frequency of the Fourier transform, summing the results of multiplication by a set of Fourier transforms of Gaussian windows of varying width. Then taking the inverse Fourier transform for each of these sums. The S-transform results in a time-frequency representation of the data. The exact code used is a C language, S-transform subroutine available from the NIMH MEG Core Facility web site (http://kurage.nimh.nih.gov/meglab/).
The output of the transform for each stimuli and electrode site was calculated by averaging the individual trials containing the time-frequency energy distributions. Rectangular regions of interest (ROI) were defined within the time-frequency analysis plane by specifying, for each ROI, a band of frequencies and a time interval relative to the stimulus onset time. To quantify S-transform magnitudes, ROIs were chosen a priori to coincide with the major EEG frequencies and the latency windows found in the rat ERP signals. The 4 ROIs were: delta band (1–4 Hz, 200–500 ms), theta band (4–7 Hz, 10–300 ms), alpha band (7–13 Hz, 0–300 ms), beta band (13–30 Hz, 0–300 ms).
Energy, or amplitude of the oscillation, is the square of the magnitude of the S-transform output in a time frequency region of interest. The S-transform output for a time/frequency ROI, for a specific EEG lead, is proportional to the input voltage of the lead over the time/frequency interval. The S-transform magnitude squared for a time/frequency interval is therefore proportional to volts squared. To account for differences between animals and electrode variance, the energy is represented by a ratio of the average energy of the rare tones divided by the average energy of the standard tone for each animal. PLI is a measure of synchrony of phase angle over trials, as a function of frequency and of time relative to the start of the stimulus for each trial. The range of PLI is from zero to 1.0, with high values at a time and frequency indicating little variation, among trials, of phase angle at that time and frequency. This definition is mathematically equivalent to the definition in [17].
Analyses were performed on data generated from trials in response to the rare tone and aimed at determining whether the energy ratio and degree of phase locking index (PLI) within each region was different across different time points (baseline, 1h test, 24h test). To determine baseline differences between electrode locations (frontal cortex, dorsal hippocampus, amygdala) we conducted a two-way ANOVA (region × group) for ERO energy ratio and PLI. Two-way ANOVAs (ROI × group) for each region were conducted to determine if baseline differences occurred between groups (Ethanol vs. Control) across the four time-frequency ROIs identified above. Due to baseline differences in ERP recordings, all further group analyses were conducted within group. Differences across sessions (baseline, 1h test, 24h test) were determined using a repeated 2-way ANOVA (ROI×session) for each brain region and exposure group. Tukey post-hoc analyses were conducted for significant analyses. Values are presented as mean ± standard error of the mean (SEM) and significances were considered at P < 0.05.
Intake was found to increase across the 15 days of intermittent 20% ethanol producing an average 24h intake of 1.22 ± 0.46 g/kg with BALs ranging from 6.9 to 35.6 mg/dl (mean = 12.53 mg/dl) when sampled 30m after the onset of the 2 bottles. As expected, there were no differences in body weight between the ethanol consumers (456.10 ± 14.18 g) and controls (465.70 ± 9.73 g) at the end of the 2-bottle choice test [F(1,17)=0.29, p=0.60].
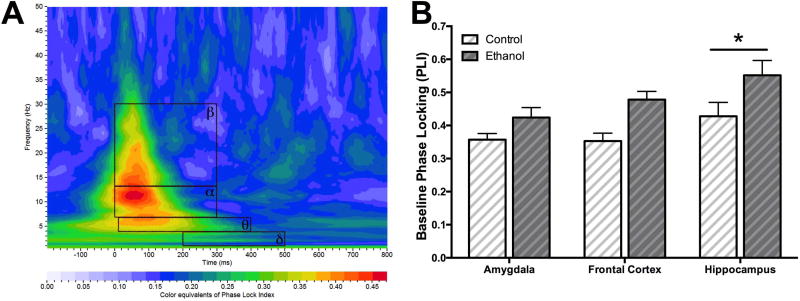
Figure 1A is an example of dorsal hippocampal phase-locking index (PLI) in a color map plot with the 4 a priori rectangular regions of interests (ROI) inlaid in black outlines. Prior to the 2-bottle choice of ethanol and/or water, animals were given the acoustic “oddball” paradigm and ERPs were recorded as a baseline test. This paradigm elicited an increase in beta energy ratio (rare tone normalized) compared to all other ROIs in the frontal cortex [F(3,68)=6.234, p < 0.001] and amygdala [F(3,56)=8.832, p < 0.0001], but not hippocampus. There was also a significant increase in beta band PLI of the frontal cortex compared to all other bands [F(3,68)=9.251, p < 0.0001]. Hippocampal PLI beta and alpha bands were each significantly higher compared to delta [F(3,56)=4.631, p < 0.01]. However, no significant differences were seen in amygdala.
Figure 1.
Baseline phase-locking across electrode locations. (A) An example of hippocampal PLI in a color map plot with inlaid black outlines of the 4 a priori rectangular regions of interests (ROI) chosen (B) Baseline PLI values (mean ± SEM) for rats who would be given access to consume 20% ethanol (ethanol group) and/or water (control group) across the three electrode locations. There was significantly main effect of region with overall more phase-locking in the hippocampus compared to the amygdala. There was also a significant main effect of group with higher baseline PLI in the ethanol group compared to control group.
During baseline recordings, a 2-way ANOVA (group×region) revealed no significant effect of group (ethanol vs. control) or region in ERO energy ratio. However, baseline hippocampal PLI was significantly higher compared to the amygdala and frontal cortex [region effect, F(2,40)=5.036, p < 0.05; Figure 1B]. Additionally, baseline PLI was overall significantly increased in the ethanol group compared to controls [group effect, F(1,40)=16.19, p < 0.001]. Variability in absolute amplitude between animals is a highly stable factor that is caused by a number of factors, such a skull thickness, that influence electrode impedance. To account for these baseline difference in activity between ethanol consuming and water controls, all further analyses were conducted within exposure groups.
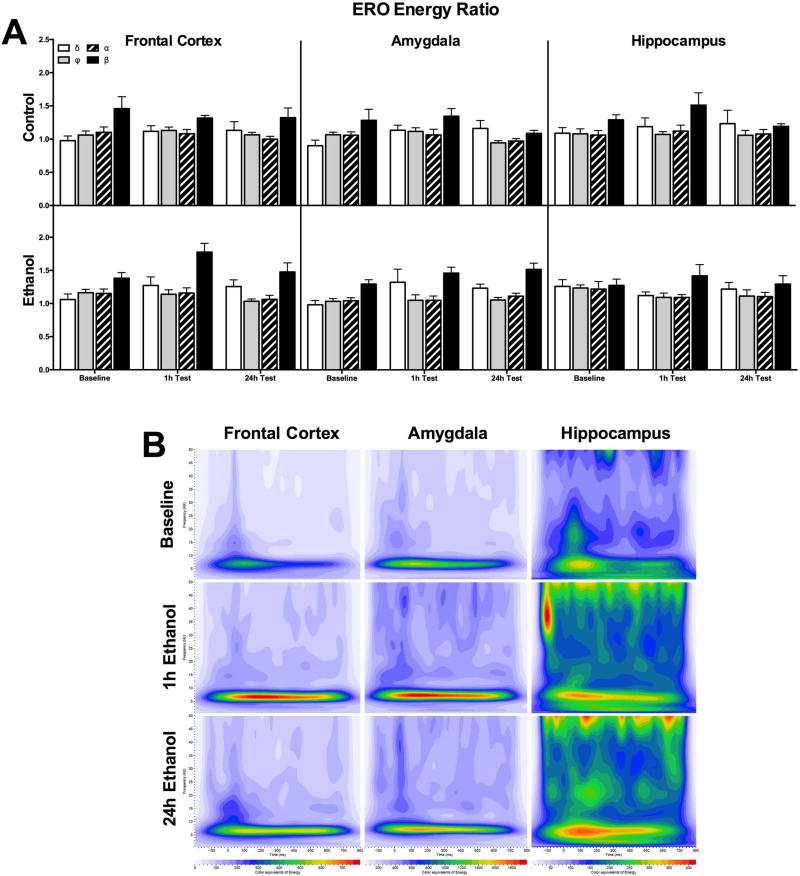
Figure 2 portrays differences across the three time points between the water only control group (top) and the ethanol drinking group (bottom) in ERO energy from the four ROIs for each electrode location. There was no effect of session in ERO energy ratio using a 2-way ANOVA (session×ROI) for all three brain regions for controls. However, there was an effect of ROI with beta band energy significantly higher than theta and alpha bands in the amygdala [F(3,24)=3.959, p < 0.05], higher than theta in the hippocampus [F(3,28)=3.393, p < 0.05], and all other bands in the frontal cortex [F(3,28)=4.599, p < 0.01]. There was no effect of session for ERO energy in ethanol consuming rats across all regions. However, there was a significant ROI effect with higher ERO energy in the beta band compared to all other bands in both the amygdala [F(3,24)=3.959, p < 0.05] and frontal cortex [F(3,28)=4.599, p < 0.01], and significantly higher than only theta in the hippocampus [F(3,28)=3.393, p < 0.05].
Figure 2.
Energy ratio of EROs for rats consuming ethanol or water only controls. Mean values (± SEM) ERO energy ratio for control and ethanol groups for the 4 ROIs across the three target locations. For the control rats that were given two bottles of water during each session (top graph), there was no significant differences in energy across the baseline and two test sessions in any of the three brain regions. There were also no significant differences in ERO energy after ethanol access for either test session compared to baseline.
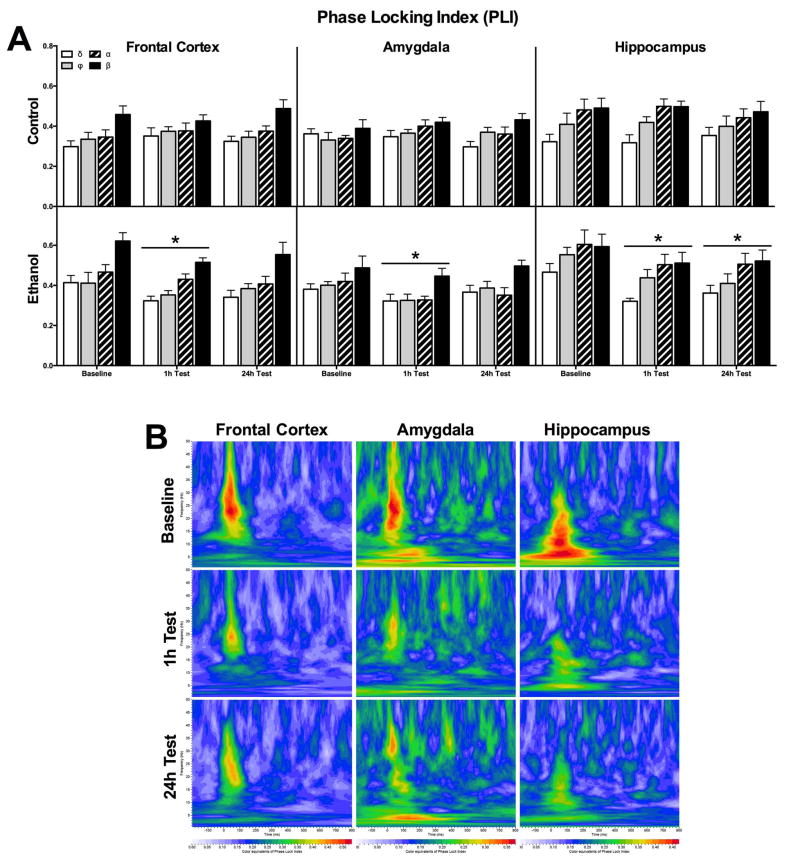
Figure 3A depicts the PLI for the same brain regions and ROIs as in Figure 2. Using a 2-way ANOVA (session×ROI), there was no effect of PLI between sessions (top). However, there was higher ERO energy in the beta band of control rats compared to all other bands in both the amygdala [F(3,24)=16.28, p < 0.0001] and frontal cortex [F(3,28)=14.90, p < 0.0001], but no ROI effect in the hippocampus. In the ethanol consuming animals (bottom), there was a significantly lower PLI in the hippocampus after the 1h and 24h test sessions [session effect, F(2,48)=13.25, p < 0.0001] and an effect of ROI but no significant pot-hoc analysis [F(3,24)=3.339, p < 0.05]. In the frontal cortex, ethanol rats had lower PLI after the 1h test session compared to baseline [session effect, F(2, 56)=5.044, p < 0.01] and an overall higher PLI in the beta band ROI compared to all others [ROI effect, F(3,28)=13.80, p < 0.0001]. Lastly, there was a significant effect of session with significantly lower PLI in the amygdala after the 1h test session compared to baseline [F(2,48)=6.549, p < 0.01] and a significantly higher PLI in the beta band ROI compared to delta and theta [ROI effect, F(3,24)=4.215, p < 0.05] in the amygdala. Figure 3B represents a heat map of PLI for ethanol consuming animals with warmer colors equivalent to higher phase locking.
Figure 3.
Phase locking values of EROs for rats consuming ethanol or water only controls. (A) Mean values (± SEM) of PLI following rare tones across the three target locations for the 4 ROIs. For the control rats (top graph), there was no significant differences in phase locking. Ethanol consuming rats (bottom graph) had significantly lower phase locking in the hippocampus one hour after the start of the 11/12th consumption session (1h test) and at the end of the ethanol access period on the 14/15th session (24h test). There was also a significant reduction in PLI in the amygdala 1h after onset of the 2-bottle choice. * p < 0.05 vs. baseline (B) Each sub-graph depicts the grand average of PLI with a time frequency representation of the phase angle synchrony values in the ethanol group in each electrode location across probe sessions. In each sub-graph frequency (Hz) is presented on the y-axis and the time interval aligned to the onset of the tone on the x-axis (msec). The PLI is presented as a ‘heat map’ with warmer (red) colors equivalent to higher phase locking.
The main goal of the present study was to investigate whether EROs are a sensitive biomarker of low levels of alcohol consumption and to give further insight into the actions of ethanol on the brain. We found that intermittent access to a 20% ethanol solution over 15 sessions, resulting in around 1 mg/kg consumption per day, was sufficient to produce a significant reduction in overall intraregional synchrony across targeted regions. These results are consistent with previous research from our lab demonstrating that ethanol can impact phase locking within and between brain regions in both rodents [4, 8–10] and in humans [4, 11]. This is the first study to our knowledge investigating changes in the measures of EROs after voluntary alcohol consumption in rats. The results indicate that alcohol can disrupt intraregional synchronization at far lower doses than previously investigated. [4]. Prior research has demonstrated that rats injected with an acute injection of ethanol (1.5 mg/kg) and tested 1h later on the same ERP “oddball” paradigm, can diminish phase locking in select frequency bands in the frontal cortex and amygdala [4]. However, they did not find any differences in hippocampal phase locking during acute ethanol administration. This discrepancy in findings might indicate that the route or duration of ethanol administration, may play an important role in altering hippocampal oscillations. Low dose alcohol consumption was not associated with changes in ERO energy. This may indicate that while low dose consumption can desynchronize phase re- ordering, it does not significantly impact the amplitude of the response.
Prior studies suggest that the effects of low doses of ethanol on multiple brain systems are the result of increased levels of noise or randomness in neuronal processing as indexed by a decrease in nonlinear structure in cortical EEG [18]. However, the neuromolecular basis of the effects of low dose ethanol is not well understood and necessitates further research to elucidate the potential mechanisms involved. Together, these data suggest that examining phase synchrony of EROs within select brain regions may represent a sensitive biomarker of low levels of alcohol intoxication.
Highlights.
Rats will voluntarily consume 20% ethanol at low levels
Higher phase locking occurs in the hippocampus compared to amygdala or cortex
Ethanol consumption reduces phase locking of event-related oscillations
ERO energy was not changed by moderate ethanol intoxication in rats
Acknowledgments
Funding Sources
This research was supported in part by the National Institutes of Health, National Institute on Alcoholism and Alcohol Abuse (NIAAA) grants: AA006059 and AA019969 (CLE), and T32 AA007456.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services. [accessed December 5, 2016];10th Special Report to the U.S. Congress on Alcohol and Health: Highlights from current research. 2000 http://pubs.niaaa.nih.gov/publications/10Report/10thSpecialReport.pdf.
- 2.Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48:277–86. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangaswamy M, Porjesz B. Understanding alcohol use disorders with neuroelectrophysiology. Handb. Clin. Neurol. 2014;125:383–414. doi: 10.1016/B978-0-444-62619-6.00023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlers CL, Wills DN, Havstad J. Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Res. 2012;1450:67–79. doi: 10.1016/j.brainres.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Başar E, Gönder A, Ungan P. Comparative frequency analysis of single EEG-evoked potential records. [accessed December 9, 2016];J. Biomed. Eng. 1980 2:9–14. doi: 10.1016/0141-5425(80)90086-2. http://www.ncbi.nlm.nih.gov/pubmed/7359903. [DOI] [PubMed] [Google Scholar]
- 6.Makeig S. Response: event-related brain dynamics -- unifying brain electrophysiology. Trends Neurosci. 2002;25:390. doi: 10.1016/s0166-2236(02)02198-7. [DOI] [PubMed] [Google Scholar]
- 7.Begleiter H, Porjesz B. Genetics of human brain oscillations. Int. J. Psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Criado JR, Ehlers CL. Effects of adolescent ethanol exposure on event-related oscillations (EROs) in the hippocampus of adult rats. Behav. Brain Res. 2010;210:164–70. doi: 10.1016/j.bbr.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Criado JR, Ehlers CL. Event-related oscillations as risk markers in genetic mouse models of high alcohol preference. Neuroscience. 2009;163:506–23. doi: 10.1016/j.neuroscience.2009.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Criado JR, Ehlers CL. Event-related oscillations in the parietal cortex of adult alcohol-preferring (P) and alcohol-nonpreferring rats (NP) Alcohol. 2010;44:335–42. doi: 10.1016/j.alcohol.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlers CL, Wills DN, Phillips E, Havstad J. Low voltage alpha EEG phenotype is associated with reduced amplitudes of alpha event-related oscillations, increased cortical phase synchrony, and a low level of response to alcohol. Int. J. Psychophysiol. 2015;98:65–75. doi: 10.1016/j.ijpsycho.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yener GG, Başar E. Brain oscillations as biomarkers in neuropsychiatric disorders: following an interactive panel discussion and synopsis. Suppl. Clin. Neurophysiol. 2013;62:343–63. doi: 10.1016/b978-0-7020-5307-8.00016-8. [DOI] [PubMed] [Google Scholar]
- 13.Ehlers CL, Criado JR. Event-related oscillations in mice: effects of stimulus characteristics. J. Neurosci. Methods. 2009;181:52–7. doi: 10.1016/j.jneumeth.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehlers CL, Somes C, Li TK, Lumeng L, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin studies in alcohol naive, preferring and non-preferring rats. Neuroscience. 1999;93:227–236. doi: 10.1016/s0306-4522(99)00113-x. [DOI] [PubMed] [Google Scholar]
- 15.Gabor D. Theory of communication. J. Inst. Electr. Eng. - Part III Radio Commun. Eng. 1946;93:429–441. [Google Scholar]
- 16.Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S transform. IEEE Trans. Signal Process. 1996;44:998–1001. [Google Scholar]
- 17.Schack B, Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci. Lett. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- 18.Ehlers CL, Havstad J, Prichard D, Theiler J. Low Doses of Ethanol Reduce Evidence for Nonlinear Structure in Brain Activity. J. Neurosci. 1998;18 doi: 10.1523/JNEUROSCI.18-18-07474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]