Abstract
Poor maternal diet can lead to metabolic disease in offspring, whereas maternal exercise may have beneficial effects on offspring health. In this study, we determined ifmaternal exercise could reverse the detrimental effects of maternal high-fat feeding on offspring metabolism of female mice. C57BL/6 female mice were fed a chow (21%) or high-fat (60%) diet and further divided by housing in static cages or cages with running wheels for 2 weeks prior to breeding and throughout gestation. Females were bred with chow-fed sedentary C57BL/6 males. High fat–fed sedentary dams produced female offspring with impaired glucose tolerance compared with offspring of chow-fed dams throughout their first year of life, an effect not present in the offspring from high fat–fed dams that had trained. Offspring from high fat–fed trained dams had normalized glucose tolerance, decreased fasting insulin, and decreased adiposity. Liver metabolic function, measured by hepatic glucose production in isolated hepatocytes, hyperinsulinemic-euglycemic clamps, liver triglyceride content, and liver enzyme expression, was enhanced in offspring from trained dams. In conclusion, maternal exercise negates the detrimental effects of a maternal high-fat diet on glucose tolerance and hepatocyte glucose metabolism in female offspring. The ability of maternal exercise to improve the metabolic health of female offspring is important, as this intervention could combat the transmission of obesity and diabetes to subsequent generations.
Introduction
Obesity and type 2 diabetes are increasing at epidemic rates in the U.S. and worldwide (1). One reason for the escalation in the prevalence of diabetes is that risk patterns for both obesity and type 2 diabetes are now known to originate because of poor maternal diet and maternal obesity (2–13). Thus, obesity, prediabetes, and diabetes in individuals of reproductive age can initiate a vicious cycle, increasing the risk of development of type 2 diabetes and obesity to subsequent generations (2–13). Understanding the mechanisms underlying the transmission of disease risk to offspring, as well as means to prevent these detrimental effects on offspring health, are important challenges for biomedical research.
Investigations of both rodents and nonhuman primates have shown that maternal overnutrition results in offspring with increased rates of obesity (6,11–13), increased adiposity (11–13), and increased food intake (14,15). In addition, maternal overnutrition can result in impaired glucose tolerance (16) and increased rates of cardiovascular disease (15) in offspring. The increased rates of obesity and impaired glucose tolerance in offspring from overfed dams have been linked to impaired β-cell (11) and liver (6) function. Relevant to altered liver function, it has been hypothesized that excess fuels in the intrauterine environment are taken up by the fetal liver (6), likely a consequence of white adipose tissue not developing until later in pregnancy, leading to whole-body insulin resistance (17,18).
In contrast to the detrimental effects of maternal obesity and overnutrition on offspring metabolic health, maternal exercise in rodents has been shown to have beneficial effects on the metabolic phenotype of adult offspring (19–25), including enhanced glucose tolerance and/or insulin sensitivity (19–23,25). We and other groups (21,23–25) have found that maternal exercise before and during pregnancy negates the detrimental effects of a maternal high-fat diet on the metabolic health of the male offspring. The tissue or tissues responsible for the improved metabolism in offspring of exercise-trained dams is not well defined or investigated. In some reports, improved skeletal muscle function has been proposed (19,20,22), although our own studies do not support a role for increased skeletal muscle glucose uptake as a mechanism for improved glucose tolerance in male offspring from exercise-trained dams that were fed a chow or high-fat diet (21). In addition to investigations of skeletal muscle, there is at least one report in rodents demonstrating that gestational exercise protected high-fat diet–fed male offspring from hepatic steatosis (23). Given the fundamental role of the liver in glucose homeostasis as measured by glucose tolerance, we hypothesized that maternal exercise may affect liver function in the adult offspring.
There has been increased recognition that numerous metabolic insults and disease states can differentially affect male and female health, and there are now strong efforts to understand the effects of interventions and treatments in both sexes (26). In regards to pregnancy, studies investigating the effects of maternal overnutrition on both male and female offspring have frequently shown that the male offspring have a more pronounced detrimental phenotype (8,13,15,27–29). In one report, maternal obesity in a rat model resulted in impaired glucose tolerance at 6 months of age in the male offspring but had no effect on female offspring (28). In studies examining maternal protein restriction, skeletal muscle insulin resistance was present earlier in male offspring than female offspring (27,29). These studies indicate that the metabolic response to maternal diet may be different in male and female offspring. In contrast to a considerable number of investigations examining the effects of maternal diet on both male and female offspring metabolism, with the exception of one study (22), the effects of maternal exercise have almost exclusively been studied in male offspring. The sole study that examined the effects of maternal exercise in female offspring demonstrated that voluntary wheel exercise in chow-fed rats resulted in increased whole-body insulin sensitivity in the offspring (22). The effect of maternal exercise in the presence of a maternal high-fat diet in female offspring, as well as investigation into the effects of maternal exercise on female offspring tissue phenotype, have not been studied.
In the current study, we investigated the effects of maternal exercise on the metabolic health of female offspring and determined if maternal exercise could attenuate the detrimental effects of maternal high-fat feeding in female offspring. In addition, we investigated the role of the liver in offspring metabolic health. We found that maternal exercise prevents the detrimental effects of high-fat feeding in adult female offspring and that the improved whole-body metabolic health of the offspring is associated with improved liver function.
Research Design and Methods
Mice and Training Paradigm
Six-week-old C57BL/6 virgin female mice were fed a chow (21% kcal from fat; 9F5020; PharmaServ) or high-fat diet (60% kcal from fat; Research Diets Inc.) for 2 weeks preconception, during gestation, and until pup weaning. Mice were additionally divided into four subgroups: trained (mice housed with running wheels preconception and during gestation); prepregnancy trained (housed with wheels preconception); gestation trained (housed with wheels during gestation); or sedentary (housed in static cages). All male breeders were 10-week-old C57BL/6 mice maintained on a chow diet and were sedentary. To control for potential differences in sires, breeding was done as harems. Litters were culled to five mice, and offspring were chow-fed and housed in static cages (sedentary) from birth onwards.
In Vivo Metabolic and Body Fat Assessments
For intraperitoneal glucose tolerance tests (ipGTTs), mice were fasted for 11 h (2200 to 0900 h) with free access to drinking water. A baseline blood sample was collected from the tails of fully conscious mice, followed by intraperitoneal injection of glucose (2 g glucose/kg body weight), and blood was taken from the tails for glucose measurements at 0, 15, 30, 60, and 120 min. For oral GTTs (oGTTs), mice were fasted for 11 h (2200 to 0900 h) with free access to drinking water. A baseline blood sample was collected from the tail of fully conscious mice, followed by oral gavage of glucose (1 g glucose/kg body weight), and blood was taken from the tail for glucose measurements at 0, 15, 30, 60, and 120 min. For insulin tolerance tests, mice were fasted for 4 h (1000 to 1400 h), and baseline blood samples were collected from the tails of fully conscious mice. Insulin (1 unit/kg body weight) (Humulin; Eli Lilly and Company, Indianapolis, IN) was administered by intraperitoneal injection, and blood samples were taken from the tail at 10, 15, 30, 45, and 60 min postinjection. Hyperinsulinemic-euglycemic clamps were performed at Sanford-Burnham Medical Research Institute as previously described, with some minor modifications as described below (Cardiometabolic Phenotyping Core Facility, Sanford-Burnham Medical Research Institute) (30–32). The clamps were performed over an ∼6-week period with an equal number of mice from each group clamped on a given week. The mice were catheterized 5 days prior to the hyperinsulinemic-euglycemic clamps. Catheters were surgically placed in the carotid artery and jugular vein for sampling and infusions, respectively. On the day of the clamp, mice were fasted for 5 h starting at 0800 h, with the clamp (insulin and glucose infusion) beginning at 1300 h [3-3H]Glucose was primed and continuously infused between −90 and 0 min at a rate of 2.5 μCi prime and 0.04 μCi/min continuous. The clamp was initiated at 0 min with a continuous insulin infusion (2.5 mU/kg/min) and maintained for 120 min. Arterial glucose was monitored every 10 min to provide feedback to adjust the glucose infusion rate (GIR). All groups were clamped at the same glucose level (∼150 mg/dL). This concentration was selected as the target glucose value because historically, this is the typical 5-h fasting glucose level for a C57BL/6 mouse. [3-3H]Glucose (0.06 μCi/μL) was added to the glucose infusate to clamp both arterial glucose and glucose-specific activity. Plasma insulin concentrations were measured at basal (−5 min) and at the end of the clamp (120 min). Assessment of fat and lean mass were determined by DEXA (Lunar PIXImus2 mouse densitometer; GE Healthcare) and offspring anesthetized with ketamine/xylazine (50 mg/mL; injected 0.1 cc/10 g body weight).
Biochemical Methods
Blood was collected from the retro-orbital sinus after an overnight fast (2200 to 0900 h). Plasma insulin and leptin were measured using mouse ELISA kits (Crystal Chem Inc., and triglyceride, cholesterol, and free fatty acid were measured by colorimetric assay (Stanbio Laboratory). mRNA levels of various liver genes were measured by quantitative RT-PCR using primers shown in Supplementary Table 1. Liver triglycerides were measured as previously described (33). Tissue processing and immunoblotting were performed as previously described (34). Antibodies used were GLUT4 (AB1346; Millipore) and HKII (AB37593; Abcam).
Glucose Production in Isolated Hepatocytes
Glucose production was measured from primary hepatocytes as previously described (35). Briefly, primary hepatocytes were isolated by liver perfusion with type II collagenase, grown on collagen-coated plates, and subjected to glucose production assays (36–38). Glucose in culture medium was measured and normalized to total protein levels, and the normalized values were used as an index to estimate glucose production.
Statistical Analysis
The data are means ± SEM. Statistical significance was defined as P < 0.05 and determined by one- or two-way ANOVA, with Tukey and Bonferroni post hoc analysis. For experiments that were carried out at various ages (12, 24, and 52 weeks of age), statistical analyses were determined based on the control group at that specific time point, and comparisons among ages were not analyzed.
Results
Characterization of Dams
The pregnant dams ran an average of 6 km/day during the prepregnancy period and 4 km/day during gestation, and there was no effect of high-fat feeding of dams on running distance (Supplementary Table 2). The dams responded to the wheel running with training adaptations to the triceps muscle as indicated by significant increases in protein expression of hexokinase II (arbitrary units: chow-fed, sedentary = 1.0 ± 0.2; chow-fed, trained = 2.5 ± 0.3 [P < 0.05]; high fat–fed, sedentary = 1.0 ± 0.3; and high fat–fed, trained = 2.6 ± 0.3 [P < 0.05]) and total GLUT4 (arbitrary units: chow-fed, sedentary = 1.0 ± 0.1; chow-fed, trained = 1.5 ± 0.4 [P < 0.05]; high fat–fed, sedentary = 0.8 ± 0.1; and high fat–fed, trained = 1.7 ± 0.3 [P < 0.05]) (21). Body weights of the high fat–fed dams were lower in dams that were trained before and during gestation (trained) and in gestation-only trained dams (Supplementary Table 2). The high fat–fed dams had impaired glucose tolerance at gestational day 15 (P < 0.01) with the prepregnancy-trained high fat–fed dams having significantly impaired glucose tolerance compared with all other groups (Supplementary Fig. 1A). There was no difference among pregnant chow-fed dams in rate of conception (data not shown), sex distribution, or litter size, but there was an overall effect of high-fat feeding to decrease litter size (Supplementary Table 3). Many of the dams and male breeders used in the current study were parents to offspring reported in our previous investigation (21).
Detrimental Effects of a Maternal High-Fat Diet on Female Offspring Are Negated by Maternal Exercise
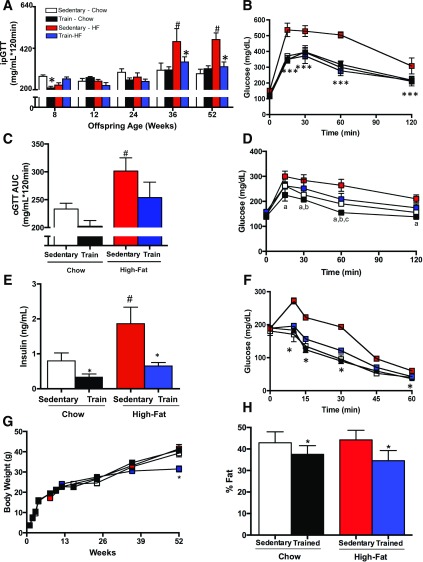
Although several investigations have determined that maternal exercise improves the metabolic health of adult male offspring (19–21,23–25), the effects of maternal exercise on adult female offspring have been less studied. In order to determine if maternal exercise affects the metabolic health of female offspring, we compared offspring from dams that were sedentary or trained and fed a chow or high-fat diet. At 8 weeks of age, the offspring from chow-fed trained dams had an improved glucose tolerance measured by ipGTT compared with offspring from chow-fed sedentary dams; although as the offspring aged, this effect was no longer present (Fig. 1A). High-fat feeding of the dams resulted in a marked impairment in glucose tolerance in female offspring, an effect evident as the mice reached 36 and 52 weeks of age (Fig. 1A). Strikingly, maternal exercise completely negated the detrimental effects of a maternal high-fat diet on offspring impaired glucose tolerance, as shown by area under the glucose excursion curve (Fig. 1A) and, as an example, the glucose excursion curve at 52 weeks of age (Fig. 1B). oGTTs were also performed in a subset of female offspring aged 46–56 weeks. Similar to the results of the ipGTT, offspring from dams that were sedentary and fed a high-fat diet had an impaired oral glucose tolerance, an effect reversed if dams were exercise-trained. There was also a main effect of maternal high-fat diet to impair oral glucose tolerance (Fig. 1C and D).
Figure 1.
Maternal exercise negates the detrimental effects of a maternal high-fat diet on offspring metabolic health. A and B: ipGTT was measured over a 52-week period in offspring of dams that were sedentary or trained and fed a chow or high-fat diet. Offspring were injected with 2 g glucose/kg body weight. Glucose area under the curve (AUC) (A) and glucose excursion curve (B) of female offspring from sedentary and trained dams fed a chow or high-fat diet. Data are expressed as means ± SEM (n = 10–20/group). Symbols represent differences compared with sedentary control groups (*P < 0.05; ***P < 0.001; #P < 0.05 high fat–fed sedentary vs. chow-fed sedentary). C and D: For oGTTs, 46- to 56-week-old female offspring were gavaged with 1 g glucose/kg body weight. Glucose AUC (C) and glucose excursion curve (D) of female offspring from sedentary and trained dams fed a chow or high-fat diet. Data are expressed as means ± SEM (n = 3–7/group). Symbols and letters represent statistical differences (#P < 0.05 high fat–fed vs. all chow-fed groups; aP < 0.05 chow-fed trained vs. high fat–fed sedentary; bP < 0.05 chow-fed sedentary vs. high fat–fed sedentary; cP < 0.05 high fat–fed trained vs. high fat–fed sedentary). E: Fasting serum insulin concentrations at 52 weeks of age. Data are expressed as means ± SEM (n = 23–25/group). F: For insulin tolerance tests, mice were injected with 1 unit insulin/kg i.p. and data expressed as glucose for female offspring. Data are expressed as means ± SEM (n = 10–20/group). Symbols represent differences compared with sedentary control groups (*P < 0.05, #P < 0.05 high fat–fed sedentary vs. chow-fed sedentary). Body weight (G) and percent fat mass (H) of female offspring at 52 weeks. Data are expressed as means ± SEM (n = 23–25/group). Asterisks represent differences compared with sedentary control groups (*P < 0.05, high fat–fed sedentary vs. chow-fed sedentary).
Fasting plasma insulin concentrations measured at 52 weeks of age were significantly lower in female offspring from trained dams regardless of the maternal diet. High-fat feeding of dams resulted in significantly higher insulin concentrations in the offspring at 52 weeks, an effect that was not present if the dams were trained (Fig. 1E). In contrast to the changes in offspring insulin with dam high-fat feeding, serum concentrations of triglycerides, cholesterol, free fatty acids, or leptin were not different among offspring groups (Table 1). There were no differences in food intake among offspring from chow or high fat–fed dams (Table 1).
Table 1.
Physiological and metabolic profile of offspring at 52 weeks
| Female offspring |
||||||||
|---|---|---|---|---|---|---|---|---|
| Dams fed chow diet | Dams fed high-fat diet |
|||||||
| Dam treatment | Sedentary (n = 23) | Trained (n = 25) | Prepregnancy trained (n = 24) | Gestation trained (n = 23) | Sedentary (n = 24) | Trained (n = 24) | Prepregnancy trained (n = 25) | Gestation trained (n = 23) |
| Triglycerides (mg/dL) | 64 ± 5 | 79 ± 8 | 72 ± 4 | 98 ± 8 | 68 ± 10 | 67 ± 8 | 72 ± 7 | 66 ± 7 |
| Cholesterol (mg/dL) | 100 ± 6 | 92 ± 7 | 94 ± 6 | 102 ± 10 | 95 ± 6 | 103 ± 10 | 89 ± 8 | 92 ± 9 |
| FFA (mEq/L) | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.2 | 0.8 ± 0.2 | 1.2 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| Leptin (ng/mL) | 35 ± 2 | 36 ± 4 | 28 ± 6 | 37 ± 9 | 34 ± 9 | 33 ± 2 | 42 ± 12 | 46 ± 9 |
| Food intake (g/2 weeks) | 24.2 ± 0.8 | 21.3 ± 0.7 | 24.6 ± 1.2 | 24.0 ± 1.4 | 22.3 ± 1.2 | 23.2 ± 0.8 | 21.9 ± 1.3 | 24.2 ± 1.0 |
Serum lipid concentrations and food intake data for female offspring at 52 weeks of age (n = 23–25/group).
FFA, free fatty acid.
High-fat feeding of dams resulted in impaired insulin tolerance in offspring at 52 weeks of age, and training of high fat–fed dams reversed this effect (Fig. 1F). Offspring from high fat–fed trained dams had decreased body weight compared with all other groups, but only at 52 weeks of age (Fig. 1G). Maternal exercise, regardless of diet, resulted in decreased percentage body fat in female offspring at 52 weeks of age compared with offspring from sedentary controls (Fig. 1H). Taken together, these data indicate that maternal exercise fully ablates the pronounced glucose intolerance and insulin resistance in female offspring of high fat–fed dams.
Enhanced Glucose Tolerance in Female Versus Male Offspring
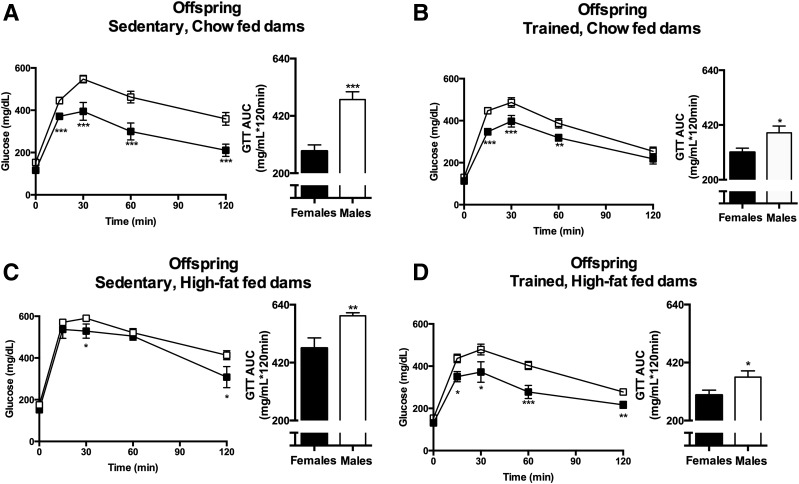
To determine if there are sex differences in glucose tolerance, ipGTT data from female offspring at 52 weeks of age were compared with our previously reported (21) ipGTT data from their male siblings (Fig. 2) (21). For offspring from sedentary chow-fed dams, the glucose excursion curve in female offspring was significantly lower at all time points postglucose injection compared with male offspring, and glucose area under the curve was ∼40% lower in the females (Fig. 2A). Training of chow-fed dams resulted in improved glucose tolerance in male offspring. Female offspring of trained chow-fed dams did not have improved glucose tolerance, most likely because aging during the first year of life did not result in any deterioration of glucose tolerance in female offspring (Fig. 1A and B). High-fat feeding of dams resulted in impaired glucose tolerance in both female and male offspring, although glucose tolerance was significantly worse in male offspring (Fig. 2C). Exercise training of dams fed a high-fat diet resulted in a similar degree of improvement in glucose tolerance in female (∼38%) and male (∼40%) offspring (Fig. 2D), although female mice still maintained an enhanced glucose tolerance compared with males. These data demonstrate that female offspring are more glucose tolerant compared with male offspring under all maternal conditions, and both female and male offspring respond to maternal exercise training.
Figure 2.
Maternal exercise negates the detrimental effects of a maternal high-fat diet on the metabolic health of both male and female offspring metabolic health. A–D: Glucose tolerance was measured at 52 weeks of age in offspring of dams that were sedentary or trained and fed a chow or high-fat diet. For GTTs, offspring were injected with 2 g glucose/kg body weight, intraperitoneal. Glucose excursion curve and glucose area under the curve (AUC) of male and female offspring from sedentary, chow-fed dams (A); trained, chow-fed dams (B); sedentary, high fat–fed dams (C); and trained, high fat–fed dams (D). Data are expressed as means ± SEM (n = 10–20/group). Asterisks represent differences compared with sedentary control groups (*P < 0.05, **P < 0.01; ***P < 0.001).
Maternal Exercise Before and During Pregnancy Is Necessary for Improved Metabolic Health in Female Offspring
The effects of maternal exercise to improve metabolic health of female offspring were observed in offspring from dams who exercised both prior to and during gestation. To determine if the timing of maternal exercise was critical for metabolic improvements in the female offspring, we also compared offspring from dams that exercised prepregnancy only, during gestation only, and both prepregnancy and during gestation. Because exercise training of chow-fed dams had minimal effects on glucose tolerance of female offspring, we only report data from offspring of fat-fed dams. Female offspring from sedentary high fat–fed dams had a significant worsening of glucose tolerance with age, and this was negated only in offspring from dams that trained before and during pregnancy (Supplementary Fig. 2A). Circulating insulin concentrations and insulin tolerance were improved in offspring from high fat–fed trained and gestation-trained dams but not in offspring from high fat–fed dams that were only trained pregestation (Supplementary Fig. 2B and C). Body weight and percentage fat mass were significantly decreased only in offspring from high fat–fed dams that were trained both before and during gestation (Supplementary Fig. 2D and E). Taken together, these data indicate that maternal exercise before and during pregnancy is essential to observe the improvements in insulin sensitivity and metabolic health of the female offspring.
Maternal Exercise During Pregnancy Improves Liver Function and Alters Gene Expression in Offspring
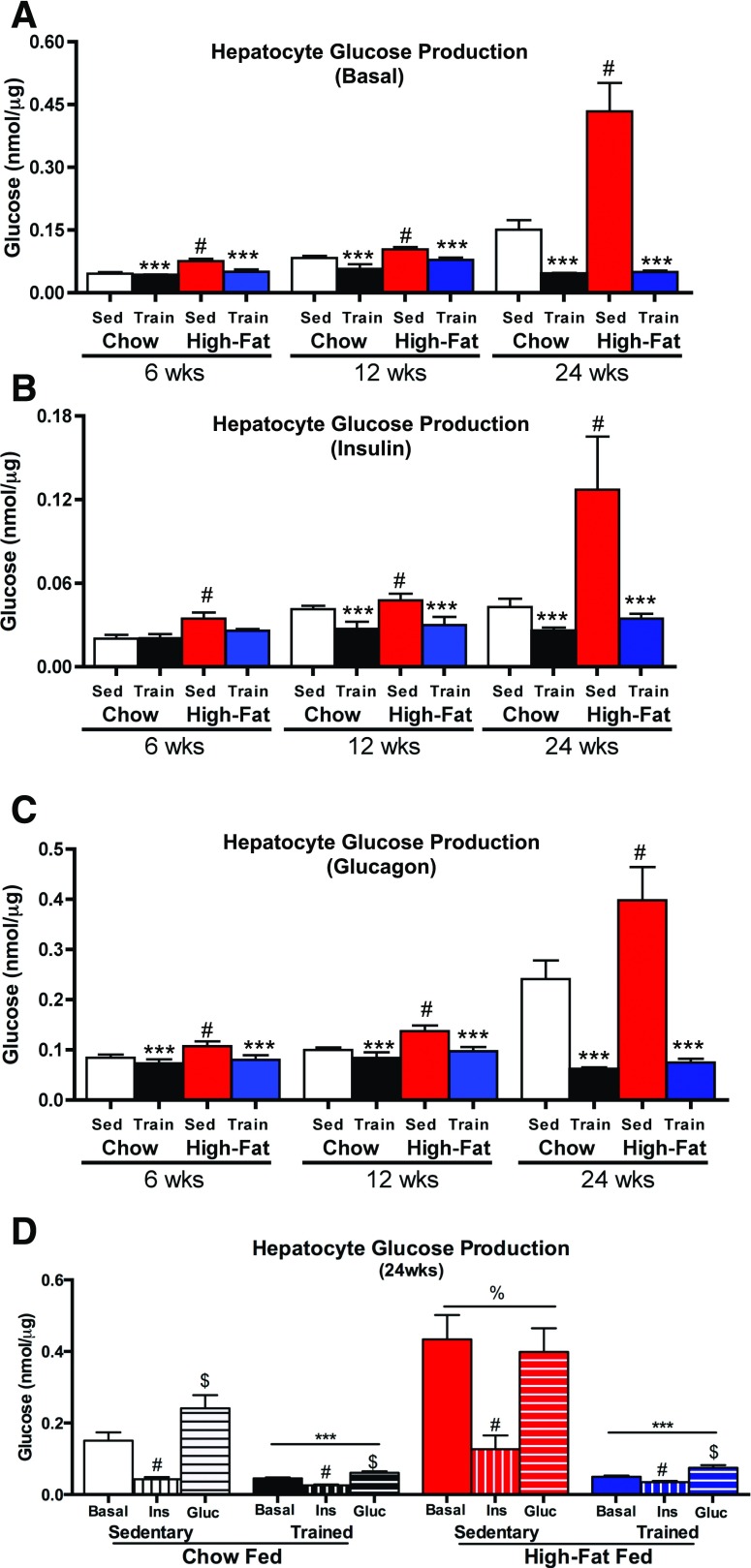
Changes in glucose tolerance are often the consequence of differences in liver metabolism (39). This coupled with previous studies showing that maternal diet and obesity can alter liver metabolic function of offspring (6,23) led us to hypothesize that maternal exercise results in improved liver metabolism in offspring. To determine the effects of maternal exercise on liver function, we initially measured hepatocyte glucose production in isolated hepatocytes from female offspring at 6, 12, and 24 weeks of age (Fig. 3A and D). Hepatocyte glucose production was not measured in 36- and 52-week-old mice because hepatocytes isolated from the older offspring failed to culture. Basal rates of hepatocyte glucose production were lower in offspring of chow-fed dams that had exercise-trained compared with offspring of sedentary chow-fed dams. High-fat feeding of sedentary dams resulted in marked increases in basal hepatic glucose production in the offspring, especially at 24 weeks of age. Remarkably, basal hepatocyte glucose production was not impaired in hepatocytes isolated from offspring of high fat–fed dams that were exercise trained. Importantly, these improvements in hepatocyte glucose production in offspring from trained dams occurred several weeks before the improvements in whole-body metabolic health and glucose tolerance were observed, suggesting that alterations in the liver could be responsible for the improved metabolic health as the offspring age.
Figure 3.
Maternal exercise improves hepatic function in isolated hepatocytes regardless of diet. Hepatic glucose production was measured in isolated hepatocytes after 4 h in the basal state (A), after incubation with insulin (B), or after stimulation with glucagon (C). Data are expressed as means ± SEM (n = 12/group). Symbols represent differences compared with sedentary control groups (***P < 0.001; #P < 0.05 high fat–fed sedentary vs. chow-fed sedentary). D: Data represent basal, insulin-suppressed, and glucagon-stimulated data at the 24-week time point. Symbols represent differences compared with basal state (#P < 0.001; $P < 0.05 vs. basal) or sedentary control groups (***P < 0.001) or versus all other groups (%P < 0.001).
Insulin’s ability to decrease hepatic glucose production was impaired in hepatocytes isolated from offspring of high fat–fed sedentary dams (Fig. 3B and D). In fact, hepatocytes isolated from high fat–fed sedentary offspring had increased glucose production in the presence of insulin compared with all other groups at 6, 12, and 24 weeks of age. Hepatocytes isolated from offspring from sedentary chow-fed dams had increased glucose production in the presence of insulin compared with offspring from chow- or high fat–fed trained dams (Fig. 3B and D). These data demonstrate that offspring from sedentary dams have increased insulin resistance in isolated hepatocytes compared with offspring from trained dams fed a chow or high-fat diet.
Glucagon stimulates gluconeogenesis in the liver and in isolated hepatocytes. Glucagon-mediated hepatocyte glucose production was significantly higher in offspring from chow-fed sedentary dams compared with offspring from chow- or high fat–fed trained dams at 24 weeks of age (Fig. 3C and D). Isolated hepatocytes from offspring of high fat–fed sedentary dams exhibited significantly higher rates of glucagon-stimulated hepatic glucose production at 12 weeks of age and dramatically higher rates at 24 weeks of age. The detrimental effects of maternal high-fat feeding on offspring hepatocytes were not present if the dams had exercise-trained. These data indicate that maternal exercise protects against impaired hepatic function and hepatic insulin resistance in offspring from sedentary dams.
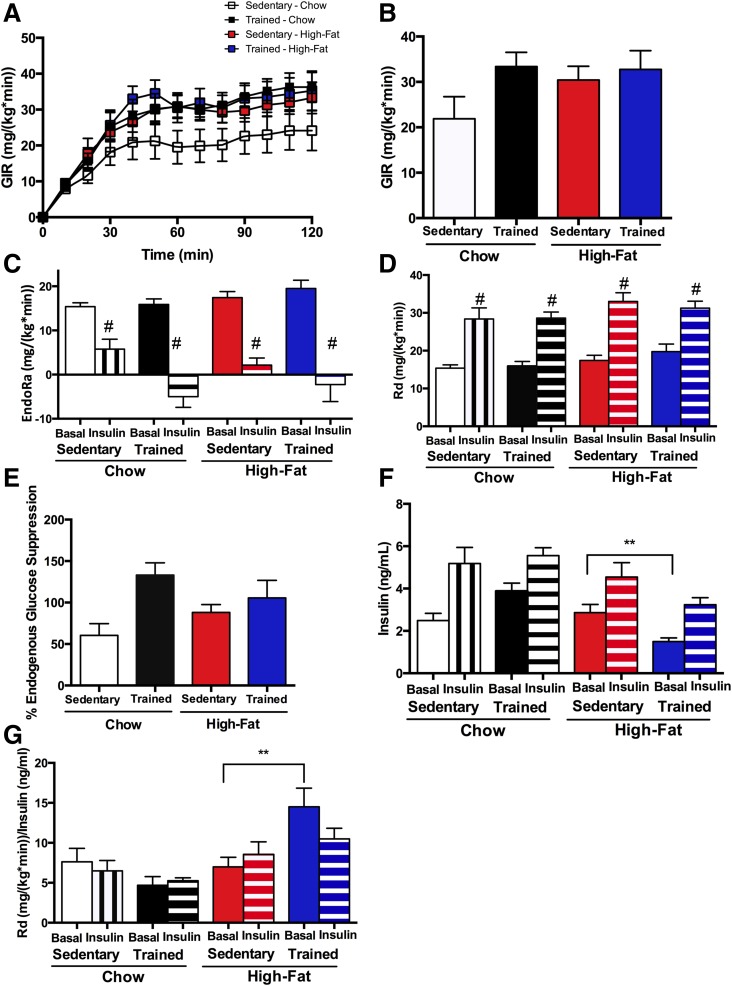
Hyperinsulinemic-euglycemic clamps were performed to measure insulin sensitivity and hepatic glucose production in vivo in offspring at 52 weeks of age (Table 2). Consistent with the GTT data in which there was no difference between offspring from sedentary and trained chow-fed dams, there were no differences in GIRs (Fig. 4A and B), rates of glucose appearance (EndoRa) (Fig. 4C), basal rates of glucose disappearance (Rd), or insulin-stimulated rates of glucose disappearance (Fig. 4D). Endogenous glucose suppression was higher in offspring from chow-fed trained dams (Fig. 4E), and there was an overall effect of maternal exercise to increase percentage endogenous glucose suppression.
Table 2.
Hyperinsulinemic-euglycemic clamps in offspring at 64 weeks of age
| Dams fed chow |
Dams fed high fat |
|||
|---|---|---|---|---|
| Sedentary | Trained | Sedentary | Trained | |
| Body weight (g) | 48.5 ± 3.2 | 47.4 ± 2.5 | 43.4 ± 4.2 | 38.8 ± 2.7* |
| Basal glucose (mg/dL) | 130 ± 7 | 148 ± 7 | 138 ± 7 | 138 ± 7 |
| Clamped glucose (mg/dL) | 154 ± 3 | 146 ± 3 | 155 ± 3 | 147 ± 3 |
| Basal insulin (ng/mL) | 2.5 ± 0.3 | 3.9 ± 0.4 | 2.9 ± 0.4 | 1.5 ± 0.2**,##,$,% |
| Clamped insulin (ng/mL) | 5.2 ± 0.8 | 5.6 ± 0.4 | 4.5 ± 0.7$ | 3.2 ± 0.3$ |
| GIR (mg · kg−1 · min−1) | 22.0 ± 4.9 | 33.3 ± 3.1 | 30.5 ± 3.1 | 32.7 ± 4.2 |
| Basal EndoRa (mg · kg−1 · min−1) | 15.4 ± 0.9$ | 15.9 ± 1.2$ | 17.5 ± 1.4 | 19.8 ± 2.0 |
| Clamp EndoRa (mg · kg−1 · min−1) | 5.7 ± 2.2 | −5.1 ± 2.4## | 2.1 ± 1.7 | −2.2 ± 3.9## |
| Rd (mg · kg−1 · min−1) | 28.3 ± 3.0 | 28.6 ± 1.6 | 32.9 ± 2.3 | 31.2 ± 1.8 |
| Percent EndoRa suppression | 60.4 ± 14.1 | 132.9 ± 15.0# | 88.2 ± 9.5 | 105.6 ± 21.1# |
| Basal Rd/insulin | 7.7 ± 1.7 | 4.7 ± 1.1*,% | 7.0 ± 1.2$ | 14.5 ± 2.3*,$ |
| Clamp Rd/insulin | 6.5 ± 1.3$ | 5.2 ± 0.4$ | 8.6 ± 1.6 | 10.5 ± 1.3 |
Body weight, basal, and insulin-stimulated data during a hyperinsulinemic-euglycemic clamp in adult female offspring (n = 7 to 8/group).
*P < 0.05; **P < 0.01, sedentary chow vs. trained chow or sedentary high fat vs. trained high fat.
#P < 0.05; ##P < 0.01, sedentary vs. trained.
$P < 0.05, chow vs. high fat.
%P < 0.05, trained chow vs. trained high fat.
Figure 4.
Hyperinsulinemic-euglycemic clamps in offspring. A–E: Hyperinsulinemic-euglycemic clamps were performed in 52-week-old female offspring from sedentary or trained dams fed a chow or high-fat diet. There was no difference in GIR (A and B), EndoRa (C), Rd (D), or percentage of endogenous glucose suppression (E). F: Fasting insulin was significantly reduced in the basal state in offspring from trained high fat–fed dams. When normalized for fasting insulin, rate of glucose disappearance (Rd/Insulin) (G) was significantly increased in offspring from trained high fat–fed dams. Data are expressed as means ± SEM (n = 8/group). Symbols represent differences compared with sedentary control groups (**P < 0.05) or to basal state (#P < 0.01).
There was no change in GIRs or basal or insulin-stimulated rates of glucose disappearance in offspring from high fat–fed sedentary dams compared with offspring from high fat–fed trained dams (Fig. 4A, B, and D). There was, however, a significant decrease in basal insulin concentrations (P < 0.001) and a trend toward a decrease in insulin concentrations during the clamped state (P = 0.09) in offspring from high fat–fed trained dams compared with offspring from high fat–fed sedentary dams (Fig. 4F). Because of this large difference in insulin concentrations, we normalized Rd by insulin concentrations (Fig. 4G) and found that offspring from high fat–fed trained dams had a significant increase in Rd/insulin compared with offspring from high fat–fed sedentary dams. This applies to the basal EndoRa values as well, because basal EndoRa equals basal Rd. The increase in Rd/insulin from the clamp data are consistent with the increased glucose tolerance and increased insulin sensitivity in isolated hepatocytes in the offspring of high fat–fed trained dams. Rd/insulin was not different between offspring from chow-fed sedentary and trained dams (Fig. 4G).
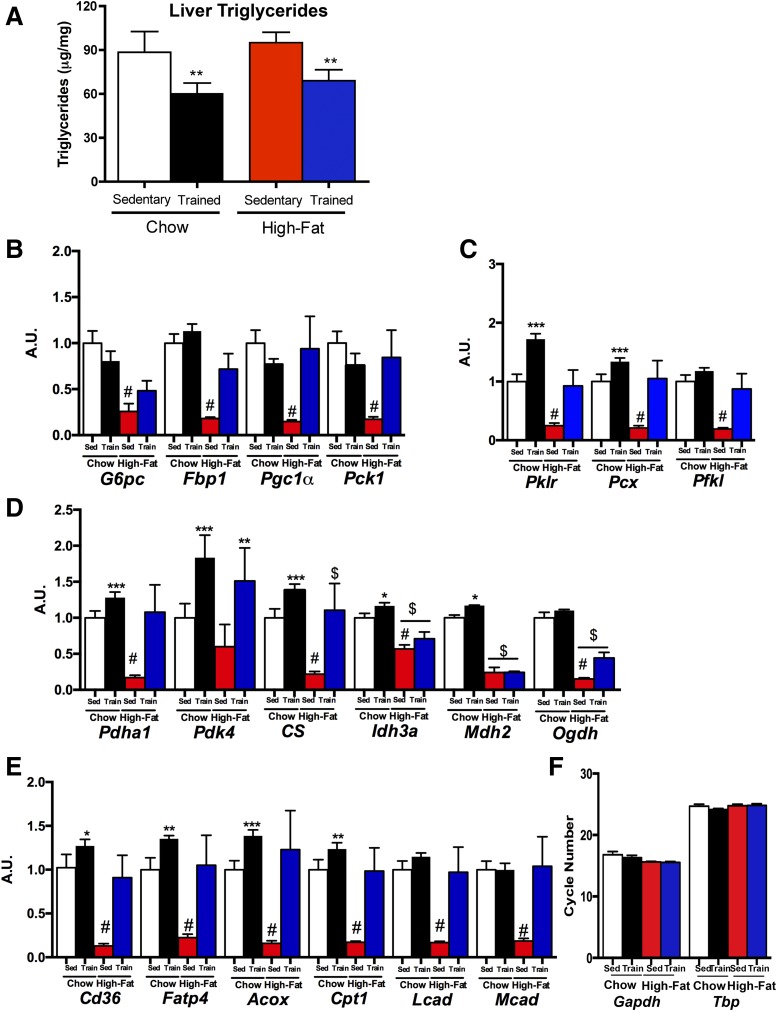
Elevated lipid concentrations in tissues can affect metabolic function; therefore, we determined the effects of maternal exercise training on triglyceride concentrations in livers from offspring at 52 weeks of age. Interestingly, offspring from high fat–fed dams did not have an elevation in liver triglyceride concentrations, but there was a significant decrease in liver triglycerides in offspring from trained chow- and high fat–fed dams (Fig. 5A). Decreased liver triglycerides in the offspring from the trained dams may also play a role in improved hepatic function and protect against the development of metabolic disease during adulthood.
Figure 5.
Maternal exercise alters hepatic composition and gene expression. Liver triglyceride concentration (A) was measured at 52 weeks of age in female offspring. Gene expression of hepatic genes involved in gluconeogenesis (B), pyruvate metabolism (C), Krebs cycle activity (D), fatty acid transport and oxidation (E), and liver housekeeping genes were measured at 52 weeks of age (F). Data are expressed as means ± SEM (n = 8/group). Symbols represent differences compared with sedentary control groups (*P < 0.05; **P < 0.01; ***P < 0.001; #P < 0.05 high fat–fed sedentary vs. chow-fed sedentary; $P < 0.05 high fat–fed sedentary vs. high fat–fed trained). A.U., arbitrary units.
To determine the effects of maternal exercise on hepatic gene expression, we measured several genes involved in gluconeogenesis (Fig. 5B), Krebs cycle activity (Fig. 5C and D), and oxidative phosphorylation (Fig. 5E) in offspring livers at 52 weeks of age. Offspring from chow-fed trained dams had significantly increased expression of several genes involved in pyruvate metabolism (Fig. 5C), Krebs cycle activity (Fig. 5D), and fatty acid transport and oxidation (Fig. 5E) compared with all other groups. There were dramatic effects of high-fat feeding of dams on offspring gene expression with almost every gene measured reduced by ∼50% (Fig. 5B–E) compared with offspring from chow-fed sedentary dams. Importantly, training of dams fed a high-fat diet rescued these dramatic effects of maternal diet on gene expression, as offspring from high fat–fed trained dams had similar gene expression to offspring from chow-fed sedentary dams (Fig. 5B–E). Expression of housekeeping genes (both Gapdh and Tbp) was not different among groups (Fig. 5F). These data demonstrate that maternal exercise can prevent the detrimental effects of a maternal high-fat diet on offspring hepatic gene expression.
Discussion
It is well established that maternal obesity is a major factor in the development of obesity and diabetes in offspring as they age, initiating a vicious cycle that likely contributes to the current rise in rates of obesity and diabetes (2–13). Regular exercise is a primary therapeutic tool to combat obesity and improve metabolic health, but the role that exercise during pregnancy may play on the health of the offspring is poorly understood, in part because of the difficulty of performing exceptionally complex longitudinal research investigations. Fortunately, rodent models of exercise during pregnancy have recently been used to begin to address these questions, with most studies showing that maternal exercise results in health benefits to male offspring (21,23–25). In the current investigation, we find that maternal exercise also confers benefits to female offspring. Moreover, the disturbances in metabolic health of the female offspring in response to maternal high-fat feeding are fully prevented if the dams are simultaneously exercise-trained. In fact, maternal exercise fully compensated for the detrimental effects of a maternal high-fat diet on glucose metabolism, insulin tolerance, circulating insulin, and body weight in female offspring. These data demonstrate that maternal exercise is critical to improve the metabolic health of adult female offspring.
There may be adaptations to numerous tissues in the offspring that mediate the beneficial effects of maternal exercise and the detrimental effects of poor maternal nutrition on offspring glucose tolerance and insulin sensitivity. Given the marked effects of maternal high-fat feeding and exercise on glucose tolerance in the offspring in our study and the critical role the liver plays in glucose homeostasis, we hypothesized that there were adaptations to the offspring liver. Indeed, we found that maternal high- fat feeding resulted in liver metabolic dysfunction in offspring and that exercise training of dams reversed these detrimental effects. A striking finding was that basal, insulin-suppressed, and glucagon-stimulated glucose production were all improved in hepatocytes from offspring of trained dams and impaired with dam high-fat feeding. These effects, measured in isolated hepatocytes, occurred as early as 6 weeks of age, whereas changes in glucose tolerance in vivo were not present until later in life. It is likely that multiple factors contribute to changes in glucose tolerance in the intact animals and that the whole-body metabolic homeostasis can be maintained by compensation from many other organs. Nevertheless, these findings suggest that defects in offspring liver metabolism may be an early phenotype that contributes to the pronounced changes in glucose tolerance that occur in vivo as the animals age.
Maternal high-fat diet feeding has been suggested to increase lipid transfer to the fetus regardless of the level of maternal obesity (6,40–43). Because the maternal high-fat diet in the current study consisted of 60% kcal from fat, it is likely that increasing maternal lipid as a source of calories in the diet contributed to changes in the fetus, and these changes may have been independent of increases in adiposity or insulin resistance in the dams. In adult humans, diet-induced obesity inundates the white adipose tissue, and as a result, fat accumulates in liver and skeletal muscle, causing insulin resistance (6,44–50). It is possible that maternal exercise negated the increase in lipid transfer to the fetus because the excess maternal lipids were used during exercise. It is important to note that in rodents, adipose tissue develops mainly during the postnatal weaning period, whereas in humans and nonhuman primates, adipose tissue develops during the third trimester. Thus in humans, it is possible that surplus fatty acids might be stored in the developing adipose tissue depots instead of liver, skeletal muscle, or brown adipose tissue. Our findings showing effects of maternal high-fat feeding are consistent with a study in primates in which high-fat feeding of mothers resulted in 26-week-old offspring with impaired liver function as measured by increased lipid accumulation and impaired gene expression (6). In addition, high-fat feeding of dams in a mouse model resulted in impaired expression of hepatic genes involved in peroxisome proliferator–activated receptor signaling, fatty acid metabolism, Krebs cycle activity, and mitochondrial biogenesis in 7-day-old male and female offspring (51). Our findings are also consistent with a recent study in rats that found that maternal exercise reduced susceptibility to metabolic impairments in offspring liver (23). Taken together, we conclude that a maternal high-fat diet impairs liver function in female offspring, whereas maternal exercise restores liver function in these animals. Opposite of poor maternal nutrition, maternal exercise must alter the intrauterine environment in a way that mediates beneficial effects on offspring liver metabolism. In future studies, it will be important to investigate epigenetic changes in the liver, which may be essential to fully understand the effects of maternal exercise on offspring health.
In contrast to male offspring from chow-fed sedentary dams, we found that as female offspring of chow-fed dams age, there is little worsening of glucose tolerance (Fig. 1A). The lack of impairment in glucose tolerance with aging in the female offspring from chow-fed dams might explain why there was no effect of dam exercise training in these offspring. Although glucose tolerance was not changed as the female offspring of trained, chow-fed dams aged, we found that these mice had lower circulating insulin concentrations at 1 year of age. This demonstrates that exercise training of dams results in enhanced insulin sensitivity in female offspring in adulthood. As has been previously reported in male offspring (19,21,22,25), we found that high-fat feeding of sedentary dams increased overnight-fasted insulin concentrations of female offspring. Not surprisingly then, the effects of dam training on offspring insulin concentrations were even more pronounced if the dams were fed a high-fat diet. Because insulin is altered in both male and female offspring in response to maternal exercise, insulin sensitivity may be a critical factor in determining how maternal exercise influences the metabolic health of adult offspring. These data have significant translational ramifications suggesting that maternal exercise, even in the presence of a high-fat diet, improves offspring insulin sensitivity. Maternal exercise before and during pregnancy may lead to enhanced pancreatic function and peripheral insulin sensitivity in the offspring.
In summary, maternal exercise during pregnancy significantly improves the metabolic health of female offspring and counteracts the detrimental effects of a maternal high-fat diet. This improvement in metabolic health is likely because of several mechanisms, including improvements in liver metabolism and decreased circulating insulin in the adult offspring. These findings suggest that maternal exercise before and during pregnancy could be an important tool to combat obesity and type 2 diabetes in future generations.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Julio Ayala from the Sanford-Burnham Medical Research Institute and Maura Mulvey, Allen Clermont, and Geetha Sankaranarayan from the Joslin Diabetes Center Diabetes and Endocrinology Research Center Physiology and Complex Assay cores for technical assistance.
Funding. This work was supported by National Institutes of Health grants R01-DK101043 (to L.J.G.), K01-DK-105109 (to K.I.S.), and 5P30-DK-36836 (to Joslin Diabetes Center Diabetes and Endocrinology Research Center) and an American College of Sports Medicine Research Endowment Grant (to K.I.S.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.I.S. designed experiments, performed experiments, analyzed the data, and wrote and edited the manuscript. H.T., K.S., A.B.A.-W., N.B.P., A.C.L., K.M.G., M.-Y.L., and M.F.H. performed experiments. L.J.G. designed experiments, analyzed the data, and wrote and edited the manuscript. L.J.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0098/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.National Task Force on the Prevention and Treatment of Obesity Overweight, obesity, and health risk. Arch Intern Med 2000;160:898–904 [DOI] [PubMed] [Google Scholar]
- 2.Isganaitis E, Jimenez-Chillaron J, Woo M, et al. Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice. Diabetes 2009;58:1192–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isganaitis E, Woo M, Ma H, et al. Developmental programming by maternal insulin resistance: hyperinsulinemia, glucose intolerance, and dysregulated lipid metabolism in male offspring of insulin-resistant mice. Diabetes 2014;63:688–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez-Chillaron JC, Hernandez-Valencia M, Reamer C, et al. Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes 2005;54:702–711 [DOI] [PubMed] [Google Scholar]
- 5.Woo M, Isganaitis E, Cerletti M, et al. Early life nutrition modulates muscle stem cell number: implications for muscle mass and repair. Stem Cells Dev 2011;20:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009;119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991;303:1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phipps K, Barker DJ, Hales CN, Fall CH, Osmond C, Clark PM. Fetal growth and impaired glucose tolerance in men and women. Diabetologia 1993;36:225–228 [DOI] [PubMed] [Google Scholar]
- 9.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976;295:349–353 [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–128 [PubMed] [Google Scholar]
- 11.Gniuli D, Calcagno A, Caristo ME, et al. Effects of high-fat diet exposure during fetal life on type 2 diabetes development in the progeny. J Lipid Res 2008;49:1936–1945 [DOI] [PubMed] [Google Scholar]
- 12.Masuyama H, Hiramatsu Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology 2012;153:2823–2830 [DOI] [PubMed] [Google Scholar]
- 13.Bayol SA, Simbi BH, Stickland NC. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 2005;567:951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muhlhausler BS, Ong ZY. The fetal origins of obesity: early origins of altered food intake. Endocr Metab Immune Disord Drug Targets 2011;11:189–197 [DOI] [PubMed] [Google Scholar]
- 15.Khan I, Dekou V, Hanson M, Poston L, Taylor P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation 2004;110:1097–1102 [DOI] [PubMed] [Google Scholar]
- 16.Cerf ME, Louw J. High fat programming induces glucose intolerance in weanling Wistar rats. Horm Metab Res 2010;42:307–310 [DOI] [PubMed] [Google Scholar]
- 17.Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol 2003;179:293–299 [DOI] [PubMed] [Google Scholar]
- 18.Stephenson T, Budge H, Mostyn A, Pearce S, Webb R, Symonds ME. Fetal and neonatal adipose maturation: a primary site of cytokine and cytokine-receptor action. Biochem Soc Trans 2001;29:80–85 [DOI] [PubMed] [Google Scholar]
- 19.Carter LG, Lewis KN, Wilkerson DC, et al. Perinatal exercise improves glucose homeostasis in adult offspring. Am J Physiol Endocrinol Metab 2012;303:E1061–E1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laker RC, Lillard TS, Okutsu M, et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes 2014;63:1605–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanford KI, Lee MY, Getchell KM, So K, Hirshman MF, Goodyear LJ. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 2015;64:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter LG, Qi NR, De Cabo R, Pearson KJ. Maternal exercise improves insulin sensitivity in mature rat offspring. Med Sci Sports Exerc 2013;45:832–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheldon RD, Nicole Blaize A, Fletcher JA, et al. Gestational exercise protects adult male offspring from high-fat diet-induced hepatic steatosis. J Hepatol 2016;64:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasinski F, Bacurau RF, Estrela GR, et al. Exercise during pregnancy protects adult mouse offspring from diet-induced obesity. Nutr Metab (Lond) 2015;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raipuria M, Bahari H, Morris MJ. Effects of maternal diet and exercise during pregnancy on glucose metabolism in skeletal muscle and fat of weanling rats. PLoS One 2015;10:e0120980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014;509:282–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol 2005;288:R368–R373 [DOI] [PubMed] [Google Scholar]
- 28.Samuelsson AM, Morris A, Igosheva N, et al. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension 2010;55:76–82 [DOI] [PubMed] [Google Scholar]
- 29.Ozanne SE, Olsen GS, Hansen LL, et al. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J Endocrinol 2003;177:235–241 [DOI] [PubMed] [Google Scholar]
- 30.Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH, Drucker DJ. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology 2009;150:1155–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayala JE, Bracy DP, James FD, Burmeister MA, Wasserman DH, Drucker DJ. Glucagon-like peptide-1 receptor knockout mice are protected from high-fat diet-induced insulin resistance. Endocrinology 2010;151:4678–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burmeister MA, Ferre T, Ayala JE, King EM, Holt RM, Ayala JE. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am J Physiol Endocrinol Metab 2012;302:E334–E343 [DOI] [PubMed] [Google Scholar]
- 33.Toyoda T, An D, Witczak CA, et al. Myo1c regulates glucose uptake in mouse skeletal muscle. J Biol Chem 2011;286:4133–4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lessard SJ, Rivas DA, Alves-Wagner AB, et al. Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise-regulated signaling networks. Diabetes 2013;62:2717–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanford KI, Middelbeek RJ, Townsend KL, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015;64:2002–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho KW, Zhou Y, Sheng L, Rui L. Lipocalin-13 regulates glucose metabolism by both insulin-dependent and insulin-independent mechanisms. Mol Cell Biol 2011;31:450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Jiang L, Rui L. Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. J Biol Chem 2009;284:11152–11159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto T, Kanemoto N, Ban T, Sudo T, Nagano K, Niki I. Establishment and characterization of a novel method for evaluating gluconeogenesis using hepatic cell lines, H4IIE and HepG2. Arch Biochem Biophys 2009;491:46–52 [DOI] [PubMed] [Google Scholar]
- 39.Björntorp P, Sjöström L. Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism 1978;27(Suppl. 2):1853–1865 [DOI] [PubMed] [Google Scholar]
- 40.McMillen IC, Adam CL, Mühlhäusler BS. Early origins of obesity: programming the appetite regulatory system. J Physiol 2005;565:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMillen IC, Mühlhäusler BS, Duffield JA, Yuen BS. Prenatal programming of postnatal obesity: fetal nutrition and the regulation of leptin synthesis and secretion before birth. Proc Nutr Soc 2004;63:405–412 [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan M, Katewa SD, Palaniyappan P, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab 2006;291:E792–E7929 [DOI] [PubMed] [Google Scholar]
- 43.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003;111:e221–e226 [DOI] [PubMed] [Google Scholar]
- 44.Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth–a review. Placenta 2002; 23(Suppl. A):S28–S38 [DOI] [PubMed] [Google Scholar]
- 45.Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev 2000;16:202–210 [DOI] [PubMed] [Google Scholar]
- 46.Herrera E, Amusquivar E, López-Soldado I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Horm Res 2006;65(Suppl. 3):59–64 [DOI] [PubMed] [Google Scholar]
- 47.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem 2000;275:8456–8460 [DOI] [PubMed] [Google Scholar]
- 48.Kim YB, Shulman GI, Kahn BB. Fatty acid infusion selectively impairs insulin action on Akt1 and protein kinase C lambda/zeta but not on glycogen synthase kinase-3. J Biol Chem 2002;277:32915–32922 [DOI] [PubMed] [Google Scholar]
- 49.Pajvani UB, Trujillo ME, Combs TP, et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med 2005;11:797–803 [DOI] [PubMed] [Google Scholar]
- 50.Trujillo ME, Pajvani UB, Scherer PE. Apoptosis through targeted activation of caspase 8 (“ATTAC-mice”): novel mouse models of inducible and reversible tissue ablation. Cell Cycle 2005;4:1141–1145 [DOI] [PubMed] [Google Scholar]
- 51.Thompson MD, Cismowski MJ, Trask AJ, et al. Enhanced steatosis and fibrosis in liver of adult offspring exposed to maternal high-fat diet. Gene Expr 2016;17:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.