Abstract
Pima Indians living in Arizona have a high prevalence of obesity, and we have previously shown that a relatively lower energy expenditure (EE) predicts weight and fat mass gain in this population. EE is a familial trait (heritability = 0.52); therefore, in the current study, we aimed to identify genetic variants that affect EE and thereby influence BMI and body fatness in Pima Indians. Genotypic data from 491,265 variants were analyzed for association with resting metabolic rate (RMR) and 24-h EE assessed in a whole-room calorimeter in 507 and 419 Pima Indians, respectively. Variants associated with both measures of EE were analyzed for association with maximum BMI and percent body fat (PFAT) in 5,870 and 912 Pima Indians, respectively. rs11014566 nominally associated with both measures of EE and both measures of adiposity in Pima Indians, where the G allele (frequency: Pima Indians = 0.60, Europeans <0.01) associated with lower 24-h EE (β = −33 kcal/day per copy), lower RMR (β = −31 kcal/day), higher BMI (β = +0.6 kg/m2), and higher PFAT (β = +0.9%). However, the association of rs11014566 with BMI did not directionally replicate when assessed in other ethnic groups. rs11014566 tags rs144895904, which affected promoter function in an in vitro luciferase assay. These variants map to GPR158, which is highly expressed in the brain and interacts with two other genes (RGS7 and CACNA1B) known to affect obesity in knockout mice. Our results suggest that common ethnic-specific variation in GPR158 may influence EE; however, its role in weight gain remains controversial, as it either had no association with BMI or associated with BMI but in the opposite direction in other ethnic groups.
Introduction
Obesity often aggregates in families.Household members typically share lifestyle factors including food choices, daily habits, and cultural views that may affect body weight; however, studies in twins reared apart have provided evidence that approximately two-thirds of the variability of BMI is attributable solely to genetics (1,2). BMI is influenced by both energy intake and energy expenditure (EE). In Pima Indians living in Arizona, we have shown that a relatively low EE predicts increases in body weight (3–5) and fat mass (FM) (4) over time. However, this inverse relationship is not observed in all populations (6–8), and a positive relationship has been reported in an African population (9). EE varies by age and sex, but its largest determinant is body size and composition, particularly fat-free mass (FFM) as an indicator of the metabolically active tissue, which accounts for ∼80% of the variance in resting metabolic rate (RMR) and 24-h EE (10). However, in addition to age, sex, and body composition, twin studies have also demonstrated that genetics contributes to the interindividual variance in EE (11,12). Taken together, these prior studies indicate that genetics has a small but measurable effect on EE, and in Pima Indians a lower EE predicts weight gain. Therefore, identification of genetic variants that influence EE may uncover new metabolic pathways that affect body weight/fatness. The aim of the current study is to estimate the heritable portion of EE and BMI in a family-based sample of Pima Indians and then perform a genome-wide analysis of variants in Pima Indians without diabetes to identify genetic variation that associates with EE and BMI/body fatness in a fashion consistent with the putative mechanistic relationship (e.g., low EE and high BMI).
Research Design and Methods
Population-Based Subjects With Outpatient Longitudinal Measures of BMI
The study subjects reside in an American Indian community near Phoenix, Arizona, where most individuals are of Pima Indian heritage. From 1965 to 2007, volunteers from this community participated in a longitudinal study of type 2 diabetes where anyone aged ≥5 years was invited for biennial health examinations (13). Subjects were asked to fast prior to these exams, and glucose tolerance was assessed by a 75-g oral glucose tolerance test. Height and weight were also measured to calculate BMI. Data on maximum BMI, defined as the highest BMI recorded at a medical exam when the subject was ≥15 years of age and was determined to be free from diabetes according to American Diabetes Association diagnostic criteria (14), was available for 5,870 subjects (Table 1). Among these subjects, 2,920 were full-heritage Pima Indian (defined as eight-eighths Pima Indian heritage by self-report) and the remaining 2,950 were mixed heritage, on average, six-eighths American Indian (typically four-eighths Pima Indian and an additional two-eighths from other related tribes). Before participation, volunteers were fully informed of the nature and purpose of the studies, and written informed consent was obtained. The protocols were approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases.
Table 1.
Anthropometric and metabolic measures of the study groups
| All | Males | Females | |
|---|---|---|---|
| Population-based longitudinal outpatient study | |||
| N | 5,870 | 2,572 | 3,298 |
| Birth year | 1,966 ± 16 | 1,967 ± 16 | 1,966 ± 16 |
| Maximum BMI (kg/m2) | 35.2 ± 8.4 | 33.9 ± 8.1 | 36.1 ± 8.5 |
| Age (years) | 29.6 ± 11.4 | 28.9 ± 11.3 | 30.1 ± 11.4 |
| Body composition inpatient study | |||
| N | 917 | 506 | 411 |
| Age (years) | 28.0 ± 8.0 | 28.1 ± 8.3 | 28.0 ± 7.6 |
| PFAT (%) | 33.4 ± 8.5 | 28.4 ± 7.0 | 39.7 ± 5.7 |
| FM (kg) | 33.0 ± 14.6 | 29.3 ± 14.0 | 37.6 ± 13.9 |
| FFM (kg) | 62.5 ± 14.2 | 68.8 ± 13.4 | 54.7 ± 10.9 |
| Height (cm) | 166.6 ± 8.4 | 172.1 ± 6.2 | 159.9 ± 5.2 |
| Respiratory chamber inpatient study | |||
| N | 419 | 254 | 165 |
| Age (years) | 27.8 ± 6.4 | 27.8 ± 6.6 | 27.8 ± 6.2 |
| Body weight (kg) | 95.3 ± 22.3 | 98.6 ± 22.7 | 90.2 ± 20.7 |
| BMI (kg/m2) | 34.2 ± 7.5 | 33.4 ± 7.3 | 35.3 ± 7.7 |
| PFAT (%) | 32.6 ± 8.2 | 28.7 ± 6.9 | 38.8 ± 6.0 |
| FM (kg) | 32.0 ± 13.0 | 29.5 ± 12.9 | 35.8 ± 12.2 |
| FFM (kg) | 63.3 ± 12.6 | 69.0 ± 10.9 | 54.4 ± 9.5 |
| Fasting plasma glucose concentration (mg/dL) | 88.8 ± 10.0 | 87.3 ± 9.9 | 91.2 ± 9.7 |
| 2-h plasma glucose concentration (mg/dL) | 123.0 ± 30.5 | 115.8 ± 30.0 | 134.0 ± 28.0 |
| 24-h EE (kcal/day) | 2,354 ± 396 | 2,531 ± 347 | 2,083 ± 303 |
| Adjusted 24-h EE (kcal/day)# | 0 ± 142.9 | 0 ± 147.0 | 0 ± 136.9 |
| SPA (%) | 7.5 ± 2.5 | 7.7 ± 2.5 | 7.1 ± 2.5 |
| Sleeping EE (kcal/day) | 1,672 ± 284 | 1,776 ± 271 | 1,513 ± 223 |
| Adjusted sleeping EE (kcal/day)# | 0 ± 135.3 | 0 ± 144.4 | 0 ± 120.3 |
| AFT (kcal/14 h) | 263 ± 122 | 288 ± 129 | 223 ± 99 |
| Adjusted AFT (kcal/14 h)# | 0 ± 114.8 | 0 ± 124.2 | 0 ± 98.6 |
| Ventilated hood inpatient study | |||
| N | 509 | 301 | 208 |
| Age (years) | 26.9 ± 6.1 | 26.8 ± 6.4 | 26.9 ± 5.8 |
| Body weight (kg) | 93.4 ± 23.0 | 97.3 ± 24.2 | 87.8 ± 19.9 |
| BMI (kg/m2) | 33.5 ± 7.6 | 32.9 ± 7.6 | 34.5 ± 7.4 |
| PFAT (%) | 32.3 ± 8.5 | 28.2 ± 7.4 | 38.2 ± 6.4 |
| FM (kg) | 31.2 ± 13.5 | 28.9 ± 13.9 | 34.5 ± 12.2 |
| FFM (kg) | 62.2 ± 12.9 | 68.4 ± 11.6 | 53.3 ± 8.8 |
| Fasting plasma glucose concentration (mg/dL) | 89.3 ± 10.0 | 87.4 ± 9.5 | 92.1 ± 10.0 |
| 2-h plasma glucose concentration (mg/dL) | 122.8 ± 30.3 | 115.7 ± 28.1 | 132.9 ± 30.5 |
| RMR (kcal/day) | 1,758 ± 326 | 1,878 ± 322 | 1,587 ± 247 |
| Adjusted RMR (kcal/day)# | 0 ± 189.6 | 0 ± 212.4 | 0 ± 151.4 |
Data are mean ± SD unless otherwise noted.
#All four EE measures (24-h EE, sleeping EE, AFT, and RMR) are adjusted for age, sex, FM, and FFM by linear regression analysis; 24-h EE and AFT are further adjusted for SPA and for fasting glucose levels, respectively.
Inpatient Subjects With Measures of Body Composition and EE
Among the community members from the longitudinal study, a subset of 917 adults who were confirmed to not have diabetes by the oral glucose tolerance test also participated in inpatient studies in our Clinical Research Section and had undergone detailed measures of body composition. Among these inpatient volunteers, 509 also underwent a measurement of RMR by a ventilated hood system and 419 underwent a 24-h session in a whole-room indirect calorimeter (352 subjects underwent both measures of RMR and 24-h EE during the same admission).
Following admission to the Clinical Research Section, subjects were given a standard weight-maintaining diet (50% carbohydrates, 30% fats, and 20% proteins) for 3 days before any metabolic test was performed (15,16). Subjects were weighed daily, and food intake was adjusted to maintain body weight within ±1% of the weight measured the second day of admission. Percent body fat (PFAT), FM, and FFM were estimated by underwater weighing until August 1993 and thereafter by total body dual-energy X-ray absorptiometry (DPX-1; Lunar Radiation Corp., Madison, WI). A conversion equation was used to make measurements of body composition comparable between the two methods (17).
RMR was measured upon awakening after an overnight fast using a respiratory hood system, as previously described (18). After 10 min of acclimation to the plastic hood, the subject’s EE was calculated every 5 min using the equations of Lusk (19), and RMR was calculated as the average EE over 40 min while the subject was instructed to stay awake and motionless and then extrapolated to 24 h.
Twenty-four–hour EE and substrate oxidation were measured in a whole-room calorimeter (respiratory chamber), as previously described (10). The volunteers entered the chamber at 08:00 and remained in the chamber for 23 h and 15 min. The rate of EE was measured continuously, calculated for each 15-min interval, averaged, and then extrapolated to the 24-h interval (24-h EE). Four meals were provided at 08:00, 11:00, 16:00, and 19:00, and the total energy content was calculated using previously described equations (20). Spontaneous physical activity (SPA) was detected by radar sensors and expressed as the percentage of time over the 15-min interval in which activity was detected (21). The EE in the inactive awake state was calculated as the intercept of the regression line between EE and SPA between 11:00 and 01:00 (22). Sleeping metabolic rate was defined as the average EE of all 15-min nightly periods between 01:00 and 05:00 during which SPA was <1.5% (23). The “awake and fed” thermogenesis (AFT) was calculated as the difference between the EE in the inactive awake state and the sleeping metabolic rate (23).
Genotypic Data for Genome-Wide Association Analysis
Genotypes for association analyses were generated using a custom Pima Indian Axiom genome-wide array (Affymetrix, Santa Clara, CA) in 7,701 Pima Indian samples. This array was designed to capture common variation (minor allele frequency [MAF] ≥ 0.05, or ≥ 0.01 for coding variants) detected in whole-genome sequence (WGS) data of 266 full-heritage Pima Indians from different nuclear families. We estimated that genotypes for the 491,265 array markers that passed quality control metrics (i.e., call rate ≥90%, discrepant rate ≤2 pairs among 100 blind duplicate pairs, and lack of deviation from Hardy-Weinberg equilibrium with a P > 10−4) tag 92% of the 4.9 million common variants with a MAF ≥ 0.05 detected in the genomes of full-heritage Pima Indians (tag defined as r2 ≥ 0.85 within 300 kb).
Functional Analysis of GPR158 Variants
DNA fragments containing each allele homozygous at rs11014566, rs144895904, rs34673593, and rs16925884 were PCR amplified (rs11014566, primers forward 5′-ACAGGTACCATTTGTGTTAACGGCTAGA-3′ and reverse 5′-TCACTCGAGGTATAAACAATTTTGCCAT-3′; rs144895904, forward 5′-ATAGGTACCAGAGATAACCGCTGTTCA-3 and reverse 5′-TCACTCGAGAGGCACAAATTACATAAC-3′; rs16925884/rs34673593, forward 5′-ACGGTACCTACTATTTGTTGTGAG-3′ and reverse 5′-AGCTCGAGATATAAATGAATGAATTG-3′). The amplicons were inserted at KpnI and XhoI sites (underlined, respectively) upstream of the pGL3 promoter firefly luciferase reporter vector (Promega, Madison, WI). DNA constructs were sequenced to confirm the nucleotide variants.
Murine N-42 hypothalamus cell line (Cellutions Biosystems, Inc., Burlington, ON, Canada) was maintained in DMEM medium supplemented with 10% FBS, 1% penicillin-streptomycin (ATCC) at 37°C, 5% CO2, and 95% air atmosphere. One microgram of DNA construct and 125 ng of pGL4 Renilla luciferase reporter vector (Promega, Madison, WI) were transiently transfected into the cells with Lipofectamine LTX (Invitrogen, Life Technologies, Carlsbad, CA). At 48 h posttransfection, cells were harvested and a dual-luciferase reporter assay was performed using a standard protocol (Promega, Madison, WI). Three separate transfections were conducted, each transfection was repeated two to three times (for a total of eight), and data were averaged. Firefly luciferase activity was normalized to Renilla luciferase activity and further normalized to pGL3 promoter luciferase activity.
Statistical Analysis
The variances in 24-h EE and maximum BMI attributable to family membership were estimated in families with at least two siblings by mixed-model analysis and quantified by the root-mean-square deviation (RMSD) and by the intraclass correlation coefficient (ICC). Heritability was estimated in a linear mixed model from a random effect that utilized the empirical genetic relatedness matrix (see below). Linear mixed effects analysis using the maximum likelihood method was conducted to assess association of genotypes with 24-h EE, RMR, maximum BMI, and PFAT with covariates of age, sex, body composition measures (FM and FFM, only for 24-h EE and RMR analyses), SPA (only for 24-h EE analysis), birth year (only for BMI analysis), and the first five genetic principal components calculated from 19,991 variants randomly selected from 200-kb windows across the genome (one variant per window). Genotype was included as a fixed effect and analyzed as a numeric variable representing 0, 1, or 2 copies of a given allele (additive model), and effects were expressed per allele copy. Missing genotypes were imputed using WGS data of 266 full-heritage Pima Indians. The models were fitted using a variance components covariance structure to account for genetic relatedness among individuals. The genetic relatedness matrix was estimated as the proportion of the genome shared identical by descent between each pair of individuals who had been genotyped (a total of 29,648,850 pairs). Genomic segments shared identical by descent were identified with the fastIBD function of Beagle package (24) using 482,616 autosomal markers with MAF > 0.05. Mixed models were fit using the SOLAR package (25). Values of BMI were log-transformed before analysis to approximate a Gaussian distribution. Linkage disequilibrium was determined using the Haploview program (version 4.2, Broad Institute, Cambridge, MA). Tag variants were selected based on the sequence data of 266 Pima genomes using the Tagger algorithm (Haploview) with a pairwise r2 ≥ 0.85 taken as indicative of redundancy. The statistical difference in mean luciferase activity detected in the functional study was analyzed by unpaired Student t test.
To estimate the statistical power to detect a physiologically meaningful effect of a common genetic variant on 24-h EE that meets genome-wide statistical significance, we estimated power for a sample size of 419 unrelated individuals, a two-sided α = 5 × 10−8, a clinically significant effect size β = −50 kcal/day per risk allele copy, and a residual SD = 143 kcal/day (after adjustment for age, sex, FM, FFM, and SPA) (Table 1). Using these parameters, we estimated the power to be 0.03 or 0.38 to detect a risk allele frequency (RAF) (defined for the allele associated with lower EE) of 0.15 or 0.50, respectively. To reduce the chance of spurious findings (type 1 error) without undue reliance on a single EE measure in a setting of low statistical power, we selected variants with consistent evidence of association in two separate EE assessments, namely, 24-h EE and RMR, where each P value was <0.01 and the direction of risk was consistent (i.e., the risk allele associated with lower 24-h EE being associated with lower RMR). Variants meeting these criteria were then analyzed for association with BMI and PFAT.
Replication Cohort
Replication of selected variants for their association with standardized values of BMI was done in individuals without diabetes of the Slim Initiative in Genomic Medicine for the Americas (SIGMA) Consortium (26) after adjustment for age, sex, and first two genetic principal components. This replication sample consisted of four studies from Mexico or Mexicans living in the U.S. comprising a total of 4,364 individuals without diabetes. All participants provided informed consent for conducting this study. Their respective local ethics committees approved all contributing studies.
Results
Estimates of Familial Effect on 24-Hour EE and BMI in Pima Indians
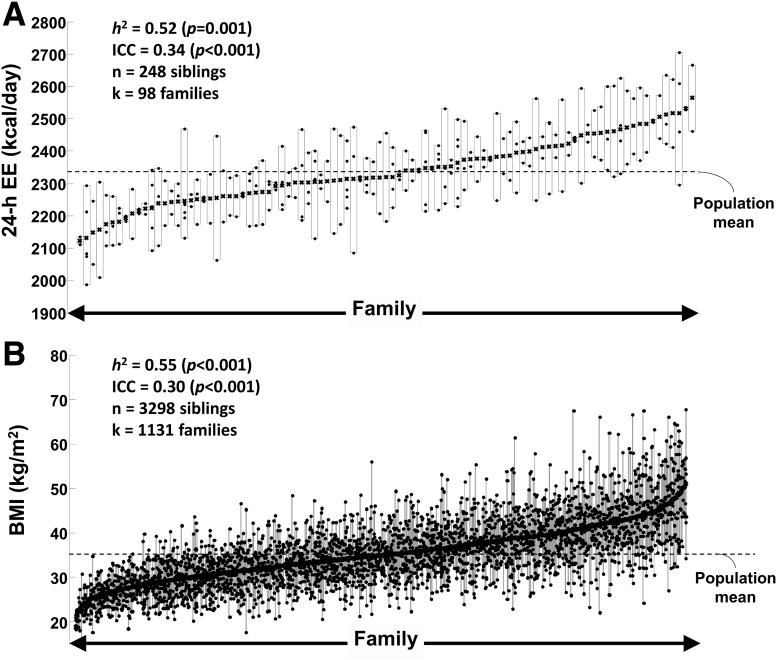
In 248 siblings from 98 Pima Indian families, family membership explained 41% of variance in unadjusted 24-h EE (RMSD = 250 kcal/day, P < 0.001). After adjustment for each subject’s age, sex, FM, FFM, and SPA, family membership was still an independent determinant of 24-h EE (RMSD = 77 kcal/day, P < 0.001), accounting for 34% of the unexplained variance in 24-h EE (Fig. 1A). In 3,298 siblings from 1,131 Pima Indian families, family membership was the largest determinant of maximum BMI, accounting for one-third of BMI variance among individuals (RMSD = 4.4 kg/m2, P < 0.001) (Fig. 1B). Inclusion of age, sex, birth year, and Pima heritage did not alter the estimate of family variance and slightly increased the explained variance of maximum BMI from 30 to 36% (P < 0.001). Heritability of 24-h EE and maximum BMI was 0.52 (95% CI 0.20–0.80, P = 1.5 × 10−3, adjusted for age, sex, FM, FFM, SPA, and first five principal components) and 0.55 (95% CI 0.51–0.60, P = 8.2 × 10−148, adjusted for age, sex, birth year, and first five principal components), respectively.
Figure 1.
Familial effects on 24-h EE and maximum BMI in American Indians. Individual values of 24-h EE (adjusted for age, sex, FM, FFM, and SPA) (A) and maximum BMI (adjusted for age, sex, birth year, and self-reported Pima heritage) (B) of family members from the American Indian community. Individual siblings are shown as dots while families are depicted as vertical rectangles identified by the highest and lowest sibling value. Each family includes at least two siblings, and families are ranked according to mean value of 24-h EE (A) and BMI (B) of siblings, e.g., families with relatively low mean EE/BMI are located on the left side of x-axis. Heritability (h2) was estimated in a linear mixed model from a random effect that utilized the empirical genetic relatedness matrix estimated as the proportion of the genome shared identical by descent between each pair of siblings who had been genotyped. Mixed models for estimating heritability included age, sex, birth year (only BMI analysis), FM (only 24-h EE analysis), FFM (only 24-h EE analysis), SPA (only 24-h EE analysis), and the first five principal components as fixed effects. The ICC was calculated as the ratio of the variance attributable to family membership divided the total variance and expressed as the percent of total variance in 24-h EE and BMI after adjustment for covariates. n, number of siblings; k, number of families with at least two siblings.
Association Analysis for Two Independent Measures of EE
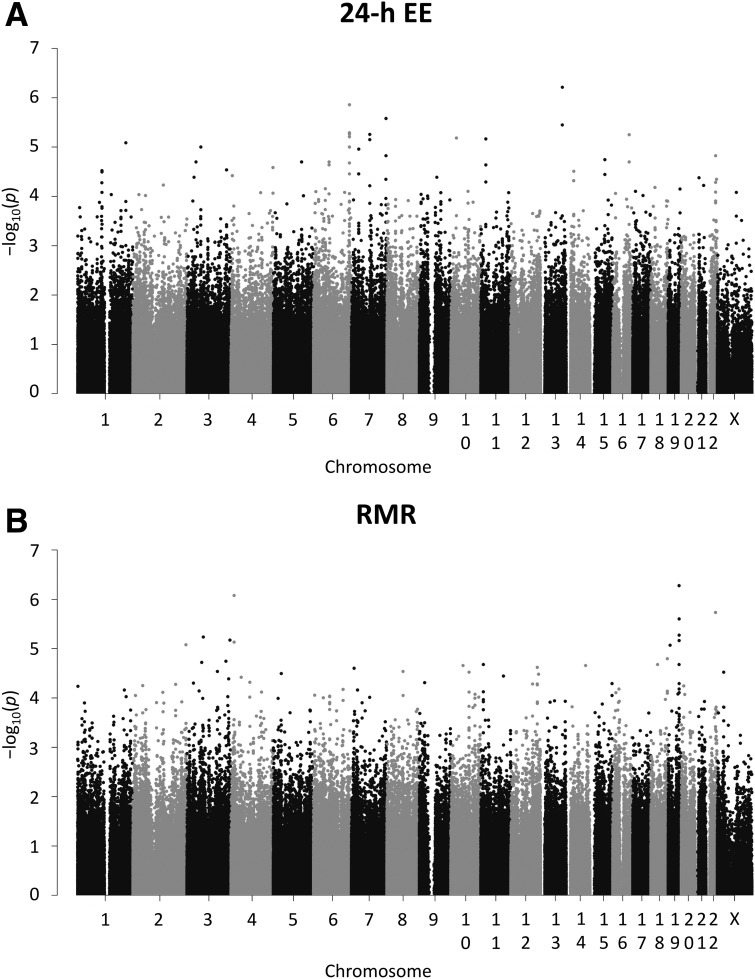
The results of the genome-wide association analyses for 24-h EE and RMR are shown in Fig. 2. The lists of variants with P < 0.01 for each EE measure are reported in Supplementary Tables 1 and 2 (effect size for 24-h EE and RMR ranging from −96 to −21 kcal/day and from −132 to −25 kcal/day, respectively). As anticipated, no variant achieved genome-wide statistical significance (P < 5 × 10−8) with either EE measure. However, 138 variants had nominal (P < 0.01), directionally consistent associations with both 24-h EE and RMR (Supplementary Table 3).
Figure 2.
Manhattan plots of genome-wide association results for 24-h EE and RMR in American Indians. The negative base-10 logarithm of the P value for the association of each genetic variant (n = 491,265, MAF ≥ 0.05) with 24-h EE (A) and RMR (B) after adjustment for age, sex, FM, FFM, SPA (only 24-h EE), and the first five principal components in a mixed model that accounted for genetic relationships among individuals is plotted against chromosome and position according to Build 37.
Association Analysis for Two Measures of Body Adiposity
The 138 variants that associated with both 24-h EE and RMR in a directionally consistent manner were further analyzed for association with maximum BMI (defined as the highest BMI recorded at a longitudinal outpatient exam) in a population-based sample of 5,870 individuals and with PFAT in 917 subjects who had undergone metabolic testing as inpatients. Although seven variants had an allele that associated with a reduced EE and were nominally associated with higher maximum BMI (Table 2) and five variants had an allele that associated with reduced EE and higher PFAT, only the variant with the strongest association with maximum BMI (rs11014566 in GPR158, P = 4.7 × 10−4) also associated with increased PFAT (P = 2.9 × 10−3).
Table 2.
Variants associated with lower 24-h EE, lower RMR, and higher maximum BMI in American Indians
| Chr. | Position | Variant ID | Gene(s) | R/NR allele | RAF | 24-h EE (n = 419) |
RMR (n = 509) |
Maximum BMI (n = 5,870) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | ||||||
| 10 | 25735858 | rs11014566 | GPR158 | G/A | 0.60 | −33.0 (10.5) | 1.7 × 10−3 | −31.1 (12.1) | 9.9 × 10−3 | 0.017 (0.005) | 4.7 × 10−4 |
| 21 | 30662480 | — | LINC00189, BACH1 | —/AAG | 0.30 | −30.6 (11.1) | 6.0 × 10−3 | −34.5 (13.3) | 9.4 × 10−3 | 0.014 (0.005) | 3.9 × 10−3 |
| 12 | 97806525 | rs12424131 | NEDD1, RMST | A/G | 0.88 | −42.8 (15.5) | 5.6 × 10−3 | −55.8 (19.2) | 3.6 × 10−3 | 0.018 (0.007) | 9.9 × 10−3 |
| 12 | 97810465 | rs17026922 | NEDD1, RMST | G/A | 0.92 | −46.8 (17.8) | 8.5 × 10−3 | −65.7 (22.6) | 3.6 × 10−3 | 0.020 (0.008) | 1.2 × 10−2 |
| 14 | 21113898 | rs56069351 | OR6S1, ANG | T/C | 0.25 | −33.0 (11.7) | 4.9 × 10−3 | −42.1 (14.1) | 2.7 × 10−3 | 0.012 (0.005) | 1.5 × 10−2 |
| 20 | 4157857 | rs1538072 | SMOX | G/C | 0.77 | −35.9 (12.7) | 4.6 × 10−3 | −52.1 (15.0) | 5.4 × 10−4 | 0.012 (0.006) | 3.4 × 10−2 |
| 3 | 77645020 | rs80275771 | ROBO2 | A/T | 0.16 | −38.8 (14.8) | 8.9 × 10−3 | −45.9 (16.9) | 6.7 × 10−3 | 0.014 (0.007) | 3.9 × 10−2 |
Variant position is based on human genome Build 37. Variant identification number (rs no.) is based on dbSNP version 141. Risk (R) allele is defined as the allele associated with lower 24-h EE, lower RMR, and higher maximum BMI. RAF was calculated in full-heritage Pima Indians. β-Coefficient (β) is expressed per copy of the risk allele in kcal/day (24-h EE and RMR) and loge (BMI) units. Results are adjusted for age, sex, FM and FFM (only for 24-h EE and RMR analyses), SPA (only for 24-h EE), birth year (only for BMI analysis), and the first five genetic principal components in a mixed model that accounted for genetic relationships among individuals. Variants are sorted by their P value for the association with maximum BMI. Chr., chromosome; NR, nonrisk.
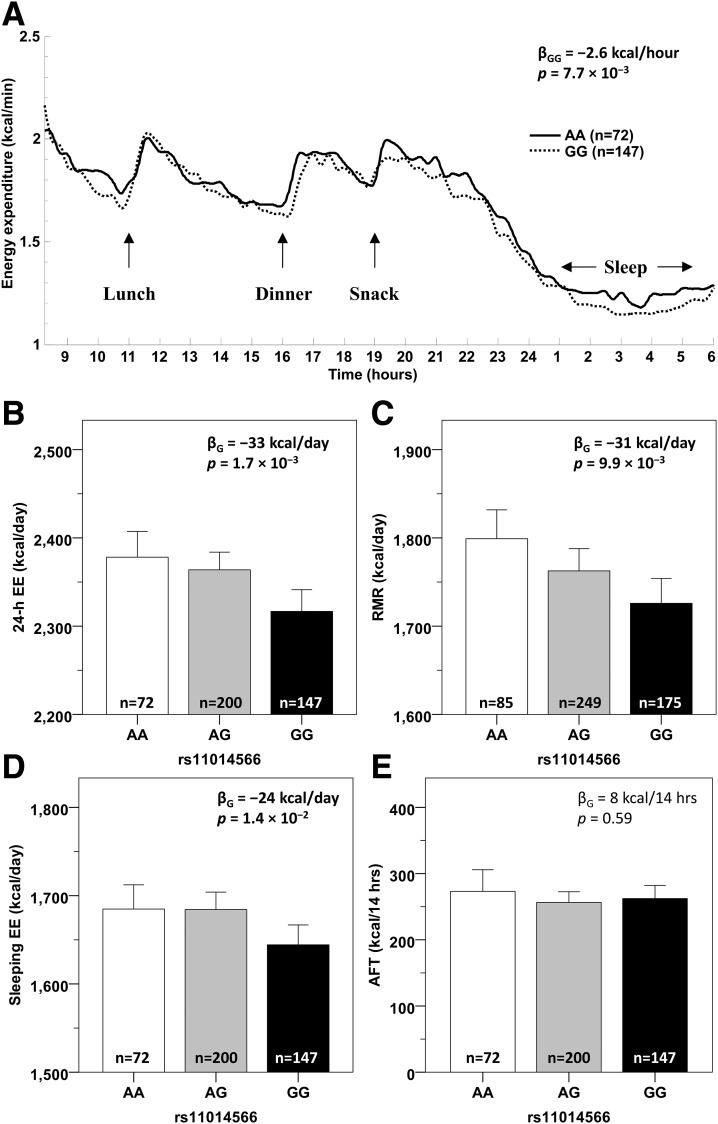
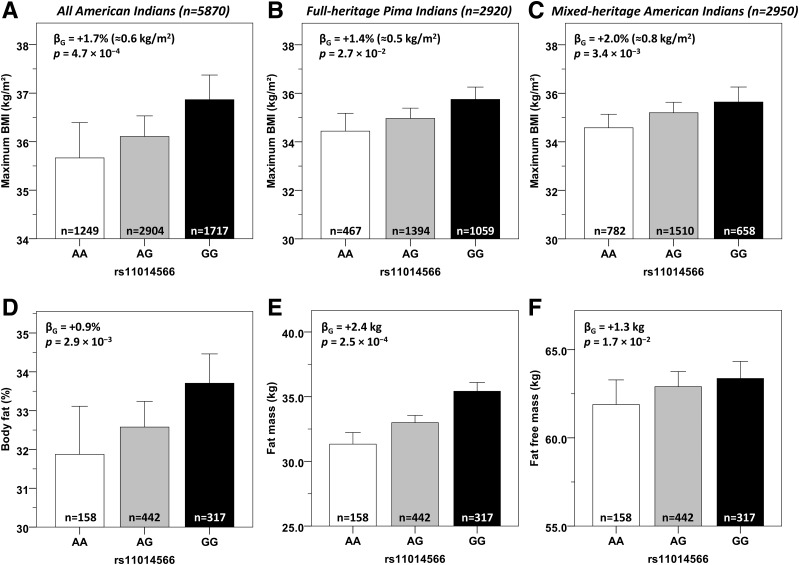
The G allele at rs11014566 (frequency in full-heritage Pima Indians = 0.60) associated with a lower 24-h EE (β = −33 kcal/day, 95% CI −54 to −12) (Fig. 3B) and a lower RMR (β = −31 kcal/day, 95% CI −55 to −7) (Fig. 3C). Compared with subjects homozygous for the A allele, subjects carrying two copies of the G allele had lower EE over the course of 24 h inside the metabolic chamber (∆ = −2.6 kcal/h, P = 7.7 × 10−3), and this was more evident in the sleeping state (Fig. 3A). Accordingly, single nucleotide polymorphism (SNP) rs11014566 was associated with sleeping EE (β = −24 kcal/day, P = 1.4 × 10−2) (Fig. 3D) but not with AFT (P = 0.59) (Fig. 3E) or SPA (P = 0.59). In the larger population-based sample of 5,870 Pima Indians, the G allele also associated with a higher maximum BMI (β = +1.7% ≈ 0.6 kg/m2 per copy, 95% CI 0.7 to 2.6, P = 4.7 × 10−4) (Fig. 4A), and this association was observed both in the 2,920 individuals who were full-heritage Pima Indians (β = +1.4%, P = 2.7 × 10−2) (Fig. 4B) and in the 2,950 individuals who were mixed-heritage American Indians (β = +2.0%, P = 3.4 × 10−3) (Fig. 4C) in this population. This variant also associated with PFAT in 917 subjects with body composition measures (β = +0.9%, 95% CI 0.3 to 1.5, P = 2.9 × 10−3) (Fig. 4D) with no difference between sexes (P = 0.39). Specifically, the G allele was associated with higher FM (β = +2.4 kg, P = 2.5 × 10−4, adjusted for age, sex, and height) (Fig. 4E) and, to a lesser extent, with higher FFM (β = +1.3 kg, P = 1.7 × 10−2) (Fig. 4F).
Figure 3.
EE measures by genotypes of SNP rs11014566 in GPR158. A: Average time courses of 24-h EE in the respiratory chamber for subjects carrying AA (solid line) and GG (dotted line) genotypes of rs11014566. Values of EE measured every 15 min are adjusted for age, sex, FM, FFM, and SPA and corrected after and accounting for repeated measures using an AR (1) covariance structure by mixed-model analysis. Mean 24-h EE inside the metabolic chamber (B), RMR assessed by ventilated hood system (C), sleeping EE (D), and AFT (E) by genotypes of rs11014566. All EE measures are adjusted for age, sex, FM, FFM, and the first five principal components in a mixed model that accounted for genetic relationships among individuals; 24-h EE and AFT are further adjusted for SPA and fasting glucose levels, respectively. β-Coefficients are expressed per copy of the G allele. Error bars represent mean with 95% CI.
Figure 4.
Anthropometric measures by genotypes of SNP rs11014566 in GPR158. Maximum BMI as derived from the longitudinal data from outpatient visits (entire population [A], full-heritage Pima Indians [B], and mixed-heritage American Indians [C]) and PFAT (D), FM (E), and FFM (F) in 917 subjects who had a body composition measure in inpatient visits by genotypes of rs11014566. BMI, PFAT, FM, and FFM values are adjusted for age, sex, and the first five principal components in a mixed model that accounted for genetic relationships among individuals. BMI is further adjusted for the subject’s birth year to account for secular changes in obesity prevalence during the course of the longitudinal study in the Pima Indian community. FM and FFM are further adjusted for the subject’s height. β-Coefficients are expressed per copy of the G allele and expressed as % (PFAT) or as kg (FM and FFM). For maximum BMI, β-coefficients are expressed per loge BMI units as percentage and, as an approximation, are converted to kg/m2 units by multiplication with the average population BMI (i.e., a β-coefficient of 0.017 is equivalent to 1.7% × 35.2 kg/m2 = 0.60 kg/m2). Error bars represent mean with 95% CI.
Analysis of Additional Variation at the GPR158 Locus
Analyses of WGS data from 266 full-heritage Pima Indians showed that rs11014566 tags (r2 > 0.85) three other variants: rs144895904 (C/T, frequency T = 0.61, r2 = 0.99), rs34673593 (−/AT, frequency AT = 0.57, r2 = 0.87), and rs16925884 (C/T, frequency T = 0.60, r2 = 0.91), all in intron 4 of GPR158. Analysis of 74 tagging variants with a MAF ≥ 0.05 across the GPR158 gene (50 kb flanking each side, chr10:25,414,290–25,941,157) determined that rs11014566 (and its three tags) had the strongest association with maximum BMI, and conditional analyses demonstrated no variant in this region associated with maximum BMI after conditioning on rs11014566 (all conditioned P > 0.05).
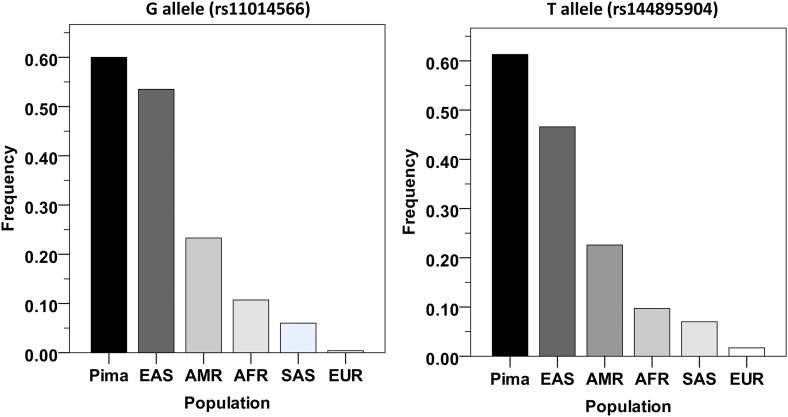
The BMI risk alleles for rs11014566 and its tags show large differences among our data and populations in the 1000 Genomes Project (1000G). For example, in our data the G allele at rs11014566 attains the highest frequency of 0.60 in full-heritage Pima Indians and 0.48 in mixed-heritage American Indians, whereas in the 1000G its frequency was 0.23 in Americans, 0.11 in Africans, and <0.01 in Europeans (Fig. 5).
Figure 5.
Frequencies of the G allele at rs11014566 and of the T allele at rs144895904 in American Indians and in the 1000G populations. Population allele frequencies are based on 1000G phase 3. Pima, full-heritage Pima Indians; EAS, East Asians; AMR, Americans; AFR, Africans; SAS, South Asians; EUR, Europeans.
Replication Analysis in SIGMA
As no other data sets exist with genotypic data on individuals with measures of EE, we sought to replicate our modest association with BMI in the SIGMA consortium. In the BMI meta-analysis of the SIGMA consortium including 4,364 Mexican individuals without diabetes (mean ± SD age 57.9 ± 8.4 years, BMI 27.5 ± 4.2 kg/m2, 1,755 males), the BMI risk alleles at both rs11014566 (β = −0.05 SD units per copy of the G allele, frequency = 0.27, P = 0.04) and rs144895904 (β = −0.05 SD units per copy of the T allele, frequency = 0.28, P = 0.01) were associated with lower BMI in this cohort. Similar results were obtained in sensitivity analyses including only subjects with a BMI greater than the median value of this cohort (27 kg/m2) or including only obese subjects with a BMI>30 kg/m2 (data not shown).
In Vitro Functional Analyses of GPR158 Variants
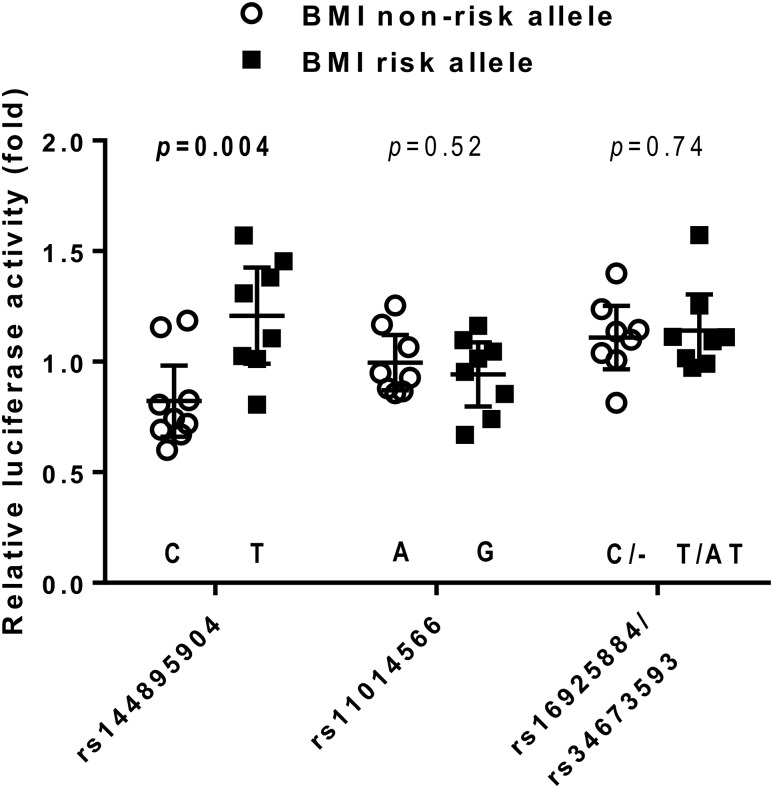
To determine whether rs11014566 in GPR158 and the three variants tagged by rs11014566 had a functional impact on promoter activity, these variants were analyzed in an in vitro luciferase reporter assay. DNA regions containing either the risk or the nonrisk allele for each variant were PCR amplified. Because of the proximity of rs16925884 (chr10:25,740,897) and rs34673593 (chr10:25,741,140), single PCR products containing either the risk alleles or the nonrisk alleles for both variants were amplified. The effect of the cloned GPR158 variants on promoter activity was assessed in a murine hypothalamus cell line, as GPR158 is endogenously expressed in the human hypothalamus at high levels (Supplementary Fig. 1). The largest difference in luciferase activity was observed when comparing constructs that differed for alleles at rs144895904 (Fig. 6), where the BMI risk allele T had on average 48% higher activity as compared with the nonrisk allele C (mean ± SEM, 1.21 ± 0.09 vs. 0.82 ± 0.07, P = 0.004). There was no significant difference in luciferase activity between alleles at rs11014566 (P = 0.52) or rs16925884/rs34673593 (P = 0.74).
Figure 6.
In vitro functional analyses of GPR158 variants in murine hypothalamus cells. Relative luciferase activity (fold change) was expressed as a ratio of firefly luciferase activity to Renilla luciferase activity and further normalized to pGL3 promoter luciferase activity. Raw data are presented along with mean and 95% CI. The statistical difference in the averaged activity was analyzed by unpaired Student t test.
Discussion
The current study was conducted in a geographically confined population of Pima Indians and, among these community members, we estimated that family membership accounts for approximately one-third of BMI variance among siblings from different families. Siblings share, on average, half of their genes; therefore, nearly approximately 60% of BMI variance in this Pima Indian population is genetically determined, as confirmed by our empirical heritability estimate (h2 = 0.55). This estimate is consistent with that reported in other ethnic groups (27) as well as prior studies in twins (1,2,28), which similarly estimated that 60% of the variability in BMI among individuals of a given population living in the same environment is genetically determined and potentially ascribable to the additive effects of genetic variants. We furthered showed in Pima Indians that 24-h EE, a determinant of BMI in this population, is also an inherited characteristic (h2 = 0.52). After adjustment for differences in body composition, family membership accounted for 34% of the variance in 24-h EE among siblings from different families, which is consistent with previous calculations done in much smaller cohorts of Pima Indians (3,18).
Given that BMI, body fat (29), and EE (3,18) are genetically determined and body weight and FM gain in Pima Indians is at least partially attributable to a relatively lower EE (3,4,23), we sought to identify genetic variants that influence body fatness and BMI in adulthood via a modest but life-long effect on EE. We performed a genome-wide association study (GWAS) for EE utilizing genotypic data from our custom Pima Indian–specific array. Although our sample of 419 Pima Indians with measures of 24-h EE and genotypes represents one of the largest existing samples, it was nonetheless underpowered to detect the modest effect sizes typically observed in GWAS at genome-wide statistical significance (P = 5 × 10−8). Therefore, rather than rely solely on statistical significance to discern true from false positives, we prioritized variants that showed physiologically supportive associations with reduced EE (assessed by separate measurements of 24-h EE and RMR) and increased body adiposity (assessed by independent measurements of BMI and body fatness). This strategy led us to identify common variation in the GPR158 gene that satisfied these criteria. Specifically, despite higher FFM (+2.6 kg), which would generally confer higher EE because of the well-documented positive association with FFM (10), Pima individuals carrying two copies of the G allele at rs11014566 in GPR158 had instead on average roughly a 70-kcal deficit in daily EE (of which 48 kcals were ascribable solely to sleeping EE) and approximately 5 kg more FM and a BMI increase of 1.2 kg/m2 as compared with subjects homozygous for the A allele. The effect of rs11014566 on BMI in Pima Indians (1.2 kg/m2 difference between individuals homozygous for the risk vs. nonrisk allele, RAF = 0.60) is comparable to the effects exerted by other variants near well-established obesity genes including rs8050136 in FTO (1.6 kg/m2, RAF ≈ 0.15) (30), rs74861148 near MC4R (1.36 kg/m2, RAF ≈ 0.15) (31), and rs2025804 in LEPR (1.0–1.9 kg/m2, RAF ≈ 0.70) (32) in this same population. Similarly, the effect of rs11014566 in GPR158 on 24-h EE (−33 kcal/day per allele copy) is comparable to that of rs11208654 in LEPR (−28 kcal/day) (Supplementary Table 1), which tags rs2025804 previously shown to affect 24-h EE in Pima Indians (32).
Given our low statistical power due to the modest sample size, our GWAS results for EE must be interpreted with caution. Nevertheless, the high heritability of 24-h EE in the Pima Indian population increases the likelihood that variants exerting true effects on EE could be uncovered using a GWAS strategy. To identify true from false positives among variants that did not achieve genome-wide significance, we considered variants that showed directional consistency for their associations with two independent assessments of EE, including precise and reproducible measures obtained at rest while fasting (18) and over 24 h during energy balance (10), assuming that true genetic associations with EE will display weak but consistent results in both settings.
Although the strength of our study is that it provides the first genome-wide screen for genetic variants that affect EE, it also has a major weakness in that there are no other genetic databases available for EE to directly assess replication. Because EE has a modest but measurable effect on weight gain in Pima Indians (4) and in Pima Indians variants in GPR158 nominally associated with adiposity, we sought replication for the association of GPR158 with BMI, as a surrogate of EE, in other ethnic groups. The G allele at rs11014566, which predicts lower EE and higher FM and BMI, has a frequency of 0.60 in full-heritage Pima Indians, which is higher than the frequency for this allele observed among any of the 1000G populations. Notably, this allele is uncommon in Europeans (MAF < 0.01), and thus its assessment for association with BMI in the GIANT (Genetic Investigation of ANthropometric Traits) consortium (33) is not optimal. Therefore, we assessed association with BMI in data sets collected from Asians (34), Africans (35) and Hispanics (26). rs11014566 did not associate with BMI in Asians or Africans (K.E. North, M.C.Y. Ng, M. Graff, and X.O. Shu, personal communication); however, modest associations with BMI were observed in the SIGMA meta-analysis of BMI whose Hispanic population more closely resembles the Pima Indians from an environmental perspective, although from a genetic perspective the frequency of the G (rs11014566) and T (rs144895904) alleles are 0.27 and 0.28, respectively, in Hispanics as compared with 0.60 in Pima Indians. However, in the SIGMA sample, the direction of the association with BMI was opposite to that observed in the Pima Indians (β = −0.05 SD units per copy of the G allele at rs11014566, P = 0.04) and (β = −0.05 SD units per copy of the T allele at rs144895904, P = 0.01). Given that a relationship between EE and BMI has not been shown in the SIGMA sample and that metabolic studies in other ethnic groups have reported no relationship (6–8) and even a positive relationship between EE and future weight gain (9) (as opposed to the inverse association observed in American Indians [3–5]), it is unclear whether an association with BMI in the opposite direction indicates that this SNP has no role in EE (i.e., our result is a false positive) or whether it indicates the complexity of feedback loops between EE and food consumption (36–38), where an imbalance predicts either weight gain or weight loss, among individuals with different body habitus and living in different environments. As additional data for genotype and EE become available in other populations, meta-analyses may be helpful to assess the extent to which our findings transfer to other populations and to boost power to detect additional variants.
The GWAS lead SNP rs11014566 maps to an intron of the GPR158 gene that encodes the G-protein–coupled receptor 158, a transmembrane protein highly expressed in brain cells. Our tissue expression profiling confirmed that GPR158 is highly expressed in the brain. In vitro functional analysis of rs11014566 and three variants tagged by rs11014566 (rs144895904, rs16925884, and rs34673593) in murine hypothalamic cells showed that intronic SNP rs144895904 had a statistically significant effect on promoter activity. Human GPR158 is involved in neurotransmitter signaling and regulation of neuronal excitability (39,40). Although there is no direct evidence that human GPR158 is involved in the pathophysiology of obesity, a previous study of mouse Gpr158 has demonstrated its role in the regulation of energy balance (41). GPR158 binds to the regulator of G-protein signaling 7 (RGS7) in the nervous system (42,43). RGS7-deficient mice are protected from obesity (44), and previous studies have provided evidence that RGS7 may constitute an obesity locus in humans (33,45). In addition to RGS7, GPR158 also binds to an N-type voltage-gated calcium channel (CACNA1B) in the rat brain (34). Homozygous CACNA1B-deficient mice gain less weight during 8 weeks of high-fat diet despite similar food intake of wild-type mice (35), implying a compensatory increase in EE that may mitigate weight gain during high-fat feeding. Because GPR158, RGS7, and CACNA1B are all expressed in the central nervous system, as are the well-established human obesity genes FTO, MC4R, and TMEM18 (46), one could speculate that they too exert an effect on hypothalamic signaling to regulate energy balance. However, additional mechanistic studies are needed to clarify the physiological pathway whereby GPR158 may affect EE and obesity in humans.
In conclusion, analysis of genotypes from a custom Pima Indian array identified a novel genetic locus in GPR158 affecting EE and predisposing Pima Indians to weight gain. The frequency of the risk allele is higher in Pima Indians as compared with other populations studied as part of the 1000G, and the risk allele demonstrated increased promoter activity in in vitro experiments. Results of this study support the hypothesis that Pima Indians may carry some genetic variants affecting EE that are enriched in this particular ethnic group; however, the effect of these variants on higher rates of weight gain remains controversial because an association with BMI was only observed in Pima Indians. We propose that studies of GPR158, as well as other EE-associated genes that will be identified in the future, may shed light into the pathophysiological mechanisms that affect EE, which could eventually lead to prevention and/or possible treatments of human obesity.
Supplementary Material
Article Information
Acknowledgments. The authors would like to thank Kari North (Department of Epidemiology, University of North Carolina at Chapel Hill), Maggie Ng (Center for Diabetes Research, Wake Forest School of Medicine), and Misa Graff (Department of Epidemiology, University of North Carolina at Chapel Hill) for providing look-up data in their African American cohort and Xiao Ou Shu (Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center and Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine) for providing look-up data in an East Asian cohort. The authors would also like to thank the nursing, clinical, and dietary staff and laboratory technicians of the Phoenix Epidemiology and Clinical Research Branch for conducting this study.
Funding. This research was supported by the Intramural Research Program of the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. This study utilized the computational resources of the Biowulf system at the National Institutes of Health, Bethesda, MD.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.P. designed the study, analyzed and interpreted data, and wrote the manuscript. I.M. and Y.L.M. performed the in vitro experiments and wrote the related procedures in the manuscript. J.M. performed statistical analyses for replication in the SIGMA Type 2 Diabetes Consortium. G.B.W. and M.M. performed the in vitro experiments. P.C., S.K., W.-C.H., and R.L.H. analyzed data and provided statistical advice. W.C.K., J.K., R.L.H., and C.B. obtained the clinical and physiological data, reviewed the manuscript, and assisted with the interpretation of the data. L.J.B. designed the study, assisted with the interpretation of the data, and edited and reviewed the manuscript. All authors critically revised the draft and approved the final manuscript. P.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Society of Human Genetics 2015 Annual Meeting, Baltimore, MD, 6–10 October 2015.
Footnotes
Clinical trial reg. no. NCT00340132, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1565/-/DC1.
Members of the SIGMA Type 2 Diabetes Consortium are provided in the Supplementary Data online.
References
- 1.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med 1990;322:1483–1487 [DOI] [PubMed] [Google Scholar]
- 2.Price RA, Gottesman II. Body fat in identical twins reared apart: roles for genes and environment. Behav Genet 1991;21:1–7 [DOI] [PubMed] [Google Scholar]
- 3.Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med 1988;318:467–472 [DOI] [PubMed] [Google Scholar]
- 4.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab 2013;98:E703–E707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tataranni PA, Harper IT, Snitker S, et al. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. Int J Obes Relat Metab Disord 2003;27:1578–1583 [DOI] [PubMed]
- 6.Anthanont P, Jensen MD. Does basal metabolic rate predict weight gain? Am J Clin Nutr 2016;104:959–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord 1992;16:667–674 [PubMed]
- 8.Weinsier RL, Nelson KM, Hensrud DD, Darnell BE, Hunter GR, Schutz Y. Metabolic predictors of obesity. Contribution of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain of post-obese and never-obese women. J Clin Invest 1995;95:980–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luke A, Durazo-Arvizu R, Cao G, Adeyemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. Am J Clin Nutr 2006;83:1076–1081 [DOI] [PubMed] [Google Scholar]
- 10.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 1986;78:1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchard C, Tremblay A, Nadeau A, et al. Genetic effect in resting and exercise metabolic rates. Metabolism 1989;38:364–370 [DOI] [PubMed] [Google Scholar]
- 12.Fontaine E, Savard R, Tremblay A, Després JP, Poehlman E, Bouchard C. Resting metabolic rate in monozygotic and dizygotic twins. Acta Genet Med Gemellol (Roma) 1985;34:41–47 [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Pettitt DJ, Saad MF, et al. Obesity in the Pima Indians: its magnitude and relationship with diabetes. Am J Clin Nutr 1991;53(Suppl.):1543S–1551S [DOI] [PubMed] [Google Scholar]
- 14.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed]
- 15.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr 2007;86:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penesova A, Venti CA, Bunt JC, Bonfiglio SM, Votruba SB, Krakoff J. Short-term isocaloric manipulation of carbohydrate intake: effect on subsequent ad libitum energy intake. Eur J Nutr 2011;50:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr 1995;62:730–734 [DOI] [PubMed] [Google Scholar]
- 18.Bogardus C, Lillioja S, Ravussin E, et al. Familial dependence of the resting metabolic rate. N Engl J Med 1986;315:96–100 [DOI] [PubMed] [Google Scholar]
- 19.Lusk G. Animal calorimetry: analysis of oxidation of mixtures of carbohydrates and fat. J Biol Chem 1924;59:41–42 [Google Scholar]
- 20.Abbott WG, Howard BV, Christin L, et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol 1988;255:E332–E337 [DOI] [PubMed] [Google Scholar]
- 21.Schutz Y, Ravussin E, Diethelm R, Jequier E. Spontaneous physical activity measured by radar in obese and control subject studied in a respiration chamber. Int J Obes 1982;6:23–28 [PubMed] [Google Scholar]
- 22.Schutz Y, Bessard T, Jéquier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr 1984;40:542–552 [DOI] [PubMed] [Google Scholar]
- 23.Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes 2013;62:4043–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Browning BL, Browning SR. A fast, powerful method for detecting identity by descent. Am J Hum Genet 2011;88:173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 1998;62:1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams AL, Jacobs SB, Moreno-Macías H, et al.; SIGMA Type 2 Diabetes Consortium . Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature 2014;506:97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allison DB, Kaprio J, Korkeila M, Koskenvuo M, Neale MC, Hayakawa K. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int J Obes Relat Metab Disord 1996;20:501–506 [PubMed]
- 28.Haworth CM, Plomin R, Carnell S, Wardle J. Childhood obesity: genetic and environmental overlap with normal-range BMI. Obesity (Silver Spring) 2008;16:1585–1590 [DOI] [PubMed] [Google Scholar]
- 29.Sakul H, Pratley R, Cardon L, Ravussin E, Mott D, Bogardus C. Familiality of physical and metabolic characteristics that predict the development of non-insulin-dependent diabetes mellitus in Pima Indians. Am J Hum Genet 1997;60:651–656 [PMC free article] [PubMed] [Google Scholar]
- 30.Rong R, Hanson RL, Ortiz D, et al. Association analysis of variation in/near FTO, CDKAL1, SLC30A8, HHEX, EXT2, IGF2BP2, LOC387761, and CDKN2B with type 2 diabetes and related quantitative traits in Pima Indians. Diabetes 2009;58:478–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller YL, Thearle MS, Piaggi P, et al. Common genetic variation in and near the melanocortin 4 receptor gene (MC4R) is associated with body mass index in American Indian adults and children. Hum Genet 2014;133:1431–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traurig MT, Perez JM, Ma L, et al. Variants in the LEPR gene are nominally associated with higher BMI and lower 24-h energy expenditure in Pima Indians. Obesity (Silver Spring) 2012;20:2426–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aissani B, Wiener HW, Zhang K. Fine mapping of the body fat QTL on human chromosome 1q43. PLoS One 2016;11:e0153794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller CS, Haupt A, Bildl W, et al. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci USA 2010;107:14950–14957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi E, Ito M, Miyamoto N, Nagasu T, Ino M, Tanaka I. Increased glucose tolerance in N-type Ca2+ channel alpha(1B)-subunit gene-deficient mice. Int J Mol Med 2005;15:937–944 [PubMed] [Google Scholar]
- 36.Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher daily energy expenditure and respiratory quotient, rather than fat-free mass, independently determine greater ad libitum overeating. J Clin Endocrinol Metab 2015;100:3011–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blundell JE, Caudwell P, Gibbons C, et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Dis Model Mech 2012;5:608–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dulloo AG, Jacquet J, Miles-Chan JL, Schutz Y. Passive and active roles of fat-free mass in the control of energy intake and body composition regulation. Eur J Clin Nutr 2017;71:353–357 [DOI] [PubMed] [Google Scholar]
- 39.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev 2005;85:1159–1204 [DOI] [PubMed] [Google Scholar]
- 40.Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell 1995;80:249–257 [DOI] [PubMed] [Google Scholar]
- 41.Wagner F, Bernard R, Derst C, French L, Veh RW. Microarray analysis of transcripts with elevated expressions in the rat medial or lateral habenula suggest fast GABAergic excitation in the medial habenula and habenular involvement in the regulation of feeding and energy balance. Brain Struct Funct 2016;221:4663–4689 [DOI] [PubMed] [Google Scholar]
- 42.Orlandi C, Xie K, Masuho I, Fajardo-Serrano A, Lujan R, Martemyanov KA. Orphan receptor GPR158 is an allosteric modulator of RGS7 catalytic activity with an essential role in dictating its expression and localization in the brain. J Biol Chem 2015;290:13622–13639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orlandi C, Posokhova E, Masuho I, et al. GPR158/179 regulate G protein signaling by controlling localization and activity of the RGS7 complexes. J Cell Biol 2012;197:711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shim H, Wang CT, Chen YL, et al. Defective retinal depolarizing bipolar cells in regulators of G protein signaling (RGS) 7 and 11 double null mice. J Biol Chem 2012;287:14873–14879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aissani B, Perusse L, Lapointe G, et al. A quantitative trait locus for body fat on chromosome 1q43 in French Canadians: linkage and association studies. Obesity (Silver Spring) 2006;14:1605–1615 [DOI] [PubMed] [Google Scholar]
- 46.Locke AE, Kahali B, Berndt SI, et al.; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium . Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.